Abstract
Colorectal cancer (CRC) is a significant cause of mortality and morbidity in the United States, much of which could be prevented through adequate screening. Consensus guidelines recommend that high-risk groups initiate screening earlier with colonoscopy and more frequently than average risk persons. However, a large proportion of high risk individuals do not receive regular colonoscopic screening. The Family Health Promotion Project (FHPP) is a randomized-controlled trial to test the effectiveness of a telephone-based counseling intervention to increase adherence to risk-appropriate colonoscopy screening in high risk individuals. Unaffected members of CRC families from two national cancer family registries were enrolled (n=632) and randomized to receive either a single session telephone counseling intervention using Motivational Interviewing techniques or a minimal mail-out intervention. The primary endpoint, rate of colonoscopy screening, was assessed at 6, 12 and 24 months post-enrollment. In this paper, we describe the research design and telephone counseling intervention of the FHPP trial, and report baseline data obtained from the two high risk cohorts recruited into this trial. Results obtained at baseline confirm the need for interventions to promote colonoscopy screening among these high risk individuals, as well as highlighting several key opportunities for intervention, including increasing knowledge about risk-appropriate screening guidelines, and providing both tailored risk information and barriers counseling.
Keywords: colonoscopy, high risk, intervention, randomized trial
1. Introduction
Colorectal cancer (CRC) is the third most common cancer and third leading cause of cancer death in the United States [1] Much of the morbidity and mortality from CRC can be prevented through effective screening. Earlier stage diagnosis of CRC through regular screening could lead to survival rates of up to 90% for CRC and 80% for rectal cancer; a strong rationale for efforts to promote screening among average risk individuals over age 50 [1–2] Additionally, removal of colon polyps by colonoscopy can substantially decrease CRC incidence.3 The small but steady decline in CRC incidence and mortality over recent decades has been largely attributed to increasing rates of CRC screening [3]
Having a family history of CRC is one of the strongest risk factors for CRC and provides a salient cue for screening. First-degree relatives of CRC patients have a two- to four-fold increased risk for CRC when compared to the general population. Moreover, having a single relative diagnosed with CRC under age 50 or two or more relatives with CRC increases risk three- to six-fold [4–5] Members of families with Lynch Syndrome, a rare hereditary syndrome also known as hereditary non-polyposis colorectal cancer (HNPCC) have an even higher lifetime risk of CRC approaching 80% [6–7] Greater risk based on family history has led to recommendations to screen these groups earlier and more frequently than average risk persons and to use colonoscopy as the preferred screening modality [8]
Available data indicate that colonoscopy screening is effective in individuals with Lynch Syndrome; screened individuals have markedly lower CRC incidence and mortality than those who do not receive regular screening [9–10] Moreover, at least one observational study reported a significant reduction in the progression from adenomas to CRC among family members of CRC patients screened with colonoscopy [11] Despite the evidence that a family history confers significant risk for CRC and that screening is effective in these groups, a large proportion (27–46%) of individuals with familial risk, including those with known or suspected gene mutations, do not receive regular colonoscopy [12–15] The relatively few studies that have examined predictors of adherence to CRC screening among individuals with familial risk suggest that socio-demographic factors, degree of family history, lack of regular provider or provider recommendation, perceived barriers, risk perception, and lack of belief that screening is effective significantly predict adherence [16–20] Few prospective studies have assessed how modifying these factors may impact adherence in high risk groups [12–13,16]
The Family Health Promotion Project (FHPP) is a randomized-controlled trial designed to test the effectiveness of a telephone counseling intervention to increase adherence to risk-appropriate colonoscopy screening in members of high risk families FHPP used an innovative approach, tapping into two national family cancer registries, and novel methods including Motivational Interviewing techniques, to address this important public health issue. We describe here the design of the FHPP trial, the counseling intervention used as well as the baseline characteristics of the study population, including their knowledge, attitudes, beliefs, risk perceptions and self-reported barriers to CRC screening.
2. Methods
Overview of Study Design
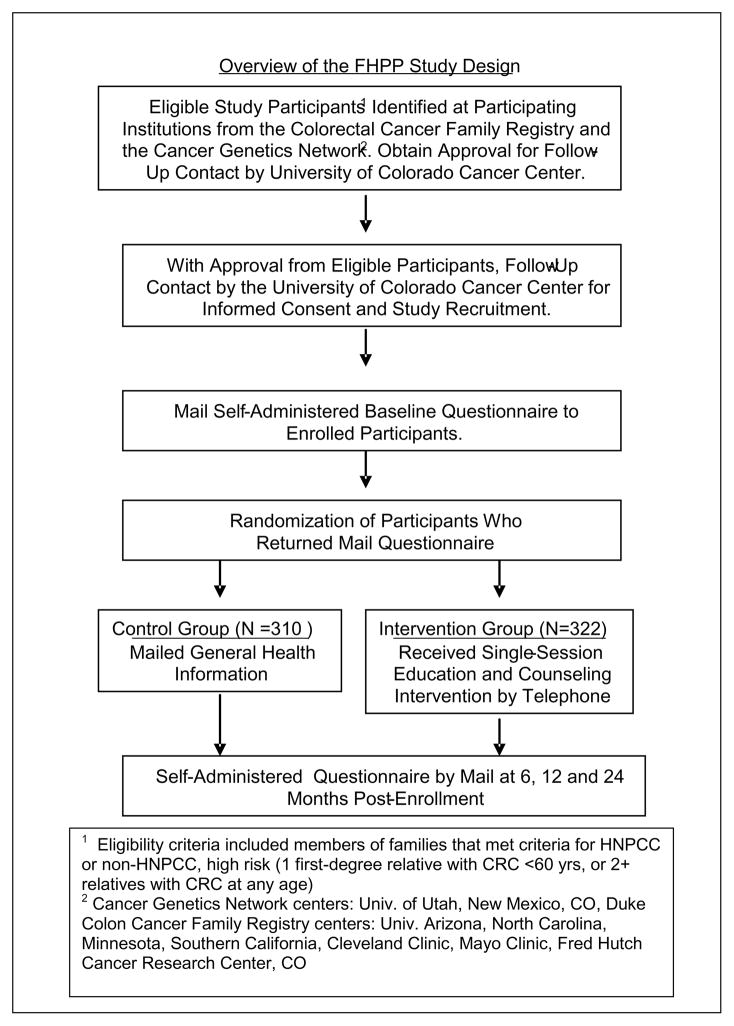
Figure 1 provides an overview of the FHPP trial. Enrolled participants were unaffected at-risk members of families that met criteria for either HNPCC or non-HPNCC high risk (HR) families that were due for colonoscopy screening within the 2-year study period. High risk participants were recruited from two national cancer registries; the Colorectal Family Registry (CFR) and the Cancer Genetics Network (CGN) [21–22] Upon providing consent and completion of a baseline questionnaire, participants were randomized using a block design by risk level (HNPCC vs. high risk), gender and family unit to the intensive or minimal intervention group. The minimal intervention group received a mailed packet that contained general health information and encouraged participants to talk with their doctor about appropriate CRC screening. The intensive intervention group received a single education and counseling session via telephone that utilized motivational interviewing (MI) techniques [23–24] The intervention was tailored to the participant’s age, gender, risk level and self reported barriers to CRC screening as reported on the baseline survey. The primary endpoint of the trial was colonoscopy screening within 24 months following the intervention, validated by endoscopy report. Assessment of CRC screening, knowledge of screening recommendations, attitudes and beliefs about screening and perceived CRC risk occurred by self-administered questionnaires at baseline and at 6, 12 and 24 month’s follow-up. This study was approved by the Colorado Multiple Institutional Review Board (Protocol #03-858).
