Abstract
Storage at room temperature is limited to 5 days because of the risk of bacterial growth and loss of platelet functionality. Platelet refrigeration remains impossible, because once chilled, platelets are rapidly removed from circulation. Chilling platelets (<4 h) clusters glycoprotein (GP) Ibα receptors, and β2 integrins on hepatic macrophages recognize clustered βGlcNAc residues leading to rapid clearance of acutely chilled platelets. Prolonged refrigeration increases the exposure of galactose residues such that, unexpectedly, hepatocytes remove platelets using their asialoglycoprotein receptors. Here we review current knowledge of the mechanisms of platelet removal, the existing knowledge of refrigerated platelet function, and methods to preserve platelet concentrates long-term for transfusion.
The invention of platelet transfusion has been a tremendous advancement in medicine, allowing hematopoetic progenitor cell transplantations and/or chemotherapy, procedures often accompanied by severe thrombocytopenia and bleeding. However, the increased advancement of therapies led to a disproportional demand for platelet transfusions [1] perpetuating the demands of platelet transfusion agencies. Improved platelet storage has permitted millions of transfusions per year. However, current blood banking practice for platelets is far from ideal. In 1969, Murphy and Gardner demonstrated that transfused human platelets stored at 4°C are rapidly cleared from the circulation [2, 3]. They and others proved that platelets stored at room temperature (22°C) show significantly better recovery and survival, with a typical life span of 7–9 days versus only 2–4 days for refrigerated platelets [2, 3] [4, 5]. This odd phenomenon of chilled platelet clearance has had profound consequences for blood banking. For decades, all platelet products have been stored at room temperature, and because of the attendant risk of bacterial growth, platelet storage has been limited to five days. This review will summarize the clearance mechanisms for refrigerated platelets and examine existing data addressing whether platelets might actually work better if refrigerated.
Refrigerated storage of platelet products would be predicted to dramatically reduce the risk of bacterial growth [6, 7], permitting extended storage of platelets and bolstering product inventories. Competing technologies now under development might ultimately undermine these potential benefits [8]. For example, platelet bacterial testing may lead to the safe extension of room temperature storage of platelets [9]. Pathogen reduction technology could virtually eliminate not only bacteria, but viral and protozoal pathogens as well [10, 11]. Furthermore, improvements in platelet storage, such as second-generation storage containers with better O2/CO2 exchange and bigger platelet storage volumes stabilize pH values, resulted in better platelet viability [4, 12–14]. Storage of platelets in synthetic media (platelet additive solutions) may further reduce platelet metabolic rate and inhibit platelet activation during storage [15–17]. Although the usage of platelet additive solutions may be successful, this assessment is mostly based on in vitro studies. Only limited clinical evaluations have been published that either studied the effectiveness of platelets in initially developed platelet additive solutions, or were specifically done in combination with pathogen reduction technologies. More clinical studies are needed to substantiate in vitro results before platelet additive solutions may safely replace plasma, and allow extended storage with maintenance of quality, however more clinical studies are needed to substantiate the in vitro result [16, 18].
Does platelet refrigeration offer any unique advantages? It is possible, although yet unproven, that 4°C storage could yield platelet products with clinically superior hemostatic function relative to their room temperature counterparts after extended storage (i.e > 5 days).
Platelet function: cold versus warm platelets
To be clinically effective, transfused platelets must (1) circulate and (2) function in clotting (i.e. prevent or stop bleeding). Currently, the gold standard test to evaluate platelet products is in vivo survival and count increment of transfused radiolabeled platelets [19, 20]. It is assumed that if a platelet product circulates normally, it should function appropriately. However, both parameters fail to assess the functional quality of transfused platelets. Furthermore, efficacy of a transfused platelet product is clearly patient dependent, complicating the assessment of platelet functionality [21]. Unequivocally, clinical experience shows that platelet transfusions work. Improvements in treatment have rendered fatal hemorrhage considerably less common today, although thrombocytopenic patients receiving prophylactic transfusions in randomized platelet “trigger” trials have had clinically significant bleeding complications (WHO Grades 2–4), at rates of 17–21.5%, irrespective of study arm [22, 23]. It seems therefore reasonable to question if current bleeding-rates could be reduced by better functioning platelet products.
Platelets are metabolically active during room temperature storage, a factor which continuously diminishes platelet function. Metabolic products, such as lactate, accumulate during room temperature storage, leading to a fall in pH [24]. pH levels below 6.0–6.2 have been associated with severely diminished platelet viability [20, 25–28]. In contrast, there is minimal lactate accumulation, and the pH does not decrease at 4°C [29, 30]. Platelets undoubtedly become activated during room temperature storage [31–35]. Key mediators of thrombosis stored in the α-granules of resting platelets, such as β-thromboglobulin (β-TG) and platelet factor 4, accumulate in the platelet storage medium over time [36]. Moreover, platelet surface P-selectin expression increases during room temperature storage [37], indicating that platelets continuously release their granule content, i.e become activated.
Whether cooling itself causes platelet activation is controversial. In many ways, platelets stored at 4°C seem to be less activated than platelets stored at room temperature. For example, 4°C platelet storage does not lead to β-TG release into the storage medium [36]. On the other hand, platelet chilling induces rapid irreversible disc-to-sphere shape changes [38] and P-selectin exposure indicates α-granule content release [39–42]. P-selectin exposure had been suggested to accelerate platelet clearance [31, 33, 41]. However, despite initial enthusiasm for the use of P-selectin as a marker of platelet quality in transfusion settings, P-selectin levels do not predict platelet survival after transfusion [33, 34, 43, 44]. Studies using platelets lacking P-selectin [43] or platelets activated with thrombin [44, 45] revealed that P-selectin exposure or loss of platelet discoid shape had no effect on platelet clearance. Conversely, spherical platelets characteristic for mice lacking β1-tubulin circulate normally [40, 46, 47]. Uncapping and fragmentation of actin filaments is one prerequisite for conversion of platelet discs to spheres following cooling [48]. However, preservation of discoid shape using actin-remodeling inhibitors did not diminishe refrigerated platelet clearance [40, 46, 47]. Taken together, these studies show that preservation of platelet discoid shape does not predict platelet survival. Phosphatidyl serine exposure and loss of the von Willebrand factor receptor complex α-chain (GPIbα) following storage occur independent of the storage temperature [33, 39, 41, 49–52]. Unfortunately, it appears that until today none of the measured surface markers has been shown to reliably predict platelet survival.
