Abstract
Tissue engineering holds immense potential for treatment of cardiovascular diseases by creating living structures to replace diseased blood vessels, heart valves, and cardiac muscle. In a traditional approach, scaffolds are seeded with stem cells and subjected to stimuli in bioreactors that mimic physiologic conditions or are directly implanted into target sites in animal models. The expected results are significant cell changes, extensive remodeling of the scaffolds and creation of surrogate structures that would be deemed acceptable for tissue regeneration. Histochemical techniques are increasingly becoming essential tools in tissue engineering research. In our studies, we used lectin and antibody-based techniques to characterize novel collagen and elastin scaffolds and to ensure efficient removal of xenoantigens. Scaffolds were implanted in animals and infiltrated host cells were identified using antibodies to activated fibroblasts, macrophages, and lymphocytes. Stem cell-seeded scaffolds were subjected to mechanical strains and tested for differentiation into cardiovascular cells using antibody-based double immunofluorescence methods. Finally, living heart valves were constructed from scaffolds and stem cells, subjected to conditioning in a bioreactor and stem cell differentiation evaluated by immunofluorescence. Overall, these techniques have proven to be outstanding companions to biochemical, molecular biology and cell analysis methods used in tissue engineering research and development.
Keywords: Heart valves, Scaffolds, Stem cells, Vascular grafts, Xenografts
Introduction
Estimates from American Heart Association statistics specify that over 81 million people in the United States have one or more forms of cardiovascular (CV) disease, including coronary heart disease, heart valve disease, heart failure, and stroke.1 CV disease is the leading cause of mortality in the United States and Western countries. Optimal replacements for failed CV components would be biocompatible tissues that have the potential to regenerate and fully restore lost functions. Tissue engineering (TE), aided by emerging stem cell technologies, holds immense potential for the treatment of CV diseases. Progress has been made in engineering various components of the CV system, including blood vessels, heart valves, and cardiac muscle.2 A typical TE strategy consists of seeding appropriate cells on biodegradable scaffolds engineered with the desired mechanical properties; cell differentiation is then stimulated and cell growth sustained under optimal conditions in a specifically designed 3D bioreactor. For pre-clinical studies, tissue engineered constructs are implanted in animals (rats, rabbits, dogs, sheep, or pigs), and their biocompatibility, remodeling, and maturation into fully functional tissue are evaluated.3
Immunohistochemistry (IHC) is one of the most important tools used in TE research laboratories. By using antibodies labeled with an enzyme or a fluorescent dye, scaffold composition and remodeling, stem cell differentiation, and host cell infiltration in TE constructs implanted in animals can be evaluated.
Scaffolds derived from xenogenic extracellular matrix have been used in numerous TE applications. The extracellular matrix is the natural scaffold for tissue and organ morphogenesis, maintenance, and reconstruction following injury, and is associated with constructive tissue remodeling. The 3D organization of its components distinguishes extracellular matrices from synthetic scaffolds.4 To produce biomaterials for medical use, the main processing step involves decellularization of mammalian tissues in such a way as to render the scaffolds minimally immunogenic. Thus the main aim of decellularization is to remove all epitopes associated with cells, especially the terminal disaccharide galactose-alpha-1-3-galactose (Gal-alpha), expressed on the cell surface of mammalian cells except those of humans and old world primates.5 Gal-alpha is considered the main epitope responsible for hyper acute rejection of xenografts and is considered the major hurdle that stands in the way of using the pig as a ‘universal organ donor’.5 The presence of the xenoantigen Gal-alpha in scaffolds of interest is detected using histochemical staining techniques based on specific lectins.6
For heart valve TE, the greatest advances using natural scaffolds have occurred with decellularized xenografts or homograft valve matrices.7 There is an increased interest in the use of acellular xenogenic scaffolds since the biomechanical properties of the valve can potentially be preserved with an optimal decellularization technique that removes the cells without damaging the matrix.8 In re-endothelialization studies of detergent decellularized heart valves, the integrity of collagen type IV, a main protein of the basement membrane, was proved by IHC staining.9,10 In our previous studies, xenogenic scaffolds were prepared from porcine tissues and implanted subdermally in rats and infiltrated cells were identified by IHC.11,12
For vascular TE, cell-seeded gels, cell-seeded polymer scaffolds, and acellular techniques have been employed in various laboratories.13 While some polymers are suitable for large diameter grafts, neither polymers nor biological scaffolds are adequate for small diameter blood vessels which occlude rapidly upon implantation.14 Elastin has been investigated in recent years as a scaffold for vascular TE due to its favorable native mechanical and biological properties.15-17 Tubular elastic structures obtained from decellularized aorta showed inflammation-resistant properties after implantation as vascular grafts in rat abdominal aortas18 and in porcine carotid arteries.19
Potential cellular sources for seeding heart valve and vascular scaffolds include differentiated cells (endothelial cells, smooth muscle cells, and myofibroblasts) collected from autologous sources (skin and blood vessels) and stem cells, autologous or allogenic multipotent cells capable of self-renewal and differentiation into several types of cells.20 IHC together with gene expression and protein expression was utilized extensively to characterize potential TE valve cell sources such as veins and arteries and compared to native aortic and pulmonary valve cells.21 Moreover, the usefulness of bone marrow derived stem cells as sources for CV TE was investigated by IHC and immunofluorescence techniques.22
The predominant cell populations in heart valves, valvular interstitial cells continuously secrete collagen types I and III, glycosaminoglycans, and other matrix components as well as matrix metalloproteinases (MMPs) and other matrix degrading enzymes such as glycosaminoglycan-degrading enzymes that mediate remodeling.23-25 Valvular interstitial cells exhibit a dynamic phenotypic spectrum ranging from quiescent fibroblast-like cells (characterized by expression of vimentin, fibroblast surface antigen, and low expression of alpha-smooth muscle cell actin and MMP-13), to activated fibroblasts, also named myofibroblasts (characterized by high expression of vimentin, alpha-smooth muscle cell actin, and MMP-13).26-29 These markers can be demonstrated in vitro and in vivo using IHC techniques.