Figure 1.
Study Population
Participants were recruited from 8 CFR and 4 CGN registry sites. These registries were established by the National Cancer Institute in 1997–1998 as resources to support studies on the etiology, prevention and clinical management of cancer with a particular emphasis on the genetic basis of cancer susceptibility [21–22] The CFR and CGN registries recruited individuals diagnosed with CRC (i.e. probands) between 1997 and 2001. With the exception of the Cleveland Clinic that enrolled patients from their high risk clinic, all other CFR and CGN registry sites participating in FHPP recruited CRC cases from population-based state cancer registries. Once enrolled in these registries, the proband was asked for permission to invite one or more of their first-degree relatives to also enroll in the registry. As part of their consent to enroll, all participants agreed to be contacted about future studies for which they might be eligible. Unaffected family members enrolled in CFR and CGN were targeted for recruitment for FHPP.
Eligibility
Individuals were eligible for FHPP if they had no personal history of CRC, were at least 21 years of age, were English speaking and were members of a family that met clinical criteria for being at high risk for CRC. The definition of high risk families was guided by those used to stratify CRC screening recommendations [3] and was separated into two high risk groups. Participants were classified as HNPCC if their families met the Amsterdam II criteria: 3 biological relatives with CRC (or other HNPCC-related cancers: endometrial, small bowel, ureter or renal pelvis) with one being a first degree relative of the other two, at least 2 generations affected, and 1 cancer diagnosis under 50 years of age [25] Participants were classified as non-HNPCC, high risk (HR) if their families did not meet the Amsterdam II criteria but they had at least one first-degree relative diagnosed with CRC under age 60 or two or more first-degree relatives with CRC diagnosed at any age. HNPCC and HR families within the registries were identified on the basis of the validated diagnosis of CRC in the proband from the state cancer registry and self-reported family history information provided by the proband upon enrollment into the CFR and CGN. This information was confirmed with participants upon enrollment into FHPP.
Eligible participants must also have been due for screening colonoscopy during the 24-month study period. Given that the recommendation for HNPCC family members is to have colonoscopy screening every 1–2 years, no HNPCC participants were excluded based on this criteria (i.e., all of these participants would be due for colonoscopy within the two year follow-up period). HR participants who had undergone colonoscopy within 3 years prior to enrollment were excluded from enrollment into FHPP as these participants would not be due for screening during the 2-year study period (i.e., screening recommendation for HR participants is colonoscopy no less than every 5 years).
Recruitment
Lists of registrants meeting inclusion criteria for FHPP were identified by the data coordinating centers for CFR and CGN and sent to the respective sites who contacted their local participants to obtain their permission to be contacted by study staff at the University of Colorado Cancer Center (UCCC). Once consent for contact was obtained, the local sites uploaded the participant’s contact information into a centralized database housed at Massachusetts General Hospital, the data coordinating center for the CGN and for FHPP. UCCC staff then approached potential participants to invite them to participate and obtain written consent. In some families, more than one first-degree relative was identified as potentially eligible and consented. Recruitment was conducted from September 2004 to May 2006.
Telephone Counseling Intervention
Consenting participants were randomized to the minimal intervention or telephone counseling groups. The counseling intervention was grounded in several complementary theoretical models to promote health behavior change, including the Health Belief Model [26–27], the Theory of Planned Behavior [28–30], and the Transtheoretical Model [31–33] Motivational Interviewing (MI) served as the counseling framework. The main premise of MI is to facilitate behavior change by helping people to explore and resolve their ambivalence [34–35] MI is thought to elicit the motivation required to move individuals through the different stages of change, and is particularly effective with individuals at early stages of readiness [36] Brief forms of MI have been used successfully in health behavior interventions [37–38] Brief MI consists of a set or menu of techniques which follow the spirit and practice of motivational interviewing but do not necessarily demand a command of the overall method or understanding of underlying theory to be effective [37], although clinical skillfulness is certainly deepened through more advanced training and practice. Classic exemplars of brief MI techniques include readiness rulers and decisional balance sheets [35] These techniques were standardized in the FHPP intervention and applied after stages of readiness assessment. In Brief MI, the non-judgmental reflective responses and well-timed summaries serve to mirror and reinforce the pros/benefits of behavior change and the client’s self-efficacy to do so. The discussion of the barriers to change is contained, specific and solutions focused. Although there is an emerging literature on the efficacy of Brief MI in managing and modifying chronic behaviors, the FHPP study is one of a few published studies to focus specifically on cancer screening [39–40]
Four interviewers were trained to deliver the FHPP brief MI intervention. None had previous training or experience in Brief MI techniques. Interviewer training began with an overview of the project, followed by a specific training for the single counseling call. Throughout the study, supervisory staff frequently monitored live calls and provided real-time feedback for quality control and improvement.
The telephone counseling session was conducted using computer-assisted telephone interviewing (CATI) software, which allowed the interviewer to receive pop-up screens appropriate to the participant’s level of risk, recommended screening interval based on risk and response to stage of change questions for colonoscopy screening and family advocacy. Based upon the participants response to the readiness question, the CATI provided one of three behavioral counseling tracks: Track 1 for those were not ready to be screened (focused on the cons of getting a colonoscopy and solutions for obstacles to screening); Track 2 for those who were ambivalent about screening (focused equally on pros and cons, using conversational interviewer probes designed to differentially tip the scale toward pros); and Track 3 for those who were ready to be screened (strongly reinforced pros). A schema of the full telephone counseling protocol can be found in Figure 2.
Figure 2.
