Abstract
Atrazine is the most commonly detected pesticide contaminant of ground water, surface water, and precipitation. Atrazine is also an endocrine disruptor that, among other effects, alters male reproductive tissues when animals are exposed during development. Here, we apply the nine so-called “Hill criteria” (Strength, Consistency, Specificity, Temporality, Biological Gradient, Plausibility, Coherence, Experiment, and Analogy) for establishing cause–effect relationships to examine the evidence for atrazine as an endocrine disruptor that demasculinizes and feminizes the gonads of male vertebrates. We present experimental evidence that the effects of atrazine on male development are consistent across all vertebrate classes examined and we present a state of the art summary of the mechanisms by which atrazine acts as an endocrine disruptor to produce these effects.
Atrazine demasculinizes male gonads producing testicular lesions associated with reduced germ cell numbers in teleost fish, amphibians, reptiles, and mammals, and induces partial and/or complete feminization in fish, amphibians, and reptiles. These effects are strong (statistically significant), consistent across vertebrate classes, and specific. Reductions in androgen levels and the induction of estrogen synthesis – demonstrated in fish, amphibians, reptiles, and mammals – represent plausible and coherent mechanisms that explain these effects. Biological gradients are observed in several of the cited studies, although threshold doses and patterns vary among species. Given that the effects on the male gonads described in all of these experimental studies occurred only after atrazine exposure, temporality is also met here. Thus the case for atrazine as an endocrine disruptor that demasculinizes and feminizes male vertebrates meets all nine of the “Hill criteria”.
Keywords: Atrazine, Gonads, Endocrine disruptor
1. Introduction
Atrazine is a triazine herbicide used primarily on corn [1]. Atrazine is the most commonly detected pesticide contaminant of ground, surface, and drinking water [1-12], and can even be found in rainwater [13-18]. As early as 1997, Crain et al. [19] suggested that atrazine is an endocrine disruptor capable of inducing aromatase and leading to inappropriate and excess estrogen production and in 1998 Reeder et al reported an association between atrazine and intersex gonads in amphibians in the wild [20]. Shortly after this initial report, in 2000, Sanderson et al. [21-23] characterized the effect of atrazine on aromatase in more detail and suggested that “a logical concern would be that exposure of wildlife and humans to triazine herbicides, which are produced and used in large quantities, and are ubiquitous environmental contaminants, may similarly contribute to estrogen-mediated toxicities and inappropriate sexual differentiation” [23]. In this same year, an EPA study concluded that “atrazine tested positive in the pubertal male screen that the Endocrine-Disrupter Screening and Testing Advisory Committee (EDSTAC) is considering as an optional screen for endocrine disrupters” [24].
Several studies in amphibians have suggested that atrazine is associated with feminized males in the wild [25-27]. In field studies, atrazine has repeatedly been associated with the presence of testicular oocytes [20,25-27] as well as feminized secondary sex characteristics in male frogs [28]. As recognized by Sir Austin Bradford Hill in 1965, however, “diseases can have more than one cause” [29] and other endocrine disrupters with mechanisms consistent with demasculinization and feminization of animals have been identified [30-32]. In fact, a retrospective study in amphibians showed that testicular oocytes were detected in museum specimens in Illinois prior to the introduction of atrazine [33], so atrazine may only be responsible for a subset of the recent elevations in such findings. Indeed other environmental contaminants have been shown to feminize amphibians also [34-37]. Because of the complexity of exposures to chemical and other stressors in the field, such eco-epidemiological studies must be coupled with controlled laboratory investigations to ensure reliable attribution of observed changes to specific causes. To establish a cause–effect relationship, Hill described nine “criteria” that should be examined [29]: Experimentation, Consistency, Strength, Specificity, Temporality, Biological Gradient, Plausibility, Coherence, and Analogy. Below, we evaluate the evidence for a cause–effect relationship between atrazine and demasculinization and feminization of male vertebrates using these nine criteria and summarize the many documented mechanisms by which atrazine demasculinizes and feminizes exposed vertebrate males.
1.1. Experimentation
Experimentation was Hill’s eighth criteria. We examine this criterion first because it provides the strongest support for atrazine as an endocrine disruptor. In fact, regarding experimentation, Hill wrote: “Occasionally it is possible to appeal to experimental, or semi-experimental, evidence” [29]. “Here the strongest support for the causation hypothesis may be revealed” [29]. Experimental evidence is the strongest (according to Hill) because controlled experiments allow scientists to address the strength of the association, consistency, specificity, temporality, biological gradients, and to examine potential mechanisms for plausibility, coherence, and analogy: the remaining eight of the nine so-called “Hill criteria”. Below, we present experimental evidence that shows strong, consistent, specific effects of atrazine on male gonadal development and present a plausible coherent mechanism.
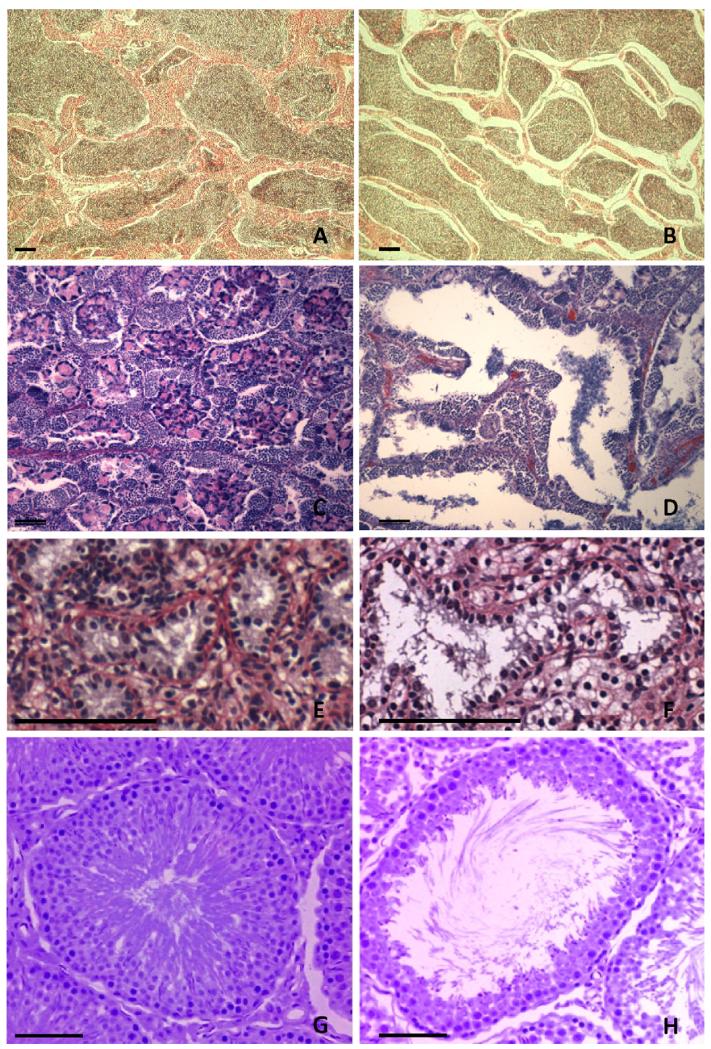
Atrazine is a gonadotoxin in males. Atrazine demasculinizes the gonads of exposed male teleost fish, amphibians, reptiles and mammals. We define “demasculinization” of male gonads as a decrease in male gonadal characteristics including decreases in testicular size, decreases in Sertoli cell number, decreases in sperm production, and decreases in androgen production. Atrazine exposure has been reported to disrupt testicular development, resulting in testicular lesions (loss of testicular tissue) in all vertebrates classes examined except birds. In fish, atrazine causes degeneration of interstitial tissue in the testes, but it has not been reported whether germ cells or Sertoli cells are the also targets [38] (Fig. 1). In amphibians [39], reptiles [40] and mammals [41,42], however, atrazine has nearly identical (specific) effects (Fig. 1). Atrazine exposure in these vertebrate classes results in increases in the size of testicular tubules, loss of Sertoli cells, and a marked loss of germ cells, often leaving the testicular tubules empty or only with what appears to be cellular debris ([39-42], Fig. 1).
Fig. 1.
Atrazine-induced histologic lesions in testes of vertebrates. Histologic sections from a fish (A and B), an amphibian (C and D), a reptile (E and F) and a mammal (G and H) are shown. Histologic sections of testes of goldfish (Carassius auratus) controls (A) and after 21 days of exposure to water containing atrazine at 1,000 μg/L (B). Note the progressive increase in gaps in the interstitium between lobules. Sections were stained with Regaud’s hematoxylin, phloxine and light green. For details see Spano et al. [38]. Histological section of testes in African clawed frogs (Xenopus laevis) controls (C) and after exposure to atrazine at 2.5 μg/L throughout larval and postmetamorphic development (D). Sections were stained in Harris’ hematoxylin and eosin. For details see Hayes et al. [39]. Photomicrographs showing seminiferous tubules from a vehicle (E) and an atrazine-treated (F) caiman. Tissue sections were stained with Picrosirius solution and counterstained with Harris hematoxylin. For details, see Rey et al. [40]. Testicular tubules of control rats (G) and tubule of rats given atrazine at 200 mg/kg by gavage for 15 days (H). Atrazine-exposed rats were characterized by luminal dilation. Extended dosing up to 40 days resulted in testicular atrophy, which was mostly formed by Sertoli-only tubules (not shown). Sections were stained with hematoxylin and eosin. For details see Victor-Costa et al. [41]. Bar = 100 μ in all panels.
