Abstract
Purpose of review
Blood platelets are involved in primary and, secondary hemostasis, and thus maintain the integrity of the vasculature. They circulate with an average lifespan of five to nine days in humans. Thus, the body must generate and clear platelets daily to maintain normal physiological blood platelet counts. Known platelet clearance mechanisms include antibody-mediated clearance by spleen macrophages, as in Immune Thrombocytopenia (ITP), and platelet consumption due to massive blood loss.
Recent findings
New concepts in the clearance mechanisms of platelets have recently emerged. New evidence shows that platelets desialyted due to chilling or sepsis are cleared in the liver by macrophages, i.e. Kupffer cells, as well as hepatocytes through lectin-mediated recognition of platelet glycans. On the other hand, platelet-associated antibodies normalize the clearance of platelets in a mouse model for Wiskott-Aldrich Syndrome (WAS).
Summary
The goal of this review is to summarize the latest findings in platelet clearance mechanisms with a focus on lectin-mediated recognition of platelet glycans. Transfusion medicine and treatments of hematopoietic disorders associated with severe thrombocytopenia may benefit from a better understanding of these mechanisms.
Keywords: Immune Thrombocytopenia (ITP), lectins, platelet clearance, glycans, Wiskott-Aldrich Syndrome (WAS)
Introduction
The crucial role of platelets in hemostasis is evident in patients with thrombocytopenia accompanied by serious bleeding complications. Thrombocytopenia is a serious side effect of myelosuppressive/myeloablative chemoradiotherapy and the hematopoietic disorder Immune Thrombocytopenia (ITP). The development of platelet transfusion was therefore a milestone in cancer treatment.
Although platelet transfusion has been improved over the years, a major complication remains their storage at room temperature, which leads to bacterial growth and deterioration of platelet function. To be clinically effective, transfused platelets must circulate and function i.e. prevent or stop bleeding. Currently, the gold standard test to evaluate transfused platelet products is in vivo circulation and count increment of transfused radiolabeled platelets [1]. It is assumed that if a platelet product circulates normally, it should function appropriately. However, both parameters fail to assess the functional quality of transfused platelets.
Our understanding of factors that dictate platelet survival remains poor, as the discovery of novel and unexpected platelet clearance mechanisms shows. This review will focus on new lectin-carbohydrate mediated platelet clearance mechanisms.
The classical pathway: antibody-mediated platelet clearance
Until recently the only well established platelet clearance mechanisms were antibody-mediated clearance and platelet consumption due to massive blood loss. In ITP, an autoantibody (usually of the IgG class) binds to circulating platelets with specificity for membrane glycoproteins [2–4]. In children, most cases of ITP are acute, manifesting a few weeks after a viral illness [5, 6]. In adults, most cases of ITP are chronic, manifesting with an insidious onset [7]. These clinical presentations suggest different triggering events. In persons with chronic ITP, the majority of autoantibodies are directed against the integrin αIIbβ3 (GPIIb-IIIa) or the Von Willebrand Factor (VWF) receptor GPIbα-IX-V [2, 3, 8].