Survey Assessments
All participants were asked to complete mailed surveys at baseline and at 6, 12 and 24 months post randomization. In addition to collecting information on colonoscopy screening history (had recent screening, yes/no; date of most recent test; reason for having test and intentions to screen), the surveys assessed several key constructs that were targeted in the counseling intervention, including knowledge of risk-appropriate CRC screening guidelines, attitudes and beliefs regarding colonoscopy (perceived efficacy and need for colonoscopy), self-reported barriers to CRC screening, perceived risk of CRC, and awareness and uptake of genetic counseling and testing. Specific questions included the survey assessments were adopted from similar assessments tested and utilized in a previous randomized trial to promote screening among first-degree relatives of breast and colorectal cancer survivors [41]. CRC screening history reported at baseline was based on self-report. Self-reported CRC screening has an overall high level of concordance (with medical record data) in studies conducted in primary care settings [42,43] and individuals with a family history of CRC have an even higher concordance than that of individuals without a family history [43]. Moreover, telephone and self-administered surveys have been found to be more accurate then face-to-face interviews for endoscopic cancer screening behavior [44]. The baseline survey also captured socio-demographic information such as gender, age, race/ethnicity education, income, health insurance and access to a regular doctor or clinic (see baseline questionnaire in supplemental data for details of all questions). A description of the survey components is provided below.
Knowledge of risk-appropriate CRC guidelines
This section elicited subjects’ knowledge of CRC screening guidelines by asking ‘how often do you think you should be screened?’ and ‘at what age do you think a person with a family history like yours should start being screened?’ The colonoscopy-specific questions were imbedded in similar questions for other cancer screening tests such as mammography, PSA testing and skin exams so as to minimize the reactivity of the assessments to affect study outcomes. For colonoscopy, responses consistent with current screening guidelines were ‘every 1–2 years’ for HNPCC and no less than every 5 years for HR.
Attitudes, Beliefs and Barriers
To assess attitudes and beliefs about CRC screening, participants were asked how effective they thought screening tests were in detecting cancer and were asked whether several statements about CRC screening specifically were applicable to them (response options: agree/disagree/undecided). Examples of two such statements were, “If I eat a healthy diet, I don’t need to be screened”, and “I won’t have screening unless I have bowel or abdominal symptoms” (see supplemental data for entire baseline questionnaire). Similarly, seventeen potential barriers or reasons for not having CRC screening including being “too young”, “ too busy”, and “disgusted by the tests”, were given and participants were asked to report whether any of these barriers were reasons for them not be screened (yes/no/unsure). The complete list of measures is presented in Tables 2 and 3.
Table 2.
Baseline data: Colonoscopy screening history, intentions to screen and knowledge about frequency of screening
| Baseline survey question | Overall N=632 |
Risk Level
|
|
|---|---|---|---|
| HNPCC† N=165 |
High Risk N=467 |
||
|
| |||
| Ever had colonoscopy? | |||
| Yes | 477 (75%) | 157 (95%) | 320 (69%) |
| No | 155 (25%) | 8 (5%) | 147 (31%) |
|
| |||
| When was your last colonoscopy? | |||
| 2 years ago or less | 95 (15%) | 92 (56%) | 0* |
| >2–5 years ago | 250 (39%) | 43 (26%) | 207 (44%) |
| More than 5 years ago | 131 (21%) | 21 (13%) | 110 (24%) |
| Never had one | 155 (25%) | 8 (5%) | 147 (31%) |
|
| |||
| Intend to have colonoscopy in next 1–2 years? | |||
| Yes | 325 (52%) | 90 (55%) | 232 (50%) |
| No | 307 (48%) | 75 (45%) | 235 (50%) |
|
| |||
| How often should you have colonoscopy? Every… | |||
| 1 or 2 years | 75 (12%) | 36 (22%)† | 39 (8%) |
| >2–5 years | 355 (56%) | 113 (68%) | 242 (52%)† |
| >5–9 years | 129 (20%) | 11 (7%) | 118 (25%) |
| 10+ years | 20 (3%) | 2 (1%) | 18 (4%) |
| Never | 3 (<1%) | 0 | 3 (1) |
| Don’t know | 42 (7%) | 1 (1%) | 41 (9%) |
HNPCC = hereditary non-polyposis colon cancer
HR excluded from FHPP if had colonoscopy less than 3 years prior to enrollment
Bolded represents recommended routine screening interval for this group
Table 3.
Baseline data: Attitudes and beliefs about colon cancer screening overall and by risk level
| Statement | Overall % agree |
Risk Level | |
|---|---|---|---|
| HNPCC† (N=165) | HR† (N=467) | ||
| % agree | |||
| If I eat a healthy diet, I don’t need to be screened. | 4 | 3 | 4 |
| If I have a rectal exam, I don’t need to be screened. | 1 | 0 | 1 |
| Once I have had a couple of negative tests I do not need any. | 3 | 1 | 3 |
| I won’t have screening unless I have bowel/abdominal symptoms. | 16 | 8 | 18 * |
| Colon cancer screening is part of good overall health care. | 94 | 95 | 93 |
| People who tell me not to bother being screened are right. | 0 | 0 | 0 |
| If screening finds something, it will be too advanced to cure. | 1 | 1 | 1 |
| Colon cancer screening is not a useful test for people my age. | 1 | 1 | 1 |
| Colon cancer screening is not reliable. | 1 | 0 | 1 |
HNPCC = hereditary non-polyposis colon cancer; HR = High Risk
p<0.05 for difference between risk groups
Perceived risk and knowledge of familial CRC risk
Participants were asked what they thought their risk of CRC was compared to others their age that do not have close relatives with CRC (response choices: much higher, a little higher, the same, a little lower, much lower, don’t know). Participants were also asked how concerned they were about getting CRC (very, moderately, not very or not at all, don’t know) and how the diagnosis of CRC in their family made them feel about their own chances of getting CRC (chances were a lot more, a little more, didn’t change chances, less, don’t know). In addition, several questions were asked to elicit participants’ perception about the role of family history and genetics in causing CRC and about their knowledge of and experience with genetic counseling and testing for genetic predisposition to CRC.
All assessments were completed by mail and then scanned for upload into the project database that was designed and maintained by the data coordinating center at Massachusetts General Hospital.
Data Analysis
The primary objective of FHPP is to test the effectiveness of a telephone-based, counseling intervention as compared to a mailed packet containing general health information to increase adherence to colonoscopy screening in high risk individuals. The main hypothesis of greater adherence in the counseling intervention group will be tested using survival analysis techniques so as to account for variations in follow-up time. Trial successes will include participants who had a colonoscopy during the 24-month study confirmed by endoscopy report and trial failures will be participants who did not have colonoscopy. Participants will be censored at the time of colonoscopy or last completed follow-up. Cox-proportional hazards models will be used to assess the intervention effect while adjusting for potential confounders and other explanatory variables such as age, gender, risk level and insurance status. A conservative sample size of 240 in each intervention arm was selected to account for drop-outs and familial clustering and to achieve at least 80% power to detect a 15% difference in screening adherence between intervention groups.
