Abstract
The tyrosine phosphatase SHP-2 has been implicated in a variety of signaling pathways, including those mediated by neurotrophins in neurons. To examine the role of SHP-2 in the development of sympathetic neurons, we inhibited the function of SHP-2 in transgenic mice by overexpressing a catalytically inactive SHP-2 mutant under the control of the human dopamine β-hydroxylase promoter. Expression of mutant SHP-2 did not influence the survival, axon initiation, or pathfinding abilities of the sympathetic neurons. However, mutant SHP-2 expression resulted in an overproduction of sympathetic fibers in sympathetic target organs. This was due to interference with SHP-2 function, as overexpression of wild type SHP-2 had no such effect. In vitro, NGF-dependent neurite growth was inhibited in neurons expressing mutant SHP-2 but not in those expressing wild type SHP-2. Mutant (but not wt) SHP-2 expression also inhibited NGF-stimulated ERK activation. The NGF-dependent survival pathway was less affected than the neurite growth pathway. Our results suggest that NGF-regulated axon growth signals, and to a lesser degree survival signals, are mediated through a SHP-2-dependent pathway in sympathetic neurons. The increased sympathetic innervation in target tissues of neurons expressing mutant SHP-2 may result from interference with normal “stop” signals dependent on signaling by gradients of NGF.
Keywords: tyrosine phosphatases, neurotrophins, ERKs, transgenic mouse
Introduction
Protein tyrosine phosphorylation, regulated by the opposing effects of protein tyrosine kinases (PTKs) and protein tyrosine phosphatases (PTPs), controls many cellular events, including the growth and guidance of axons during development (Chisholm and Tessier-Lavigne, 1999; Desai et al., 1997). Efforts to understand the signals underlying the regulation of axon growth have historically focused mainly on PTKs (Holland et al., 1998; Paves and Saarma, 1997). Recently, the group of transmembrane PTPs known as receptor protein tyrosine phosphatases has been implicated in the regulation of axon growth decisions (Bixby, 2000). In contrast, little is known about how any cytosolic PTP influences axonal patterning.
Nerve growth factor (NGF) and other neurotrophins promote neuronal survival, differentiation, and plasticity (Henderson, 1996; Lo, 1995; Snider, 1994). In particular, NGF is necessary for the normal development of sympathetic neurons, including their survival and axon growth (Hayashi et al., 1985; Hendry, 1977; Levi-Montalcini, 1976; Thoenen et al., 1971). Binding of NGF to trkA, a receptor PTK, stimulates several signaling pathways, including at least one that results in the activation of extracellular signal-regulated kinases (ERKs; Kaplan and Miller, 2000; Sofroniew et al., 2001; see also Watson et al., 2001). Evidence both from sympathetic neurons and from PC12 cells suggests that activation of ERK is an important step in the induction of axon growth by NGF (Cowley et al., 1994; Fukuda et al., 1995). This is in line with recent findings implicating ERK activation as a central event in the initiation of neuronal process outgrowth (Perron and Bixby, 1999).
SHP-2 is a ubiquitously expressed cytosolic PTP containing two tandemly linked SH2 domains (Ahmad et al., 1993; Feng et al., 1993; Freeman et al., 1992; Pluskey et al., 1995; Vogel et al., 1993). SHP-2 activity is required for appropriate transduction of signals originating from a number of cell surface receptors, including many receptor PTKs (Feng, 1999). In particular, SHP-2 acts downstream of receptors for EGF, FGF, PDGF, NGF, and insulin and is required for activation of ERK by these receptors (Bennett et al., 1994, 1996; Milarski and Saltiel, 1994; Noguchi et al., 1994; Rivard et al., 1995; Yamauchi et al., 1995; Zhao et al., 1995). Futhermore, interference with SHP-2 activity, in vitro, inhibits NGF-induced neuronal differentiation of PC12 cells (Wright et al., 1997). It is therefore reasonable to hypothesize that SHP-2 could regulate survival and/or axon growth of sympathetic neurons in vivo, especially those aspects dependent on NGF.