It is assumed that both the retention of platelet discoid shape, as measured photometrically by the extent of shape change (ESC), and hypotonic shock response (HSR) indicating metabolic efficacy, report on platelet viability [24, 53–55]. Not surprisingly, platelets stored at room temperature perform better in both tests because platelets rapidly loose their “discoid shape” when chilled, implying that refrigerated platelets are not metabolically efficient and viable [3, 38, 55]. Most investigators however agree that 4°C stored platelet products show better pH stability [29, 30], a reduced rate of glycolysis, and a better response when stimulated by ADP, epinephrine or collagen than room temperature stored platelets [3, 56], implying that refrigerated platelets function better.
Two studies have used platelet in vitro “bleeding time devices” (Thrombostat-4000 or PFA-100) to evaluate the effectiveness of room temperature platelet concentrates transfusions [57] [58]showing that in some patients closure times were shortened following transfusion, and more effectively when fresh platelets were transfused. Unsurprisingly, both studies showed that the closure times do not correlate very well with platelet count increments. Some patients failed to have good platelet count increment, but showed closure times shortening, suggesting that platelet function was improved. On the other hand, these results may also suggest that “super-functional” platelets are removed rapidly from the circulation. PFA-100 or Hepcon HMS tests have also been used to evaluate platelet in vivo and in vitro function in both, platelet products and the platelet donors. These studies showed that both platelet donor and patients have platelet dysfunctions, often due to surreptitious intake of cyclooxygenase inhibitors (e.g., aspirin), [21, 59, 60] severely complicating assessments of platelet function.
In vivo studies of refrigerated human platelets
One key concept that has emerged from recent refrigerated platelet studies is that a platelet’s ability to survive normally in the circulation may be entirely separable from its ability to function in blood clotting [40]. This concept challenges the “old” conclusion that refrigerated platelets are removed from the circulation because they do not function.
A small number of human studies performed in the early 1970s suggest that 4°C platelets have better in vivo function than room temperature platelets despite having poor survival in the circulation. Becker, Aster and colleagues [3] tested the effects of platelets refrigerated for ≤ 72 h in 68 thrombocytopenic patients. The patients, with pre-transfusion bleeding times of >15 minutes, were given fresh platelet concentrates, room temperature platelet concentrates that had been stored for 24, 48 or 72 hours, or 4°C platelet concentrates that had been stored for equivalent periods. Bleeding times were measured one hour after transfusion. Platelets refrigerated for 48 or 72 hours corrected the bleeding time in 63% of cases, while room temperature platelets stored for an equivalent period corrected the bleeding time in only 24% of patients. In similar studies performed in aspirin-treated volunteers, bleeding times were corrected almost to baseline within four hours after transfusion on 4°C platelets. In contrast, almost no effect was seen at this early time point in subjects receiving platelets stored at room temperature for 72 hours. At 24 hours post-transfusion, substantial correction of the bleeding time was seen in the recipients of room temperature platelets [3] [5]. Another study showed that refrigerated (≤ 72hrs) platelets transfused into 41 leukemia patients were clinically effective (i.e stopped bleeding) and were considered as “safe” in over 100 transfused patients [61]. Similar observations were made by Handin and Valeri [62], who found that room temperature platelets corrected the bleeding time of aspirin treated volunteers 24 hours post-transfusion, but had no effect immediately post-transfusion. Together, this evidence suggests that loss of in vitro platelet function may be reversible upon transfusion [62]. Based on these studies, the suggestion was made that platelets specifically intended to treat acute/active bleeding (i.e trauma or surgical patients) should be stored at 4°C, while platelets used for bleeding prophylaxis should be stored at room temperature [55, 63].
However, this approach never gained acceptance, as studies performed subsequently failed to confirm that transfused room temperature platelets showed a significant delay in hemostatic function. A study by Slichter and Harker showed that room temperature platelets consistently corrected the bleeding times of aplastic thrombocytopenic patients within 2 and ½ hours. In contrast refrigerated platelets (< 72 hours) corrected the bleeding time in 6/8 cases shortly post-transfusion, but the effect was not sustained beyond 2 1/2 hours [4] [64]. Filip and Aster repeated the bleeding time experiments done previously in their laboratory [64], administering either room temperature or 4°C platelets to 41 thrombocytopenic patients and reported that platelets stored at room temperature for 24 hours produced the greatest bleeding time correction one hour post-transfusion, while platelets stored at 4°C for 72 hours yielded the least bleeding time correction, although the difference was not statistically significant. The investigators attributed the relative ineffectiveness of room temperature platelets in their earlier study to inadequate low storage volume [4].
The sole reason platelets are currently stored at room temperature is that chilled platelets do not circulate for an acceptable period of time in the recipient. It appears that the mechanisms dictating platelet removal from the circulation, including refrigerated platelets, are independent of platelet function and remain poorly understood.
Clearance mechanisms for refrigerated platelets: Dual roles for hepatic lectins
An effort to address the clinically relevant problem of why refrigerated platelets fail to circulate led us to define two previously unsuspected, carbohydrate-dependent platelet clearance mechanisms.