Cell phenotypes after bioreactor conditioning of cell seeded scaffolds or after animal implantation as well as remodeling and matrix synthesis have been characterized by IHC techniques including enzyme-based IHC, immunofluorescence, and immuno-electron microscopy.30,31
In this paper, we present four studies where IHC techniques proved invaluable for development of scaffolds, cell sourcing and development of a novel approach to heart valve TE.
Two biological scaffolds used in CV TE are presented in this paper: a collagen scaffold and an elastin scaffold. Collagen scaffolds were chosen for heart valve TE because of their mechanical strength and long term durability, while elastin scaffolds were selected for vascular TE because of their natural resilience. The scaffolds were prepared by complete decellularization of porcine tissues, i.e. treatment with solutions that dissolve and extract away cells while leaving the major extracellular matrix components intact. Since these scaffolds are known to degrade rapidly in vivo unless stabilized, both scaffolds were further treated with pentagalloyl glucose (PGG), a matrix-binding polyphenol. Scaffolds were used as 3D supports for human mesenchymal stem cell cultures and differentiation into CV cells as a response to biochemical and mechanical cues. Comparative properties of native and PGG-stabilized biological scaffolds are described below.
Methods
Preparation and characterization of biological scaffolds
Collagen scaffolds
To prepare collagen scaffolds, porcine pericardium was collected at a slaughterhouse, cleaned, rinsed in sterile saline, cut into strips, and decellularized using detergents and enzymes as follows. Tissues were first stored in double-distilled water overnight at 4°C to induce cell lysis. Tissues were rinsed, and treated with 0.25% sodium-deoxycholate, 0.15% Triton X-100, 0.1% ethylene-diamine-tetra-acetic acid, and 0.02% sodium azide (NaN3), in 50 mM Tris hydrochloric acid (HCl) buffer (pH 7.8) for 6 days. Tissues were then treated with a deoxyribonuclease/ribonuclease mixture (360 mU/ml for each enzyme) at 37°C for 24 hours to fully digest away nucleic acids. This was followed by incubation in ultrapure elastase (10 U/ml) in 50 mM Tris buffer, 1 mM calcium chloride, and 0.02% NaN3 (pH 8), at 37°C for 6 days to remove elastin. Finally tissues were rinsed in double-distilled water and 70% ethanol and then stored in sterile saline supplemented with 0.02% NaN3 until use. Collagen scaffolds were shown to be completely free of cells, confirmed by histology stains and DNA analysis.
Elastin scaffolds
Fresh porcine carotid arteries collected at a slaughter-house were incubated in 0.1M of sodium hydroxide solution at 37°C for exactly 24 hours and then extensively rinsed with deionized water until pH dropped to neutral. This treatment removes all cells and most of the collagen, leaving vascular elastin intact. Histology and DNA analysis confirmed lack of resident cells (not shown).