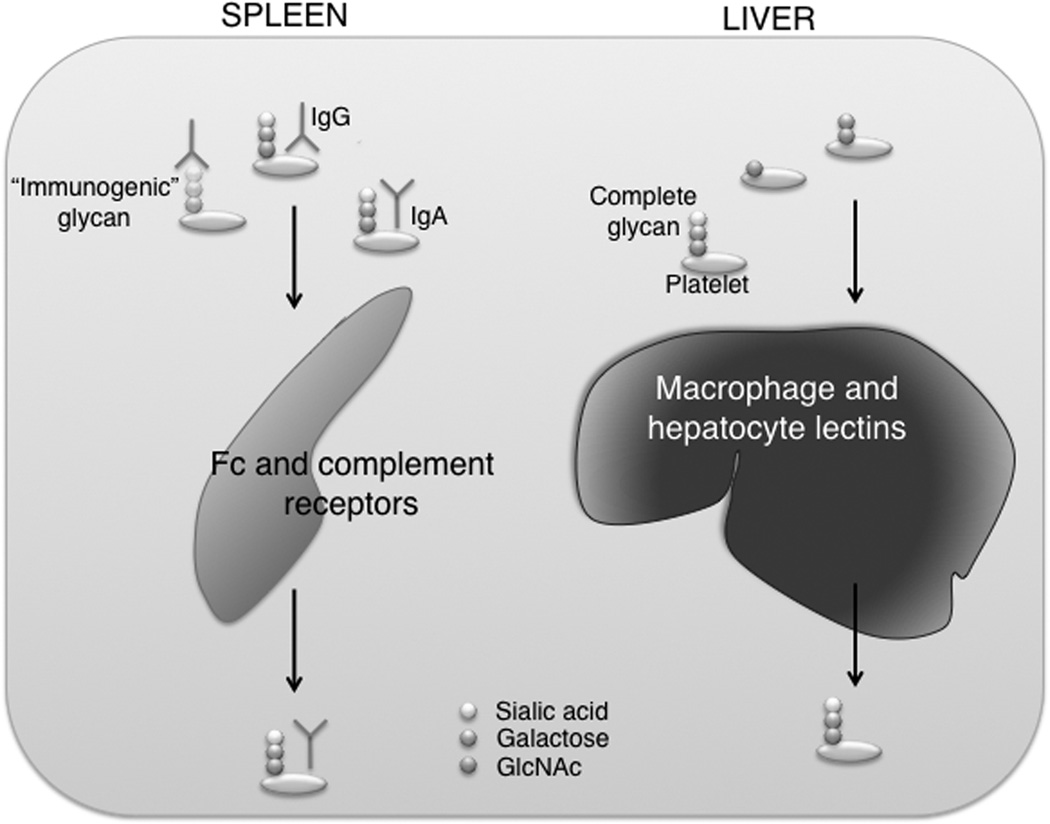
The coating of platelets with IgGs renders them susceptible to opsonization and Fc receptor-mediated phagocytosis by mononuclear macrophages, primarily but not exclusively in the spleen [9]. It is assumed that platelet autoantibodies are formed in the white pulp of the spleen and mononuclear macrophages in the red pulp destroy IgG-coated platelets [10]. The slow passage of platelets through splenic sinusoids with a high local concentration of antibodies and low-affinity macrophage Fc (FcγRIA, IIA, and IIIA) or complement (CR1 and CR3) receptors promotes platelet phagocytosis and destruction [FIG]. The best evidence that the spleen plays an important role in the removal of autoantibody-coated platelets comes from ITP patients who have undergone splenectomy, a procedure which results in restoration of normal platelet counts in most, but not all cases [11].
Fig. 1. Platelet clearance pathways.
Legend: Platelets bearing incomplete glycans are recognized by either liver macrophages or hepatocyte lectins, which leads to their clearance. IgG-coated platelets are phagocytized by both the Fc and complement receptors in the spleen. IgGs may recognize both amino acid and glycan residues. On the other hand, the binding of immune complexes, e.g. IgAs, to platelets prevents their rapid clearance.
The IgG autoantibodies are also thought to damage megakaryocytes, the platelet precursors. However, this mechanism may only slightly contribute to the decrease in platelet counts in ITP [12]. The stimulus for autoantibody production in ITP is probably due to abnormal T cell activity [13]. The exact mechanisms of antibody mediated platelet clearance remain unclear.
The novel pathway: lectin-carbohydrate mediated platelet clearance
For decades, all platelet products have been stored at room temperature, limiting platelet storage to five days because of the risk of bacterial growth and loss of platelet functionality [14]. Platelet refrigeration remains impossible because once chilled platelets are rapidly removed from the circulation. This odd clearance phenomenon has had profound consequences for blood banking. We have been investigating this clinically relevant problem of why refrigerated platelets fail to circulate for almost a decade and defined two previously unsuspected, carbohydrate-dependent platelet clearance mechanisms (for review see [15]).
We disproved the notion that chilled platelets are cleared because they undergo an extensive shape change when exposed to low temperatures and become trapped in the vasculature [16]. Cooling of platelets induces progressive clustering of glycan-bearing receptors, which causes lectins on macrophages and, unexpectedly, on hepatocytes to recognize chilled platelets.
