Abstract
Background
Increasingly, women with stage 2 and 3 breast cancers receive neoadjuvant therapy, after which many are eligible for breast-conserving surgery (BCS). The question often arises as to whether BCS, if achievable, provides adequate local control. We report the results of local recurrence (LR) from the I-SPY 1 Trial in the setting of maximal multidisciplinary treatment where approximately 50 % of patients were treated with BCS.
Methods
We analyzed data from the I-SPY 1 Trial. Women with tumors ≥3 cm from nine clinical breast centers received neoadjuvant doxorubicin, cyclophosphamide and paclitaxel followed by definitive surgical therapy, and radiation at physician discretion. LR following mastectomy and BCS were analyzed in relation to clinical characteristics and response to therapy as measured by residual cancer burden.
Results
Of the 237 patients enrolled in the I-SPY 1 Trial, 206 were available for analysis. Median tumor size was 6.0 cm, and median follow-up was 3.9 years. Fourteen patients (7 %) had LR and 45 (22 %) had distant recurrence (DR). Of the 14 patients with LR, nine had synchronous DR; one had DR > 2 years later. Only four (2 % of evaluable patients) had LR alone. The rate of LR was low after mastectomy and after BCS, even in the setting of significant residual disease.
Conclusions
Overall, these patients at high risk for early recurrence, treated with maximal multidisciplinary treatment, had low LR. Recurrence was associated with aggressive biological features such as more advanced stage at presentation, where LR occurs most frequently in the setting of DR.
Breast cancer is a heterogeneous disease, and some patients have a higher risk of recurrence than others. Patients who present with large palpable tumors are known to have higher risk of recurrence relative to those with tumors found by screening.1–3 Increasingly, other biological features of the tumor are also known to predict recurrence risk and affect response to therapy.4–6 For many high-risk patients, neoadjuvant chemotherapy is used both to downstage patients and enable breast-conserving surgery (BCS)7 and evaluate response to therapy.
Patients who have a good response to neoadjuvant chemotherapy and have minimal residual disease have improved survival compared with those who have considerable residual disease present.2,3,8–13 Data regarding the outcome of BCS in downstaged patients are needed to inform choices of local therapy after neoadjuvant therapy. Small studies suggest low ipsilateral breast tumor recurrence (IBTR) in the neoadjuvant setting.14 Other studies [e.g. National Surgical Adjuvant Breast and Bowel Project (NSABP)-18] suggest increased IBTR after neoadjuvant chemotherapy, although this did not persist with time and was related to younger age.7
The I-SPY 1 Trial is a multicenter neoadjuvant chemotherapy observational study of women with histologically confirmed breast cancer. We report the local recurrence (LR) in the context of the distant recurrence (DR) rate in this group of patients treated with maximal multidisciplinary treatment, and assess the recurrence rates associated with clinical and biological characteristics in the context of surgical treatment (BCS vs. mastectomy).
METHODS
Study Design and Patient Selection
The I-SPY 1 Trial was a collaboration of the American College of Radiology Imaging Network (ACRIN), Cancer and Leukemia Group B (CALGB), and Specialized Program of Research Excellence (SPORE). Details of the trial have been published previously.5,6,15 Briefly, eligible patients with histologically-confirmed invasive breast cancer ≥3 cm were treated with an anthracycline-based chemotherapy regimen plus optional taxane. Axillary surgery was conducted post-chemotherapy, although sentinel node alone was allowed for patients who presented with clinically node-negative disease. Choice of mastectomy or BCS was at the physicians’ discretion. Radiation after breast conservation was standard but post-mastectomy radiation was determined on an individual basis.
Clinical and Molecular Biomarkers
Hormone receptor (HR) and human epidermal growth factor receptor 2 (HER2)-neu status and Ki-67 scoring were determined on pretreatment core biopsies as previously described.6 The presence of lymphovascular invasion (LVI) was recorded on case-report forms and taken from the pathology report.
Evaluation of Response to Therapy Using Pathologic Data
Residual cancer burden (RCB) was determined using the dimensions of the primary tumor bed, proportion of primary tumor bed that is invasive cancer, the number of positive nodes, and the size of the largest nodal metastasis, as previously described.16 In addition, the MD Anderson Prognostic Index (MDAPI) score, derived to predict local and ipsilateral recurrences after BCS following neoadjuvant chemotherapy, was computed based on initial node status, pathologic tumor size, morphology of residual disease, and LVI as previously described.17,18
Statistical Analysis
The endpoints of interest were LR and DR. Following DR, LR is often not reported; however, we queried every site to determine whether an LR had occurred after the time of DR up to the point of last follow-up. We used the Chi-square test to assess the association between clinical parameters and surgical treatment type for categorical variables, and the Mann–Whitney U test for continuous variables. Kaplan–Meier curves and the log-rank test were used to assess the association between clinical variables and recurrence over all cases, and stratified by surgical treatment type. Time to LR and time to first recurrence (local and/or distant) were censored at 5 years in the survival analysis.