Secondary analyses will seek to identify mediating factors as defined by four criteria developed by Baron & Kenny (1986) and Holmbeck (1997) [45–46]; (i) the counseling intervention when compared to the control condition should increase screening adherence in the study population at 24 months follow-up, (ii) the intervention significantly modulates the hypothesized mediating variables at 6 and 12 months in a positive direction, (iii) the mediating variables at 6 and 12 months are predictive of screening adherence in the study population at 24 months follow-up, and (iv) controlling for mediating variables at 6 and 12 months substantially reduces or eliminates the intervention effects observed on screening adherence at 24 months follow-up. As noted previously, potential mediating factors include knowledge of risk-appropriate CRC screening guidelines, attitudes, beliefs and reported barriers to screening and perceived risk related to CRC in the family. These analyses will utilize general estimating equations (GEE) within logistic regression. In defining a candidate set of mediating variables by criterion (iv) above, stepwise procedures will be used for model selection using the variables that are significant by univariate analysis.
Results
A total of 1068 participants were identified by participating CFR and CGN sites as potentially eligible for FHPP and contacted for enrollment. Of these, 156 were found to be ineligible; of the remaining 912, 632 consented and 280 refused (or did not respond) for a participation rate of 69 percent. There were no significant differences between responders (n=632) and non-responders (n=280) with respect to gender, age, race/ethnicity or risk level (data not shown). Among the 156 individuals deemed ineligible, 75 were due to the individual not being due for their next colonoscopy within the 2 year study period (as described above, this exclusion criteria was applicable to HR participants), 49 did not confirm a family history at baseline that was consistent with the high-risk history previously reported by the affected family member initially enrolled in the registry (the proband), 9 had a recent CRC diagnosis and 7 reported they had tested negative for the HNPCC genes (indicating lower risk). The most common reasons were refusal were ‘not interested’ (53%) or ‘too busy’ (25%). There were no significant differences between potential participants who refused and those who consented to participate with respect to gender, age, race/ethnicity or risk level.
Characteristics of enrolled study participants, overall and according to risk level, are presented in Table 1. Approximately 60% of the participants were women. Participants were predominately middle-aged and older (70% were 50 years or older), educated (78% above high school), Caucasian (93%), and reported having health insurance and a regular doctor (92%). Sixty-one percent of participants reported incomes at or above $45,000 per year. Twenty-six percent of participants met criteria for HNPPC (n=165), 74% for HR (n=467). There were no differences in demographic factors by risk level. The 632 participants enrolled represented 541 families; 468 families (87%) had one member enrolled, 60 families (11%) had two members enrolled and the remaining 13 families (2%) had three or more members enrolled.
Table 1.
Characteristics of study participants overall and according to risk level
| Characteristic | Overall (N=632) N (%) |
Risk Level
|
|
|---|---|---|---|
| HNPCC† (N=165) N (%) |
High Risk (N=467) N (%) |
||
|
| |||
| Gender | |||
| Male | 261 (41) | 81 (49) | 180 (39) |
| Female | 371 (59) | 84 (51) | 287 (61) |
|
| |||
| Age | |||
| < 40 | 36 (1) | 12 (7) | 24 (5) |
| 40 – 49 | 152 (24) | 33 (20) | 119 (25) |
| 50 – 64 | 272 (43) | 83 (50) | 189 (40) |
| 65+ | 172 (27) | 37 (22) | 135 (29) |
|
| |||
| Race | |||
| African American | 11 (2) | 2 (1) | 9 (2) |
| Caucasian | 589 (93) | 155 (94) | 434 (93) |
| Other | 23 (4) | 5 (3) | 18 (4) |
| Missing | 9 (1) | 3 (2) | 6 (1) |
|
| |||
| Ethnicity | |||
| Hispanic | 15 (2) | 3 (2) | 12 (3) |
| Non-Hispanic | 605 (96) | 161 (98) | 444 (95) |
| Missing | 12 (2) | 1 (1) | 11 (2) |
|
| |||
| Education | |||
| Post college | 117 (19) | 29 (18) | 88 (19) |
| College graduate | 175 (28) | 49 (30) | 126 (27) |
| Some college/tech school | 195 (31) | 50 (30) | 145 (31) |
| High school/GED | 121 (19) | 30 (18) | 91 (19) |
| Less than high school | 20 (3) | 6 (4) | 14 (3) |
| Missing | 4 (<1) | 1 (1) | 3 (1) |
|
| |||
| Household Income | |||
| $70,000 or more | 235 (37) | 65 (39) | 170 (36) |
| $45,000 – $69,999 | 149 (24) | 45 (27) | 114 (24) |
| $30,000 – $44,999 | 106 (17) | 23 (14) | 83 (18) |
| $15,000 – $29,999 | 68 (11) | 12 (7) | 56 (12) |
| < $15,000 | 28 (4) | 7 (4) | 21 (4) |
| Missing/Don’t know | 36 (6) | 13 (8) | 23 (5) |
|
| |||
| Health Insurance Status | |||
| Insured | 603 (95) | 158 (96) | 445 (95) |
| Not insured | 25 (4) | 6 (4) | 19 (4) |
| Missing | 4 (<1) | 1 (1) | 3 (1) |
|
| |||
| Have regular doctor or clinic | |||
| Yes | 582 (92) | 152 (92) | 430 (92) |
| No | 48 (8) | 13 (8) | 35 (7) |
| Missing | 2 (<1) | 0 | 2 (<1) |
HNPCC = hereditary non-polyposis colon cancer
Data from select baseline measures are presented in Tables 2–5. Data are presented for the overall study population and according to risk level as several measures including what denotes accurate knowledge of risk-appropriate screening recommendations, will differ between these two risk groups. Overall, seventy five percent of participants reported ever having a colonoscopy (Table 2). The proportion of those who ever had a colonoscopy was higher in HNPCC participants (95%) than in the high-risk group (69%). Fifty-six percent of HNPCC participants were adherent with colonoscopy screening guidelines at baseline as they reported having had colonoscopy within 2 years of enrollment. Because we excluded HR participants that had colonoscopy within 3 years of enrollment, we cannot estimate overall baseline adherence in the HR group. However, within our sample of 467 HR participants who were due for colonoscopy, the majority (55%), had either had their last colonoscopy more than 5 years or prior had never had a colonoscopy. When asked when they planned to have colonoscopy, only a little over half of the participants said that they planned on having a colonoscopy with the next 1–2 years though based on eligibility criteria for the study, all would be due for their next screening within this time frame. There was considerable variability among participants as to how often they thought they should be screened. Only 22% of the HNPCC group thought they should be screened every 1–2 years which is the current recommendation for these individuals. Among the HR group, 52% of participants thought they should be screened with colonoscopy every 3–5 years (which is consistent with the current recommendation for this group to be screened no less frequently than every 5 years), 29% thought they that should be screened at intervals longer than 5 years and 9% were unsure how often they should have colonoscopy. Knowledge of guidelines appeared to correlate with intentions to screen. Among participants who were aware of the current guidelines in the HNPCC group, 83% reported intention to screen within 1–2 years, compared to 46% who were not aware of the current guidelines. The corresponding figures for the HR group were 61% and 35%, respectively (data not shown).