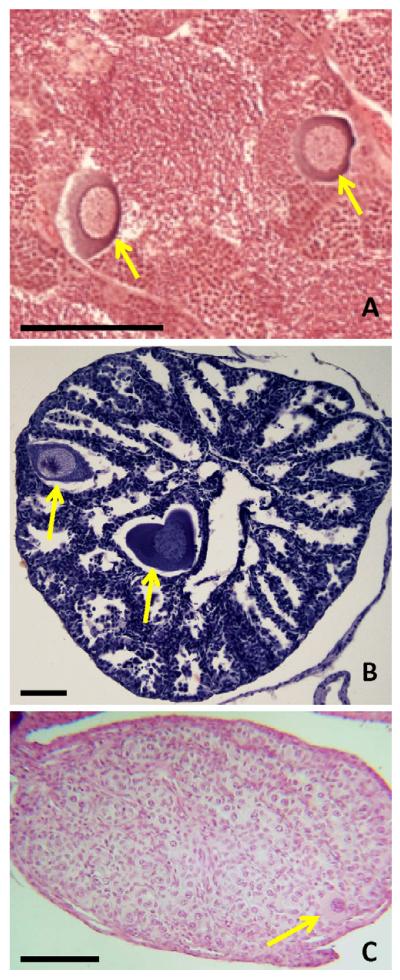
In addition to the demasculinization effects described above (testicular lesions), atrazine feminizes the gonads of developing male teleost fish (hereafter referred to simply as “fish”) [43] (Fig. 2), amphibians [26,44-46], and reptiles [47]. “Feminization” of male gonads is defined as the development of oocytes in the testes or complete ovarian differentiation in genetic males leading to decreases in the frequency of morphologic males in the exposed population. The loss of male germ cells, described above, is accompanied by the development of female germ cells (testicular oocytes) in the testes in some cases ([25,26,43,45,47], Fig. 2). This effect has been reported in fish [43], amphibians [26,44,45], and reptiles [47], but has not been reported in birds or mammals.
Fig. 2.
Partial feminization by atrazine in vertebrates. Testicular oocytes are induced by atrazine in fish (A), amphibians (B), and reptiles (C). Testes from an adult fathead minnow (Pimephales promelas) (A) exposed to water containing atrazine at 5 μg/L for 14 days presenting multiple testicular oocytes within the gametogenic and supportive cellular structures of the testes (Photo courtesy Diana Papoulias, U.S. Geological Survey, Columbia Environmental Research Center, Columbia, MO, USA). See Tillitt et al. [43] for details of experimental conditions. Testicular oocytes in the testes of a male Rana pipiens [B] exposed to atrazine at 0.1 μg/L. Section stained in Harris’ hematoxylin and eosin. For details see Hayes et al. [26]. Testicular oocyte (stained in Harris’ hematoxylin and eosin) in a snapping turtle (Chelydra serpentine) exposed to soil treated with atrazine at a typical application rate (3.1 L/ha). For details see de Solla et al. [47]. Bar = 100 μ in all panels.
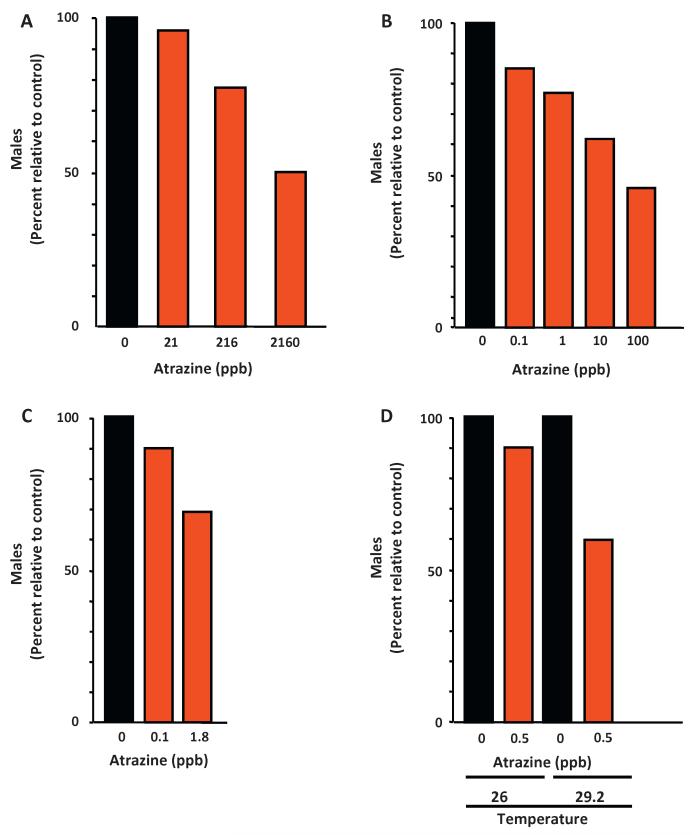
Atrazine can also result in complete feminization of males. At least one study in fish [48], three studies in amphibians [39,49,50], and two studies in reptiles [47,51] have causes significant shifts in sex ratios toward females (Fig. 3). In fish, the effect is manifested by skewed sex ratios in zebrafish (Danio rerio), which have no distinguishable sex chromosomes [48]. In amphibians, shifts in the sex ratio have been documented [49,50], and one study used genetic markers in Xenopus laevis to show that indeed genetic males were converted into complete functional females after exposure to atrazine [39]. Finally, in reptiles, two studies in two different species (of different genera and families) with temperature-dependent sex determination, examined the effect of atrazine on sex differentiation. Atrazine caused female biased sex ratios compared to controls near the transitional male–female temperatures [51], and turtles with testicular oocytes were found only in atrazine treatments [47]. Thus, experimental data support the hypothesis that atrazine both demasculinizes male gonads in all vertebrate classes examined with the possible exception of birds and feminizes the gonads of male vertebrate ectotherms (fish, amphibians, and reptiles).
Fig. 3.
Complete sex reversal by atrazine in vertebrates. Atrazine exposure causes a loss of males in exposed fish (A), amphibians (B and C), and reptiles (D). In fish, atrazine exposure produced a concentration-dependent decrease in the frequency of males [48]. (A) Similarly atrazine produced a concentration-dependent decrease in the frequency of males in African clawed frogs (Xenopus laevis) in the laboratory [49] (B) and in leopard frogs (Rana pipiens) exposed in semi-field conditions [50] (C). In red-eared sliders (Trachemys scripta elegans), atrazine seemed to reduce the number of males slightly at 26°, and a statistically significant reduction was evident at 29.2° [51] (D). In all cases, data are shown as percent males in experimental groups relative to controls. Figures A and B were adapted from Hayes et al. [39]. Original data for panel C from Langlois et al. [50] and original data were from panel D from Willingham et al. [51].
1.2. Consistency
With regards to consistency (Hill’s second criterion), Hill wrote, “Whether chance is the explanation or whether a true hazard has been revealed may sometimes be answered only by a repetition of the circumstances and observations” [29]. Hill queried further, “Has it (the effect) been repeatedly observed by different persons, in different places, circumstances and times?” Moreover, he stated, “I would myself put a good deal of weight upon similar results reached in quite different ways.” In fact, this scenario is revealed here: demasculinizing effects of atrazine on developing male gonads across vertebrate classes (fish, amphibians, reptiles, and mammals), as well as partial and complete feminization of male gonads across three vertebrate classes (fish, amphibians, and reptiles). These studies used different routes of exposure, varying doses, and widely varying experimental conditions (see references cited in Figs. 1-3), yet all found similar effects.
Echoing Hill’s recommendations, Glen Fox wrote: “In ecoepidemiology, the occurrence of an association in more than one species and species population is very strong evidence for causation” [52]. Kniewald et al. [42] and Victor-Costa et al. [41] independently reported identical effects of atrazine on testes in both Fischer [42] and Wistar [41] rats and, indeed, testicular lesions and feminization of male gonads have been consistently observed across vertebrate classes. These effects on the gonads are both specific and consistent and do not occur merely across populations, species, or even genera or orders, but across vertebrate classes: although the loss of testicular tissue may occur primarily in the interstitium in fish, the loss of Sertoli cells and male germ cells in testicular tubules, development of female germ cells (testicular oocytes) in exposed males and complete feminization of males across three classes of vertebrates are specific and consistent effects described by independent laboratories in eight different countries on five continents (see references cited in Figs. 1-3 and work described herein). These effects are also consistent with earlier studies in African clawed frogs (Xenopus laevis) which showed a loss of germ cells and nursing (Sertoli) cells in both males and females exposed during larval stages [53,54].