Role of the macrophage αMβ2-integrin in refrigerated platelet clearance
Earlier, we reported that the macrophage carbohydrate-binding αMβ2 recognizes clustered GPIbα subunits of the von Willebrand factor receptor (vWfR) complex following short-term refrigeration (2 hours, 0°C), resulting in the phagocytosis and clearance of platelets in vivo in mice and in vitro by human THP-1 macrophages [40, 65–67]. Experiments using αMβ2-deficient but not vWf-deficient, complement- or P-selectin-deficient mice[43] markedly improved the survival of refrigerated platelets. Removal of GPIbα ’s ligand binding-domain using the O-sialoglycoprotein endopeptidase restored the circulation of refrigerated murine wild-type platelets indicating that the external domain of GPIbα initiates clearance [40]. Subsequent work narrowed carbohydrate recognition by αMβ2 to Yexposed βGlcNAc (β-N-acetylglucosamine) residues on N-linked GPIbα glycans (Fig. 1) [65, 66]. GPIbα associated N-linked glycans are complex-type, branched carbohydrates covalently attached to asparagine residues. When completely assembled, they are capped by sialic acid. Removal of sialic acid (desialylation) exposes galactose and degalactosylation reveals βGlcNAc. The exposure of individual sugars is detectable by their binding to specific lectins, e.g., ricinus communis agglutinin (RCA) I binds galactose and succinylated wheat germ agglutinin (sWGA) binds βGlcNAc. Although resting platelets bind some sWGA, refrigerated platelets have markedly increased binding, suggesting that altered epitope presentation of exposed βGlcNA on GPIbα can facilitate lectin binding to refrigerated platelets. Since altered presentation correlates with a clustering of GPIbα on the surface of refrigerated platelets, we believe clustering plays an important role in the increased lectin recognition [40, 65]. The βGlcNAc residues recognized by αMβ2 represent either incomplete glycan processing on platelets or result from a degradative process that occurs in blood. In an attempt to remedy their poor circulation, we galactosylated refrigerated platelets and were surprised to learn that both human and murine platelets have functional platelet galactosyltransferase(s) on their surface. These enzyme(s) respond to the simple addition of UDP-galactose by transferring galactose onto exposed βGlcNAc residues of human or mouse GPIbα [65]. Galactosylation markedly improved the survival of mouse platelets refrigerated for 2 hours [65]. Galactsylation provided a simple approach to improve refrigerated platelet circulation.
Figure 1. Schematic diagram of the human von Willebrand factor receptor subunit GPIbα.
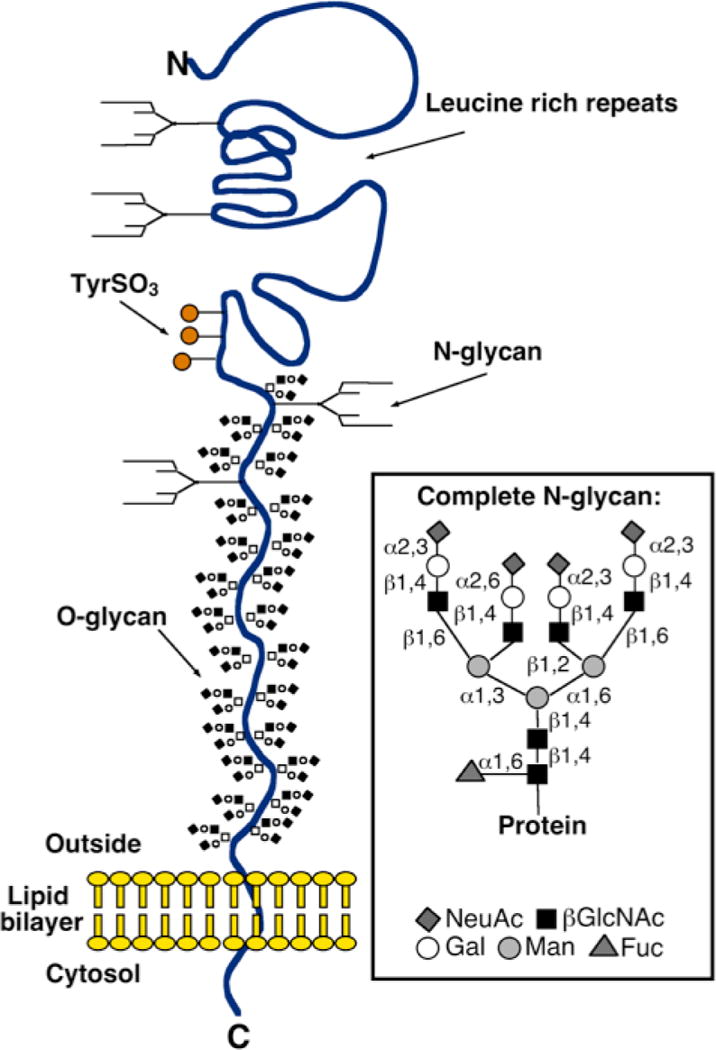
A typical, complete N-glycan identified on GIPbα is shown (inserted box).
While depriving the αMβ2 lectin-domain of its βGlcNAc ligand on refrigerated platelets, galactosylation theoretically provides a new ligand for asialoglycoprotein receptors (Asgpr). Hence, it was surprising that refrigerated mouse platelet circulation could be improved by galactosylation. We postulated that the number of exposed βGlcNAc residues on GPIbα was small, such that even after clustering and galactosylation, the galactose density was insufficient to engage galactose-recognizing lectins [65].
Therefore, a clinical trial was performed using apheresis platelets refrigerated for 48 h. This phase I clinical trial administering autologous, radiolabeled galactosylated apheresis platelets refrigerated for 48 h into human volunteers clearly showed that the galactosylation procedure did not extend their circulation time [50]. For logistical reasons, in our early studies we used isolated platelets and had not stored mouse platelets for clearance studies longer than hours. In contrast, platelets for transfusion were stored for days as platelet rich plasma. Using a mouse transfusion system we subsequently found that just as with human platelets stored in plasma, galactosylation has no effect on the survival of > 48 h long-term refrigerated mouse platelets [50].
Evidently, different mechanisms are involved in the clearance of platelets refrigerated for days.
The role of the hepatic Asialoglycoprotein (Ashwell-Morell) receptor in refrigerated platelet clearance
Using the murine transfusion model, we have dissected the clearance mechanism for platelets refrigerated for > 48 h in plasma. We have found that as with short-term cooling, biotinylated or radiolabeled platelets refrigerated for 48 h in plasma (designated throughout the following text as long-term refrigerated platelets) are removed from the recipients’ circulation by the liver [68, 69] but, unexpectedly by hepatocytes [69] and not by Kupffer cells (macrophages), as we previously reported for short-term cooled isolated platelets [40]. The in situ finding that hepatocytes can ingest long-term refrigerated platelets was confirmed using cultured hepatocyte cell lines (HepG2 cells) and human platelet concentrates refrigerated for up to 10 days. Further evidence that long-term refrigerated platelets are ingested by hepatocytes and not by macrophages was obtained by purging recipient mice of mature hepatic and splenic macrophages by injecting toxic clodronate-encapsulated liposomes [70] prior to transfusions of fresh room temperature platelets or short- or long-term refrigerated and/or galactosylated platelets. As expected, removal of macrophages greatly improved recoveries and survivals of short-term cooled platelets, confirming our earlier results. In contrast, the recoveries and survivals of long-term refrigerated platelets were not affected by macrophage depletion, showing that removal of long-term refrigerated platelets is macrophage-independent and rather mediated by hepatocytes [69]. Interestingly, these studies also revealed that macrophages rapidly remove a large fraction of transfused fresh room temperature, short-or long-term refrigerated platelets (~40%) [69], possibly reflecting the detection of damage inflicted during isolation, a loss of ~30–40% platelet recovery is consistently observed following the transfusion of fresh platelets into healthy volunteers [50].