Table 5.
Baseline data: Perceived risk of CRC overall and by risk level
| Baseline Question | Overall N (%) |
Risk Level
|
|
|---|---|---|---|
| HNPCC† (165) | High Risk (467) | ||
|
| |||
| N (%) | |||
|
| |||
| What do you think your risk of getting CRC is compared to people your age who do NOT have a family history? | |||
| Much higher | 275 (44) | 113 (68) | 162 (35) * |
| A little higher | 241 (38) | 37 (22) | 204 (44) |
| The same | 63 (10) | 6 (4) | 57 (12) |
| A little or much lower | 25 (4) | 6 (4) | 19 (4) |
| Don’t know | 26 (4) | 3 (2) | 23 (5) |
|
| |||
| How concerned are you about getting colon cancer? | |||
| Very concerned | 132 (21) | 56 (34) | 76 (16) * |
| Moderately concerned | 312 (49) | 76 (46) | 236 (51) |
| Not very/not at all concerned | 170 (27) | 31 (19) | 139 (30) |
| Don’t know | 7 (1) | 1 (1) | 6 (1) |
|
| |||
| How did the diagnosis of colon cancer in your family make you feel about your own chances of getting CRC? | |||
| My chances were a lot more | 300 (47) | 120 (73) | 180 (39) * |
| My chances were a little more | 250 (40) | 40 (24) | 210 (45) |
| No change/chances were less | 67 (11) | 5 (3) | 62 (13) |
| Don’t know | 12 (2) | 0 | 12 (3) |
HNPCC = hereditary non-polyposis colon cancer
p<0.001 for difference between risk groups
Attitudes and beliefs about CRC screening
Participants decidedly agreed (90%) that colonoscopy was effective at detecting CRC (data not shown). As indicated in Table 3, the majority (>90%) endorsed statements affirming the benefits of colon screening as part of good general health care, and disagreed (>90%) with statements that refuted the need for screening due to such factors as age, having a healthy diet, or a history of negative screening exams. The only statement that resulted in any disparate response between risk groups pertained to symptoms, “I won’t have screening unless I have bowel or abdominal symptoms.” Overall, about 16% of all participants agreed with this statement and HR participants were more likely than HNPCC participants to agree (18% vs. 8%).
Barriers to CRC screening
Self-reported barriers to CRC screening are shown in Table 4. About 70% of participants reported at least one barrier to screening. The median number of barriers reported was two (range=0 to 14). The most commonly cited barriers were anxiety over results (20%), cost (22%), lack of symptoms (34%), fear of pain (21%), busy schedule (26%), worry about test preparation (25%), fear of the test (23%), and lack of a physician recommendation (16%). Seventy-two percent of the HR participants and 54% of the HNPCC participants reported one or more barrier to CRC screening. HR participants were more likely than HNPCC participants to report lack of a physician referral, cost, fear, pain, lack of symptoms, and being too busy as important barriers to screening.
Table 4.
Baseline data: Self-Reported Barriers to colon cancer screening overall and by risk level
| Statement | Overall (632) |
Risk Level | |
|---|---|---|---|
| HNPCC† (165) | HR† (467) | ||
| % yes | % yes | ||
| I am too young or too old. | 5 | 4 | 6 |
| I feel anxious about the results. | 20 | 19 | 20 |
| The cost is too high. | 22 | 15 | 24* |
| I don’t have a doctor. | 6 | 5 | 6 |
| The tests are embarrassing. | 11 | 7 | 13 |
| My doctor hasn’t recommended it. | 16 | 7 | 20* |
| The tests are frightening or intimidating. | 23 | 16 | 25* |
| I have other health problems. | 13 | 6 | 16 |
| I have no symptoms or problems. | 34 | 19 | 40* |
| I think that the tests could be painful. | 21 | 13 | 24* |
| I fear that I could be injured. | 9 | 3 | 10 |
| I have a busy schedule. | 26 | 16 | 30* |
| I feel it is unnecessary. | 4 | 2 | 5 |
| I feel the FOBT is disgusting. | 11 | 10 | 11 |
| I am worried about the preparation for endoscopy | 25 | 21 | 26 |
| I don’t have insurance that covers it. | 15 | 11 | 16 |
| I feel the screening doesn’t work. | 1 | 1 | 1 |
| Total reporting any barrier N (%) | 423 (67%) | 89 (54%) | 334 (72%)** |
HNPCC = hereditary non-polyposis colon cancer; HR = High Risk
p<0.05 for difference between risk groups
p<0.01 for difference between risk groups
Perceived risk of CRC and knowledge of familial risk
Over 80% of participants believed that their risk of developing CRC was ‘a little’ or ‘much higher’ than people the same age without similar family history of CRC (Table 5). Nearly 70% of the HNPCC group felt their risk was ‘much higher’ than others (only 35% in HR). The majority of participants also indicated they were moderately or very concerned about getting CRC (70%) and felt their own chances of getting CRC were greater given the diagnosis of CRC in a family member (87%). A substantial proportion of participants (34% of HNPCC and 16% of HR) were very concerned about getting CRC and felt that their chances of getting CRC were a lot more given the diagnosis of CRC in their family (73% and 39%).
When asked how important genetics and/or family history of CRC is in causing CRC, over half (53%) of participants thought it was ‘very’ important and another 40% thought it was somewhat important (data not shown). Only 40% of participants had ever heard of genetic testing for CRC (63% HNPCC; 32% HR; p<0.05);14% had been advised to have testing and 3% reported having had genetic testing.
Discussion
Previous research, largely in average risk individuals, has shown that various attitudes, beliefs, risk perceptions, and self-reported barriers predict adherence to CRC screening, reflecting key theory-based constructs or targets for educational interventions [16,47–51] Relatively few studies have examined the frequency of these factors and how they relate to screening adherence among individuals at increased risk for CRC due to family history [16–20] Moreover, very few prospective studies have assessed how modifying these factors, for example knowledge of genetic risk, may affect screening adherence in these high risk groups [12–13,16] The FHPP is a novel prospective trial designed to test the effectiveness of a telephone counseling intervention to promote colonoscopy screening among two high risk cohorts: HNPCC and non-HNPCC high risk (HR) family members. Using Motivational Interviewing techniques, the single-session counseling intervention incorporates educational messages about familial risk and appropriate screening intervals and mitigates barriers to screening in order to increase colonoscopy adherence. Information from the baseline assessment reported here, is used to tailor the intervention to individual participants.