That is not to say, however, that these effects have been documented in all species in these classes or even in all populations (or studies) within a species. Variation is to be expected and as Hill pointed out: “the different results of a different inquiry certainly cannot be held to refute the original evidence.” [29]. To quote Hill, “The lesson here is that broadly the same answer has been reached in quite a wide variety of situations and techniques. In other words, we can justifiably infer that the association is not due to some constant error or fallacy that permeates every inquiry.” [29]
1.3. Temporality, Specificity, and Strength
The effects described here meet all three of these components of Hill’s criteria. Regarding temporality, Hill believed that the “cause” should precede the effect. In the current case, atrazine exposure should precede demasculinization and feminization. In the experimental evidence presented here, this of course is the case.
In addition, the lesions of the gonads (demasculinization), partial feminization (testicular oogenesis) and complete feminization (sex reversal resulting in female-biased sex ratios) are specific effects. It is important to realize, as Hill pointed out, that diseases may have more than one cause, and a given factor (such as atrazine) may produce more than one disease (effect). This observation is certainly true for atrazine. More than one chemical may induce the gonadal malformations that atrazine induces (see references in Section 1), and atrazine affects more than gonadal development (see Section 2). In the cases presented here, however, atrazine’s effects are supported by controlled experiments in fish, amphibians, reptiles, and mammals, and produce similar specific effects across studies. Thus, the complications associated with identifying cause and effect in epidemiological and eco-epidemiological studies are not of general concern here.
The effects are also strong associations, in the species examined. Hill listed “strength” as his first criterion. Despite the importance of strength in establishing cause and effect, Hill wrote: “In thus putting emphasis upon the strength of an association, we must, nevertheless look at the obverse of the coin. We must not be too ready to dismiss a cause–effect hypothesis merely on the grounds that the observed association appears to be slight” [29]. Hill later goes on, regarding statistics, to caution, “…far too often we deduce ‘no difference’ from ‘no significant difference”’. In the cases presented here, all of the effects are statistically significant, with the exception of some of the findings in snapping turtles (Chelydra serpentina) [47]. But as Hill espoused, statistics are not even necessary in obvious cases, such as here, where the malformations described do not occur in controls in any of the experiments. In the few studies where malformed gonads were observed in controls, the authors reported atrazine contamination in controls above the biologically effective dose [55-57], with the exception of Orton et al. who found intersex animals in control Rana pipiens [46].
1.4. Biological gradient
Here, Hill suggested that some dose–response relationship would lend support for the cause–effect relationship. Although Hill focused on a monotonic linear dose response, he readily acknowledged that in some cases “a much more complex relationship to satisfy the cause and effect hypothesis” must be envisaged. With regard to feminization of males, several studies show just the type of dose response that Hill suggested would support cause and effect. In both zebrafish and African clawed frogs, the frequency of males declines in a dose-dependent fashion in response to increasing doses of atrazine (Fig. 3). In most studies, the proportion of affected males increases with atrazine concentration [26,44-46,56]. With regard to demasculinization (testicular lesions) and partial feminization (testicular oogenesis), quantitative methods are yet to be standardized, thus parametric dose–response analyses of this type are not available.
Regarding the effective doses, the demasculinization effects of atrazine were produced at low ecologically relevant doses (e.g. 2.5 ppb or below) in amphibians. Doses in reptiles are more difficult to interpret because the doses reported in some of these studies are the doses applied to the egg shell and it is not known how much atrazine reached the affected tissues. Nonetheless, snapping turtle eggs readily absorb current-use pesticides, including atrazine, from soil treated with typical application rates (de Solla et al., unpublished data). We hypothesize, however, that the barrier created by the egg shell and membranes may explain why effects in reptiles are less pronounced. In addition, it is possible that the exposure time to atrazine was insufficient in some reptile studies. In rats, the atrazine was delivered by gavage and the higher metabolism [58] in small endotherms likely explains why seemingly higher doses are required for effects in rodents, but comparative studies of atrazine uptake, distribution in the body, and metabolism are not available.
1.5. Plausibility and Coherence
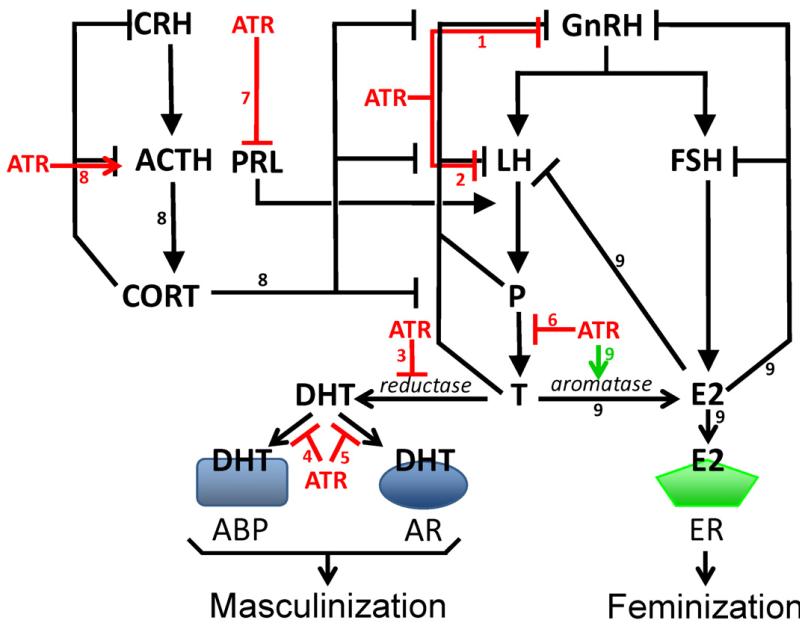
Plausible, coherent mechanisms are available to explain gonadal demasculinization and feminization. Atrazine exposure significantly reduces synthesis, secretion and circulating levels of androgens across vertebrate classes including fish [38,59], amphibians [26,39], reptiles [40], and mammals [24,60] with lesser effects in birds [61]. Androgen production is critical for germ cell differentiation, development and maturation. Thus, limiting androgen production and availability provides a plausible mechanism to explain the demasculinization of gonads in exposed males. Multiple mechanisms likely account for the decreased androgens and decreased androgen activity (summarized in Fig. 4): (1) atrazine inhibits luteinizing hormone (LH) and follicle stimulating hormone (FSH) peaks and surges by inhibiting pulsatile release of gonadotropin releasing hormone (GnRH) from the hypothalamus which leads to decreased androgen synthesis [24,62-66]; (2) atrazine inhibits LH release from the pituitary directly which leads to decreased androgen production [62,64,67,68]; (3) atrazine inhibits the enzyme 5α reductase which leads to decreased levels of the potent androgen dihydrotestosterone (DHT) and leaves more testosterone as substrate for aromatase to convert to estradiol which negatively feeds back on the hypothalamus and pituitary [69,70]; (4) atrazine inhibits binding of DHT to the androgen binding protein [71]; (5) atrazine inhibits interactions between DHT and the androgen receptor(AR) [69,70,72] but perhaps not by directly binding to the receptor [73], but perhaps not by directly inhibiting binding to the receptor (see [78]); (6) atrazine inhibits androgen synthesis in the testes [60,74,75]; (7) atrazine decreases prolactin secretion [63,78,79]. Prolactin promotes LH receptor expression, and thus a decrease in prolactin would lead to a decrease in LH receptors, impairing normal LH-stimulation of testosterone production; (8) atrazine increases adreno-corticotropic hormone (ACTH) secretion from the pituitary leading to increased progesterone and increased corticosteroid (cortisol or corticosterone) secretion [76,77]. Progesterone negatively feeds back on GnRH, LH and FSH and thus decreases androgen production and reproductive function, whereas corticosteroids inhibit the reproductive axes at the hypothalamo (GnRH), anterior pituitary (LH and FSH) and the gonads (androgen production).
Fig. 4.
Multiple mechanisms of action have been identified for atrazine’s demasculinizing and feminizing effects on male gonads. Arrows indicate processes that are increased; bars indicate processes that are inhibited. Red lines indicate demasculinizing pathways that are directly affected by ATR and green lines indicate feminizing pathways that are directly affected by ATR. Numbers on pathways, refer to mechanisms listed in the text (see Section 1.5). ABP = androgen binding protein, ACTH = adrenocorticotropic hormone, AR = androgen receptor, ATR = atrazine, CORT = cortisol/corticosterone, CRH = corticotrophin-releasing hormone, DHT = dihydrotestosterone, E2 = 17β estradiol, ER = estrogen receptor, FSH = follicle stimulating hormone, GnRH = gonadotropin stimulating hormone, LH = luteinizing hormone, P = progesterone, PRL = prolactin., and T = testosterone (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of the article.)
