Abstract
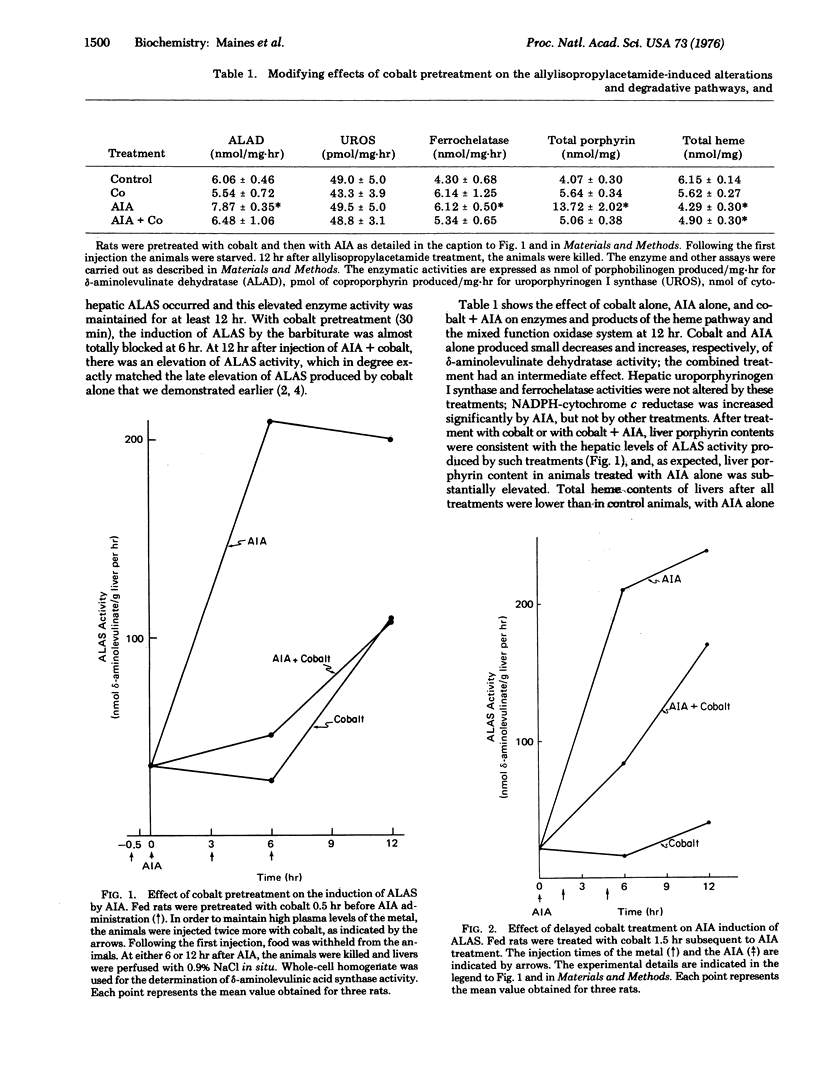
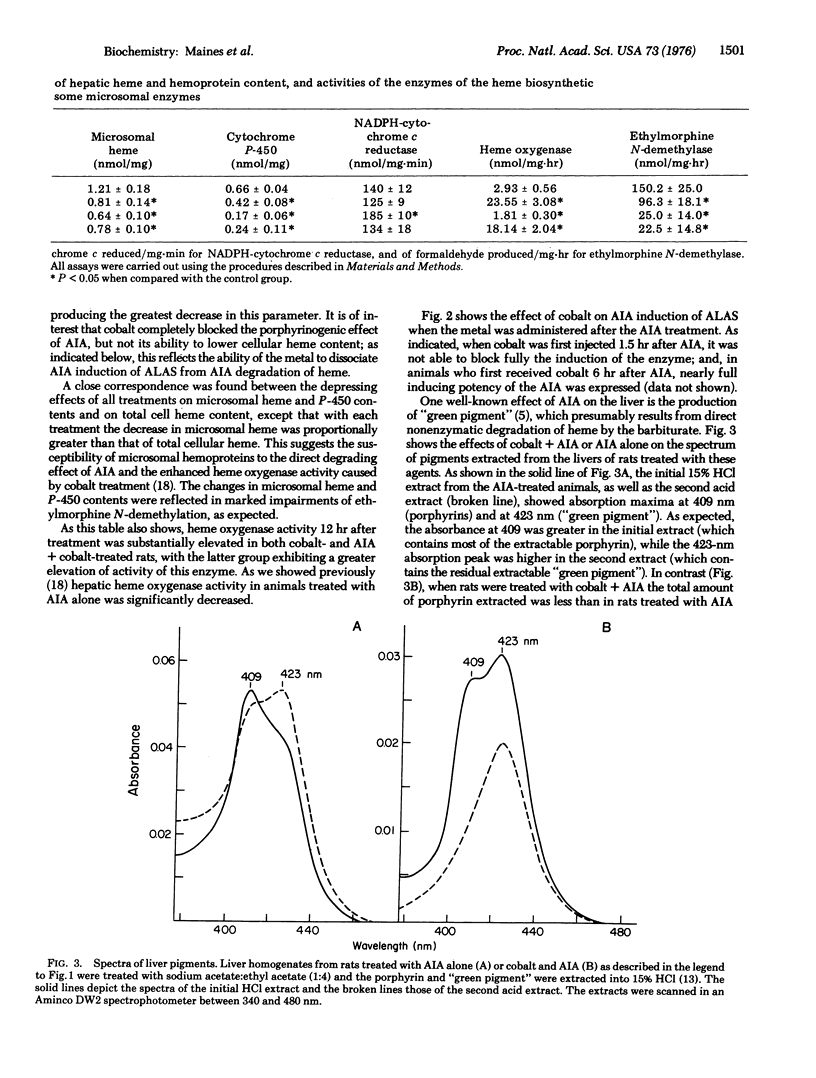
Cobalt has complex actions on the metabolism of heme in the liver. In this organ the metal potently induces heme oxygenase (EC 1.14.99.3), and decreases cellular heme and hemoprotein content. The metal also displays biphasic effects on hepatic heme synthesis. These effects are reflected in the ability of cobalt to initially inhibit synthesis of delta-aminolevulinate synthase [succinyl-CoA:glycine C-succinyltransferase (decarboxylating) EC 2.3.1.37], the rate limiting enzyme of the heme pathway, following which a later enhanced rate of formation of this enzyme occurs. In this study, cobalt was shown to block almost entirely the ability of the barbiturate analogue allylisopropylacetamide to induce delta-aminolevulinate synthase in liver. The blocking effect of cobalt on the otherwise potent enzyme inducing action of this drug was time-dependent; if the metal was injected 30 min prior to allylisopropylacetamide, inhibition of enzyme induction was complete. When the metal was administered 1.5 or more hours after allylisopropylacetamide, inhibition of enzyme induction was incomplete. Cobalt did not block the ability of the drug to directly degrade heme to "green pigment" thus the enzyme inducing action of allylisopropylacetamide and its degradative action on heme are separately mediated.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Copp D. H., Greenberg D. M. Studies in Mineral Metabolism with the Aid of Artificial Radioactive Isotopes: VI. Cobalt. Proc Natl Acad Sci U S A. 1941 Mar 15;27(3):153–157. doi: 10.1073/pnas.27.3.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Matteis F. Rapid loss of cytochrome P-450 and haem caused in the liver microsomes by the porphyrogenic agent 2-allyl-2-isopropylacetamide. FEBS Lett. 1970 Feb 25;6(4):343–345. doi: 10.1016/0014-5793(70)80094-1. [DOI] [PubMed] [Google Scholar]
- GRANICK S., MAUZERALL D. Pbrphyrin biosynthesis in erythrocytes. II. Enzymes converting gamma-aminolevulinic acid to coproporphyrinogen. J Biol Chem. 1958 Jun;232(2):1119–1140. [PubMed] [Google Scholar]
- GRANICK S., URATA G. Increase in activity of alpha-aminolevulinic acid synthetase in liver mitochondria induced by feeding of 3,5-dicarbethoxy-1,4-dihydrocollidine. J Biol Chem. 1963 Feb;238:821–827. [PubMed] [Google Scholar]