RESULTS
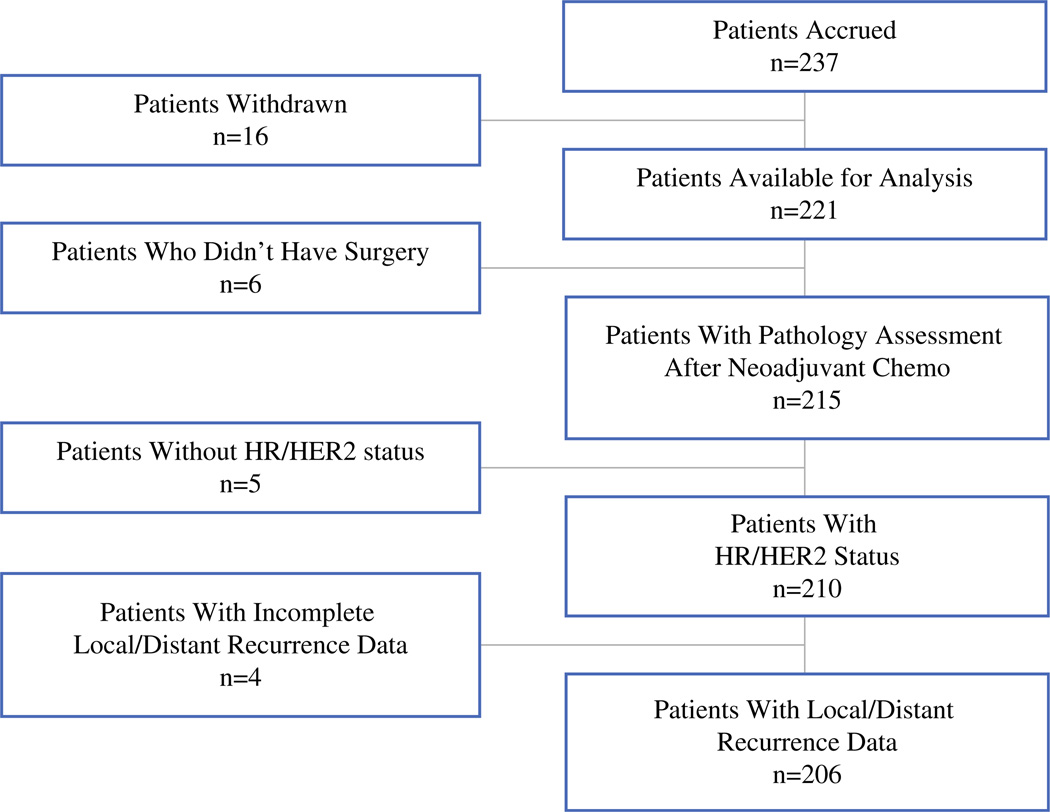
A total of 237 patients enrolled in the I-SPY 1 Trial between May 2002 and March 2006. As shown in the CONSORT diagram (Fig. 1), 206 patients with complete information were available for this analysis. Patient characteristics are shown in Table 1. After a median follow-up of 3.9 years, 14 patients (7 %) had LR, and 45 patients (22 %) had DR. Of the patients with LR, 10 (22 %) had both an LR and DR (Table 2), four of which were synchronous, five of which occurred less than 1 year prior to the diagnosis of metastatic disease, and one that preceded the metastatic diagnosis within 2 years (Table 2). LR alone occurred in only four patients (2 %). A 2 × 2 table of distant and LR is shown in supplemental Table 1 (see electronic supplementary material).
FIG. 1.
CONSORT diagram of patients available for analysis. HR hormone receptor, HER2 human epidermal growth factor receptor 2
TABLE 1.
Patient characteristics and comparison between the BCS and mastectomy groups
| Patient characteristics | All cases | BCS group | Mastectomy group | p-Value |
|---|---|---|---|---|
| Total patients [n (%)] | 206 | 90 (44) | 116 (56) | |
| Age [years; median (range)] | 49.1 (26–68) | 50.0 (27–68) | 47.6 (26–67) | 0.04 |
| Race [% (n)] | ||||
| Caucasian | 75 (155) | 73 (66) | 77% (89) | 0.92 |
| African American | 18 (37) | 20 (18) | 21% (24) | |
| Asian | 4 (9) | 4 (4) | 4% (5) | |
| Other | 2 (5) | 1 (1) | 1% (3) | |
| Clinical tumor size [cm; median (range)] | 6.0 (0–25) | 5.0 (1–14) | 7.0 (0–25) | <0.0001 |
| Clinical T stage (n = 204) [% (n)] | ||||
| 1a | 2 (5) | 2 (2) | 3 (3) | 0.0001 |
| 2 | 35 (73) | 51 (46) | 23 (27) | |
| 3 | 50 (104) | 41 (37) | 58 (67) | |
| 4 | 11 (22) | 4 (4) | 16 (18) | |
| Clinically node positive [% (n)] | 64 (131) | 61 (55) | 66 (76) | 0.47 |
| Clinical stage (n = 205) [% (n)] | ||||
| Ia | 1 (3) | 1 (1) | 2 (2) | 0.0006 |
| II | 47 (96) | 60 (54) | 36 (42) | |
| III | 44 (91) | 37 (33) | 49 (57) | |
| Inflammatory | 8 (16) | 1 (1) | 13 (15) | |
| Histologic grade [% (n)] | ||||
| I | 7 (15) | 10 (9) | 5 (6) | 0.36 |
| II | 47 (96) | 47 (42) | 44 (51) | |
| III | 46 (95) | 41 (37) | 50 (58) | |
| Indeterminate | 1 (3) | 2 (2) | 1 (1) | |
| Receptor status [% (n)] | ||||
| HR (+) | 60 (123) | 54 (49) | 64 (74) | 0.20 |
| HER2 (+) | 32 (66) | 31 (28) | 33 (38) | 0.88 |
| HR (+) HER2 (−) | 44 (90) | 40 (36) | 47 (54) | 0.53 |
| HR (−) HER2 (−) | 24 (50) | 29 (26) | 21 (24) | |
| HR (−) HER2 (+) | 16 (33) | 17 (15) | 16 (18) | |
| HR (+) HER2 (+) | 16 (33) | 14 (13) | 17 (20) | |
| Ki-67 [% (n)] | ||||
| Low (<10 %) | 24 (49) | 24 (19) | 29 (26) | 0.35 |
| Medium (10–25 %) | 30 (61) | 31 (24) | 36 (32) | |
| High (>25 %) | 34 (70) | 45 (35) | 34 (31) | |
| Indeterminate | 13 (26) | |||
| Residual cancer burden [% (n)] | n = 192 | n = 109 | n = 83 | |
| 0 | 26 (54) | 34 (28) | 24 (26) | 0.45 |
| 1 | 9 (18) | 10 (8) | 9 (10) | |
| 2 | 41 (84) | 41 (34) | 46 (50) | |
| 3 | 7 (14) | 16 (13) | 21 (23) | |
| Lymphovascular invasion [% (n)] | 17 (36) | 15 (13) | 20 (23) | 0.46 |
| Total receiving radiation [% (n)] | 83 (170) | 87 (78) | 79 (92) | 0.20 |
| Total receiving taxane [% (n)] | 5 (11) | 6 (5) | 5 (6) | 1 |
BCS breast-conserving surgery, HR hormone receptor, HER2 human epidermal growth factor receptor 2
As of data lock in February 2012
TABLE 2.