The partial and complete feminization of gonads in fish, amphibians and reptiles are analogous to the effects of estrogen in these vertebrate classes (see Section 1.6). Atrazine could feminize animals by increasing estrogen synthesis, decreasing degradation of endogenous estrogen, or acting as an estrogen receptor agonist. No available evidence suggests that atrazine decreases degradation of endogenous estrogen and this is not a plausible mechanism anyway, because developing males would not have adequate circulating estrogens to stabilize. In addition, atrazine does not bind the estrogen receptor [23,80], so this is not a plausible mechanism either. Several studies, however, suggest that atrazine increases estrogen synthesis. Atrazine induces aromatase in the gonads of fish [48], amphibians [39], and reptiles (in vitro) [19], and in human cell lines [21-23,48,81-83]. Also, atrazine increases circulating estrogens in fish [38,59], amphibians [39], and in mammals (laboratory rats) [24]. Estrogens induce partial and complete feminization in fish [84,85], amphibians [86,87], and reptiles [86,88], so the induction of aromatase and subsequent increases in estrogen synthesis represent a plausible mechanism for the feminization effects.
In addition, atrazine and the atrazine metabolite, deethylatrazine, also inhibit 5α-reductase [66]. Reducing the availability of 5α-reductase in atrazine-exposed male rats, decreases the conversion of testosterone to the potent androgen 5α-dihydrotestosterone [72]. Dihydrotestosterone is not convertible to estrogen; thus, the inhibition of 5α-reductase by atrazine may increase the pool of testosterone available for conversion to estrogen. For example exposure to exogenous testosterone, though not dihydrotestosterone, can cause feminization in turtles [89], through the conversion to estrogen via aromatase. Given the similarity between the effects of exogenous estrogen in vertebrates (see Section 1.6) and atrazine, this plausible mechanism (the induction of aromatase) is coherent.
1.6. Analogy
Hill wrote, “…the cause-and-effect interpretation of our data should not seriously conflict with the generally known facts of the natural history and biology of the disease”. Androgens are necessary for testicular development and maintenance of male germ cells in all vertebrates. Thus, given that atrazine reduces androgen production and stability, it is reasonable to expect the demasculinization effect in all vertebrates. On the other hand, partial or complete sex reversal of gonads by estrogens is limited to fish and amphibians, which lack morphological distinguishable sex chromosomes, and to reptiles with environmental sex determination, which lack sex chromosomes altogether [86]. Birds and mammals, which have genetic sex determination and highly differentiated sex chromosomes are not susceptible to estrogen-induced sex reversal of the gonads [86,90]. As such, while depleting androgens will impair testicular development and induce testicular lesions (such as the effects described here) in all vertebrates, increasing estrogen production (via atrazine) would not be expected to induce feminization of the gonads in birds and mammals, but would do so in fish, amphibians, and reptiles with environmental sex determination.
2. Conclusions
All nine of the Hill criteria are met in the case for atrazine as an endocrine disruptor that alters male gonadal development. Furthermore, effects occur in all vertebrate classes examined with the possible exception of birds, suggesting a ubiquitous problem. Published studies assessing the effects of atrazine on the developing gonads of birds are limited to one study in Japenese quail (Coturnix coturnix japonica) [91] and another in chickens (Gallus gallus domesticus) [92]. When injected into quail eggs at 123, 246, and 504 μg/kg, atrazine had no effect on the sex ratio as determined on day 14 post-hatching, but 504 μg/kg caused a decrease in ovarian weight and in progesterone levels in females [91]. Atrazine had no effect on gonadal weight or circulating estradiol, testosterone, or progesterone in males and no incidences of left ovotestes were observed [91]. In contrast, when atrazine was injected into chicken eggs [92], 0.1 to 3 mg/egg caused retention of the right gonad in female chicks [92]. No effects were observed in males [92]. So based on these two studies, atrazine produces much more subtle effects in birds, relative to other vertebrate taxa and only at high doses. Birds are also probably less likely to be exposed to atrazine in the wild. Given that atrazine is mostly an aqueous contaminant, water fowl maybe more likely affected by atrazine and studies are warranted. Similarly, the absence of data in the only two remaining classes of vertebrates (Agnatha and Chondricthyes) reflects the fact that no published studies on effects of atrazine are available for these taxa.
While morphologic changes found in studies of atrazine-exposed vertebrates are of concern, functional impairments are likely of greatest importance. In fact, male salmon (Salmo salar) exposed to atrazine showed decreased androgen levels, decreased mating behavior, and decreased milt production [59]. Nearly identical reproductive impairments were observed in amphibians (decreased androgens, decreased mating behavior and decreased fertility) [39]. Similarly, atrazine induced poor reproductive performance in rodents [42] and resulted in as much as a 50% decrease in epididymal sperm number and decreased sperm motility [42]. Low fertility, low sperm count, and poor semen quality were also associated with atrazine exposure (as measured by atrazine metabolites in the urine) in humans living in agricultural areas where atrazine was widely used [93]. Furthermore, atrazine is also associated with follicular atresia in females of two species of fish [38,43], limiting the reproductive potential of females as well.
Atrazine is prevalent and persistent in the environment. There are many other documented reproductive effects of atrazine in laboratory rodents: induced abortion [62,64], impaired mammary development [94-96], the induction of reproductive and hormone-dependent cancers [97-102] as well as other non-reproductive effects including impaired immune function (also observed in multiple studies across vertebrate classes) [103-127] and impaired neural development [117,128-132]. Thus, with the additional the indirect effects of atrazine on habitats [133-142], atrazine can have dramatic effects on ecosystems, environmental health and public health.
Acknowledgements
We thank Donald Tillitt and Diana Papoulias, USGS, for the photograph featured in Fig. 2, panel A. Primary research on atrazine was supported as follows: TBH (Novartis, Syngenta Crop Protection, Ecorisk, the National Science Foundation, the World Wildlife Fund, the Alton Jones Foundation, the Homeland Foundation, the Rose Foundation, Park-Water Company, the Biofaculty Award [UCB], the Distinguished Mentor Award [UCB], the Distinguished Teaching Award [UCB], the Mitchell Kapor Foundation, the David Foundation, the Cornell-Douglas Foundation, the Wallace Global Fund, the Class of ’43 Endowed Chair (UCB), the Toxic Substances Research and Teaching Program (UCB), Hewlett Packard, the Biology Fellows Program (Howard Hughes Medical Institute), the McNair Scholars Program (UCB), the Amgen Scholars Program (UCB), and the Mentored Research fellowship program (UCB); LA (USDA grant through the Center for Designing Foods to Improve Nutrition; VRB (John G. Shedd Aquarium Funding through support from the Dr. Scholl Foundation); SRdS (Environment Canada); TI & TO (Ministry of the Environment, Japan); HI & MS (National Institutes of Health), PK (National Science Funds, FNRS, Belgium); JK & ZK (Ministry of Science and Technology of the Republic of Croatia, Former Yu-USA Joint Fund for S&T Coop, and the Ford Foundation; VSL (NSERC-PGSD program), EHL & MMT (Universidad Nacional del Litoral, CAI+D program, the Argentine National Council for Science and Technology (CONICET), and the Argentine National Agency for the Promotion of Science and Technology; KAM (National Institute of Environmental Health Sciences to L. Guillette, Rewald, Olowo, Society for Integrative and Comparative Biology, Sigma Xi grants in aid of research and the University of Florida Institute of Food and Agricultural Sciences Women’s Club Fellowship; CO & ABV-C (Conselho Nacional de Desenvolvimento Cientifico e Tecnológico, CNPq/Brazil, grant and research fellowship to CAO, Master fellowship to ABVC supported by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, CAPES/Brazil); FO (Declining Amphibian Population Task Force and Depertment for Environmental, Food and Rural Affairs, UK); SR & LET-M (Toxic Substances Research Initiative, Government of Canada; VLT (Environment Canada and the Canadian Water Network); EW (Texas State University, San Marcos).