The Asialoglycoprotein (Ashwell-Morell) receptor
How does the hepatic galactose lectin or the Ashwell-Morell receptor recognize long-term refrigerated platelets? We reported that short-term cooled platelets have exposed/clustered βGlcNAc residues that are recognized and phagocytozed by the αMβ2 hepatic macrophage receptors [40, 65]. In contrast, platelets refrigerated for long periods have severely increased galactose exposure, as evidenced by the galactose-binding lectins (RCA I and ECA), suggesting a ligand for the hepatic Asiologycoprotein receptor (Asgpr1/2). Consistent with this hypothesis, experiments using mice lacking Asgr-1 or Asgr-2 subunits of the Asgpr1/2 showed a significantly improved recovery and circulation of long-term refrigerated platelets. Similarly co-injection of asialofetuin, a competitive inhibitor of the Asgpr1/2, restored the recovery and circulation of long-term refrigerated platelets, but not of short-term refrigerated platelets. Conversely, Asialofetuin inhibited phagocytosis of human platelets refrigerated for up to 10 days by HepG2 cells in vitro [69]. Not surprisingly, long-term refrigerated and galactosylated platelets are cleared slightly faster independent of macrophage depletion [69]. Our studies, combined with a recent report by Grewal et al, point out the importance of a hepatic-based platelet removal system that uses its Asgr1/2 to recognize defectively sialylated proteins and remove platelets expressing desialylated glycans on their surface [71, 72] [69]. Whether hepatocytes also remove senile/desialylated platelets remains to be established.
Desialylated glycans reside on GPIbα following long-term refrigeration
We investigated whether GPIbα also plays a role in the removal of long-term refrigerated platelets, as we previously reported for short-term cooled platelets. Two of the putative N-linked glycans are localized within the 45 kDa of GPIbα extracellular domain (Fig. 1) [73]. Removal of the N-terminal 282 residues of GPIbα from human platelets using the snake venom protease mocarhagin [74] or O-sialoglycoprotein endopeptidase eliminates two putative N-glycan residues, as well as the vWf-binding region of GPIbα [74]. Removal of GPIbα’s 45 kDa domain from mouse or human platelets significantly restored the circulation of long-term refrigerated wild-type mouse platelets and prevented human platelet ingestion by HepG2 cells in vitro, pointing to the fact that most exposed galactose and βGlcNAc residues reside within the external domain of GPIbα initiating clearance [69]. It is tempting to speculate that most of the exposed βGal residues reside within N-linked glycans on GPIbα, as shown previously for βGlcNAc[65]. However, it remains to be determined whether N-linked glycans are solely involved in this interaction.
Does GPIbα presentation influence platelet clearance? Platelets from ST3GalIV−/− mice have exposed galactose on surface glycoproteins as they lack α2–3-sialyltransferase activity [71, 75]. In long-term refrigerated and rewarmed platelets, the mechanism of galactose exposure remains to be determined. Release, or activation, of sialidase activity during storage could cleave terminal sialic acid residues, revealing underlying galactose residues. However, the extent of galactose exposure on refrigerated platelets is less than that present on the surface of ST3GalIV+/– platelets that retain their ability to circulate with normal lifetimes [71, 72, 75]. We observed increased clustering of GPIbα with prolonged refrigeration [69]. Hence, clustering of GPIbα subunits in the platelet with cooling might amplify the galactose signal enhancing lectin avidity and binding, as reported earlier for βGlcNAc [65]. However, the functional relationship between GPIbα clustering and platelet clearance by hepatocyte Ashwell-Morell remains to be established, particularly, if proteolytic removal of GPIbα ’s N-Terminal 45 kDa portion alters clustering.
Are other glycoproteins than GPIbα glycans involved in the clearance of cooled platelets? Von Willebrand factor (vWf) binding increases with storage [69]. It appears that preferentially desialylated vWf binds to long-term refrigerated platelets, indicating that desialylation of vWf molecules may promote binding to GPIbα. In support of this notion that desialylated vWf binds better to platelets, we found that despite a 50% reduction of vWf in ST3Gal-IV−/− plasma, binding of deficiently sialylated vWf to the vWfR on WT platelets is enhanced in vitro and in vivo [72]. Proteolytic removal of the GPIbα N-terminal region deprives GPIbα of its vWf-binding domain and bound vWf Whether vWf-glycans contribute to recognition of platelets by Ashwell-Morell receptor remains to be determined.
Conclusions
The sole reason platelets are currently stored at room temperature is that they do not circulate for an acceptable period of time when transfused. Chilling does cause platelets to lose their discoid shape, but shape change per se does not appear to adversely affect either platelet function or survival. It is tempting to speculate that refrigerated platelets might function more effectively than room temperature platelets especially after prolonged storage, i.e > 5 days. In vitro data and early studies in humans suggest that 4°C platelets appear “healthier” than room temperature platelets. Most compelling is the observation at 4°C platelets have better-preserved in vitro aggregation responses than room temperature platelets, specifically after 5 days of storage. Obviously, the ultimate approach would be to evaluate chilled galactosylated platelets in controlled trials in thrombocytopenic patients, using peritransfusion bleeding as an endpoint. This strategy was used effectively to compare the hemostatic efficacy of pathogen inactivated platelets with standard platelets [11, 76, 77]. However, before performing clinical trials refrigerated platelets must be able to circulate.