The macrophage αMβ2 integrin
The macrophage αM-lectin recognizes clustered GPIbα subunits of the VWF receptor complex following hours of cooling, which results in the phagocytosis and clearance of platelets by liver macrophages, i.e. Kupffer cells, in vivo in mice and in vitro by human THP-1 macrophages. Subsequent work narrowed recognition of chilled platelet by αMβ2 receptors to GPIbα glycans with exposed β-N-acetylglucosamine (βGlcNAc) residues [17]. The finding of exposed incomplete glycans on surfaces of circulating platelets is surprising, as sialic acid generally caps glycans. Removal of sialic acid (desialylation) exposes galactose and de-galactosylation reveals βGlcNAc. Although resting platelets contain some glycans with exposed βGlcNAc residues, refrigerated platelets have markedly increased βGlcNAc exposure. This altered epitope presentation, possibly induced by cooling and clustering, of exposed βGlcNAc on GPIbα facilitates lectin recognition and removal of refrigerated platelets [16, 17]. The mechanism of βGlcNAc exposure on circulating platelets remains unclear. However, mice deficient for αM-lectin have slightly higher platelet counts [16] which suggests that 1) the platelets lose sialic acid and galactose residues while circulating, and 2) the αM-lectin removes platelets with incomplete glycans from the circulation, thereby controlling platelet survival in vivo.
We attempted to rescue the clearance of chilled platelets by covering exposed βGlcNAc residues with galactose. Indeed, survival of platelets chilled for hours was markedly increased following galactosylation by functional galactosyltransferase(s) expressed by platelets [17]. Galactosylation therefore provided a simple approach to improve refrigerated platelet circulation. However, while depriving the αMβ2 lectin-domain of its βGlcNAc ligand on refrigerated platelets, galactosylation theoretically provides a new ligand for asialoglycoprotein receptors. Hence, it was surprising that refrigerated mouse platelet circulation could be improved by galactosylation. However, the small amounts of added galactose does not engage galactose lectin expressed on phagocytes.
Subsequently, a phase I clinical trial administering autologous, radiolabeled galactosylated apheresis platelets refrigerated for two days to human volunteers clearly showed that the galactosylation procedure did not extend platelet survival [18]. Similar data were found for mice. Evidently, different mechanisms are involved in the clearance of platelets refrigerated for hours or days.
The Ashwell-Morell receptor
We found that platelets refrigerated for days (designated below as long-term refrigerated platelets) are also removed from the recipients’ circulation by the liver [19, 20]. Unexpectedly, hepatocytes, not Kupffer cells, ingested long-term refrigerated platelets [20]. How do hepatocytes recognize and ingest platelets? Masking of βGal terminating oligosaccharide chains by sialic acid prevents recognition by galactose recognizing lectin receptors, lectins abundantly expressed on macrophages and hepatocytes. The hepatic lectin (Ashwell-Morell receptor) mediates the binding and removal of glycoconjugates with exposed βgalactose (βGal) and GalNAc.
Long-term refrigerated platelets have increased βGal exposure, which provides a ligand for hepatic lectin receptors. Indeed, long-term refrigerated platelet recovery and survival are significantly improved in mice lacking hepatic lectin receptors [20]. Not surprisingly, long-term refrigerated and galactosylated platelets are cleared slightly faster independent of macrophage depletion [20]. In long-term refrigerated platelets, the mechanism of βGal exposure remains undetermined. However, it appears that most glycans with exposed βGal are associated with GPIbα. Does the βGal bearing GPIbα presentation influence platelet clearance? We observed increased clustering of GPIbα with prolonged refrigeration [20]. Hence, clustering of GPIbα glycans on the surface of chilled platelets might amplify the galactose signal, thus enhancing Ashwell-Morell receptor avidity and binding, as reported earlier for βGlcNAc [17]. However, the functional relationship between GPIbα clustering and platelet clearance by hepatic lectin remains unclear.
Platelet sialic acid
These results point to the critical role of glycans, specifically on GPIbα, in platelet clearance. Experiments using mice lacking α2-3-sialyltransferase (ST3GalIV) activity further support this notion. ST3GalIV-null platelets have exposed βGal on their surface [21–23] and are also cleared by Ashwell-Morell receptors [20]. GPIbα on ST3GalIV-null platelets is the major counter receptor for galactose-recognizing lectins. Taken together, our studies, as well as a recent report by Grewal et al, point out the importance of a hepatic-based platelet removal system that uses Ashwell-Morell receptors to remove platelets expressing desialylated glycans on their surface [20, 21].