Local versus DR by time of recurrence (total n = 206)
| Time from original cancer to first recurrence | No LR | Time between LR and DR | ||||
|---|---|---|---|---|---|---|
| DR only | Synchronous | Within 1 year | Within 2 years | Within 5 years | No DR | |
| 0–2 years | 19 | 2 | 4 | 1 | 3 | |
| 2–5 years | 15 | 2 | 1 | |||
| >5 years | 1 | 1 | ||||
| Total recurrences | 35 | 10 | 4 | |||
| No recurrence | 157 | |||||
DR distant recurrence, LR local recurrence
Ninety patients (44 %) had BCS, and 116 patients (56 %) had mastectomy. Not unexpectedly, BCS patients had a lower overall clinical (presenting) stage and clinical T stage than the mastectomy group. However, these groups did not differ in other biological characteristics (Table 1).
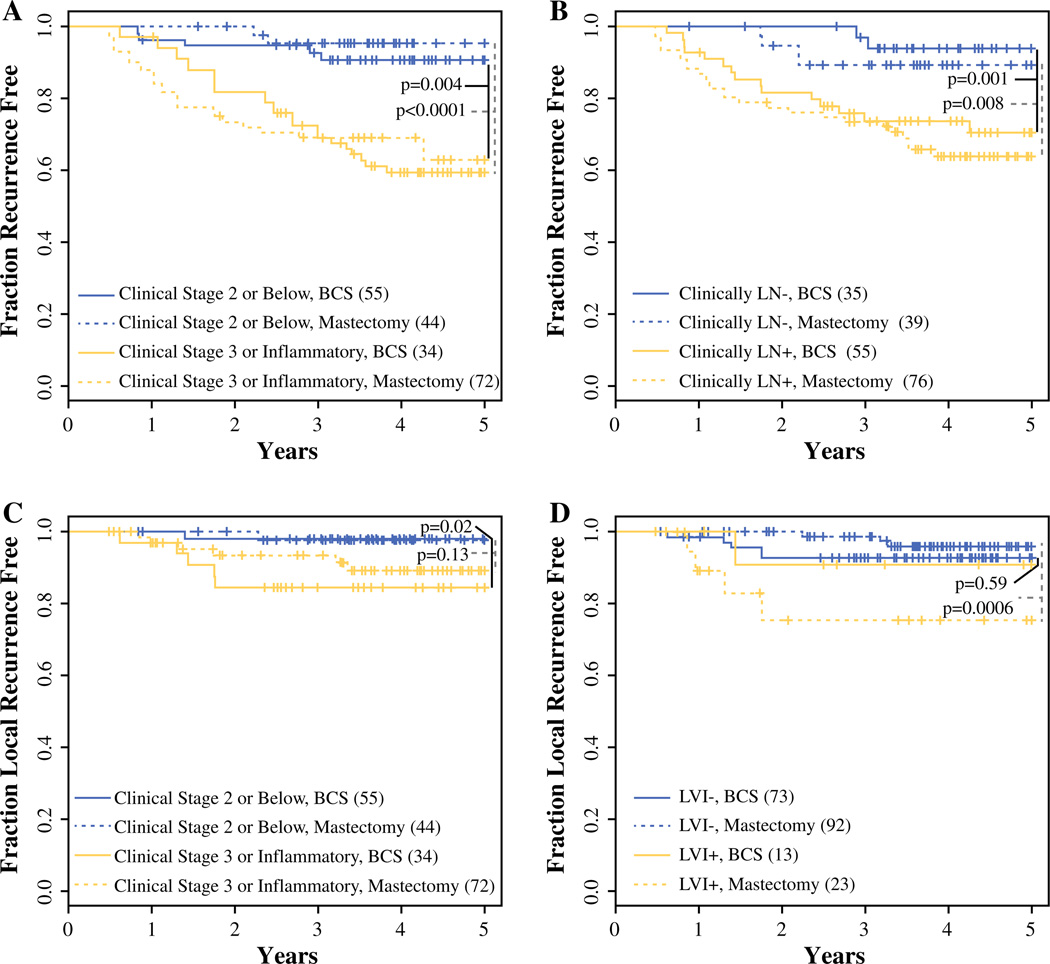
As published previously,6 clinical variables associated with recurrence included clinical stage, nodal status, RCB and LVI (LR and/or DR) with log rank p < 0.0001, 0.0002, 0.03, <0.0001, respectively. Kaplan–Meier curves of time to first recurrence (LR and/or DR) within the mastectomy and BCS groups, stratified by dichotomized clinical stage and node positivity, are shown in Fig. 2a and b. Patients presenting with clinical stage 3 or inflammatory disease have higher overall 5-year recurrence risk (41 and 37 % for the mastectomy and BCS groups) when compared with lower-stage patients (5 and 9 % in the mastectomy and BCS groups, respectively). Overall 5-year recurrence risks for node-positive disease at presentation were 36 and 29 %, and for node-negative disease were 11 and 6 % in the mastectomy and BCS groups, respectively.