References
- [1].Solomon K, et al. Ecological risk assessment of atrazine in North American surface waters. Environ. Toxicol. Chem. 1996;15:31–76. doi: 10.1002/etc.2050. [DOI] [PubMed] [Google Scholar]
- [2].Boyd R. Herbicides and herbicide degradates in shallow groundwater and the Cedar River near a municipal well field, Cedar Rapids, Iowa, Sci. Total Environ. 2000;248:241–253. doi: 10.1016/s0048-9697(99)00546-x. [DOI] [PubMed] [Google Scholar]
- [3].Capel P, Larson S. Effect of scale on the behavior of atrazine in surface waters. Environ. Sci. Technol. 2001;35(4):648–657. doi: 10.1021/es001220f. [DOI] [PubMed] [Google Scholar]
- [4].Du Preez LH, et al. Seasonal exposures to triazine and other pesticides in surface waters in the western Highveld corn-production region in South Africa. Environ. Pollut. 2005;135(1):131–141. doi: 10.1016/j.envpol.2004.09.019. [DOI] [PubMed] [Google Scholar]
- [5].Fenelon J, Moore R. Transport of agrichemicals to ground and surface waters in a small central Indiana watershed. J. Environ. Qual. 1998;27:884–894. [Google Scholar]
- [6].Fischer J, Apedaile B, Vanclief L. Seasonal loadings of atrazine and metolachlor to a southeastern Ontario river from surface runoff and groundwater discharge, Water Qual. Res. J. Canada. 1995;30(3):533–553. [Google Scholar]
- [7].Frank R, Logan L. Pesticide and industrial chemical residues at the mouth of the Grand, Saugeen and Thames rivers, Ontario, Canada, 1981-85. Arch. Environ. Contam. Toxicol. 1988;17:741–754. doi: 10.1007/BF01183882. [DOI] [PubMed] [Google Scholar]
- [8].Frank R, Logan L, Clegg B. Pesticide and polychlorinated biphenyl residues in waters at the mouth of the Grand, Saugeen and Thames rivers, Ontario, Canada, 1986-1990. Arch. Environ. Contam. Toxicol. 1991;21:585–595. doi: 10.1007/BF01183882. [DOI] [PubMed] [Google Scholar]
- [9].Frank R, et al. Survey of farm wells for pesticides, Ontario, Canada. Arch. Environ. Contam. Toxicol. 1987;16:1–8. doi: 10.1007/BF01701223. [DOI] [PubMed] [Google Scholar]
- [10].Frank R, Sirons G. Atrazine: its use in corn production and its loss to stream waters in southern Ontario. Sci. Total Environ. 1979;12:223–239. [Google Scholar]
- [11].Graymore M, Stagnitti F, Allinson G. Impacts of atrazine in aquatic ecosystems. Environ. Int. 2001;26(7-8):483–495. doi: 10.1016/s0160-4120(01)00031-9. [DOI] [PubMed] [Google Scholar]
- [12].Rudolph D, Goss M. Ontario farm groundwater quality survey—summer 1992, A report prepared for Agriculture Canada, Agri-Food Development Branch, Guelph, Ontario, Agriculture Canada. 1993 [Google Scholar]
- [13].Hatfield JL, et al. Herbicide and nitrate distribution in central Iowa rainfall. J. Environ. Qual. 1996;25(2):259–264. [Google Scholar]
- [14].Lode O, et al. Pesticides in precipitation in Norway. Sci. Total Environ. 1995;160-161:421–431. [Google Scholar]
- [15].Mast MA, Foreman WT, Skaates SV. Current-use pesticides and organochlorine compounds in precipitation and lake sediment from two high-elevation national parks in the Western United States. Arch. Environ. Contam. Toxicol. 2007;52(3):294–305. doi: 10.1007/s00244-006-0096-1. [DOI] [PubMed] [Google Scholar]
- [16].Miller S, et al. Atrazine and nutrients in precipitation: results from the Lake Michigan mass balance study. Environ. Sci. Technol. 2000;34(1):55–61. [Google Scholar]
- [17].Vogel JR, Majewski MS, Capel PD. Pesticides in rain in four agricultural watersheds in the United States. J. Environ. Qual. 2008;37(3):1101–1115. doi: 10.2134/jeq2007.0079. [DOI] [PubMed] [Google Scholar]
- [18].Thurman E, Cromwell A. Atmospheric transport, deposition, and fate of triazine herbicides and their metabolites in pristine areas at Isle Royale National Park. Environ. Sci. Technol. 2000;34:3079–3085. [Google Scholar]
- [19].Crain D, et al. Alterations in steroidogenesis in alligators (Alligator mississippiensis) exposed naturally and experimentally to environmental contaminants. Environ. Health Perspect. 1997;105:528–533. doi: 10.1289/ehp.97105528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Reeder A, et al. Forms and prevalence of intersexuality and effects of environmental contaminants on sexuality in cricket frogs (Acris crepitans) Environ. Health Perspect. 1998;106:261–266. doi: 10.1289/ehp.98106261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Sanderson J, et al. Induction and inhibition of aromatase (CYP19) activity by various classes of pesticides in H295R human adrenocortical carcinoma cells. Toxicol. Appl. Pharmacol. 2002;182:44–54. doi: 10.1006/taap.2002.9420. [DOI] [PubMed] [Google Scholar]
- [22].Sanderson JT, et al. Effects of chloro-s-triazine herbicides and metabolites on aromatase activity in various human cell lines and on vitellogenin production in male carp hepatocytes. Environ. Health Perspect. 2001;109:1027–1031. doi: 10.1289/ehp.011091027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Sanderson JT, et al. 2-Chloro-triazine herbicides induce aromatase (CYP19) activity in H295R human adrenocortical carcinoma cells: a novel mechanism for estrogenicity? Toxicol Sci. 2000;54:121–127. doi: 10.1093/toxsci/54.1.121. [DOI] [PubMed] [Google Scholar]
- [24].Stoker T, et al. The effect of atrazine on puberty in male Wistar rats: an evaluation in the protocol for the assessment of pubertal development and thyroid function. Toxicol. Sci. 2000;58(1):50–59. doi: 10.1093/toxsci/58.1.50. [DOI] [PubMed] [Google Scholar]
- [25].Hayes TB, et al. Feminization of male frogs in the wild. Nature. 2002;419:895–896. doi: 10.1038/419895a. [DOI] [PubMed] [Google Scholar]
- [26].Hayes TB, et al. Atrazine-induced hermaphroditism at 0.1 ppb in American leopard frogs (Rana pipiens): laboratory and field evidence. Environ. Health Perspect. 2002;111:568–575. doi: 10.1289/ehp.5932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Murphy MB, et al. Atrazine concentrations, gonadal gross morphology and histology in ranid frogs collected in Michigan agricultural areas. Aquat. Toxicol. 2006;76(3-4):230–245. doi: 10.1016/j.aquatox.2005.09.010. [DOI] [PubMed] [Google Scholar]
- [28].McCoy KA, et al. Agriculture alters gonadal form and function in the toad Bufo marinus. Environ. Health Perspect. 2008;116(11):1526–1532. doi: 10.1289/ehp.11536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Hill A. The environment and disease: association or causation. Proc. R. Soc. Med. 1965;58:295–300. doi: 10.1177/003591576505800503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].International Program of Chemical Safety. World Health Organization; Geneva: 2002. IPCS global assessment of the state-of-the-science of endocrine disruptors. [Google Scholar]
- [31].Daxenberger A. Pollutants with androgen-disrupting potency. Eur. J. Lipid Sci. Technol. 2002;104(2):124–130. [Google Scholar]
- [32].Markey CM, et al. Endocrine disruptors: from Wingspread to environmental developmental biology. J. Steroid Biochem. Mol. Biol. 2002;83(1-5):235–244. doi: 10.1016/s0960-0760(02)00272-8. [DOI] [PubMed] [Google Scholar]
- [33].Reeder AL, et al. Intersexuality and the cricket frog decline: historic and geographic trends. Environ. Health Perspect. 2005;113(3):261–265. doi: 10.1289/ehp.7276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Sowers AD, Mills MA, Klaine SJ. The developmental effects of a municipal wastewater effluent on the northern leopard frog, Rana pipiens. Aquatic Toxicology. 2009;94(2):145–152. doi: 10.1016/j.aquatox.2009.06.013. [DOI] [PubMed] [Google Scholar]
- [35].Howe C, et al. Toxicity of glyphosate-based pesticides to four North American frog species. Environ. Toxicol. Chem. 2004;23(8):1928–1938. doi: 10.1897/03-71. [DOI] [PubMed] [Google Scholar]
- [36].Hogan NS, et al. Estrogenic exposure affects metamorphosis and alters sex ratios in the northern leopard frog (Rana pipiens): Identifying critically vulnerable periods of development. General and Comparative Endocrinology. 2008;156(3):515–523. doi: 10.1016/j.ygcen.2008.03.011. [DOI] [PubMed] [Google Scholar]
- [37].Jofre MB, Karasov WH. Effect of mono-ortho and di-ortho substituted polychlorinated biphenyl (PCB) congeners on leopard frog survival and sexual development. Chemosphere. 2008;70(9):1609–1619. doi: 10.1016/j.chemosphere.2007.08.002. [DOI] [PubMed] [Google Scholar]