Transfused fresh mouse wild type platelets circulate longer in mice deficient for Ashwell-Morell receptors [20], which suggests that hepatocytes also remove senile, desialylated platelets. As platelets lose sialic acid from membrane glycoproteins during aging and circulation, enhanced exposure of non-sialylated glycan chains may represent a physiological phenomenon which triggers clearance of senescent blood cells [24]. Further supporting this notion, mice lacking hepatic lectin receptors have higher platelet counts and their platelets are deficient for sialic acid (unpublished data). Similar to erythrocytes, in vitro desialylated platelets are cleared rapidly from the circulation [25, 26].
Are glycoproteins other than GPIbα involved in the clearance of chilled platelets? VWF binding increases during platelet storage [20]. It appears that preferentially desialylated VWF binds to long-term refrigerated platelets, indicating that desialylation of VWF molecules may promote binding to GPIbα. In support of this idea, we found that binding of deficiently sialylated VWF, derived from ST3Gal-IV-null plasma, to WT platelets is enhanced both in vitro and in vivo [23]. Proteolytic removal of the GPIbα N-terminal region deprives GPIbα of its VWF-binding domain and bound VWF. Whether VWF glycans contribute to recognition of platelets by Ashwell-Morell receptor is unclear.
The role of macrophages
Macrophages do not phagocytize long-term refrigerated desialylated platelets. However, our studies revealed that macrophages rapidly (hours) remove ~40% of transfused fresh room temperature platelets, implying that macrophages detect “damage” inflicted during platelet isolation, a loss which is consistently observed following transfusion of fresh human platelets into healthy volunteers [18]. It is possible that macrophages recognize damaging events acutely inflicted to platelets, including antibody binding. On the other hand, hepatocytes could control the removal of senile platelets and daily platelet turnover. In support of this hypothesis, we showed that transfused fresh platelets circulate normally in macrophage-depleted mice [20]. It is tempting to speculate that platelet membrane or associated glycoproteins, e.g. antibodies or other plasma proteins, mediate clearance through both Fc-receptors and lectins on macrophages. On the other hand, bound glycoproteins could shield platelets from recognition through phagocytic receptors.
Wiskott-Aldrich Syndrome
Wiskott-Aldrich Syndrome (WAS) is a recessive hematopietic disorder characterized by immunodeficiency, eczema and severe microthrombocytopenia [27]. X-linked thrombocytopenia (XLT) is a milder phenotype [28]. The WASP gene implicated in WAS and XLT encodes a protein of 502 amino acids and 64 kDa, called WAS protein (WASp), a key regulator of actin assembly in hematopoietic cells. The mechanisms of WAS-associated thrombocytopenia are poorly understood, although increased platelet destruction by the spleen is believed to play a major role. Platelets isolated from WAS patients or WASp-null mice function normally [29, 30]. Furthermore, megakaryocytes isolated from WAS patients form proplatelets normally and produce platelets of normal size in vitro [31]. Premature proplatelet formation and platelet production are observed in the bone marrow compartment of WASp-null mice [32]. On the other hand, WAS platelets are cleared rapidly from circulation when transfused autologously [33, 34]. Similarly, mice lacking WASp or the WASp-interacting protein (WIP) have moderate thrombocytopenia due in part to increased platelet clearance [35, 36]. WIP and WASp form a tight complex in platelets. WIP-null platelets lack WASp and thus are double deficient [36]. WIP-null mice have a phenotype closer to that of WAS than WASp-null mice [37].
Studies suggest an antibody-mediated platelet clearance in WAS, similar to that of ITP. Platelet-associated antibodies are often observed in WAS patients, a phenotype which improves after splenectomy, as do low platelet count and small platelet size [38, 39]. However, most WIP-null, but not WASp-null, mice evolve platelet-associated antibodies of the IgA class, which diminish their biological functions initiated by the collagen receptor GPVI but normalize their platelet survival [36]. Thus, platelet-associated antibodies protect against rather than mediate the increased clearance of WIP-null platelets. The reason for IgA binding to WIP-null platelets is unclear. WIP-null mice evolve IgA-mediated glomerular nephropathy [37], as do WAS and XLT patients [40, 41], and may have circulating IgA complexes that bind to platelets. We speculate that IgAs may directly block binding sites for immune cells and/or the clearance system becomes initially saturated and therefore inhibited by high levels of platelet-bound IgAs.