Lectin histochemistry
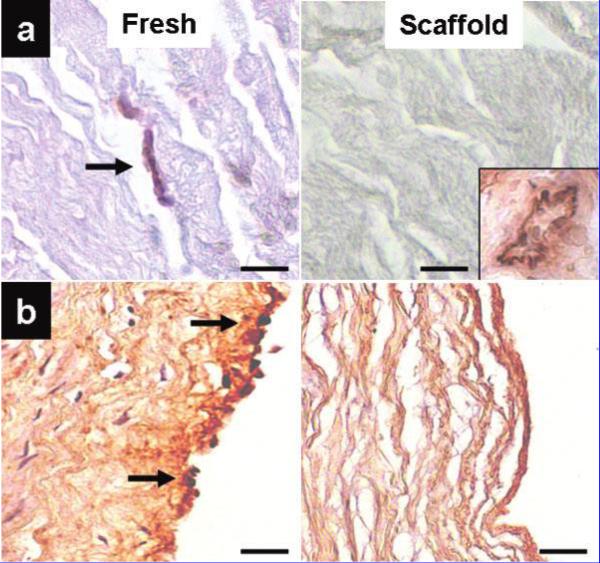
To detect the presence of the xenoantigen Gal-alpha, we performed lectin histochemistry using biotinylated Griffonia simplicifolia on fresh tissues and both the collagen and elastin scaffolds. Tissue and scaffold samples were placed in formalin, and paraffin sections (5 μm) were deparaffinized and exposed to 0.1% proteinase K solution (0.05 U/ml, Qiagen DNeasy Tissue Kit (Germantown, MD, USA)) in Tris buffered saline, pH 7.5, at 22°C for 30 seconds for antigen retrieval. Endogenous peroxidases were blocked with 0.3% hydrogen peroxide in 0.3% normal sera (Vectastain Elite ABC Kit; Vector Laboratories, Burlingame, CA, USA). Sections were incubated for 1 hour at 22°C witha 20 mg/ml dilution of biotinylated Griffonia simplicifolia lectin. Negative staining controls were performed with the omission of the lectin. A diaminobenzidine tetra-hydrochloride peroxidase substrate kit was used to visualize the specific staining (Vector Laboratories), and sections were lightly counterstained with hematoxylin. As positive controls we always seek tissues which are known or expected to yield strong positive reactions. In this example, we show that porcine kidney sections serve well as positive reaction tissues, where small arterioles yielded excellent staining (Fig. 1).
Figure 1.
Identification of Gal-alpha porcine xenoantigens using Griffonia simplicifolia lectin as the primary binding reagent. (a) Porcine pericardium showing a capillary stained positively for Gal-alpha. At right, decellularized porcine pericardium scaffold showing absence of the antigen. Inset shows staining of porcine kidney (arteriole) used as a positive control. (b) Porcine carotid artery showing extensive Gal-alpha staining of the arterial endothelium. At right, decellularized porcine carotid artery scaffold shows lack of staining. Sections were counterstained with hematoxylin (nuclei dark blue). Bars are 50 μm in all micrographs. Inset: original magnification, ×25.
Biocompatibility testing
Animal surgery
To test biocompatibility of collagen and elastin scaffolds and to evaluate their potential for remodeling, scaffold samples were implanted subdermally in male juvenile Sprague–Dawley rats as previously described.11,12 Sterile collagen and elastin scaffolds were prepared as described above and cut into 10×10 mm squares. Samples were collagen and elastin scaffold controls (no PGG treatment, n=58 per group) and PGG-treated scaffolds (0.3% PGG in phosphate buffer for 24 hours at 22°C, n=58 per group). All samples were rinsed overnight in sterile saline before the day of surgery. For implantation, a small incision was made on the backs of the rats, and two subdermal pouches were created by blunt dissection. Samples were inserted into the subdermal pouches and incisions were closed using surgical staples. After surgery, the rats were allowed to recover and permitted free access to water and food. After 4 weeks, the rats were humanely euthanized using CO2 asphyxiation and samples retrieved for analysis. The Animal Research Committee at Clemson University approved the animal protocol, and National Institutes of Health (NIH) guidelines for the care and use of laboratory animals (NIH publication no. 86-23, revised 1996) were observed throughout the experiment.
IHC
Samples were placed in formalin, and paraffin sections (5 μm) were first stained with hematoxylin and eosin for general morphology. For identification of infiltrating cell types, samples tagged for IHC were fixed in formalin and paraffin embedded. Sections (5 μm) were deparaffinized and exposed to 0.1% proteinase K solution (0.05 U/ml, Qiagen DNeasy Tissue Kit) in Tris buffered saline, pH 7.5, at 22°C for 30 seconds for antigen retrieval. Endogenous peroxidases were blocked with 0.3% hydrogen peroxide in 0.3% normal sera (Vectastain Elite ABC Kit). Sections were immunostained using mouse anti-rat monoclonal antibodies to vimentin (1 : 500 dilution; Sigma, St Louis, MO, USA), active prolyl-4-hydroxylase (1 : 200 dilution; Chemicon, Temecula, CA, USA), monocytes/macrophages (recognizes a single chain GP of 90–100 kDa, similar to CD68 in the human, 1 : 200 dilution; Chemicon), CD8 for cytotoxic lymphocytes (1 : 200; Sigma), and alpha-smooth muscle cell actin (1 : 200; Sigma) at 22°C for 1 hour. To minimize cross-reactivity, rat-absorbed biotinylated anti-mouse IgG was used in place of the biotinylated secondary antibody provided with the staining kit (Vectastain Elite ABC Kit). Negative staining controls were performed with the omission of the primary antibody. A diaminobenzidine tetra-hydrochloride peroxidase substrate kit was used to visualize the specific staining (Vector Laboratories), and sections were lightly counterstained with hema-toxylin. As positive controls, we stained paraffin sections from rat spleen (macrophage and cytotoxic T lymphocyte control) and rat skin (fibroblast control) in parallel with the explant sections.