Data from the baseline survey revealed surprisingly low colonoscopy screening rates in these high-risk groups. Despite their high risk profile, and despite the fact that all participants were members in one of two high risk registries, nearly a third of the HR group did not report a prior colonoscopy (5% in the HNPCC group), and baseline adherence to current CRC screening guidelines for the HNPCC group was only 56% (we could not estimate baseline adherence among the HR risk due to exclusion of participants that had colonoscopy within the previous 3 years). Also alarming was that only 50% of participants (HNPCC = 55%, HR = 50%) expressed intention to have colonoscopy in the next 1–2 years, though all were due for colonoscopy during this time frame.
These low rates of colonoscopy screening and intentions to screen within the recommended interval underscore a significant public health challenge, particularly since colonoscopy screening offers both primary and secondary prevention opportunities for reducing CRC burden. These findings also raise the key question of how much these high rates of non-adherence and low intentions to screen could be improved through educational interventions and outreach. Importantly, results from these analyses can help inform efforts to meet this critical challenge. For example, these results indicate that both risk groups had strongly positive attitudes toward CRC screening and were aware of the efficacy of colonoscopy screening, suggesting that screening efficacy messages may be less effective as a central theme in educational interventions to promote colonoscopy in these groups. The same conclusion can be inferred for various attitudes and beliefs regarding the need for CRC screening. For example, attitudes and beliefs that might minimize the perceived need for screening were almost universally rejected in both high risk groups (e.g., eating a healthy diet, having a rectal exam, screening not useful, diagnosis would occur too late, etc.).
Based on this study, risk perceptions regarding CRC and tailored barriers counseling would seem to offer more opportunities for intervention. A substantial percentage of participants in both risk groups (20–30%) were unaware of their elevated risk status suggesting the value of tailored risk messages to correct this misinformation. Even more opportunities to promote CRC screening in these groups may exist for tailored barriers counseling. About 55% of the HNPCC group reported at least one barrier to screening, compared to 70% of the HR group. Underscoring the need for tailored barriers counseling is the fact that no single barrier was dominant for either group, although a core set of barriers did emerge. These included no symptoms, preparation for endoscopy, fear and anxiety about the test and test results, cost, no doctor recommendation, and convenience factors, all of which have been reported previously [16,47–51] The overall prevalence of barriers was higher within the HR group and several specific barriers were also more prevalent including: having no symptoms (40% vs. 19%) and no doctor recommendation (20% vs. 7%). The fact that the HNPCC group reported fewer barriers and lower frequency of all specific barriers may reflect that this group had more experience with colonoscopy, is better educated about screening because of their stronger family history, and/or that barriers might seem less important in light of their increased risk. It is also plausible that by virtue of their exceptionally high risk status, health care providers may be more likely to stress the importance of CRC screening and to make referrals for screening to HNPCC family members.
While risk perception and barrier messages would appear to offer important opportunities for intervention, perhaps the single greatest opportunity to promote adherence to CRC may also be the easiest to remedy. Nearly 80% of the HNPCC group was not aware that they should be screened every 1–2 years, and 40% of the HR group was not aware that they should be screened no less than every 5 years. Our data also suggest that low intentions to screen according to current guidelines may be a function of not knowing what is recommended. For both risk groups, the percentage of participants who planned to undergo screening within 2 years was nearly double among those who were aware of the recommended guidelines.
Taken together, these findings suggest several key considerations for designing health education programs to promote colonoscopy screening among high risk groups. First, in light of the positive attitudes and beliefs about colonoscopy screening but the low level of intentions to get screened within recommended guidelines it would appear that one major goal should be to increase the rate of intentions to be screened. Second, the apparent widespread lack of information about current guidelines for CRC screening in these two high risk groups, suggests that providing this information could in itself serve as a catalyst to be screened. Tailored risk messages might also serve this same function, since about 20%-30% of these high risk individuals were unaware of their elevated risk status. Third, our data suggest that the optimal intervention would be one that also facilitates movement from intentions to action by addressing barriers to screening, where such efforts can anticipate a core set of barriers as described above. Thus, we conclude that the optimal intervention would seem to be one that must remain flexible and individualized, that attempts to identify knowledge gaps and to resolve ambivalence regarding intentions (if needed), and that then strategically helps in the transition from intentions to action. Importantly, this is precisely the type of intervention being tested in FHPP using a telephone-based MI intervention, the results of which will be forthcoming.
This study has several important strengths. Namely, the FHPP is one of very few prospective intervention trials designed to promote colonoscopy screening in members of high risk families. Moreover, the baseline assessment represents a large, population-based sample of high risk individuals that collected more detailed information ever reported for these high risk groups. Several important limitations of this study should also be noted. Both high risk groups were predominately Caucasian, highly educated, had health insurance and a regular source of health care and thus might not accurately represent lower socioeconomic or minority populations. Moreover, because we excluded HR participants that had had colonoscopy in the previous 3 years, participants in our HR group may not fully represent the distribution of attitudes and behaviors of HR individuals in the general population who are adherent with CRC screening. Another study limitation is that the sample consisted of self-selected participants in two high risk cancer registries. It is likely that their interest in joining these registries signals increased awareness and concern for their familial risk, which may not generalize to other comparable high risk groups in the community. Although the registries have not systematically disseminated information about colonoscopy to their participants, they send out annual newsletters and serve as an educational resource for those participants who request information. Thus, by virtue of their participation in these registries, our participants may be more motivated and educated about screening than would high risk individuals in the general population.
Finally, while these study limitations should be acknowledged, it is also important to note that these limitations are likely to yield results that underestimate the challenge of promoting CRC screening among high risk groups in general, including similar populations who are not enrolled in high risk registries and those who are likely to be more underserved based on income, education and race/ethnicity. For these populations, their knowledge, attitudes, beliefs, risk perceptions and behavioral intentions to be screened may be less supportive of CRC screening than the results of this study would indicate, and their rates of non-adherence to CRC screening guidelines may actually be greater than suggested in this study. Accordingly, while the results obtained from this study underscore the need to continue developing and testing interventions to promote CRC screening among high risk individuals, this need may be even more compelling than indicated by this analysis.