Surface glycans are possible targets for removal of WAS platelets. Glycosylation defects are associated with WASp deficiency. For example, CD43 (leukosialin, sialophorin) is abnormally O-glycosylated in lymphocytes isolated from WAS patients [42–44]. WAS lymphocytes and platelets have increased expression and activity of the core 2 GlcNAc transferase and the α2-6-sialyl transferase [45, 46]. Furthermore, the gene encoding for the UDP-galactose translocator, also impaired in WAS, is located on the Xp11.22-p11.23 locus of the X chromosome which also contains the WASP gene [47]. Whether platelet glycosylation defects are involved in the severe thrombocytopenia associated with WASp deficiency remains to be determined.
Conclusion and potential implications
By addressing the problem of platelet storage we have defined two carbohydrate-mediated platelet clearance mechanisms. One potential implication of our findings is that addition of sialic acid together with galactose may prove successful in allowing chilled platelet circulation or may ameliorate the deterioration of room temperature stored platelets. The mechanism of how platelets lose sialic acid or galactose during storage, i.e whether plasma or platelet derived glycosidases mediate the removal of glycan residues, is unclear. Inhibition of glycosidases would offer another feasible approach to prevent platelet clearance following cooling.
It is likely that hematopoietic disorders, such as ITP or WAS, are due to deteriorated glycans recognized by antibodies leading to platelet clearance. Understanding the role of glycans in platelet survival and function will provide a new dimension to platelet homeostasis under normal and disease conditions, including immune or cardiovascular disorders. Novel therapies in which glycans, rather than proteins, are modulated may become a useful tool to improve platelet circulation and function.
The potential role of hepatocytes in removal of senile/desialylated platelets is intriguing. Hepatocytes may modulate daily platelet turnover, thereby regulating or promoting platelet production in the bone marrow.
Acknowledgments
This study was supported by grants from the US National Institutes of Health: HL056949 and HL089224 (K.M.H.), and NIH HL059561 (H.F.) and the Pew Scholars Award (K.M.H.). K.M.H. received sponsored research from Velico Medical Inc. (formerly Zymequest Inc.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
- 1.Murphy S, Rebulla P, Bertolini F, et al. In vitro assessment of the quality of stored platelet concentrates. The BEST (Biomedical Excellence for Safer Transfusion) Task Force of the International Society of Blood Transfusion. Transfus Med Rev. 1994;8:29–36. doi: 10.1016/s0887-7963(94)70095-x. [DOI] [PubMed] [Google Scholar]
- 2.Stasi R, Evangelista ML, Stipa E, et al. Idiopathic thrombocytopenic purpura: current concepts in pathophysiology and management. Thromb Haemost. 2008;99:4–13. doi: 10.1160/TH07-08-0513. [DOI] [PubMed] [Google Scholar]
- 3.McMillan R. The pathogenesis of chronic immune thrombocytopenic purpura. Semin Hematol. 2007;44:S3–S11. doi: 10.1053/j.seminhematol.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 4. Cines DB, Bussel JB, Liebman HA, Luning Prak ET. The ITP syndrome: pathogenic and clinical diversity. Blood. 2009;113:6511–6521. doi: 10.1182/blood-2009-01-129155. This review provides a detailed overview on ITP, highlighting its heterogeneity for a better understanding of diagnosis and management.