Differentiation of mesenchymal stem cells into valvular interstitial cells
Mechanical/biochemical stimulation
Collagen scaffolds were prepared as described above, treated with PGG and glued onto flexible silicone membranes mounted within 6-well culture plates (Flexercell System; Flexcell International Corporation, Hillsborough, NC, USA). Scaffolds were then seeded with human bone marrow-derived stem cells (hBMSCs) and exposed to physiologic biaxial mechanical strains (14% cyclic extension at 0.6 Hz) while being maintained in a cell culture incubator with 10 ng/ml TGF-beta-1 added to the culture media. Cells seeded within same scaffolds but maintained in static conditions and without TGF-beta-1 served as controls.
Immunofluorescence
After 4 days of stimulation, cells were stained as follows. Cells were first fixed for 1 hour with warm 4% formaldehyde, rinsed, permeabilized with 0.2% Triton X-100 for 5 minutes at 22°C, and blocked for 2 hours with 5% bovine serum albumin. Cells were then incubated with mouse anti-human smooth muscle cell actin and separately with rabbit anti-human vimentin at 1 : 200 dilutions overnight at 4°C. After rinsing, sections were incubated with Alexa Fluor-tagged secondary antibodies matched for the primary anti-body species (green for actin and red for vimentin). Finally cells were stained with DAPI nuclear stain and mounted. For double immunostaining, cells were exposed to a mixture of the two antibodies and then reacted with corresponding secondaries.
Stem cell studies in tissue engineered heart valves
Mesenchymal stem cells are excellent candidates for CV TE.22 Several cell types were suggested as ‘surrogates’ for heart valve TE,21 but the real challenge is to induce stem cell differentiation into valvular interstitial cells.
Tissue engineered heart valves
Collagen scaffolds prepared from decellularized porcine pericardium were fashioned into a tri-layered aortic valve-shaped construct and treated with PGG for stabilization. hBMSCs were then seeded inside the tri-layered construct and the tissue engineered heart valve sutured inside a porcine aortic root. This in turn was mounted inside a heart valve bioreactor. This device consists of a valve mounting ring assembly encased between a ventricular chamber and an aortic chamber filled with cell culture medium. The medium is pushed through the valve by a flexible silicone membrane diaphragm connected to an air pump. Extensive testing has shown that the bioreactor ensures comparable pumping functions to a living heart (not shown here). The stem cell seeded tissue engineered valve was subjected to open–close cycles at 0.6 Hz/40 mmHg, in sterile culture medium. Similar cell seeded scaffolds were maintained in static conditions as controls.
Immunofluorescence
Cell-seeded scaffolds were removed from the bioreactor and stained for vimentin by immunofluorescence as described above.
Results and Discussion
Preparation and characterization of biological scaffolds
Sparse Gal-alpha-positive reactions were observed in fresh porcine pericardial cells (mostly capillaries) but none in fibroblasts or the fibrous collagen matrix and virtually undetectable presence of the antigen in decellularized collagen scaffolds (Fig. 1). For fresh arteries and elastin scaffolds, positive Gal-alpha reactions were detected in endothelial cells and the subendothelial matrix, but none in medial smooth muscle cells or adventitial fibroblasts (Fig. 1). Moreover, no staining was detected in elastin scaffolds, suggesting that our decellularization procedure completely removes Gal-alpha antigens from porcine arteries.
These results indicate that decellularization completely removes Gal-alpha antigens from porcine tissues, thus reducing potential biocompatibility and immune rejection issues in human applications.
Biocompatibility testing
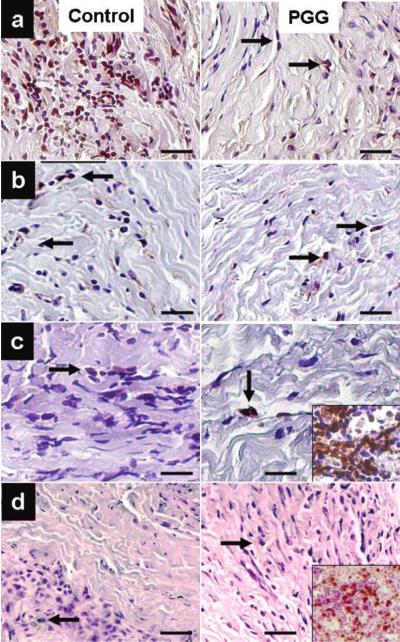
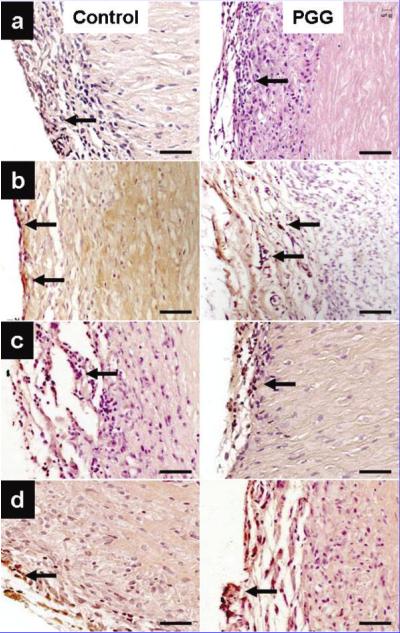
IHC analysis of implanted collagen scaffolds revealed fibroblast-like cells positive for vimentin infiltrating throughout the scaffolds. Most cells were also positive for 4-prolyl-hydroxylase, an enzyme involved in collagen synthesis. Explants were negative for macrophages and cytotoxic T lymphocytes (Fig. 2). Similarly, infiltrating cells in elastin scaffolds were positive for vimentin and alpha-smooth muscle cell actin with very few cells positive for macrophage and cytotoxic T lymphocyte antigens (Fig. 3). As proof of remodeling, a large number of infiltrating cells were also positive for prolyl-4-ghydroxylase. Semi-quantitative analysis of cell infiltration in explants obtained at 4 weeks revealed larger numbers of macrophages in untreated scaffolds than in PGG-treated scaffolds (data not shown), suggesting that PGG could slow down the rate of inflammation and scaffold degeneration in vivo.