- [38].Spano L, et al. Effects of atrazine on sex steroid dynamics, plasma vitellogenin concentration and gonad development in adult goldfish (Carassius auratus) Aquat. Toxicol. (Amsterdam) 2004;66(4):369–379. doi: 10.1016/j.aquatox.2003.10.009. [DOI] [PubMed] [Google Scholar]
- [39].Hayes TB, et al. Atrazine induces complete feminization and chemical castration in male African clawed frogs (Xenopus laevis) Proc. Natl. Acad. Sci. U.S.A. 2010;107(10):4612–4617. doi: 10.1073/pnas.0909519107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Rey F, et al. Prenatal exposure to pesticides disrupts testicular histoarchitecture and alters testosterone levels in male Caiman latirostris. Gen. Comp. Endocrinol. 2009;162(3):286–292. doi: 10.1016/j.ygcen.2009.03.032. [DOI] [PubMed] [Google Scholar]
- [41].Victor-Costa AB, et al. Changes in testicular morphology and steroidogenesis in adult rats exposed to Atrazine. Reprod. Toxicol. 2010;29(3):323–331. doi: 10.1016/j.reprotox.2009.12.006. [DOI] [PubMed] [Google Scholar]
- [42].Kniewald J, et al. Disorders of male rat reproductive tract under the influence of atrazine. J. Appl. Toxicol. 2000;20:61–68. [PubMed] [Google Scholar]
- [43].Tillitt DE, et al. Atrazine reduces reproduction in fathead minnow. Marine Environ. Res. 2008;66(1):51–151. [Google Scholar]
- [44].Hayes TB, et al. Hermaphroditic, demasculinized frogs after exposure to the herbicide atrazine at low ecologically relevant doses, Proc. Natl. Acad. Sci. U.S.A. 2002;99:5476–5480. doi: 10.1073/pnas.082121499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Hayes TB, et al. Characterization of atrazine-induced gonadal malformations and effects of an androgen antagonist (cyproterone acetate) and exogenous estrogen (estradiol 17β): support for the demasculinization/feminization hypothesis. Environ. Health Perspect. 2006;114(Suppl. 1):134–141. doi: 10.1289/ehp.8067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Orton F, Carr J, Handy R. Effects of nitrate and atrazine on larval development and sexual differentiation in the northern leopard frog Rana pipiens. Environ. Toxicol. Chem. 2006;25(1):65–71. doi: 10.1897/05-136r.1. [DOI] [PubMed] [Google Scholar]
- [47].De Solla SR, et al. Effects of environmentally relevant concentrations of atrazine on gonadal development of snapping turtles (Chelydra serpentina) Environ. Toxicol. Chem. 2006;25(2):520–526. doi: 10.1897/05-165r.1. [DOI] [PubMed] [Google Scholar]
- [48].Suzawa M, Ingraham H. The herbicide atrazine activates endocrine gene networks via non-steroidal NR5A nuclear receptors in fish and mammalian cells. PLoS One. 2008;3:2117. doi: 10.1371/journal.pone.0002117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Oka T, et al. Effect of atrazine on metamorphosis and sexual differentiation in Xenopus laevis. Aquat. Toxicol. 2008;87(4):215–226. doi: 10.1016/j.aquatox.2008.02.009. [DOI] [PubMed] [Google Scholar]
- [50].Langlois V, et al. Low levels of the herbicide atrazine alters sex ratios and reduces metamorphic success in Rana pipiens tadpoles raised in outdoor mesocosms. Environ. Health Perspect. 2009 doi: 10.1289/ehp.0901418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Willingham EJ. The effects of atrazine and temperature on turtle hatchling size and sex ratios. Front. Ecol. Environ. 2005;3(6):309–313. [Google Scholar]
- [52].Fox G. Practical causal inference for epidemiologists. J. Toxicol. Environ. Health. 1991;33:359–373. doi: 10.1080/15287399109531535. [DOI] [PubMed] [Google Scholar]
- [53].Tavera-Mendoza L, et al. Response of the amphibian tadpole (Xenopus laevis) to atrazine during sexual differentiation of the testis. Environ. Toxicol. Chem. 2002;21:527–531. doi: 10.1897/1551-5028(2002)021<0527:rotatx>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- [54].Tavera-Mendoza L, et al. Response of the amphibian tadpole Xenopus laevis to atrazine during sexual differentiation of the ovary. Environ. Toxicol. Chem. 2002;21(6):1264–1267. [PubMed] [Google Scholar]
- [55].Hayes TB. There is no denying this: defusing the confusion about atrazine. Bioscience. 2004;54(12):1138–1149. [Google Scholar]
- [56].Carr J, et al. Response of larval Xenopus laevis to atrazine: assessment of growth, metamorphosis, and gonadal and laryngeal morphology. Environ. Toxicol. Chem. 2003;22:396–405. [PubMed] [Google Scholar]
- [57].Hecker M, et al. Plasma concentrations of estradiol and testosterone, gonadal aromatase activity and ultrastructure of the testis in Xenopus laevis exposed to estradiol or atrazine. Aquat. Toxicol. (Amsterdam) 2005;72(4):383–396. doi: 10.1016/j.aquatox.2005.01.008. [DOI] [PubMed] [Google Scholar]
- [58].Gojmerac T, Kniewald J. Atrazine biodegradation in rats—a model for mammalian metabolism. Bull. Environ. Contam. Toxicol. 1989;43(2):199–206. doi: 10.1007/BF01701748. [DOI] [PubMed] [Google Scholar]
- [59].Moore A, Waring C. Mechanistic effects of a triazine pesticide on reproductive endocrine function in mature male Atlantic salmon (Salmo salar L.) parr. Pesticide Biochem. Physiol. 1998;62:41–50. [Google Scholar]
- [60].Friedmann A. Atrazine inhibition of testosterone production in rat males following peripubertal exposure. Reprod. Toxicol. 2002;16(3):275–279. doi: 10.1016/s0890-6238(02)00019-9. [DOI] [PubMed] [Google Scholar]
- [61].Wilhelms KW, et al. Effects of atrazine on sexual maturation in female Japanese quail induced by photostimulation or exogenous gonadotropin. Environ. Toxicol. Chem. 2006;25(1):233–240. doi: 10.1897/05-039r.1. [DOI] [PubMed] [Google Scholar]
- [62].Cummings A, Rhodes B, Cooper R. Effect of atrazine on implantation and early pregnancy in 4 strains of rats. Toxicol. Sci. 2000;58(1):135–143. doi: 10.1093/toxsci/58.1.135. [DOI] [PubMed] [Google Scholar]
- [63].Cooper RL, et al. Atrazine disrupts the hypothalamic control of pituitary-ovarian function. Toxicol. Sci. 2000;53:297–307. doi: 10.1093/toxsci/53.2.297. [DOI] [PubMed] [Google Scholar]
- [64].Narotsky M, et al. Strain comparisons of atrazine-induced pregnancy loss in the rat. Reprod. Toxicol. 2001;15(1):61–69. doi: 10.1016/s0890-6238(00)00111-8. [DOI] [PubMed] [Google Scholar]
- [65].Foradori CD, et al. Effects of atrazine and its withdrawal on gonadotropin-releasing hormone neuroendocrine function in the adult female Wistar rat. Biol. Reprod. 2009;81(6):1099–1105. doi: 10.1095/biolreprod.109.077453. [DOI] [PubMed] [Google Scholar]
- [66].Babic-Gojmerac T, Kniewald Z, Kniewald J. Testosterone metabolism in neuroendocrine organs in male rats under atrazine and deethylatrazine influence. J. steroid. Biochem. 1989;33(1):141–146. doi: 10.1016/0022-4731(89)90369-5. [DOI] [PubMed] [Google Scholar]
- [67].Foradori CD, et al. Atrazine inhibits pulsatile luteinizing hormone release without altering pituitary sensitivity to a gonadotropin-releasing hormone receptor agonist in female Wistar rats. Biol. Reprod. 2009;81(1):40–45. doi: 10.1095/biolreprod.108.075713. [DOI] [PubMed] [Google Scholar]
- [68].McMullin T, et al. Evidence that atrazine and diaminochlorotriazine inhibit the estrogen/progesterone induced surge of luteinizing hormone in female Sprague-Dawley rats without changing estrogen receptor action. Toxicol. Sci. 2004;79:278–286. doi: 10.1093/toxsci/kfh127. [DOI] [PubMed] [Google Scholar]
- [69].Kniewald J, et al. Effect of s-triazine compounds on testosterone metabolism in the rat prostate. J. Appl. Toxicol. 1995;15(3):215–218. doi: 10.1002/jat.2550150312. [DOI] [PubMed] [Google Scholar]
- [70].Kniewald J, Mildner P, Kniewald Z. Effects of s-triazine herbicides on hormone-receptor complex formation, 5α reductase and 3α hydroxysteroid dehydrogenase activity at the anterior pitiuitary level. J. Steroid Biochem. 1979;11(1):833–838. doi: 10.1016/0022-4731(79)90018-9. [DOI] [PubMed] [Google Scholar]
- [71].Danzo BJ. Environmental xenobiotics may disrupt normal endocrine function by interfering with the binding of physiological ligands to steroid receptors and binding proteins. Environ. Health Perspect. 1997;105(3):294–301. doi: 10.1289/ehp.97105294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Kniewald J, Mildner P, Kniewald Z. Effects of s-triazine herbicides on 5-alpha dihydrotestosterone receptor complex formation in hypothalamus and ventral prostate. In: Action E, Genazzani F, DiCarlo, Mainwaring WIP, editors. Pharmacological Modulation of Steroid Action. Raven Press; New York: 1980. pp. 159–169. [Google Scholar]