- 5.Kuhne T, Buchanan GR, Zimmerman S, et al. Intercontinental Childhood ITPSG: A prospective comparative study of 2540 infants and children with newly diagnosed idiopathic thrombocytopenic purpura (ITP) from the Intercontinental Childhood ITP Study Group. J Pediatr. 2003;143:605–608. doi: 10.1067/s0022-3476(03)00535-3. [DOI] [PubMed] [Google Scholar]
- 6.Imbach P, Kuhne T, Muller D, et al. Childhood ITP, 12 months follow-up data from the prospective registry I of the Intercontinental Childhood ITP Study Group (ICIS) Pediatr Blood Cancer. 2006;46:351–356. doi: 10.1002/pbc.20453. [DOI] [PubMed] [Google Scholar]
- 7.Segal JB, Powe NR. Prevalence of immune thrombocytopenia: analyses of administrative data. J Thromb Haemost. 2006;4:2377–2383. doi: 10.1111/j.1538-7836.2006.02147.x. [DOI] [PubMed] [Google Scholar]
- 8.Chan H, Moore JC, Finch CN, et al. The IgG subclasses of platelet-associated autoantibodies directed against platelet glycoproteins IIb/IIIa in patients with idiopathic thrombocytopenic purpura. Br J Haematol. 2003;122:818–824. doi: 10.1046/j.1365-2141.2003.04509.x. [DOI] [PubMed] [Google Scholar]
- 9.Crow AR, Lazarus AH. Role of Fcgamma receptors in the pathogenesis and treatment of idiopathic thrombocytopenic purpura. J Pediatr Hematol Oncol. 2003;25(Suppl 1):S14–S18. doi: 10.1097/00043426-200312001-00004. [DOI] [PubMed] [Google Scholar]
- 10.Sandler SG. The spleen and splenectomy in immune (idiopathic) thrombocytopenic purpura. Semin Hematol. 2000;37:10–12. doi: 10.1016/s0037-1963(00)90112-4. [DOI] [PubMed] [Google Scholar]
- 11.Kuhne T, Blanchette V, Buchanan GR, et al. Splenectomy in children with idiopathic thrombocytopenic purpura: A prospective study of 134 children from the Intercontinental Childhood ITP Study Group. Pediatr Blood Cancer. 2007;49:829–834. doi: 10.1002/pbc.21108. [DOI] [PubMed] [Google Scholar]
- 12.Ballem PJ, Segal GM, Stratton JR, et al. Mechanisms of thrombocytopenia in chronic autoimmune thrombocytopenic purpura. Evidence of both impaired platelet production and increased platelet clearance. J Clin Invest. 1987;80:33–40. doi: 10.1172/JCI113060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stasi R, Cooper N, Del Poeta G, et al. Analysis of regulatory T-cell changes in patients with idiopathic thrombocytopenic purpura receiving B cell-depleting therapy with rituximab. Blood. 2008;112:1147–1150. doi: 10.1182/blood-2007-12-129262. [DOI] [PubMed] [Google Scholar]
- 14.AuBuchon JP, Herschel L, Roger J, Murphy S. Preliminary validation of a new standard of efficacy for stored platelets. Transfusion. 2004;44:36–41. doi: 10.1046/j.0041-1132.2004.00604.x. [DOI] [PubMed] [Google Scholar]
- 15.Rumjantseva V, Hoffmeister KM. Novel and unexpected clearance mechanisms for cold platelets. Transfus Apher Sci. 2009 doi: 10.1016/j.transci.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoffmeister K, Felbinger T, Falet H, et al. The clearance mechanism of chilled blood platelets. Cell. 2003;10:87–97. doi: 10.1016/s0092-8674(02)01253-9. [DOI] [PubMed] [Google Scholar]
- 17.Hoffmeister K, Josefsson E, Isaac N, et al. Glycosylation restores survival of chilled blood platelets. Science. 2003;301:1531–1534. doi: 10.1126/science.1085322. [DOI] [PubMed] [Google Scholar]
- 18.Wandall HH, Hoffmeister KM, Sorensen AL, et al. Galactosylation does not prevent the rapid clearance of long-term, 4 degrees C-stored platelets. Blood. 2008;111:3249–3256. doi: 10.1182/blood-2007-06-097295. [DOI] [PubMed] [Google Scholar]
- 19.Valeri C, Giorgio A, Macgregor H, Ragno G. Circulation and distribution of autotransfused fresh, liquid-preserved and cryopreserved baboon platelets. Vox Sang. 2002;83:347–351. doi: 10.1046/j.1423-0410.2002.00229.x. [DOI] [PubMed] [Google Scholar]
- 20. Rumjantseva V, Grewal PK, Wandall HH, et al. Dual roles for hepatic lectin receptors in the clearance of chilled platelets. Nat Med. 2009;15:1273–1280. doi: 10.1038/nm.2030. Here the authors provide crucial evidence for the role of platelet carbohydrates and hepatic lectins i.e. Kupffer cell αM-lectins and hepatocyte Ashwell-Morrell receptors, in the clearance of chilled platelets.