FIG. 2.
Kaplan–Meier analysis of time to recurrence within mastectomy and BCSS subsets. a,b Kaplan–Meier curves of time to first recurrence (LR and/or DR) within mastectomy (dotted line) and BCSS (solid line) subsets were stratified by a dichotomized clinical stage at presentation (gold stage 2 or below; blue stage 3 or inflammatory); b nodal status at presentation (gold node negative; blue node positive). c,d Kaplan–Meier curves of time to LR within the mastectomy (dotted line) and BCSS (solid line) subsets were stratified by c dichotomized clinical stage at presentation (gold stage 2 or below; blue stage 3 or inflammatory); d LVI at surgery (gold negative; blue positive). p-Values refer to the difference in either first time to recurrence (a,b) or LR (c,d) dichotomized by clinical stage, node status, and LVI within each surgical type. LR local recurrence, DR distant recurrence, LVI lymphovascular invasion, BCS breast-conserving surgery
We then evaluated whether the factors predicting recurrence risk overall also associate with LR risk in an exploratory analysis, and found that higher clinical stage at presentation and LVI were associated with significantly higher risk of LR (log rank p: 0.007 and 0.008). Figure 2c and d show the Kaplan–Meier curves for time to LR within the mastectomy and BCS groups, stratified by dichotomized clinical stage and LVI, respectively. Patients presenting with stage 3 or inflammatory disease have higher 5-year LR risk (11 and 15 %) compared with patients presenting with stage 2 (2 and 2 %) for the mastectomy and BCS groups, respectively. Patients positive for LVI are also at a higher risk for LR, with 5-year rates of 24 and 9 % compared with 4 and 7 % when LVI is not present, for the mastectomy and BCS groups, respectively. Interestingly, patients with LVI who underwent BCS did not have a higher LR rate. Radiation may play a role; as in the BCS group, 12/13 (92 %) patients with LVI received radiation, while, in contrast, in the mastectomy group, 17/23 (74 %) patients with LVI received post-mastectomy radiation. However, the numbers are too small to make any definitive conclusions.
In patients who had radiation, the 5-year LR risk was low within both the mastectomy (6 %) and BCS (7 %) groups, and appears to be similar to that observed in patients who did not receive radiation (16 % in the mastectomy group and 9 % in the BCS group). However, we recognize that radiation treatment was not randomized and was likely influenced by tumor clinical and biological characteristics which may bias these findings.
Of the 90 BCS-treated patients, only 56 had complete information to allow MDAPI score computation for LR risk prediction. Consistent with the low overall LR risk within the I-SPY Trial, 73 % of our BCS group were classified as MDAPI low risk; 19 % were classified as immediate risk; and only 7 % were classified as high risk. No significant differences in LR risk were observed between the MDAPI risk groups (p = 0.583).
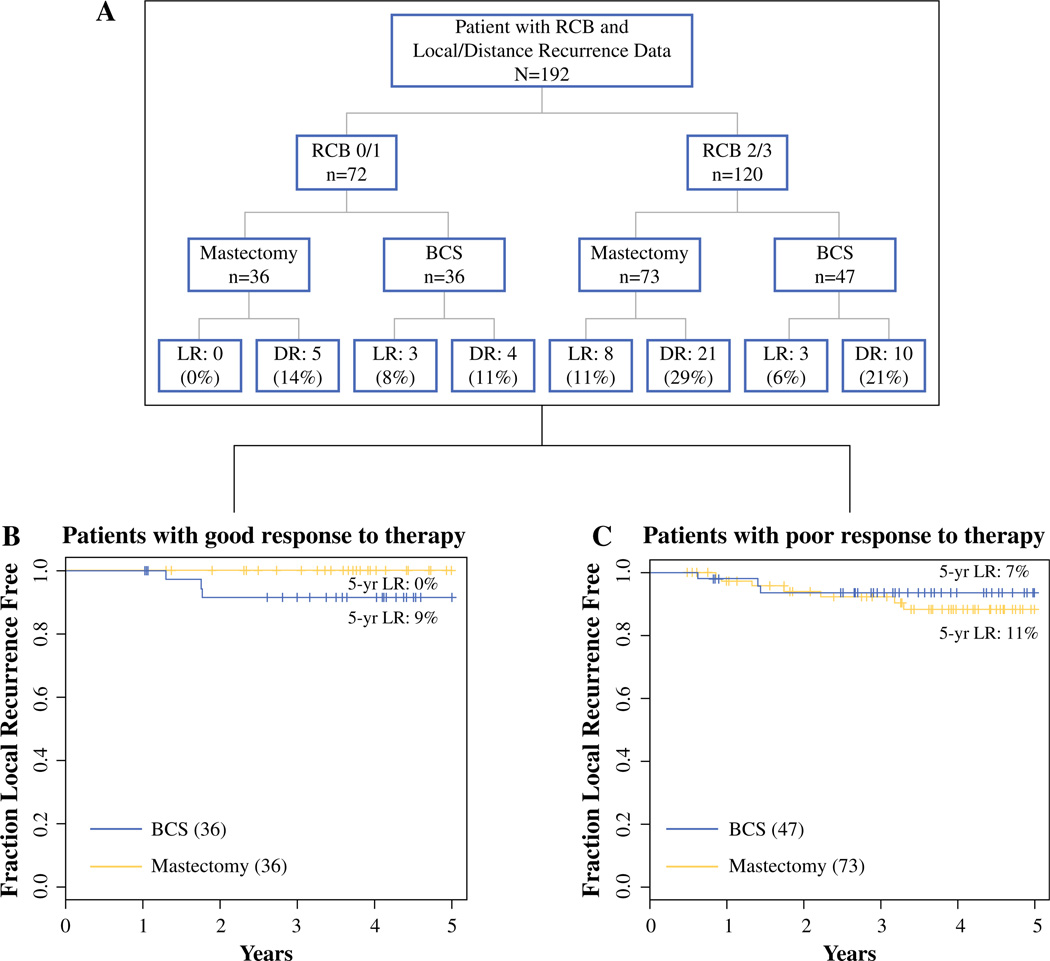
Patients were not randomized to surgical procedure following chemotherapy. Therefore, we are presenting the following data without any statistical inferences. In the setting of an excellent response to therapy (RCB 0 or RCB 1), the 5-year LR risk was 0 % for mastectomy and 9 % for breast conservation (Fig. 3b). In the setting of significant residual disease (RCB 2 or 3) (Fig. 3c), the LR rate was 12 % for mastectomy and 7 % for breast conservation. The majority of the LRs (79 %) were in the RCB 2/3 group (11/14) compared with the RCB 0/1 (3/14) group.
FIG. 3.