- [73].Orton F, et al. Endocrine disrupting effects of herbicides and pentachlorophenol: In vitro and in vivo evidence. Environmental Science & Technology. 2009;43(6):2144–2150. doi: 10.1021/es8028928. [DOI] [PubMed] [Google Scholar]
- [74].Pogrmic K, et al. Atrazine oral exposure of peripubertal male rats downregulates steroidogenesis gene expression in Leydig cells. Toxicol. Sci. 2009;111(1):189–197. doi: 10.1093/toxsci/kfp135. [DOI] [PubMed] [Google Scholar]
- [75].Pogrmic K, et al. Assesment of in vitro effects of atrazine on peripubertal rat Leydig cell steroidogenesis. FEBS J. 2008;275:462–1462. [Google Scholar]
- [76].Fraites MJP, et al. Characterization of the hypothalamic-pituitary-adrenal axis response to atrazine and metabolites in the female rat. Toxicol. Sci. 2009;112(1):88–99. doi: 10.1093/toxsci/kfp194. [DOI] [PubMed] [Google Scholar]
- [77].Laws SC, et al. Chlorotriazine herbicides and metabolites activate an ACTHdependent release of corticosterone in male Wistar rats. Toxicol. Sci. 2009;112(1):78–87. doi: 10.1093/toxsci/kfp190. [DOI] [PubMed] [Google Scholar]
- [78].Zorrilla LM, Gibson EK, Stoker TE. The effects of simazine, a chlorotriazine herbicide, on pubertal development in the female Wistar rat. Reproductive Toxicology. 2010;29(4):393–400. doi: 10.1016/j.reprotox.2010.03.010. [DOI] [PubMed] [Google Scholar]
- [79].Stoker TE, Robinette CL, Cooper RL. Maternal exposure to atrazine during lactation suppresses suckling-induced prolactin release and results in prostatitis in the adult offspring. Toxicol. Sci. 1999;52:68–79. doi: 10.1093/toxsci/52.1.68. [DOI] [PubMed] [Google Scholar]
- [80].Roberge M, Hakk H, Larsen G. Atrazine is a competitive inhibitor of phosphodiesterase but does not affect the estrogen receptor. Toxicol. Letters. 2004;154:61–68. doi: 10.1016/j.toxlet.2004.07.005. [DOI] [PubMed] [Google Scholar]
- [81].Fan W, et al. Herbicide atrazine activates SF-1 by direct affinity and concomitant co-activators recruitments to induce aromatase expression via promoter II. Biochem. Biophys. Res. Commun. 2007;355(4):1012–1018. doi: 10.1016/j.bbrc.2007.02.062. [DOI] [PubMed] [Google Scholar]
- [82].Fan W, et al. Atrazine-induced aromatase expression is SF-1-dependent: implications for endocrine disruption in wildlife and reproductive cancers in humans. Environ. Health Perspect. 2007;115(5):720–727. doi: 10.1289/ehp.9758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Holloway AC, et al. Atrazine-induced changes in aromatase activity in estrogen sensitive target tissues. J. Appl. Toxicol. 2008;28(3):260–270. doi: 10.1002/jat.1275. [DOI] [PubMed] [Google Scholar]
- [84].Piferrer F. Endocrine sex control strategies for the feminization of teleost fish. Aquaculture. 2001;197(1-4):229–281. [Google Scholar]
- [85].Guiguen Y, et al. Ovarian aromatase and estrogens: a pivotal role for gonadal sex differentiation and sex change in fish. Gen. Comp. Endocrinol. 2010;165(3 (Sp. Iss. SI):352–366. doi: 10.1016/j.ygcen.2009.03.002. [DOI] [PubMed] [Google Scholar]
- [86].Hayes TB. Hormonal regulation of sex differentiation in amphibians, reptiles, and birds. In: Knobil E, Neill J, editors. Encyclopedia of Reproduction. Academic Press; San Diego: 1998. pp. 102–109. [Google Scholar]
- [87].Hayes TB. Sex determination and primary sex differentiation in amphibians. J. Exp. Zool. 1998;281:373–399. [PubMed] [Google Scholar]
- [88].Crews D. Temperature-dependent sex determination: the interplay of steroid hormones and temperature. Zool. Sci. 1996;13(1):1–13. doi: 10.2108/zsj.13.1. [DOI] [PubMed] [Google Scholar]
- [89].Gutzke WHN, Bull JJ. Steroid hormones reverse sex in turtles. Gen. Comp. Endocrinol. 1986;64(3):368–372. doi: 10.1016/0016-6480(86)90070-5. [DOI] [PubMed] [Google Scholar]
- [90].Hayes TB. Comparative endocrinology: a tool for understanding mechanisms and predicting effects of endocrine mimicking xenobiotics. In: Guillette LJ, editor. Environmental Endocrine Disruptors: An Evolutionary Perspective. Taylor and Francis; NY: 1999. [Google Scholar]
- [91].Wilhelms KW, et al. In ovo exposure to a triazine herbicide: Effects of atrazine on circulating reproductive hormones and gonadal histology in young Japanese quail. Archives of Environmental Contamination and Toxicology. 2006;51(1):117–122. doi: 10.1007/s00244-005-0165-x. [DOI] [PubMed] [Google Scholar]
- [92].Matsushita S, et al. Effects of in ovo exposure to imazalil and atrazine on sexual differentiation in chick gonads. Poultry Science. 2006;85(9):1641–1647. doi: 10.1093/ps/85.9.1641. [DOI] [PubMed] [Google Scholar]
- [93].Swan S, et al. Semen quality in relation to biomarkers of pesticide exposure. Environ. Health Perspect. 2003;111(12):1478–1484. doi: 10.1289/ehp.6417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Rayner J, Enoch R, Fenton S. Adverse effects of prenatal exposure to atrazine during a critical period of mammary gland growth. Toxicol. Sci. 2005;87(1):255–266. doi: 10.1093/toxsci/kfi213. [DOI] [PubMed] [Google Scholar]
- [95].Rayner J, Wood C, Fenton S. Exposure parameters necessary for delayed puberty and mammary gland development in Long-Evans rats exposed in utero to atrazine. Toxicol. Appl. Pharmacol. 2004;195(23-34) doi: 10.1016/j.taap.2003.11.005. [DOI] [PubMed] [Google Scholar]
- [96].Rayner JL, et al. Atrazine-induced reproductive tract alterations after transplacental and/or lactational exposure in male Long-Evans rats. Toxicol. Appl. Pharmacol. 2007;218(3):238–248. doi: 10.1016/j.taap.2006.11.020. [DOI] [PubMed] [Google Scholar]
- [97].Pintér A, et al. Long-term carcinogenecity bioassay of the herbicide atrazine in F344 rats. Neoplasma. 1980;37:533–544. [PubMed] [Google Scholar]
- [98].Ueda M, et al. Possible enhancing effects of atrazine on growth of 7,12-dimethylbenz(a) anthracene induced mammary tumors in ovariectomized Sprague-Dawley rats. Cancer Sci. 2005;96(1):19–25. doi: 10.1111/j.1349-7006.2005.00008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Eldridge J, et al. Factors affecting mammary tumor incidence in chlorotriazine-treated female rats: hormonal properties, dosage, and animal strain. Environ. Health Perspect. 1994;102(11):29–36. doi: 10.1289/ehp.94102s1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Stevens J, et al. Hypothesis for mammary tumorigenesis in Sprague-Dawley rats exposed to certain triazine herbicides. J. Toxicol. Environ. Health. 1994;43:139–154. doi: 10.1080/15287399409531911. [DOI] [PubMed] [Google Scholar]
- [101].Wetzel LT, et al. Chronic effects of atrazine on estrus and mammary gland formation in female Sprague-Dawley and Fischer-344 rats. J. Toxicol. Environ. Health. 1994;43:169–182. doi: 10.1080/15287399409531913. [DOI] [PubMed] [Google Scholar]
- [102].Kettles MA, et al. Triazine exposure and breast cancer incidence: an ecologic study of Kentucky counties. Environ. Health Perspect. 1997;105(11):1222–1227. doi: 10.1289/ehp.971051222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Brodkin M, et al. Atrazine is an immune disruptor in adult northern leopard frogs (Rana pipiens) Environ. Toxicol. Chem. 2007;26(1):80–84. doi: 10.1897/05-469.1. [DOI] [PubMed] [Google Scholar]
- [104].Cantemir C, et al. p53 protein expression in peripheral lymphocytes from atrazine chronically intoxicated rats. Toxicol. Lett. 1987;93(2-3):87–94. doi: 10.1016/s0378-4274(97)00050-7. [DOI] [PubMed] [Google Scholar]
- [105].Christin MS, et al. Effects of agricultural pesticides on the immune system of Rana pipiens and on its resistance to parasitic infection. Environ. Toxicol. Chem. 2003;22(5):1127–1133. [PubMed] [Google Scholar]
- [106].Christin MS, et al. Effects of agricultural pesticides on the immune system of Xenopus laevis and Rana pipiens. Aquat. Toxicol. 2004;67(1):33–43. doi: 10.1016/j.aquatox.2003.11.007. [DOI] [PubMed] [Google Scholar]
- [107].Filipov N, et al. Immunotoxic effects of short-term atrazine exposure in young male C57BL/6 mice. Toxicol. Sci. 2005;86(2) doi: 10.1093/toxsci/kfi188. [DOI] [PubMed] [Google Scholar]