- 21. Grewal PK, Uchiyama S, Ditto D, et al. The Ashwell receptor mitigates the lethal coagulopathy of sepsis. Nat Med. 2008 doi: 10.1038/nm1760. This paper describes the important role of hepatocyte Ashwell-Morrell receptor in the removal of de-sialylated platelets in the acute pathology of sepsis.
- 22.Ellies L, Ditto D, Levy G, et al. Sialyltransferase ST3Gal-IV operates as a dominant modifier of hemostasis by concealing asialoglycoprotein receptor ligands. Proc Natl Acad Sci U S A. 2002;99:10042–10047. doi: 10.1073/pnas.142005099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sørensen AL, Rumjantseva V, Nayeb-Hashemi S, et al. Role of sialic acid for platelet life span: exposure of beta-galactose results in the rapid clearance of platelets from the circulation by asialoglycoprotein receptor-expressing liver macrophages and hepatocytes. Blood. 2009;114:1645–1654. doi: 10.1182/blood-2009-01-199414. This paper describes the important role of sialic acid in the turn-over of circulating platelets.
- 24.Steiner M, Vancura S. Asymmetrical loss of sialic acid from membrane glycoproteins during platelet aging. Thromb Res. 1985;40:465–471. doi: 10.1016/0049-3848(85)90283-x. [DOI] [PubMed] [Google Scholar]
- 25.Greenberg J, Packham M, Cazenave J, Reimers H, Mustard J. Effects on platelet function of removal of platelet sialic acid by neuraminidase. Lab Invest. 1975;32:476–484. [PubMed] [Google Scholar]
- 26.Bratosin D, Mazurier J, Debray H, et al. Flow cytofluorimetric analysis of young and senescent human erythrocytes probed with lectins. Evidence that sialic acids control their life span. Glycoconj J. 1995;12:258–267. doi: 10.1007/BF00731328. [DOI] [PubMed] [Google Scholar]
- 27.Ochs HD, Thrasher AJ. The Wiskott-Aldrich syndrome. J Allergy Clin Immunol. 2006;117:725–738. doi: 10.1016/j.jaci.2006.02.005. quiz 739. [DOI] [PubMed] [Google Scholar]
- 28. Albert MH, Bittner TC, Nonoyama S, et al. X-linked thrombocytopenia (XLT) due to WAS mutations: clinical characteristics, long-term outcome, and treatment options. Blood. 2010;115:3231–3238. doi: 10.1182/blood-2009-09-239087. In this manuscript the authors present a large data analysis of the mutations in patients with XLT, a mild variant related to WAS.
- 29.Gross BS, Wilde JI, Quek L, et al. Regulation and function of WASp in platelets by the collagen receptor, glycoprotein VI. Blood. 1999;94:4166–4176. [PubMed] [Google Scholar]
- 30.Falet H, Hoffmeister KM, Neujahr R, Hartwig JH. Normal Arp2/3 complex activation in platelets lacking WASp. Blood. 2002;100:2113–2122. [PubMed] [Google Scholar]
- 31.Haddad E, Cramer E, Riviere C, et al. The thrombocytopenia of Wiskott Aldrich syndrome is not related to a defect in proplatelet formation. Blood. 1999;94:509–518. [PubMed] [Google Scholar]
- 32.Sabri S, Foudi A, Boukour S, et al. : Deficiency in the Wiskott-Aldrich protein induces premature proplatelet formation and platelet production in the bone marrow compartment. Blood. 2006;108:134–140. doi: 10.1182/blood-2005-03-1219. [DOI] [PubMed] [Google Scholar]
- 33.Grottum KA, Hovig T, Holmsen H, et al. Wiskott-Aldrich syndrome: qualitative platelet defects and short platelet survival. Br J Haematol. 1969;17:373–388. doi: 10.1111/j.1365-2141.1969.tb01383.x. [DOI] [PubMed] [Google Scholar]
- 34.Murphy S, Oski FA, Naiman JL, et al. Platelet size and kinetics in hereditary and acquired thrombocytopenia. N Engl J Med. 1972;286:499–504. doi: 10.1056/NEJM197203092861001. [DOI] [PubMed] [Google Scholar]
- 35.Prislovsky A, Marathe B, Hosni A, et al. Rapid platelet turnover in WASP(-) mice correlates with increased ex vivo phagocytosis of opsonized WASP(-) platelets. Exp Hematol. 2008;36:609–623. doi: 10.1016/j.exphem.2007.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Falet H, Marchetti MP, Hoffmeister KM, et al. Platelet-associated IgAs and impaired GPVI responses in platelets lacking WIP. Blood. 2009;114:4729–4737. doi: 10.1182/blood-2009-02-202721. This study shows that IgA binding impairs platelet responses to the collagen receptor GPVI, but unexpectedly protects platelets against increased clearance in a mouse model of WAS.