When modified appropriately (e.g. galactosylated) transfused short-term cooled platelets survive in mice as well as room temperature platelets [65], a finding that is contradictory to the notion that galactose recognition by Asialoglycoprotien (Ashwell-Morell) receptors promotes platelet removal. However, duration of platelet refrigeration influences the nature of glycan exposed, i.e., either βGlcNAc or βGal. It is, therefore, not surprising that lectin receptors on macrophages and/or hepatocytes cooperate to remove refrigerated platelets. The two mechanisms mediating the clearance of cooled platelets are summarized in (Fig. 2)
Figure 2. Lectin receptors mediating the clearance of cooled platelets.
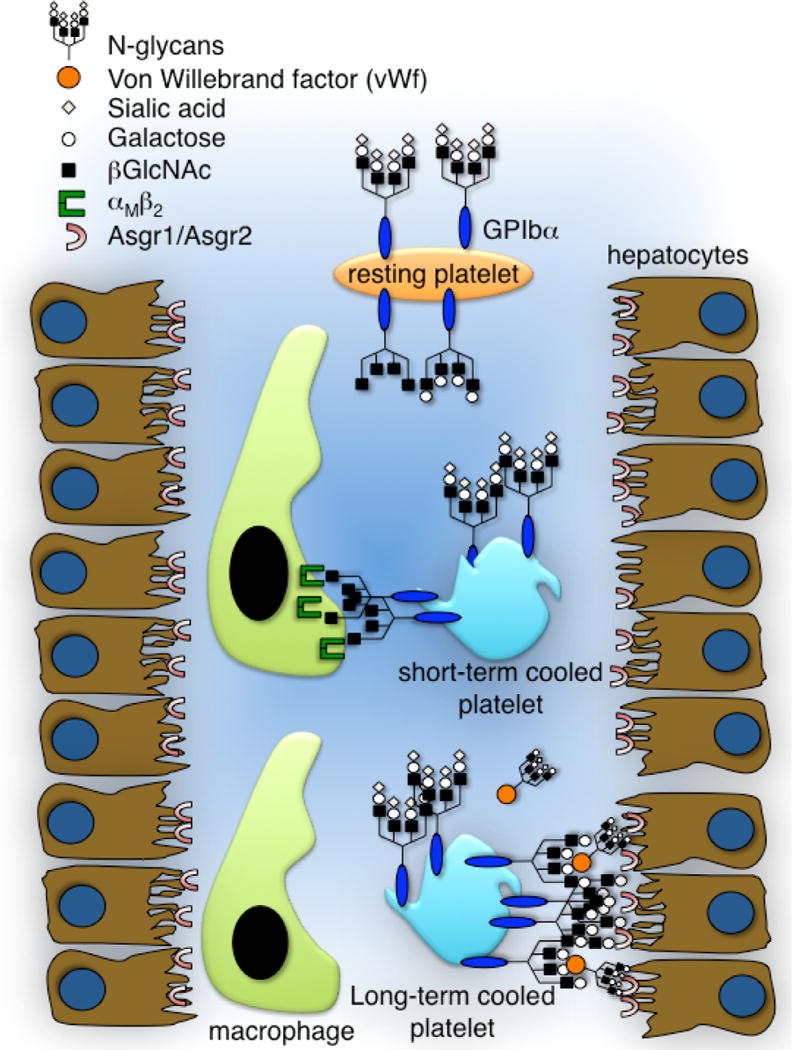
The von Willebrand factor receptor (vWfR) complex, specifically GPIbα, has complete and incomplete N-linked glycans with exposed βGlcNAc and/or galactose residues. Clustering of vWfRs and of exposed β-GlcNAc initiates recognition and phagocytosis by the macrophage αMβ2 integrin following short-term refrigeration. Extended refrigeration is followed by additional surface changes such as “hyperclustering” of receptors and galactose exposure and some vWf binding. These changes induce clearance by asialoglycoprotein receptors on hepatocytes (Ashwell-Morell receptors) and macrophages (αmβ2).
Future studies will investigate if re-glycosylation, i.e. a combination of galactosylation and sialylation of immature platelet surface glycans, can rescue refrigerated platelet survival to accommodate their refrigeration for transfusion. Inhibition of sialidase activity could also enhance survival of long-term refrigerated platelets. The surprising presence of multiple glycosyltransferases (galactosyltransferases and sialyltransferases) in and on the surface of human platelets may have implications for platelet functionality and survival.
Acknowledgments
Supported by grants from the US National Institutes of Health grant PO1 HL056949 (to KMH) and grant HL089224 (to KMH); The Pew Scholars Award to KMH; The Swedish Medical Research Council, Göteborg University Jubileums stipend to VR.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict-of-interest statement: K.M.H. is a consultant for Velico Medical (formerly ZymeQuest Inc.). K.M.H. and V.R. have received sponsored research support form ZymeQuest.