- [108].Forson D, Storfer A. Atrazine increases Ranavirus susceptibility in the tiger salamander Ambystoma tigrinum. Ecol. Appl. 2006;16(6):2325–2332. doi: 10.1890/1051-0761(2006)016[2325:airsit]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- [109].Forson D, Storfer A. Effects of atrazine and iridovirus infection on survival and life history traits of the long-toed salamander (Ambystoma macrodatylum) Environ. Toxicol. Chem. 2006;25(1):168–173. doi: 10.1897/05-260r.1. [DOI] [PubMed] [Google Scholar]
- [110].Gendron AD, et al. Exposure of leopard frogs to a pesticide mixture affects life history characteristics of the lungworm Rhabdias ranae. Oecologia. 2003;135(3):469–476. doi: 10.1007/s00442-003-1210-y. [DOI] [PubMed] [Google Scholar]
- [111].Hooghe R, Devos S, Hooghe-Peters E. Effects of selected herbicides on cytokine production in vitro. Life Sci. 2000;66(26):2519–2525. doi: 10.1016/s0024-3205(00)00586-5. [DOI] [PubMed] [Google Scholar]
- [112].Houck A, Sessions S. Could atrazine affect the immune system of the frog Rana pipiens? Bios. 2006;77(4):107–112. [Google Scholar]
- [113].Karrow NA, et al. Oral exposure to atrazine modulates cell-mediated immune function and decreases host resistance to the B16F10 tumor model in female B6C3F1 mice. Toxicology. 2005;209(1):15–28. doi: 10.1016/j.tox.2004.12.002. [DOI] [PubMed] [Google Scholar]
- [114].Kim KR, et al. Immune alterations in mice exposed to the herbicide simazine. J. Toxicol. Environ. Health A: Curr. Issues. 2003;66(12):1159–1173. doi: 10.1080/15287390306358. [DOI] [PubMed] [Google Scholar]
- [115].Langerveld AJ, et al. Chronic exposure to high levels of atrazine alters expression of genes that regulate immune and growth-related functions in developing Xenopus laevis tadpoles. Environ. Res. 2009;109(4):379–389. doi: 10.1016/j.envres.2009.01.006. [DOI] [PubMed] [Google Scholar]
- [116].Liskova A, et al. Effect of the herbicide atrazine on some immune parameters in mice. J. Trace Micropr. Tech. 2000;18(2):235–240. [Google Scholar]
- [117].Mizota K, Ueda H. Endocrine disrupting chemical atrazine causes degranulation through G(q/11) protein-coupled neurosteroid receptor in mast cells. Toxicol. Sci. 2006;90(2):362–368. doi: 10.1093/toxsci/kfj087. [DOI] [PubMed] [Google Scholar]
- [118].Pistl J, et al. Determination of the immunotoxic potential of pesticides on functional activity of sheep leukocytes in vitro. Toxicology. 2003;188(1):73–81. doi: 10.1016/s0300-483x(03)00046-5. [DOI] [PubMed] [Google Scholar]
- [119].Porter W, Jaeger J, Carlson I. Endocrine, immune, and behavioral effects of aldicarb (carbamate), atrazine (triazine) and nitrate fertilizer mixtures at groundwater concentrations. Toxicol. Ind. Health. 1999;15:133–150. doi: 10.1177/074823379901500111. [DOI] [PubMed] [Google Scholar]
- [120].Pruett S, et al. Modeling and predicting immunological effects of chemical stressors: characterization of a quantitative biomarker for immunological changes caused by atrazine and ethanol. Toxicol. Sci. 2003;75(2):343–354. doi: 10.1093/toxsci/kfg200. [DOI] [PubMed] [Google Scholar]
- [121].Rooney A, Matulka R, Luebke R. Developmental atrazine exposure suppresses immune function in male, but not female Sprague-Dawley rats. Toxicol. Sci. 2003;76:366–375. doi: 10.1093/toxsci/kfg250. [DOI] [PubMed] [Google Scholar]
- [122].Rowe A, et al. Immunomodulatory effects of maternal atrazine exposure on male Balb/c mice. Toxicol. Appl. Pharmacol. 2006;214(1):69–77. doi: 10.1016/j.taap.2005.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Rowe AM, Brundage KM, Barnett JB. Developmental immunotoxicity of atrazine in rodents. Basic Clin. Pharmacol. Toxicol. 2008;102(2):139–145. doi: 10.1111/j.1742-7843.2007.00175.x. [DOI] [PubMed] [Google Scholar]
- [124].Rymuszka A, et al. Application of immunostimulants after suppression induced byxenobiotics: effect of lysozyme dimer (KLP-602) after immunosuppression induced by atrazine in rabbits. Pol. J. Food Nutr. Sci. 2004;13(2):51–54. [Google Scholar]
- [125].Whalen M, et al. Immunomodulation of human natural killer cell cytotoxic function by triazine and carbamate pesticides. Chem.-Biol. Interact. 2003;145(3):311–319. doi: 10.1016/s0009-2797(03)00027-9. [DOI] [PubMed] [Google Scholar]
- [126].Zeljezic D, et al. Evaluation of DNA damage induced by atrazine and atrazinebased herbicide in human lymphocytes in vitro using a comet and DNA diffusion assay. Toxicol. In Vitro. 2006;20(6):923–935. doi: 10.1016/j.tiv.2006.01.017. [DOI] [PubMed] [Google Scholar]
- [127].Rohr JR, et al. Agrochemicals increase trematode infections in a declining amphibian species. Nature. 2008;455(7217):1235–1239. doi: 10.1038/nature07281. [DOI] [PubMed] [Google Scholar]
- [128].Giusi G, et al. The endocrine disruptor atrazine accounts for a dimorphic somatostatinergic neuronal expression pattern in mice. Toxicol. Sci. 2006;89(1):257–264. doi: 10.1093/toxsci/kfj012. [DOI] [PubMed] [Google Scholar]
- [129].Hossain MM, Filipov NM. Alteration of dopamine uptake into rat striatal vesicles and synaptosomes caused by an in vitro exposure to atrazine and some of its metabolites. Toxicology. 2008;248(1):52–58. doi: 10.1016/j.tox.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Martyniuk CJ, et al. Aquatic contaminants alter genes involved in neurotransmitter synthesis and gonadotropin release in largemouth bass. Aquat. Toxicol. 2009;95(1):1–9. doi: 10.1016/j.aquatox.2009.06.009. [DOI] [PubMed] [Google Scholar]
- [131].Rodriguez V, Thiruchelvam M, Cory-Slechta D. Sustained exposure to the widely used herbicide atrazine: altered function and loss of neurons in brain monoamine systems. Environ. Health Perspect. 2005;113(6):708–715. doi: 10.1289/ehp.7783. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [132].Tierney KB, et al. Relating olfactory neurotoxicity to altered olfactory-mediated behaviors in rainbow trout exposed to three currently-used pesticides. Aquat. Toxicol. 2007;81(1):55–64. doi: 10.1016/j.aquatox.2006.11.006. [DOI] [PubMed] [Google Scholar]
- [133].Alvarez MD, Fuiman LA. Environmental levels of atrazine and its degradation products impair survival skills and growth of red drum larvae. Aquat. Toxicol. 2005;74(3):229–241. doi: 10.1016/j.aquatox.2005.05.014. [DOI] [PubMed] [Google Scholar]
- [134].de Noyelles F, Kettle W, Sinn D. The response of plankton communities in experimental ponds to atrazine, the most heavily used pesticide in the United States. Ecology. 1285;63(1982):1293. [Google Scholar]
- [135].Dewey SL. Effects of the herbicide atrazine on aquatic insect community structure and emergence. Ecology. 1986;67(1):148–162. [Google Scholar]
- [136].Griggs JL, Belden LK. Effects of atrazine and metolachlor on the survivorship and infectivity of Echinostoma trivolvis trematode cercariae. Arch. Environ. Contam. Toxicol. 2008;54(2):195–202. doi: 10.1007/s00244-007-9029-x. [DOI] [PubMed] [Google Scholar]
- [137].Koprivnikar J, Baker RL, Forbes MR. Environmental factors influencing community composition of gastropods and their trematode parasites in southern Ontario. J. Parasitol. 2007;93:992–998. doi: 10.1645/GE-1144R.1. [DOI] [PubMed] [Google Scholar]
- [138].Koprivnikar J, Forbes MR, Baker RL. Contaminant effects on host-parasite interactions: atrazine, frogs, and trematodes. Environ. Toxicol. Chem. 2007;26:2166–2170. doi: 10.1897/07-220.1. [DOI] [PubMed] [Google Scholar]
- [139].Rohr J, Crumrine P. Effects of an herbicide and an insecticide on pond community structure and processes. Ecol. Appl. 2005;15:1135–1147. [Google Scholar]
- [140].Rohr JR, et al. Understanding the net effects of pesticides on amphibian trematode infections. Ecol. Appl. 2008;18(7):1743–1753. doi: 10.1890/07-1429.1. [DOI] [PubMed] [Google Scholar]
- [141].Rohr JR, et al. Parasites, info-disruption, and the ecology of fear. Oecologia. 2009;159(2):447–454. doi: 10.1007/s00442-008-1208-6. [DOI] [PubMed] [Google Scholar]
- [142].Russo J, Lagadic L. Effects of parasitism and pesticide exposure on characteristics and functions of hemocyte populations in the freshwater snail Lymnaea palustris (Gastropoda Pulmonata) Cell Biol. Toxicol. 2000;16(1):15–30. doi: 10.1023/a:1007640519746. [DOI] [PubMed] [Google Scholar]