Figure 2.
Identification of infiltrating cells in implanted collagen scaffolds. PGG-stabilized scaffolds were compared to untreated control scaffolds. (a) Vimentin stain showing fibroblast-like cells at 4 weeks after implantation. (b) Prolyl-hydroxylase immunohistochemical staining: arrows point to positively stained cells. (c) Specific staining showing few infiltrated macrophages (arrows). Inset shows intense macrophage staining of rat spleen sections used as a positive control. (d) CD8 staining for infiltrating cytotoxic T lymphocytes. Inset shows intense CD8 staining of rat spleen sections used as a positive control. Sections were counterstained with hematoxylin (nuclei dark blue). Bars are 50 μm in all micrographs.
Figure 3.
Identification of infiltrating cells in implanted elastin scaffolds. PGG-stabilized scaffolds were compared to untreated control scaffolds. (a) Vimentin stain showing small numbers of fibroblast-like cells. (b) Prolyl-4-hydroxylase immunohisto-chemical staining: arrows point to positively stained cells. (c) IHC staining showing few infiltrating macrophages (arrows). (d) Alpha-smooth muscle cell staining identifying cells at the periphery of the implants. Sections were counterstained with hematoxylin (nuclei dark blue). Bars are 50 μm in all micrographs.
Taken together these results indicate that both collagen and elastin scaffolds encourage infiltration by host cells and support scaffold remodeling in the absence of immune or inflammatory reactions in the rat. This study clearly demonstrates the over-the-top efficacy of IHC methods in identifying types of host cells associated with implanted tissue engineered scaffolds.
Differentiation of mesenchymal stem cells into valvular interstitial cells
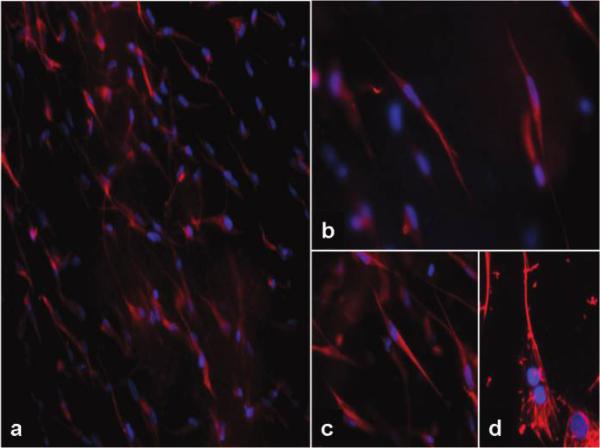
As compared to static controls, numerous hBMSCs which were exposed to biochemical and mechanical stimuli co-expressed both human alpha-smooth muscle cell actin and vimentin strongly indicating a myofibroblast-like synthetic phenotype very similar to activated valvular interstitial cells (Fig. 4). Our earlier IHC and analytical data showed similarly stimulated rat stem cells (14% cyclic extension at 0.6 Hz and 10 ng/ml TGF-beta-1 added to the culture media) also express focal adhesion kinase, beta-1 integrin, collagen type I and type III and chondroitin 6-sulfate as well as matrix metalloproteinases and their inhibitors (not shown here).
Figure 4.