References
- 1.Wallace E, et al. Collection and transfusion of blood and blood components in the United States, 1989. Transfusion. 1993;33:139–144. doi: 10.1046/j.1537-2995.1993.33293158046.x. [DOI] [PubMed] [Google Scholar]
- 2.Murphy S, Gardner F. Effect of storage temperature on maintenance of platelet viability – deleterious effect of refrigerated storage. N Engl J Med. 1969;280:1094–1098. doi: 10.1056/NEJM196905152802004. [DOI] [PubMed] [Google Scholar]
- 3.Becker G, et al. Studies of platelet concentrates stored at 22°C and 4°C. Transfusion. 1973;13:61–68. doi: 10.1111/j.1537-2995.1973.tb05442.x. [DOI] [PubMed] [Google Scholar]
- 4.Slichter S, Harker L. Preparation and storage of platelet concentrates. II. Storage variables influencing platelet viability and function. Brit J Haemat. 1976;34:403–419. doi: 10.1111/j.1365-2141.1976.tb03587.x. [DOI] [PubMed] [Google Scholar]
- 5.Valeri CR. Hemostatic effectiveness of liquid-preserved and previously frozen human platelets. N Engl J Med. 1974;290(7):353–8. doi: 10.1056/NEJM197402142900702. [DOI] [PubMed] [Google Scholar]
- 6.Kuehnert M, et al. Transfusion-transmitted bacterial infection in the United States, 1998 through 2000. Transfusion. 2001;41(12):1493–1499. doi: 10.1046/j.1537-2995.2001.41121493.x. [DOI] [PubMed] [Google Scholar]
- 7.Currie L, et al. Inhibition of cytokine accumulation and bacterial growth during storage of platelet concentrates at 4 degrees C with retention of in vitro functional activity. Transfusion. 1997;37(1):18–24. doi: 10.1046/j.1537-2995.1997.37197176946.x. [DOI] [PubMed] [Google Scholar]
- 8.Snyder E, Rinder H. Platelet storage–time to come in from the cold? N Engl J Med. 2003;348(20):2032–2033. doi: 10.1056/NEJMcibr035099. [DOI] [PubMed] [Google Scholar]
- 9.Brecher ME, Hay SN. Bacterial contamination of blood components. Clin Microbiol Rev. 2005;18(1):195–204. doi: 10.1128/CMR.18.1.195-204.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.AuBuchon JP, et al. Preliminary validation of a new standard of efficacy for stored platelets. Transfusion. 2004;44(1):36–41. doi: 10.1046/j.0041-1132.2004.00604.x. [DOI] [PubMed] [Google Scholar]
- 11.van Rhenen D, et al. Transfusion of pooled buffy coat platelet components prepared with photochemical pathogen inactivation treatment: the euroSPRITE trial. Blood. 2003;101(6):2426–2433. doi: 10.1182/blood-2002-03-0932. [DOI] [PubMed] [Google Scholar]
- 12.Murphy S. What’s so bad about old platelets? Transfusion. 2002;42(7):809–11. doi: 10.1046/j.1525-1438.2002.00197.x. [DOI] [PubMed] [Google Scholar]
- 13.Murphy S, Gardner FH. Platelet storage at 22 degrees C: role of gas transport across plastic containers in maintenance of viability. Blood. 1975;46(2):209–18. [PubMed] [Google Scholar]
- 14.White J. Ultrastructural changes in stored platelets. Blood Cells. 1992;18:461–475. [PubMed] [Google Scholar]
- 15.Gulliksson H, et al. Storage of platelets in additive solutions: a multicentre study of the in vitro effects of potassium and magnesium. Vox Sang. 2003;85(3):199–205. doi: 10.1046/j.1423-0410.2003.00356.x. [DOI] [PubMed] [Google Scholar]
- 16.Murphy S. The efficacy of synthetic media in the storage of human platelets for transfusion. Transfus Med Rev. 1999;13(3):153–63. doi: 10.1016/s0887-7963(99)80029-7. [DOI] [PubMed] [Google Scholar]
- 17.Gulliksson H. Defining the optimal storage conditions for the long-term storage of platelets. Transfus Med Rev. 2003;17(3):209–15. doi: 10.1016/s0887-7963(03)00020-8. [DOI] [PubMed] [Google Scholar]
- 18.van der Meer PF. Platelet additive solutions: a future perspective. Transfus Clin Biol. 2007;14(6):522–5. doi: 10.1016/j.tracli.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 19.Administration, F.a.D. Guidance for Industry For Platelet Testing and Evaluation of Platelet Substitute Products Draft Guidance. Rockville, MD: 1999. pp. 1–7. [Google Scholar]
- 20.Murphy S, et al. In vitro assessment of the quality of stored platelet concentrates. The BEST (Biomedical Excellence for Safer Transfusion) Task Force of the International Society of Blood Transfusion. Transfus Med Rev. 1994;8(1):29–36. doi: 10.1016/s0887-7963(94)70095-x. [DOI] [PubMed] [Google Scholar]
- 21.Ishida A, et al. Clinical factors influencing posttransfusion platelet increment in patients undergoing hematopoietic progenitor cell transplantation–a prospective analysis. Transfusion. 1998;38(9):839–47. doi: 10.1046/j.1537-2995.1998.38998409004.x. [DOI] [PubMed] [Google Scholar]
- 22.Rebulla P, et al. The threshold for prophylactic platelet transfusions in adults with acute myeloid leukemia. Gruppo Italiano Malattie Ematologiche Maligne dell’Adulto. N Engl J Med. 1997;337(26):1870–5. doi: 10.1056/NEJM199712253372602. [DOI] [PubMed] [Google Scholar]
- 23.Wandt H, et al. Safety and cost effectiveness of a 10 × 10(9)/L trigger for prophylactic platelet transfusions compared with the traditional 20 × 10(9)/L trigger: a prospective comparative trial in 105 patients with acute myeloid leukemia. Blood. 1998;91(10):3601–6. [PubMed] [Google Scholar]
- 24.Murphy S, Gardner FH. Platelet storage at 22 degrees C; metabolic, morphologic, and functional studies. J Clin Invest. 1971;50(2):370–7. doi: 10.1172/JCI106504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Holme S, Heaton WA, Whitley P. Platelet storage lesions in second-generation containers: correlation with in vivo behavior with storage up to 14 days. Vox Sang. 1990;59(1):12–8. doi: 10.1111/j.1423-0410.1990.tb02106.x. [DOI] [PubMed] [Google Scholar]
- 26.Slichter S, Harker L. Preparation and storage of platelet concentrates. I. Factors influencing the harvest of viable platelets from whole blood. Brit J Haemat. 1976;34:395–402. doi: 10.1111/j.1365-2141.1976.tb03586.x. [DOI] [PubMed] [Google Scholar]
