Abstract
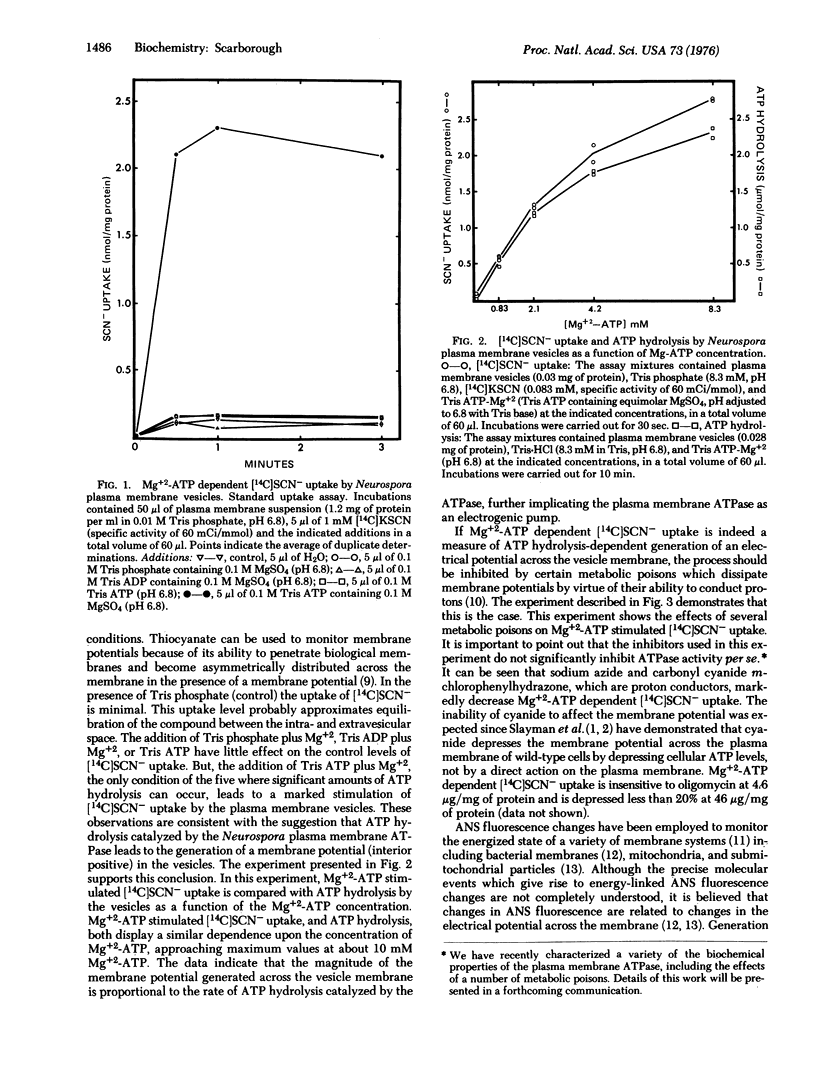
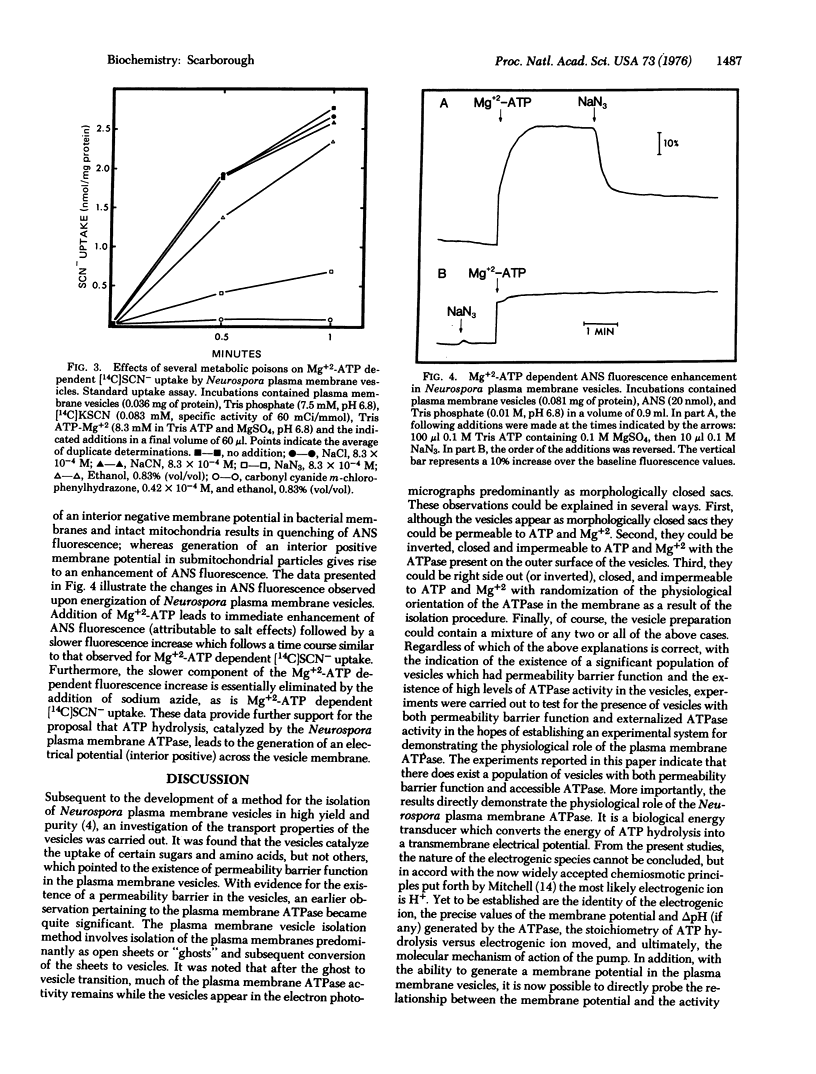
Biochemicalical evidence is presented which demonstrates that the Neurospora crassa plasma membrane ATPase (ATP phosphohydrolase, EC 3.6.1.3) is an electrogenic pump. The electrical potential across the Neurospora plasma membrane, as monitored by [14C]SCN- uptake by isolated Neurospora plasma membrane vesicles, is markedly increased interior positive under conditions of ATP hydrolysis catalyzed by plasma membrane ATPase. [14C]SCN- uptake by the vesicles is minimal in the presence of Tris phosphate, Tris phosphate plus Mg+2, Tris ADP plus Mg+2, and Tris ATP, but is markedly stimulated in the presence of Tris ATP plus Mg+2.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brand L., Gohlke J. R. Fluorescence probes for structure. Annu Rev Biochem. 1972;41:843–868. doi: 10.1146/annurev.bi.41.070172.004211. [DOI] [PubMed] [Google Scholar]
- Harold F. M. Conservation and transformation of energy by bacterial membranes. Bacteriol Rev. 1972 Jun;36(2):172–230. doi: 10.1128/br.36.2.172-230.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mitchell P. Chemiosmotic coupling in oxidative and photosynthetic phosphorylation. Biol Rev Camb Philos Soc. 1966 Aug;41(3):445–502. doi: 10.1111/j.1469-185x.1966.tb01501.x. [DOI] [PubMed] [Google Scholar]
- Reeves J. P., Lombardi F. J., Kaback H. R. Mechanisms of active transport in isolated bacterial membrane vesicles. VII. Fluorescence of 1-anilino-8-naphthalenesulfonate during D-lactate oxidation by membrane vesicles from Escherichia coli. J Biol Chem. 1972 Oct 10;247(19):6204–6211. [PubMed] [Google Scholar]