- Granick S., Sinclair P., Sassa S., Grieninger G. Effects by heme, insulin, and serum albumin on heme and protein synthesis in chick embryo liver cells cultured in a chemically defined medium, and a spectrofluorometric assay for porphyrin composition. J Biol Chem. 1975 Dec 25;250(24):9215–9225. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Maines M. D., Kappas A. Cobalt induction of hepatic heme oxygenase; with evidence that cytochrome P-450 is not essential for this enzyme activity. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4293–4297. doi: 10.1073/pnas.71.11.4293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maines M. D., Kappas A. Cobalt stimulation of heme degradation in the liver. Dissociation of microsomal oxidation of heme from cytochrome P-450. J Biol Chem. 1975 Jun 10;250(11):4171–4177. [PubMed] [Google Scholar]
- Maines M. D., Kappas A. Studies on the mechanism of induction of haem oxygenase by cobalt and other metal ions. Biochem J. 1976 Jan 15;154(1):125–131. doi: 10.1042/bj1540125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maines M. D., Kappas A. The degradative effects of porphyrins and heme compounds on components of the microsomal mixed function oxidase system. J Biol Chem. 1975 Mar 25;250(6):2363–2369. [PubMed] [Google Scholar]
- Murphy F. R., Krupa V., Marks G. S. Drug-induced porphyrin biosynthesis--XIII. Role of lipophilicity in determining porphyrin-inducing activity of aliphatic amides after blockade of their hydrolysis by bis-(rho-nitrophenyl)phosphate. Biochem Pharmacol. 1975 Apr 15;24(8):883–889. doi: 10.1016/0006-2952(75)90159-8. [DOI] [PubMed] [Google Scholar]
- OMURA T., SATO R. THE CARBON MONOXIDE-BINDING PIGMENT OF LIVER MICROSOMES. II. SOLUBILIZATION, PURIFICATION, AND PROPERTIES. J Biol Chem. 1964 Jul;239:2379–2385. [PubMed] [Google Scholar]
- SCHMID R., SCHWARTZ S. Experimental porphyria. III. Hepatic type produced by sedormid. Proc Soc Exp Biol Med. 1952 Dec;81(3):685–689. doi: 10.3181/00379727-81-19987. [DOI] [PubMed] [Google Scholar]
- Sassa S., Granick S., Bickers D. R., Bradlow H. L., Kappas A. A microassay for uroporphyrinogen I synthase, one of three abnormal enzyme activities in acute intermittent porphyria, and its application to the study of the genetics of this disease. Proc Natl Acad Sci U S A. 1974 Mar;71(3):732–736. doi: 10.1073/pnas.71.3.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tephly T. R., Hibbeln P. The effect of cobalt chloride administration on the synthesis of hepatic microsomal cytochrome P-450. Biochem Biophys Res Commun. 1971 Feb 19;42(4):589–595. doi: 10.1016/0006-291x(71)90528-6. [DOI] [PubMed] [Google Scholar]
- WILLIAMS C. H., Jr, KAMIN H. Microsomal triphosphopyridine nucleotide-cytochrome c reductase of liver. J Biol Chem. 1962 Feb;237:587–595. [PubMed] [Google Scholar]