- 37.Curcio C, Pannellini T, Lanzardo S, et al. WIP null mice display a progressive immunological disorder that resembles Wiskott-Aldrich syndrome. J Pathol. 2007;211:67–75. doi: 10.1002/path.2088. [DOI] [PubMed] [Google Scholar]
- 38.Corash L, Shafer B, Blaese RM. Platelet-associated immunoglobulin, platelet size, and the effect of splenectomy in the Wiskott-Aldrich syndrome. Blood. 1985;65:1439–1443. [PubMed] [Google Scholar]
- 39.Semple JW, Siminovitch KA, Mody M, et al. Flow cytometric analysis of platelets from children with the Wiskott-Aldrich syndrome reveals defects in platelet development, activation and structure. Br J Haematol. 1997;97:747–754. doi: 10.1046/j.1365-2141.1997.1132938.x. [DOI] [PubMed] [Google Scholar]
- 40.DeSanto NG, Sessa A, Capodicasa G, et al. IgA glomerulonephritis in Wiskott-Aldrich syndrome. Child Nephrol Urol. 1988;9:118–120. [PubMed] [Google Scholar]
- 41.Matsukura H, Kanegane H, Miya K, et al. IgA nephropathy associated with X-linked thrombocytopenia. Am J Kidney Dis. 2004;43:e7–e12. doi: 10.1053/j.ajkd.2003.11.016. [DOI] [PubMed] [Google Scholar]
- 42.Parkman R, Remold-O'Donnell E, Kenney DM, et al. Surface protein abnormalities in lymphocytes and platelets from patients with Wiskott-Aldrich syndrome. Lancet. 1981;2:1387–1389. doi: 10.1016/s0140-6736(81)92802-6. [DOI] [PubMed] [Google Scholar]
- 43.Remold-O'Donnell E, Kenney DM, Parkman R, et al. Characterization of a human lymphocyte surface sialoglycoprotein that is defective in Wiskott-Aldrich syndrome. J Exp Med. 1984;159:1705–1723. doi: 10.1084/jem.159.6.1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Greer WL, Higgins E, Sutherland DR, et al. Altered expression of leucocyte sialoglycoprotein in Wiskott-Aldrich syndrome is associated with a specific defect in O-glycosylation. Biochem Cell Biol. 1989;67:503–509. doi: 10.1139/o89-081. [DOI] [PubMed] [Google Scholar]
- 45.Higgins EA, Siminovitch KA, Zhuang DL, et al. Aberrant O-linked oligosaccharide biosynthesis in lymphocytes and platelets from patients with the Wiskott-Aldrich syndrome. J Biol Chem. 1991;266:6280–6290. [PubMed] [Google Scholar]
- 46.Piller F, Le Deist F, Weinberg KI, et al. Altered O-glycan synthesis in lymphocytes from patients with Wiskott-Aldrich syndrome. J Exp Med. 1991;173:1501–1510. doi: 10.1084/jem.173.6.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hara T, Yamauchi M, Takahashi E, et al. The UDP-galactose translocator gene is mapped to band Xp11.23-p11.22 containing the Wiskott-Aldrich syndrome locus. Somat Cell Mol Genet. 1993;19:571–575. doi: 10.1007/BF01233383. [DOI] [PubMed] [Google Scholar]