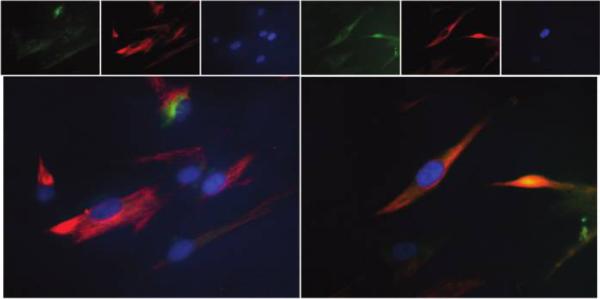
Differentiation of mesenchymal stem cells into valvular interstitial-like cell. Collagen scaffolds were glued onto Flexercell membranes seeded with hBMSCs and exposed to physiologic biaxial mechanical strains. After 4 days cells were stained by immunofluorescence methods. Stem cells expressed either smooth muscle cell actin (green) or vimentin (red) as shown in upper row; some cells also co-expressed both antigens (orange) as evidenced by double immunofluorescence (lower row). Nuclei were stained blue with DAPI and images overlapped digitally. Original magnification was ×400.
Overall, our results suggest that combination of physiologic mechanical stimuli with biochemical cues encourages differentiation of stem cells into cells that closely resemble activated valvular interstitial cells capable of matrix synthesis and degradation. Double-immunofluorescence techniques (using two antibodies on the same sample) proved invaluable for assessment of stem cell differentiation into new, desired cells which co-express two (or more) specific markers. This approach could be extended to additional markers and matrix protein synthesis for increased sensitivity.
Stem cell studies in tissue engineered heart valves
To mimic the shape and biomechanics of heart valves, three-layered tissue scaffolds were seeded with hBMSCs and mounted into a heart valve bioreactor. After 8 days, the cells became elongated significantly and stained positive for vimentin, very similar properties to those of natural valve interstitial cells. (Fig. 5).
Figure 5.
Stem cell studies in tissue engineered heart valves. hBMSCs were seeded onto collagen scaffolds and inserted into a tri-layered valve-shaped construct. The tissue engineered valve was then mounted in a heart valve bioreactor and subjected to open–close cycles at 1 Hz. After 8 days scaffolds were stained for vimentin by immunofluorescence (red). (a–c) Note the numerous slender, vimentin-positive elongated cells similar to valve cells. (d) For comparison, native heart valve interstitial cells were stained for vimentin (red). Nuclei were stained blue with DAPI and images overlapped digitally. Original magnification was ×100 for (a), and ×400 for (b–d).
Thus, physiologic mechanical stimuli offered by our 3D tissue engineered heart valve constructs and the heart bioreactor encouraged differentiation of stem cells into activated valvular interstitial cells, a much sought-after outcome of CV TE. Immunofluorescence techniques were instrumental in documenting expression of new target markers and will expand applications towards additional CV TE applications. Those include stem cell differentiation into other CV cells (endothelial cells and smooth muscle cells), evaluation of remodeling abilities in CV scaffolds (using anti-bodies to human collagen, proteoglycans, etc.), and further applications to vascular grafts and myocardial regeneration.
Conclusions
Histochemical and immunohistochemical techniques are the cornerstone of modern regenerative medicine. Our experience and that of others clearly demonstrate that each and every step of the TE process can and should benefit from these traditional methods and variations adapted to the new scenarios.
It is evident that decellularization changes tissue properties and thus monitoring of cell antigen presence as well as matrix integrity is required to ensure safety of TE biological scaffolds. The Gal-alpha staining is a unique example of how histochemical methods are used to ensure safety of biological scaffolds for human use. Additional applications related to biological scaffold preparation are: testing for completeness of decellularization procedures using antibodies to specific cell proteins and testing for retention of important matrix molecules such as collagens, proteoglycans, growth factors, and others.
Animal implantation of scaffolds is routinely performed to evaluate biocompatibility, re-population with host cells and remodeling as well as immune or inflammatory reactions. This plethora of changes can only be documented by IHC methods that can identify new and old matrix components and types of infiltrating host cells. In the current study, we showed that upon subdermal implantation, collagen and elastin scaffolds were readily infiltrated by activated fibroblasts and macrophages, but did not seem to have elicited significant immune reactions. Slow but sustained cell infiltration will permit the temperate remodeling process, which will maintain the mechanical properties demanded from a heart valve or blood vessel scaffold.
In our TE approach, scaffolds were seeded with stem cells and subjected to conditioning in bioreactors that mimic physiologic conditions or directly implanted into target sites in animal models. Stem cell differentiation into the aimed cell type was then evaluated using IHC techniques. Specifically, multiple labeling techniques proved invaluable for assessment of stem cell differentiation into new, desired cells which co-express two (or more) specific markers. Multiple labeling can be accomplished by using fluorescent dyes with different excitation and emission spectra, and fluorescent microscopy for imaging.
As with other applications of IHC (e.g. in detecting markers of different diseases or identifying structures in biological research), multiple techniques should be developed to define changes occurring in tissues and cells during remodeling. The effects of different fixation methods, tissue processing, and antibody specificity, excellently reviewed by Daneshtalab and others,32 should also be evaluated in tissue engineered samples. It is certain that the future of IHC is bright and colorful. New developments in regenerative medicine and TE will challenge and consequently, drive progress in IHC methods as well. Imminent are novel techniques of in vitro and in vivo imaging of various marker expressions using labeled antibodies for diagnostic pathology and for monitoring the fate of implanted medical devices and TE constructs.