- Scarborough G. A. Isolation and characterization of Neurospora crassa plasma membranes. J Biol Chem. 1975 Feb 10;250(3):1106–1111. [PubMed] [Google Scholar]
- Scarborough G. A. Sugar transport in Neurospora crassa. II. A second glucose transport system. J Biol Chem. 1970 Aug 10;245(15):3985–3987. [PubMed] [Google Scholar]
- Scarborough G. A. Sugar transport in Neurospora crassa. J Biol Chem. 1970 Apr 10;245(7):1694–1698. [PubMed] [Google Scholar]
- Schuldiner S., Padan E., Rottenberg H., Gromet-Elhanan Z., Avron M. Delta pH and membrane potential in bacterial chromatophores. FEBS Lett. 1974 Dec 15;49(2):174–177. doi: 10.1016/0014-5793(74)80505-3. [DOI] [PubMed] [Google Scholar]
- Slayman C. L., Gradmann D. Electrogenic proton transport in the plasma membrane of Neurospora. Biophys J. 1975 Sep;15(9):968–971. doi: 10.1016/S0006-3495(75)85877-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slayman C. L., Long W. S., Lu C. Y. The relationship between ATP and an electrogenic pump in the plasma membrane of Neurospora crassa. J Membr Biol. 1973;14(4):305–338. doi: 10.1007/BF01868083. [DOI] [PubMed] [Google Scholar]
- Slayman C. L., Lu C. Y., Shane L. Correlated changes in membrane potential and ATP concentrations in Neurospora. Nature. 1970 Apr 18;226(5242):274–276. doi: 10.1038/226274a0. [DOI] [PubMed] [Google Scholar]
- Stanton M. G. Colorimetric determination of inorganic phosphate in the presence of biological material and adenosine triphosphate. Anal Biochem. 1968 Jan;22(1):27–34. doi: 10.1016/0003-2697(68)90255-8. [DOI] [PubMed] [Google Scholar]