- 27.Schlichter S, Harker L. Preparation and storage of platelet concentrates II. Storage variables influencing platelet viability and function. Brit J Haemat. 1976;34:403–419. doi: 10.1111/j.1365-2141.1976.tb03587.x. [DOI] [PubMed] [Google Scholar]
- 28.Murphy S, Gardner FH. The effect of temperature on platelet viability. Vox Sang. 1969;17(1):22. [PubMed] [Google Scholar]
- 29.Rock G, Figueredo A. Metabolic changes during platelet storage. Transfusion. 1976;16(6):571–9. doi: 10.1046/j.1537-2995.1976.16677060241.x. [DOI] [PubMed] [Google Scholar]
- 30.Slichter SJ. In vitro measurements of platelet concentrates stored at 4 and 22° C: correlation with postrtansfusion platelet viability and function. Vox Sang. 1981;40(suppl 1):72–86. doi: 10.1111/j.1423-0410.1981.tb00741.x. [DOI] [PubMed] [Google Scholar]
- 31.Rinder H, Snyder E. Activation of platelet concentrate during preparation and storage. Blood Cells. 1992;18(3):445–456. [PubMed] [Google Scholar]
- 32.Rinder HM, Ault KA. Platelet activation and its detection during the preparation of platelets for transfusion. Transfus Med Rev. 1998;12(4):271–87. doi: 10.1016/s0887-7963(98)80003-5. [DOI] [PubMed] [Google Scholar]
- 33.Rinder H, et al. Progressive platelet activation with storage: evidence for shortened survival of activated platelets after transfusion. Transfusion. 1991;31(5):409–414. doi: 10.1046/j.1537-2995.1991.31591263195.x. [DOI] [PubMed] [Google Scholar]
- 34.Triulzi D, Kickler T, Braine H. Detection and significance of alpha granule membrane protein 140 expression on platelets collected by apheresis. Transfusion. 1992;32(6):529–533. doi: 10.1046/j.1537-2995.1992.32692367196.x. [DOI] [PubMed] [Google Scholar]
- 35.Fijnheer R, et al. Detection of platelet activation with monoclonal antibodies and flow cytometry. Changes during platelet storage. Transfusion. 1990;30(1):20–5. doi: 10.1046/j.1537-2995.1990.30190117623.x. [DOI] [PubMed] [Google Scholar]
- 36.Snyder EL, et al. Occurrence of the release reaction during preparation and storage of platelet concentrates. Vox Sang. 1981;41(3):172–7. doi: 10.1111/j.1423-0410.1981.tb01032.x. [DOI] [PubMed] [Google Scholar]
- 37.Perrotta PL, Perrotta CL, Snyder EL. Apoptotic activity in stored human platelets. Transfusion. 2003;43(4):526–35. doi: 10.1046/j.1537-2995.2003.00349.x. [DOI] [PubMed] [Google Scholar]
- 38.Zucker M, Borrelli J. Reversible alteration in platelet morphology produced by anticoagulants and by cold. Blood. 1954;28:524–534. [PubMed] [Google Scholar]
- 39.Babic A, et al. In vitro function and phagocytosis of galactosylated platelet concentrates following long-term refrigeration. Transfusion. 2006;47(3):442–451. doi: 10.1111/j.1537-2995.2007.01134.x. [DOI] [PubMed] [Google Scholar]
- 40.Hoffmeister K, et al. The clearance mechanism of chilled blood platelets. Cell. 2003;10(112):87–97. doi: 10.1016/s0092-8674(02)01253-9. [DOI] [PubMed] [Google Scholar]
- 41.Leytin V, et al. Role of platelet surface glycoprotein Ibalpha and P-selectin in the clearance of transfused platelet concentrates. Transfusion. 2004;44(10):1487–95. doi: 10.1111/j.1537-2995.2004.04042.x. [DOI] [PubMed] [Google Scholar]
- 42.Sandgren P, et al. Storage of buffy-coat-derived platelets in additive solutions at 4 degrees C and 22 degrees C: flow cytometry analysis of platelet glycoprotein expression. Vox Sang. 2007;93(1):27–36. doi: 10.1111/j.1423-0410.2007.00912.x. [DOI] [PubMed] [Google Scholar]
- 43.Berger G, Hartwell D, Wagner D. P-selectin and platelet clearance. Blood. 1998;92:4446–4452. [PubMed] [Google Scholar]
- 44.Michelson A, et al. In vivo tracking of platelets: circulating degranulated platelets rapidly lose suface P-selectin but continue to circulate and function. Proc Natl Acad Sci, USA. 1996;93:11877–11882. doi: 10.1073/pnas.93.21.11877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reimers HJ, et al. In vitro and in vivo functions of thrombin-treated platelets. Thromb Haemost. 1976;35(1):151–66. [PubMed] [Google Scholar]
- 46.Italiano JJ, et al. Mechanisms and implications of platelet discoid shape. Blood. 2003;101:4789–4796. doi: 10.1182/blood-2002-11-3491. [DOI] [PubMed] [Google Scholar]
- 47.Valeri C, et al. Effect of thrombopoietin alone and a combination of cytochalasin B and ethylene glycol bis(beta-aminoethyl ether) N,N′-tetraacetic acid-AM on the survival and function of autologous baboon platelets stored at 4 degrees C for as long as 5 days. Transfusion. 2004;44(6):865–870. doi: 10.1111/j.1537-2995.2004.03326.x. [DOI] [PubMed] [Google Scholar]
- 48.Winokur R, Hartwig J. Mechanism of shape change in chilled human platelets. Blood. 1995;85:1796–1804. [PubMed] [Google Scholar]
- 49.Scharf R, Hanfland P. Platelet storage lesions: analysis of platelet membrane glycoproteins and platelet-derived microparticles by fluorescence-activated flow cytometry. Transfus Sci. 1993;14:189–194. doi: 10.1016/0955-3886(93)90030-X. [DOI] [PubMed] [Google Scholar]
- 50.Wandall HH, et al. Galactosylation does not prevent the rapid clearance of long-term, 4{degrees}C-stored platelets. Blood. 2008;111(6):3249–56. doi: 10.1182/blood-2007-06-097295. [DOI] [PubMed] [Google Scholar]
- 51.Dijkstra-Tiekstra MJ, Pietersz RN, Huijgens PC. Correlation between the extent of platelet activation in platelet concentrates and in vitro and in vivo parameters. Vox Sang. 2004;87(4):257–63. doi: 10.1111/j.1423-0410.2004.00573.x. [DOI] [PubMed] [Google Scholar]
- 52.Holme S, et al. The expression of p-selectin during collection, processing, and storage of platelet concentrates: relationship to loss of in vivo viability. Transfusion. 1997;37(1):12–7. doi: 10.1046/j.1537-2995.1997.37197176945.x. [DOI] [PubMed] [Google Scholar]