LR in the context of response to therapy within mastectomy and BCS subsets. a Recurrence in patients with good or poor response to therapy by surgery type. b Kaplan–Meier curves of time to LR within mastectomy (dotted line) and lumpectomy (solid line) subsets in patients with good response to therapy (RCB 0/1). c Kaplan–Meier curves of time to LR within mastectomy (dotted line) and lumpectomy (solid line) subsets in patients with poor response to therapy (RCB 2/3). LR local recurrence, DR distance recurrence, BCS breast-conserving surgery, RCB residual cancer burden
DISCUSSION
LR after BCS post-neoadjuvant chemotherapy ranges from 5 to 20 %.2,19–24 After mastectomy, the range is from 4 to 28 %.24–26 Fisher et al. found that patients who have BCS after being downstaged by neoadjuvant chemotherapy had a higher recurrence than those patients undergoing BCS who were eligible for lumpectomy from the beginning, although this observation did not hold true with longer follow-up and was related to younger age.27 Some would argue that these patients would not have recurred if they had undergone mastectomy. The question remains whether mastectomy would have helped counteract aggressive biology, or whether the patients with aggressive biology would have recurred with either type of surgery.
Biologic characteristics such as advanced stage at presentation, nodal status, receptor status, LVI, multifocality, and response to therapy have been shown to affect recurrence rates after neoadjuvant chemotherapy.3,5,6,8–13,25,28,29 Paradoxically, higher-risk molecular profiles (HER2+ or TN, for example) have been shown to be associated with higher likelihood of achieving a complete pathologic response, which in turn predicts better disease-free survival, as reported by the I-SPY Trial investigators and others.5,6,30,31 Failure to achieve a complete or near complete pathologic response in these same patients is associated with increased risk of recurrence-free survival.5,6 These same factors appear to predict recurrence whether patients undergo BCS or mastectomy.
For patients who meet the I-SPY Trial eligibility criteria, the biggest risk is DR. LR alone was rare (2 %), and DR was three times more common than LR. LR strongly predicted DR. A potential flaw in these data is that once DR is reported, the procedure for assessing LR is different and less likely to be complete; however, we specifically queried all sites to determine whether patients who had DR had experienced a delayed LR.
Likely, the systemic therapy controlled not only distant but also local disease, and in this setting, when response to therapy was excellent, BCS and mastectomy both resulted in low LR. The overwhelming majority of patients received radiation therapy, and this likely contributed to local control as well. Maximal multidisciplinary treatment may also have resulted in more optimal selection of patients for breast conservation. Patients in this study underwent serial imaging and multiple visits, and had coordinated surgical and medical oncology care, which may also have contributed to better local outcomes.
The MDAPI is reported to predict a 5-year LR in BCS-treated patients following neoadjuvant chemotherapy.17,18 The MDAPI did not appear predictive of LR risk in the I-SPY Trial BCS-treated patients. It is not apparent why our results are different as systemic treatments were similar, as was high use of radiation in both the MD Anderson cohorts and the I-SPY multisite trial. This lack of association may be attributed to an important limitation in our exploratory analysis evaluating factors predicting LR risk. Our sample size was small, and given that the LR risk within our study was low, we may not be inadequately powered to detect differences between risk groups.