Acknowledgements
This work was funded in part by NIH grants no. P20RR016461 and no. HL084194. The authors wish to thank Linda Jenkins HT for help with histology and the Godley-Snell Animal Research Center at Clemson University for the animal studies.
Footnotes
One continuing education contact hour can be earned by reading this article and taking a short test; for details see www.nsh.org.
References
- 1.American Heart Association National Center Youth and cardiovascular diseases statistical fact sheet. 2004 Available from: http://www.americanheart.org/
- 2.Nugent HM, Edelman ER. Tissue engineering therapy for cardiovascular disease. Circ Res. 2003;92:1068–78. doi: 10.1161/01.RES.0000073844.41372.38. [DOI] [PubMed] [Google Scholar]
- 3.Wu X, Rabkin-Aikawa E, Guleserian KJ, Perry TE, Masuda Y, Sutherland FW, et al. Tissue-engineered microvessels on three-dimensional biodegradable scaffolds using human endothelial progenitor cells. Am J Physiol Heart Circ Physiol. 2004;287:H480–7. doi: 10.1152/ajpheart.01232.2003. [DOI] [PubMed] [Google Scholar]
- 4.Badylak SF. The extracellular matrix as a biologic scaffold material. Biomaterials. 2007;28:3587–93. doi: 10.1016/j.biomaterials.2007.04.043. [DOI] [PubMed] [Google Scholar]
- 5.Badylak SF. Xenogeneic extracellular matrix as a scaffold for tissue reconstruction. Transpl Immunol. 2004;12:367–77. doi: 10.1016/j.trim.2003.12.016. [DOI] [PubMed] [Google Scholar]
- 6.Strokan V, Molne J, Svalander CT, Breimer ME. Heterogeneous expression of Gal alpha1-3Gal xenoantigen in pig kidney: a lectin and immunogold electron microscopic study. Transplantation. 1998;66:1495–503. doi: 10.1097/00007890-199812150-00013. [DOI] [PubMed] [Google Scholar]
- 7.Breuer CK, Shinoka T, Tanel RE, Zund G, Mooney DJ, Ma PX, et al. Tissue engineering lamb heart valve leaflets. Biotechnol Bioeng. 1996;50:562–7. doi: 10.1002/(SICI)1097-0290(19960605)50:5<562::AID-BIT11>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 8.Liao J, Joyce EM, Sacks MS. Effects of decellularization on the mechanical and structural properties of the porcine aortic valve leaflet. Biomaterials. 2008;29:1065–74. doi: 10.1016/j.biomaterials.2007.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baraki H, Tudorache I, Braun M, Hoffler K, Gorler A, Lichtenberg A, et al. Orthotopic replacement of the aortic valve with decellularized allograft in a sheep model. Biomaterials. 2009;30:6240–46. doi: 10.1016/j.biomaterials.2009.07.068. [DOI] [PubMed] [Google Scholar]
- 10.Lichtenberg A, Cebotari S, Tudorache I, Hilfiker A, Haverich A. Biological scaffolds for heart valve tissue engineering. Methods Mol Med. 2007;140:309–17. doi: 10.1007/978-1-59745-443-8_17. [DOI] [PubMed] [Google Scholar]
- 11.Chuang TH, Stabler C, Simionescu A, Simionescu DT. Polyphenol-stabilized tubular elastin scaffolds for tissue engineered vascular grafts. Tissue Eng Part A. 2009;15:2837–51. doi: 10.1089/ten.tea.2008.0394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tedder ME, Liao J, Weed B, Stabler C, Zhang H, Simionescu A, et al. Stabilized collagen scaffolds for heart valve tissue engineering. Tissue Eng Part A. 2009;15:1257–68. doi: 10.1089/ten.tea.2008.0263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nerem RM, Seliktar D. Vascular tissue engineering. Annu Rev Biomed Eng. 2001;3:225–43. doi: 10.1146/annurev.bioeng.3.1.225. [DOI] [PubMed] [Google Scholar]
- 14.Vorp DA, Maul T, Nieponice A. Molecular aspects of vascular tissue engineering. Front Biosci. 2005;1:768–89. doi: 10.2741/1571. [DOI] [PubMed] [Google Scholar]
- 15.Daamen WF, Veerkamp JH, van Hest JC, van Kuppevelt TH. Elastin as a biomaterial for tissue engineering. Biomaterials. 2007;28:4378–98. doi: 10.1016/j.biomaterials.2007.06.025. [DOI] [PubMed] [Google Scholar]
- 16.Dahl SL, Koh J, Prabhakar V, Niklason LE. Decellularized native and engineered arterial scaffolds for transplantation. Cell Transplant. 2003;12:659–66. [PubMed] [Google Scholar]