- 53.Kim B, Baldini M. The platelet response to hypotonic shock. Its value as an indicator of platelet viability after storage. Transfusion. 1974;14(2):130–138. doi: 10.1111/j.1537-2995.1974.tb04504.x. [DOI] [PubMed] [Google Scholar]
- 54.Murphy S, Gardner FH. Room temperature storage of platelets. Transfusion. 1976;16(1):2–3. doi: 10.1046/j.1537-2995.1976.16176130831.x. [DOI] [PubMed] [Google Scholar]
- 55.Kattlove HE. Platelet preservation–what temperature? A rationale for strategy. Transfusion. 1974;14(4):328–30. doi: 10.1111/j.1537-2995.1974.tb04540.x. [DOI] [PubMed] [Google Scholar]
- 56.Kattlove HE, Alexander B, White F. The effect of cold on platelets. II. Platelet function after short-term storage at cold temperatures. Blood. 1972;40(5):688–96. [PubMed] [Google Scholar]
- 57.Eriksson L, et al. Evaluation of platelet function using the in vitro bleeding time and corrected count increment of transfused platelets. Comparison between platelet concentrates derived from pooled buffy coates and apheresis. Vox Sang. 1996;70(2):69–75. doi: 10.1111/j.1423-0410.1996.tb01296.x. [DOI] [PubMed] [Google Scholar]
- 58.Salama ME, et al. Platelet function testing to assess effectiveness of platelet transfusion therapy. Transfus Apher Sci. 2004;30(2):93–100. doi: 10.1016/j.transci.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 59.Jilma-Stohlawetz P, et al. Impaired platelet function among platelet donors. Thromb Haemost. 2001;86(3):880–6. [PubMed] [Google Scholar]
- 60.Harrison P, et al. High incidence of defective high-shear platelet function among platelet donors. Transfusion. 2004;44(5):764–70. doi: 10.1111/j.1537-2995.2004.00368.x. [DOI] [PubMed] [Google Scholar]
- 61.Silva VA, Miller WV. Platelet transfusion survey in a regional blood program. Transfusion. 1977;17(3):255–60. doi: 10.1046/j.1537-2995.1977.17377196361.x. [DOI] [PubMed] [Google Scholar]
- 62.Handin R, Valeri C. Hemostatic effectiveness of platelets stored at 22°C. N Engl J Med. 1971;285:538–543. doi: 10.1056/NEJM197109022851003. [DOI] [PubMed] [Google Scholar]
- 63.Valeri CR. Circulation and hemostatic effectiveness of platelets stored at 4 C or 22 C: studies in aspirin-treated normal volunteers. Transfusion. 1976;16(1):20–3. doi: 10.1046/j.1537-2995.1976.16176130832.x. [DOI] [PubMed] [Google Scholar]
- 64.Filip D, Aster R. Relative hemostatic effectiveness of human platelets stored at 4oC and 22oC. J Lab Clin Med. 1978;91:618–624. [PubMed] [Google Scholar]
- 65.Hoffmeister K, et al. Glycosylation restores survival of chilled blood platelets. Science. 2003;301(5639):1531–1534. doi: 10.1126/science.1085322. [DOI] [PubMed] [Google Scholar]
- 66.Josefsson E, et al. The macrophage alphaMbeta2 integrin alphaM lectin domain mediates the phagocytosis of chilled platelets. J Biol Chem. 2005;280(18):18025–18032. doi: 10.1074/jbc.M501178200. [DOI] [PubMed] [Google Scholar]
- 67.Badlou B, et al. Role of glycoprotein Ibalpha in phagocytosis of platelets by macrophages. Transfusion. 2006;46(12):2090–2099. doi: 10.1111/j.1537-2995.2006.01034.x. [DOI] [PubMed] [Google Scholar]
- 68.Valeri C, et al. Circulation and distribution of autotransfused fresh, liquid-preserved and cryopreserved baboon platelets. Vox Sang. 2002;83(4):347–351. doi: 10.1046/j.1423-0410.2002.00229.x. [DOI] [PubMed] [Google Scholar]
- 69.Rumjantseva V, Grewal PK, Wandall HH, Josefsson EC, Sørensen AL, Larson G, Marth JD, Hartwig JH, Hoffmeister KM. Dual roles for hepatic lectin receptors in the clearance of chilled platelets. Nature Medicine. 2009 doi: 10.1038/nm.2030. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.van Rooijen N, van Kesteren-Hendrikx E. “In vivo” depletion of macrophages by liposome-mediated “suicide”. Methods Enzymol. 2003;373:3–16. doi: 10.1016/s0076-6879(03)73001-8. [DOI] [PubMed] [Google Scholar]
- 71.Grewal PK, et al. The Ashwell receptor mitigates the lethal coagulopathy of sepsis. Nat Med. 2008;14(6):648–55. doi: 10.1038/nm1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sorensen AL, et al. Role of sialic acid for platelet lifespan: exposure of {beta}galactose results in the rapid clearance of platelets from the circulation by asialoglycoprotein receptor-expressing liver macrophages and hepatocytes. Blood. 2009 doi: 10.1182/blood-2009-01-199414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tsuji T, Osawa T. The carbohydrate moiety of human platelet glycocalicin: the structures of the major Asn-linked sugar chains. J Biochem. 1987;101(1):241–249. doi: 10.1093/oxfordjournals.jbchem.a121897. [DOI] [PubMed] [Google Scholar]
- 74.Berndt M, et al. Purification and preliminary characterization of the glycoprotein Ib complex in the human platelet membrane. Eur J Biochem. 1985;151(3):637–649. doi: 10.1111/j.1432-1033.1985.tb09152.x. [DOI] [PubMed] [Google Scholar]
- 75.Ellies L, et al. Sialyltransferase ST3Gal-IV operates as a dominant modifier of hemostasis by concealing asialoglycoprotein receptor ligands. Proc Natl Acad Sci U S A. 2002;99(15):10042–10047. doi: 10.1073/pnas.142005099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cazenave JP, Davis K, Corash L. Design of clinical trials to evaluate the efficacy of platelet transfusion: the euroSPRITE trial for components treated with Helinx technology. Semin Hematol. 2001;38(4 Suppl 11):46–54. doi: 10.1016/s0037-1963(01)90123-4. [DOI] [PubMed] [Google Scholar]
- 77.McCullough J, et al. Therapeutic efficacy and safety of platelets treated with a photochemical process for pathogen inactivation: the SPRINT Trial. Blood. 2004;104(5):1534–41. doi: 10.1182/blood-2003-12-4443. [DOI] [PubMed] [Google Scholar]