We have reported previously that most patients will have significant shrinkage of tumor after neoadjuvant therapy and many will be able to achieve breast conservation. Patients with more circumscribed masses, based on magnetic resonance imaging phenotypes, are more likely to achieve shrinkage that will enable BCS.32 In this study, we demonstrate that for those patients who are able to achieve breast conservation, the LR rates are low, even when tumors are large at presentation.
A potential limitation of the study is the length of follow-up as HR+ tumors may take longer to recur.4 However, based on data from the Early Breast Cancer Trialists’ Collaborative Group Overview data, 75–85 % of all LRs occur in the first 5 years in node-positive patients.33 The key limitation of our study is that the choices of surgery and radiation therapy were not randomized. Certainly, patients who cannot have breast conservation due to large tumor-to-breast ratio or significant residual disease will be much more likely to have mastectomy. This likely explains the particularly high LR rate in the mastectomy patients with residual disease and LVI. Additionally, most patients presenting with inflammatory breast cancer underwent mastectomy. However, we note that with the exception of clinical stage and T stage at presentation, the BCS and mastectomy groups were not different with respect to other biologic factors.
Despite these limitations, there are a few insights that merit testing in future studies. As the majority of LRs occur in the RCB 2/3 arm, perhaps local management should be driven by residual disease and LVI. The numbers of patients in the I-SPY 1 cohort are not large enough to make definitive conclusions, and we plan to re-evaluate our findings in an expanded cohort once the I-SPY 1 extension 3-year EFS data become available.
For the majority of patients in the I-SPY1 Trial, recurrence came in the form of distant metastasis. Both mastectomy and breast conservation were associated with relatively low and acceptable LR rates. High-risk factors (advanced stage at presentation, poor response to therapy, and LVI) increased the chance of LR in the setting of both mastectomy and BCS. Going forward, there may be an opportunity to reduce the extent of treatment after neoadjuvant therapy for groups of patients with less risky biological features, especially those who have a pathologic complete response and choose mastectomy. Radiation therapy may not add much value as the LR risk is already low. Larger randomized trials of neoadjuvant therapy from NSABP have resulted in the identification of patient groups who would likely not have a survival benefit from post-mastectomy radiation.34 Given that radiation after mastectomy, especially in the setting of reconstruction, is associated with higher complications and additional procedures,35–38 this could be an important contribution to improving treatment options and outcomes. We recognize that in this study, radiation was given at the physicians’ discretion, with 28 of 36 RCB 0/1 patients who received mastectomy (Fig. 3a) also receiving radiation. In comparison, 64/73 patients in the RCB 2/3 mastectomy group received radiation. As neoadjuvant trials and treatment become more common, a randomized controlled trial of radiation versus no radiation after good response to neoadjuvant therapy and/or low-risk biologic features, in the setting of mastectomy, would be of clinical importance. The information would be of great value to patients and their treating physicians.
After neoadjuvant chemotherapy, there will still be patients with large residual tumor-to-breast ratio or diffuse disease, and patients who prefer mastectomy. These are the indications for mastectomy after neoadjuvant chemotherapy, not findings of aggressive biology, as aggressive biology dictates recurrence in patients after either type of surgery. We can counsel patients that BCS and mastectomy are both oncologically safe. Patients, especially those who are likely to undergo radiation, may be able to decrease local complications by undergoing BCS with acceptable rates of local control.
Supplementary Material
ACKNOWLEDGEMENT
We wish to thank the study patients and patient advocates. There are also several people who were the champions of this I-SPY Trial, without whom the study would not have been initiated: Jorge Gomez from the National Cancer Institute (NCI); Larry Norton from CALGB; and Ken Buetow and Subha Madhavan for enabling the development of the data systems for the trial data. We also wish to thank Rachel Gomez for developing the training and systems to guarantee the quality of the pathologic data, and to Sarah Davis for her efforts to ensure the integrity of the entire data set, and to Meredith Buxton for coordinating the entire study. We would also like to thank Lisa Carey from the University of North Carolina for her valuable input. Funding was received from the NCI SPORE in Breast Cancer (CA58207), ACRIN (CA079778 and CA080098), CALGB (CA31964 & CA33601), NCI Center for Bioinformatics, The Breast Cancer Research Foundation, Bruce and Martha Atwater, The Terry Fox Foundation Postdoctoral Fellowship, and ‘Give Breast Cancer the Boot’.
Footnotes
This study was conducted on behalf of the I-SPY 1 TRIAL Investigators.
Electronic supplementary material The online version of this article (doi:10.1245/s10434-014-3721-7) contains supplementary material, which is available to authorized users.