Supplementary Material
Acknowledgments
Funded by National Cancer Institute, Grant #5R01CA68099
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ries L, Melbert D, Krapcho M, Stinchcomb D, Howlader N, Horner M, et al. SEER Cancer Statistics Review 1975–2005. Bethesda, MD: National Cancer Institute; 2008. http://seer.cancer.gov/csr/1975-2005/, based on November 2007 SEER data submission, posted to the SEER web site. [Google Scholar]
- 2.American Cancer Society. Colorectal Cancer Facts and Figures 2011–13. Atlanta: American Cancer Society; 2011. [Google Scholar]
- 3.Levin B, Lieberman DA, McFarland B, et al. American Cancer Society Colorectal Cancer Advisory Group; US Multi-Society Task Force; American College of Radiology Colon Cancer Committee. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer and the American College of Radiology. Gastroenterology. 2008;134:1570–95. doi: 10.1053/j.gastro.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 4.Fuchs CS, Giovannucci EL, Colditz GA, Hunter DJ, Speizer FE, Willet WC. A prospective study of family history and the risk of colorectal cancer. N Eng J Med. 1994;331:1669–1674. doi: 10.1056/NEJM199412223312501. [DOI] [PubMed] [Google Scholar]
- 5.Burt RW, DiSario JA, Cannon-Albright L. Genetics of colon cancer: impact of inheritance on colon cancer risk. Annu Rev Med. 1995;46:371–379. doi: 10.1146/annurev.med.46.1.371. [DOI] [PubMed] [Google Scholar]
- 6.Vasen HF, Wijnen JT, Menko FH, et al. Cancer risk in families with hereditary nonpolyposis colorectal cancer diagnosed by mutation analysis. Gastroenterology. 1996 Apr;110(4):1020–7. doi: 10.1053/gast.1996.v110.pm8612988. [DOI] [PubMed] [Google Scholar]
- 7.Aarnio M, Sankila R, Pukkala E, et al. Cancer risk in mutation carriers of DNA-mismatch repair genes. Int J Cancer. 1999 Apr 12;81(2):214–8. doi: 10.1002/(sici)1097-0215(19990412)81:2<214::aid-ijc8>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 8.National Comprehensive Cancer Network (NCCN) [Accessed August 2010];Clinical practice guidelines in oncology: colon cancer, Version 1.2011. http://www.nccn.org/professionals/physician_gls/PDF/colon.pdf.
- 9.Jarvinen HJ, Aarnio M, Mustonen H, et al. Controlled 15-year trial on screening for colorectal cancer in families with hereditary nonpolyposis colorectal cancer. Gastroenterology. 2000;118:829–834. doi: 10.1016/s0016-5085(00)70168-5. [DOI] [PubMed] [Google Scholar]
- 10.Renkonen-Sinisalo L, Aarnio M, Mecklin JP, Jarvinen HJ. Surveillance improves survival of colorectal cancer in patients with hereditary nonpolyposis colorectal cancer. Cancer Detect Prev. 2000;24:137–142. [PubMed] [Google Scholar]
- 11.Niv Y, Dickman R, Figer A, Abuksis G, Fraser G. Case-control study of screening colonoscopy in relatives of patients with colorectal cancer. Am J Gastroenterology. 2003;98:486–489. doi: 10.1111/j.1572-0241.2003.07258.x. [DOI] [PubMed] [Google Scholar]
- 12.Hadley DW, Jenkins JF, Dimond E, de Carvalho M, Kirsch I, Palmer CG. Colon cancer screening practices after genetic counseling and testing for hereditary nonpolyposis colorectal cancer. J Clin Oncol. 2004;22:39–44. doi: 10.1200/JCO.2004.06.128. [DOI] [PubMed] [Google Scholar]
- 13.Halbert CH, Lynch H, Lynch J, et al. Colon cancer screening practices following genetic testing for hereditary nonpolyposis colon cancer (HNPCC) mutations. Arch Intern Med. 2004;164:1881–1887. doi: 10.1001/archinte.164.17.1881. [DOI] [PubMed] [Google Scholar]
- 14.Kinney A, Hicken B, Simonsen S, et al. Colorectal cancer surveillance behaviors among members of typical and attenuated FAP families. Am J Gastoenterology. 2007 Jan;102(1):153–62. doi: 10.1111/j.1572-0241.2006.00860.x. [DOI] [PubMed] [Google Scholar]
- 15.Fletcher RH, Lobb R, Bauer MR, et al. Screening patients with a family history of colorectal cancer. J Gen Intern Med. 2007;22:508–513. doi: 10.1007/s11606-007-0135-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rees G, Martin PR, Macrae FA. Screening participation in individuals with a family history of colorectal cancer: a review. Eur J Cancer Care. 2007;17:221–32. doi: 10.1111/j.1365-2354.2007.00834.x. [DOI] [PubMed] [Google Scholar]
- 17.Manne S, Markowitz A, Winawer S, et al. Correlates of colorectal cancer screening compliance and stage of adoption among siblings of individuals with early onset colorectal cancer. Health Psych. 2002;21:3–15. [PubMed] [Google Scholar]
- 18.Codori AM, Petersen GM, Migliorett DL, Boyd P. Health beliefs and endoscopic screening for colorectal cancer: potential for cancer prevention. Prev Med. 2002;33:128–136. doi: 10.1006/pmed.2001.0862. [DOI] [PubMed] [Google Scholar]
- 19.Madlensky L, Esplen MJ, Galinger S, McGlaughlin JR, Goel V. Relatives of colorectal cancer patients – factors associated with screening behavior. Am J Prev Med. 2003;25:187–194. doi: 10.1016/s0749-3797(03)00202-2. [DOI] [PubMed] [Google Scholar]
- 20.Bleiker EMA, Menko FH, Taal BG, et al. Screening behavior of individuals at high risk for colorectal cancer. Gastroenterology. 2005;128:280–287. doi: 10.1053/j.gastro.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 21.Anton-Culver H, Ziogas A, Finkelstein D, et al. Cancer Genetics Network: Recruitment results and pilot studies. Community Genetics. 2003;6(3):171–177. doi: 10.1159/000078165. [DOI] [PubMed] [Google Scholar]
- 22.Newcomb PA, Baron J, Cotterchio M, et al. Colon Cancer Family Registry: an international resource for studies of the genetic epidemiology of colon cancer. Cancer Epidemiol Biomarkers Prev. 2007 Nov;16(11):2331–43. doi: 10.1158/1055-9965.EPI-07-0648. [DOI] [PubMed] [Google Scholar]
- 23.Lundahl B, Burke BL. The effectiveness and applicability of motivational interviewing; A practice-friendly review of four meta analyses. Journal of Clinical Psychology. 2009;65(11):1232–1245. doi: 10.1002/jclp.20638. [DOI] [PubMed] [Google Scholar]
- 24.Miller WR, Rose GS. Toward a theory of motivational interviewing. AM Psychol. 2009;64(6):527–537. doi: 10.1037/a0016830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bonis PA, Trikalinos TA, Chung M, et al. Hereditary non-polyposis colorectal cancer: diagnostic strategies and their implications. Evid Rep Technol Assess. 2007 May;(150):1–180. [PMC free article] [PubMed] [Google Scholar]