- 17.Simionescu DT, Lu Q, Song Y, Lee JS, Rosenbalm TN, Kelley C, et al. Biocompatibility and remodeling potential of pure arterial elastin and collagen scaffolds. Biomaterials. 2006;27:702–13. doi: 10.1016/j.biomaterials.2005.06.013. [DOI] [PubMed] [Google Scholar]
- 18.Liu SQ, Tieche C, Alkema PK. Neointima formation on vascular elastic laminae and collagen matrices scaffolds implanted in the rat aortae. Biomaterials. 2004;25:1869–82. doi: 10.1016/j.biomaterials.2003.08.044. [DOI] [PubMed] [Google Scholar]
- 19.Hinds MT, Courtman DW, Goodell T, Kwong M, Brant-Zawadzki H, Burke A, et al. Biocompatibility of a xenogenic elastin-based biomaterial in a murine implantation model: the role of aluminum chloride pretreatment. J Biomed Mater Res A. 2004;69:55–64. doi: 10.1002/jbm.a.20109. [DOI] [PubMed] [Google Scholar]
- 20.Schmidt D, Mol A, Breymann C, Achermann J, Odermatt B, Gossi M, et al. Living autologous heart valves engineered from human prenatally harvested progenitors. Circulation. 2006;114(1 Suppl):I125–31. doi: 10.1161/CIRCULATIONAHA.105.001040. [DOI] [PubMed] [Google Scholar]
- 21.Schaefermeier PK, Cabeza N, Besser JC, Lohse P, Daebritz SH, Schmitz C, et al. Potential cell sources for tissue engineering of heart valves in comparison with human pulmonary valve cells. Asaio J. 2009;55:86–92. doi: 10.1097/MAT.0b013e31818f54e4. [DOI] [PubMed] [Google Scholar]
- 22.Hoerstrup SP, Kadner A, Melnitchouk S, Trojan A, Eid K, Tracy J, et al. Tissue engineering of functional trileaflet heart valves from human marrow stromal cells. Circulation. 2002;106(12 Suppl 1):I143–50. [PubMed] [Google Scholar]
- 23.Simionescu DT, Lovekamp JJ, Vyavahare NR. Degeneration of bioprosthetic heart valve cusp and wall tissues is initiated during tissue preparation: an ultrastructural study. J Heart Valve Dis. 2003;12:226–34. [PubMed] [Google Scholar]
- 24.Simionescu DT, Lovekamp JJ, Vyavahare NR. Glycosaminoglycan-degrading enzymes in porcine aortic heart valves: implications for bioprosthetic heart valve degeneration. J Heart Valve Dis. 2003;12:217–25. [PubMed] [Google Scholar]
- 25.Simionescu DT, Lovekamp JJ, Vyavahare NR. Extracellular matrix degrading enzymes are active in porcine stentless aortic bioprosthetic heart valves. J Biomed Mater Res A. 2003;66:755–63. doi: 10.1002/jbm.a.10066. [DOI] [PubMed] [Google Scholar]
- 26.Mendelson K, Schoen FJ. Heart valve tissue engineering: concepts, approaches, progress, and challenges. Ann Biomed Eng. 2006;34:1799–819. doi: 10.1007/s10439-006-9163-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Latif N, Sarathchandra P, Thomas PS, Antoniw J, Batten P, Chester AH, et al. Characterization of structural and signaling molecules by human valve interstitial cells and comparison to human mesenchymal stem cells. J Heart Valve Dis. 2007;16:56–66. [PubMed] [Google Scholar]
- 28.Ku CH, Johnson PH, Batten P, Sarathchandra P, Chambers RC, Taylor PM, et al. Collagen synthesis by mesenchymal stem cells and aortic valve interstitial cells in response to mechanical stretch. Cardiovasc Res. 2006;71:548–56. doi: 10.1016/j.cardiores.2006.03.022. [DOI] [PubMed] [Google Scholar]
- 29.Taylor PM, Batten P, Brand NJ, Thomas PS, Yacoub MH. The cardiac valve interstitial cell. Int J Biochem Cell Biol. 2003;35:113–8. doi: 10.1016/s1357-2725(02)00100-0. [DOI] [PubMed] [Google Scholar]
- 30.Sacks MS, Schoen FJ, Mayer JE. Bioengineering challenges for heart valve tissue engineering. Annu Rev Biomed Eng. 2009;11:289–313. doi: 10.1146/annurev-bioeng-061008-124903. [DOI] [PubMed] [Google Scholar]
- 31.Sales VL, Mettler BA, Engelmayr GC, Jr, Aikawa E, Bischoff J, Martin DP, et al. Endothelial progenitor cells as a sole source for ex vivo seeding of tissue-engineered heart valves. Tissue Eng Part A. 2010;16:257–67. doi: 10.1089/ten.tea.2009.0424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Daneshtalab N, Doré JJ, Smeda JS. Troubleshooting tissue specificity and antibody selection: procedures in immunohisto-chemical studies. J Pharmacol Toxicol Methods. 2010;61:127–35. doi: 10.1016/j.vascn.2009.12.002. [DOI] [PubMed] [Google Scholar]