REFERENCES
- 1.Lin C, Buxton MB, Moore D, et al. Locally advanced breast cancers are more likely to present as interval cancers: results from the I-SPY 1 TRIAL (CALGB 150007/150012, ACRIN 6657, InterSPORE Trial) Breast Cancer Res Treat. 2012;132:871–879. doi: 10.1007/s10549-011-1670-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rouzier R, Mathieu MC, Sideris L, et al. Breast-conserving surgery after neoadjuvant anthracycline-based chemotherapy for large breast tumors. Cancer. 2004;101:918–925. doi: 10.1002/cncr.20491. [DOI] [PubMed] [Google Scholar]
- 3.Pierga JY, Mouret E, Laurence V, et al. Prognostic factors for survival after neoadjuvant chemotherapy in operable breast cancer: the role of clinical response. Eur J Cancer. 2003;39:1089–1096. doi: 10.1016/s0959-8049(03)00069-8. [DOI] [PubMed] [Google Scholar]
- 4.Esserman LJ, Moore DH, Tsing PJ, et al. Biologic markers determine both the risk and the timing of recurrence in breast cancer. Breast Cancer Res Treat. 2011;129:607–616. doi: 10.1007/s10549-011-1564-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Esserman LJ, Berry DA, Demichele A, et al. Pathologic complete response predicts recurrence-free survival more effectively by cancer subset: results from the I-SPY 1 TRIAL-CALGB 150007/150012, ACRIN 6657. J Clin Oncol. 2012;30:3242–3249. doi: 10.1200/JCO.2011.39.2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Esserman LJ, Berry DA, Cheang MC, et al. Chemotherapy response and recurrence-free survival in neoadjuvant breast cancer depends on biomarker profiles: results from the I-SPY 1 TRIAL (CALGB 150007/150012; ACRIN 6657) Breast Cancer Res Treat. 2012;132:1049–1062. doi: 10.1007/s10549-011-1895-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fisher B, Bryant J, Wolmark N, et al. Effect of preoperative chemotherapy on the outcome of women with operable breast cancer. J Clin Oncol. 1998;16:2672–2685. doi: 10.1200/JCO.1998.16.8.2672. [DOI] [PubMed] [Google Scholar]
- 8.Kuerer HM, Newman LA, Smith TL, et al. Clinical course of breast cancer patients with complete pathologic primary tumor and axillary lymph node response to doxorubicin-based neoadjuvant chemotherapy [see comments] J Clin Oncol. 1999;17:460–469. doi: 10.1200/JCO.1999.17.2.460. [DOI] [PubMed] [Google Scholar]
- 9.Kuerer HM, Newman LA, Buzdar AU, et al. Residual metastatic axillary lymph nodes following neoadjuvant chemotherapy predict disease-free survival in patients with locally advanced breast cancer. Am J Surg. 1998;176:502–509. doi: 10.1016/s0002-9610(98)00253-0. [DOI] [PubMed] [Google Scholar]
- 10.Kuerer HM, Newman LA, Fornage BD, et al. Role of axillary lymph node dissection after tumor downstaging with induction chemotherapy for locally advanced breast cancer [see comments] Ann Surg Oncol. 1998;5:673–680. doi: 10.1007/BF02303476. [DOI] [PubMed] [Google Scholar]
- 11.Kuerer HM, Newman LA, Buzdar AU, et al. Pathologic tumor response in the breast following neoadjuvant chemotherapy predicts axillary lymph node status. Cancer J Sci Am. 1998;4:230–236. [PubMed] [Google Scholar]
- 12.Pierga JY, Mouret E, Dieras V, et al. Prognostic value of persistent node involvement after neoadjuvant chemotherapy in patients with operable breast cancer. Br J Cancer. 2000;83:1480–1487. doi: 10.1054/bjoc.2000.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schwartz GF, Birchansky CA, Komarnicky LT, et al. Induction chemotherapy followed by breast conservation for locally advanced carcinoma of the breast. Cancer. 1994;73:362–369. doi: 10.1002/1097-0142(19940115)73:2<362::aid-cncr2820730221>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 14.Cance WG, Carey LA, Calvo BF, et al. Long-term outcome of neoadjuvant therapy for locally advanced breast carcinoma: effective clinical downstaging allows breast preservation and predicts outstanding local control and survival. Ann Surg. 2002;236:295–202. doi: 10.1097/01.SLA.0000027526.67560.64. discussion 302–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hylton NM, Blume J, Bernreuter W, et al. Prediction of response to neoadjuvant chemotherapy for women with locally-advanced breast cancer: results from the ACRIN 6657/ I-SPY Trial. Radiology. 2012;263:663–672. doi: 10.1148/radiol.12110748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Symmans WF, Peintinger F, Hatzis C, et al. Measurement of residual breast cancer burden to predict survival after neoadjuvant chemotherapy. J Clin Oncol. 2007;25:4414–4422. doi: 10.1200/JCO.2007.10.6823. [DOI] [PubMed] [Google Scholar]
- 17.Chen AM, Meric-Bernstam F, Hunt KK, et al. Breast conservation after neoadjuvant chemotherapy: the MD Anderson Cancer Center experience. J Clin Oncol. 2004;22:2303–2312. doi: 10.1200/JCO.2004.09.062. [DOI] [PubMed] [Google Scholar]
- 18.Chen AM, Meric-Bernstam F, Hunt KK, et al. Breast conservation after neoadjuvant chemotherapy. Cancer. 2005;103:689–695. doi: 10.1002/cncr.20815. [DOI] [PubMed] [Google Scholar]
- 19.Min SY, Lee SJ, Shin KH, et al. Locoregional recurrence of breast cancer in patients treated with breast conservation surgery and radiotherapy following neoadjuvant chemotherapy. Int J Radiat Oncol Biol Phys. 2011;81:e697–e705. doi: 10.1016/j.ijrobp.2010.10.014. [DOI] [PubMed] [Google Scholar]