- 26.Janz NK, Becker MH. The Health Belief Model: A decade later. Health Education Quarterly 1984. 1984;11(1):1–47. doi: 10.1177/109019818401100101. [DOI] [PubMed] [Google Scholar]
- 27.Janz NK, Champion VL, Strecher VJ. The Health Belief Model. In: Glanz K, Rimer BK, Lewis FM, editors. Health Behavior and Health Education: Theory, Research and Practice. 3. Jossey Bass; San Francisco, CA: 2002. pp. 45–66. [Google Scholar]
- 28.Ajzen I. Theory of planned behavior. Organizational Behavior and Human Decision Processes. 1991;50:179–211. [Google Scholar]
- 29.Ajzen I, Madden TJ. Prediction of goal-directed behavior: Attitudes, intentions and perceived behavioral control. Journal of Experimental Social Psychology. 1986;22:453–474. [Google Scholar]
- 30.Montano DE, Kasprzyk D. The Theory of Reasoned Action and The Theory of Planned Behavior. In: Glanz K, Rimer BK, Lewis FM, editors. Health Behavior and Health Education: Theory, Research and Practice. 3. Jossey Bass; San Francisco, CA: 2002. pp. 67–98. [Google Scholar]
- 31.Prochaska JO, DiClemente CC. Stages and processes of self-change of smoking: Toward an integrative model of change. Journal of Consulting and Clinical Psychology. 1983;51:390–395. doi: 10.1037//0022-006x.51.3.390. [DOI] [PubMed] [Google Scholar]
- 32.Prochaska JO. Strong and weak principles for progressing from precontemplation to action based on twelve problem behaviors. Health Psychology. 1994;13:47–51. doi: 10.1037//0278-6133.13.1.47. [DOI] [PubMed] [Google Scholar]
- 33.Prochaska JO, Redding CA, Evers KE. The Transtheoretical Model and stages of change. In: Glanz K, Rimer BK, Lewis FM, editors. Health Behavior and Health Education: Theory, Research and Practice. 3. Jossey Bass; San Francisco, CA: 2002. pp. 99–120. [Google Scholar]
- 34.Miller WR, Rollnick . Motivational interviewing: 2nd edition: Preparing people to change. The Guilford Press; New York, NY: 2002. [Google Scholar]
- 35.Rollnick S, Miller WR, Butler CC. Motivational Interviewing in Health Care: Helping Patients Change Behavior. New York, New York: The Guilford Press; 2008. [Google Scholar]
- 36.Wilson GT, Schlam TR. The transtheoretical model and motivational interviewing in the treatment of eating and weight disorders. Clinical Psychology Review. 2004;24(3):361–78. doi: 10.1016/j.cpr.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 37.Miller WR. Motivational interviewing in service to health promotion. American Journal of Health Promotion. 2004;18(3):A1–A10. [Google Scholar]
- 38.Garrett K, Heimendinger J, Barnes D, Coffey-Kluhsman B, O’neil C, Vanonni C. Healthy options and personal exploration (HOPE): Results of a telephone counseling study using motivational interviewing and print material to address the informational and healthy lifestyle concerns of breast cancer survivors. Abstracted submitted to the APOS 5th Annual Conference; Feb 28 – March 2, 2008. [Google Scholar]
- 39.Ludman EJ, Curry SJ, Meyer D, Taplin SH. Implementation of outreach telephone counseling to promote mammography participation. Health Educ Behav. 1999;26(5):689–702. doi: 10.1177/109019819902600509. [DOI] [PubMed] [Google Scholar]
- 40.Wahab S, Menon U, Szalacha L. Motivational interviewing and colorectal cancer screening: A peek from the inside out. Patient Education and Counseling. 2008;72:210–217. doi: 10.1016/j.pec.2008.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marcus A, Ahnen D, Cutter G, Calonge N, Russell S, Sedlacek S, Wood M, Manchester D, Fox L, McCaskill-Stevens W, Fairclough D, Hines S, Wenzel L, Osborn K. Promoting Cancer Screening among the First-Degree Relatives of Breast and Colorectal Cancer Patients: The Design of Two Randomized Trials. Preventive Med. 1999 Mar;28(3):229–242. doi: 10.1006/pmed.1998.0408. [DOI] [PubMed] [Google Scholar]
- 42.Vernon SW, Tiro JA, Vojvodic RW, et al. Reliability and validity of a questionnaire to measure colorectal cancer screening behaviors: does mode of survey administration matter? Cancer Epidemiol Biomarkers Prev. 2008;17:758–67. doi: 10.1158/1055-9965.EPI-07-2855. [DOI] [PubMed] [Google Scholar]
- 43.Partin MR, Grill J, Noorbaloochi S, et al. Validation of self-reported colorectal cancer screening behavior from a mixed-mode survey of veterans. Cancer Epidemiol Biomarkers Prev. 2008;17:768–76. doi: 10.1158/1055-9965.EPI-07-0759. [DOI] [PubMed] [Google Scholar]
- 44.Rauscher GH, Johnson TP, Cho YI, Walk JA. Accuracy of self-reported cancer screening histories: a meta-analysis. Cancer Epidemiol Prev. 2008;17:748–57. doi: 10.1158/1055-9965.EPI-07-2629. [DOI] [PubMed] [Google Scholar]
- 45.Baron RM, Kenny DA. The moderator-mediator distinction in social psychological research: conceptual, strategic and statistical considerations. J Pers Soc Psych. 1986;51:1173–82. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- 46.Holmbeck N. Toward terminology, conceptual and statistical clarity in the study of mediators and moderators: examples from the child-clinical and pediatric psychology literatures. J Consult Clin Psych. 1997;65:599–610. doi: 10.1037//0022-006x.65.4.599. [DOI] [PubMed] [Google Scholar]
- 47.Subramanian S, Klosterman M, Amonkar MM, Hunt TL. Adherence with colorectal cancer screening guidelines: a review. Prev Med. 2004;38:536–50. doi: 10.1016/j.ypmed.2003.12.011. [DOI] [PubMed] [Google Scholar]
- 48.Beydoun HA, Beydoun MA. Predictors of colorectal cancer screening behaviors among average-risk older adults in the United States. Cancer Causes and Control. 2008;19:339–359. doi: 10.1007/s10552-007-9100-y. [DOI] [PubMed] [Google Scholar]
- 49.Guessous I, Dash C, Lapin P, Doroshenk M, Smith R, Klabunde CN. Colorectal cancer screening barriers and facilitators in older persons. Prev Med. 2010;50:3–10. doi: 10.1016/j.ypmed.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 50.Berkowitz Z, Hawkins NA, Peipins LA, White MC, Nadel MR. Beliefs, risk perceptions, and gaps in knowledge as barriers to colorectal cancer screening in older adults. J Am Geriatr Soc. 2008 Feb;56(2):307–14. doi: 10.1111/j.1532-5415.2007.01547.x. [DOI] [PubMed] [Google Scholar]
- 51.Klabunde CN, Vernon SW, Nadel MR, Breen N, Seeff LC, Brown ML. Barriers to colorectal screening: a comparison of reports from primary care physicians and average risk adults. Med Care. 2005 Sep;43(9):939–44. doi: 10.1097/01.mlr.0000173599.67470.ba. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.