- 20.Rouzier R, Extra JM, Carton M, et al. Primary chemotherapy for operable breast cancer: incidence and prognostic significance of ipsilateral breast tumor recurrence after breast-conserving surgery. J Clin Oncol. 2001;19:3828–3835. doi: 10.1200/JCO.2001.19.18.3828. [DOI] [PubMed] [Google Scholar]
- 21.Mauriac L, MacGrogan G, Avril A, et al. Neoadjuvant chemotherapy for operable breast carcinoma larger than 3 cm: a unicentre randomized trial with a 124-month median follow-up. Institut Bergonie Bordeaux Groupe Sein (IBBGS) Ann Oncol. 1999;10:47–52. doi: 10.1023/a:1008337009350. [DOI] [PubMed] [Google Scholar]
- 22.Beriwal S, Schwartz GF, Komarnicky L, Garcia-Young JA. Breast-conserving therapy after neoadjuvant chemotherapy: longterm results. Breast J. 2006;12:159–164. doi: 10.1111/j.1075-122X.2006.00225.x. [DOI] [PubMed] [Google Scholar]
- 23.Cebrecos I, Cordoba O, Deu J, et al. Can we predict local recurrence in breast conserving surgery after neoadjuvant chemotherapy? Eur J Surg Oncol. 2010;36:528–534. doi: 10.1016/j.ejso.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 24.Huang EH, Strom EA, Perkins GH, et al. Comparison of risk of local-regional recurrence after mastectomy or breast conservation therapy for patients treated with neoadjuvant chemotherapy and radiation stratified according to a prognostic index score. Int J Radiat Oncol Biol Phys. 2006;66:352–357. doi: 10.1016/j.ijrobp.2006.04.046. [DOI] [PubMed] [Google Scholar]
- 25.Garg AK, Strom EA, McNeese MD, et al. T3 disease at presentation or pathologic involvement of four or more lymph nodes predict for locoregional recurrence in stage II breast cancer treated with neoadjuvant chemotherapy and mastectomy without radiotherapy. Int J Radiat Oncol Biol Phys. 2004;59:138–145. doi: 10.1016/j.ijrobp.2003.10.037. [DOI] [PubMed] [Google Scholar]
- 26.Huang EH, Tucker SL, Strom EA, et al. Predictors of locoregional recurrence in patients with locally advanced breast cancer treated with neoadjuvant chemotherapy, mastectomy, and radiotherapy. Int J Radiat Oncol Biol Phys. 2005;62:351–357. doi: 10.1016/j.ijrobp.2004.09.056. [DOI] [PubMed] [Google Scholar]
- 27.Fisher B, Brown A, Mamounas E, et al. Effect of preoperative chemotherapy on local-regional disease in women with operable breast cancer: findings from National Surgical Adjuvant Breast and Bowel Project B-18. J Clin Oncol. 1997;15:2483–2493. doi: 10.1200/JCO.1997.15.7.2483. [DOI] [PubMed] [Google Scholar]
- 28.Rouzier R, Pusztai L, Delaloge S, et al. Nomograms to predict pathologic complete response and metastasis-free survival after preoperative chemotherapy for breast cancer. J Clin Oncol. 2005;23:8331–8339. doi: 10.1200/JCO.2005.01.2898. [DOI] [PubMed] [Google Scholar]
- 29.Uematsu T, Kasami M, Watanabe J, et al. Is lymphovascular invasion degree one of the important factors to predict neoadjuvant chemotherapy efficacy in breast cancer? Breast Cancer. 2011;18:309–313. doi: 10.1007/s12282-010-0211-z. [DOI] [PubMed] [Google Scholar]
- 30.Fasching PA, Heusinger K, Haeberle L, et al. Ki67, chemotherapy response, and prognosis in breast cancer patients receiving neoadjuvant treatment. BMC Cancer. 2011;11:486. doi: 10.1186/1471-2407-11-486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gianni L, Zambetti M, Clark K, et al. Gene expression profiles in paraffin-embedded core biopsy tissue predict response to chemotherapy in women with locally advanced breast cancer. J Clin Oncol. 2005;23:7265–7277. doi: 10.1200/JCO.2005.02.0818. [DOI] [PubMed] [Google Scholar]
- 32.Mukhtar RA, Yau C, Rosen M, et al. Clinically meaningful tumor reduction rates vary by prechemotherapy MRI phenotype and tumor subtype in the I-SPY 1 TRIAL (CALGB 150007/150012/ACRIN 6657) Ann Surg Oncol. 2013;20:3823–3830. doi: 10.1245/s10434-013-3038-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Clarke M, Collins R, Darby S, et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;366:2087-06. doi: 10.1016/S0140-6736(05)67887-7. [DOI] [PubMed] [Google Scholar]
- 34.Mamounas EP. NSABP breast cancer clinical trials: recent results and future directions. Clin Med Res. 2003;1:309–326. doi: 10.3121/cmr.1.4.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Berry T, Brooks S, Sydow N, et al. Complication rates of radiation on tissue expander and autologous tissue breast reconstruction. Ann Surg Oncol. 2010;17(Suppl 3):202–210. doi: 10.1245/s10434-010-1261-3. [DOI] [PubMed] [Google Scholar]
- 36.Brooks S, Djohan R, Tendulkar R, et al. Risk factors for complications of radiation therapy on tissue expander breast reconstructions. Breast J. 2012;18:28–34. doi: 10.1111/j.1524-4741.2011.01182.x. [DOI] [PubMed] [Google Scholar]
- 37.Spear SL, Onyewu C. Staged breast reconstruction with salinefilled implants in the irradiated breast: recent trends and therapeutic implications. Plast Reconstr Surg. 2000;105:930–942. doi: 10.1097/00006534-200003000-00016. [DOI] [PubMed] [Google Scholar]
- 38.Peled A, Foster R, Esserman L. A comparison of oncoplastic reduction mammoplasty and mastectomy with immediate reconstruction in patients with locally advanced breast cancer. 2012 In press. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.