Targeted deletions of mouse shp-2 result in early embryonic lethality, which precludes investigation of potential abnormalities in neuronal differentiation (Saxton et al., 1997). We therefore adopted a transgenic strategy in which a catalytically inactive form of SHP-2 was expressed selectively in sympathetic neurons by using the human dopamine β-hydroxylase (hDBH) promoter. The hDBH promoter construct we used is known to direct transgene expression to a small subset of cells, including sympathetic neurons, some neurons in the CNS, and adrenal chromaffin cells. This hDBH promoter drives expression in sympathetic neurons from embryonic day 9.5 (E9.5) and is constitutively expressed in sympathetic neurons (Kapur et al., 1991; Mercer et al., 1991). This ensures that the SHP-2 transgene will be expressed in sympathetic neurons commencing when neural crest cells coalesce into ganglia and prior to axon outgrowth (Kapur et al., 1991; Rubin, 1985). Expression of a catalytically inactive SHP-2 has been shown previously to act as a dominant-interfering mutant, in vitro, in numerous cell types (Bennett et al., 1994, 1996; Guan and Dixon, 1991; Milarski and Saltiel, 1994; Noguchi et al., 1994; Rivard et al., 1995; Yamauchi et al., 1995; Zhao et al., 1995). Using transgenic mice that express this SHP-2 mutant, we demonstrate that SHP-2 activity is not required for survival, initial differentiation, or axon outgrowth of sympathetic neurons in vivo but is necessary for normal axon termination within innervated targets. Further, our data indicate that SHP-2 activity is necessary for NGF-dependent neurite growth and ERK activation in sympathetic neurons in vitro and plays a role in NGF-dependent survival. Our results are consistent with a model in which gradients of NGF determine the extent of axonal arborization in sympathetic targets via a SHP-2-dependent pathway.
Materials and Methods
Generation of SHP-2 transgenic mouse lines
A 1.9 kb SHP-2 cDNA (wtSHP-2) containing the full coding region was isolated from an adult brain cDNA library. A DN SHP-2 mutant (C459S) was generated by PCR-based site-directed mutagenesis and confirmed by sequencing. SHP-2 cDNAs were fused 3′ to a 5.8-kb hDBH promoter and a 171-bp segment spanning the first intron of the rat insulin II gene, included to boost expression (Palmiter et al., 1991). A 799-bp portion of the human growth hormone gene containing a 66-bp coding sequence and a 633-bp 3′-untranslated region (UTR) was added 3′ to the SHP-2 cDNA to provide a polyadenylation signal for mRNA stability and a unique sequence tag. The constructs were injected into mouse zygotes and transgenic founders identified by tail blots (Brinster et al., 1981), using probes corresponding to the hGH 3′ UTR. In established lines, genotypes were determined by PCR using primers corresponding to SHP-2 cDNA and the hGH 3′ UTR, respectively. Three lines that expressed wt SHP-2 [OX: TgN(DBH-SHP2)Bmas 2,9,14] and three that expressed DN SHP-2 [DN: TgN(DBH-SHP2C459S) Bmas 21,31,33] were established, and the phenotypes described were consistent across each genotype.
SHP-2 Western blot and measures of transgene expression
Western blots of paired adult mouse superior cervical ganglia homogenates were prepared as described (Perron and Bixby, 1999), using a 1:1000 dilution of monoclonal anti-SHP-2 antibody (Transduction Laboratories P54420) followed by anti-mouse alkaline phosphatase-conjugated secondary antibody (Promega) diluted 1:5000. Two different methods were used to quantify SHP-2 levels in wt and trangenic SCGs; these agreed well with each other. In the slope method, optical densities of SHP-2-immunoreactive bands were plotted against the amount of protein loaded for each SCG pair, and linear regression was performed for transgenic or wt SCG. Relative expression was determined by the ratio of the calculated slopes, transgenic:wt. In some cases, blots were normalized by reprobing to measure immunoreactive neuron-specific enolase (Fig. 1); in these cases, the SHP-2/NSE ratio was calculated. The average ratios of SHP-2 protein (tg/wt) in SCGs for the two DN lines and the two OX lines used for further experiments were: DN 33, 2.4 ± 0.63; DN 31, 2.04 ± 0.32; OX 14, 2.2 ± 0.56; OX 2, 2.15 ± 0.15. Most experiments used line 33 for DN SHP-2, and line 14 for OX SHP-2.
Fig. 1.
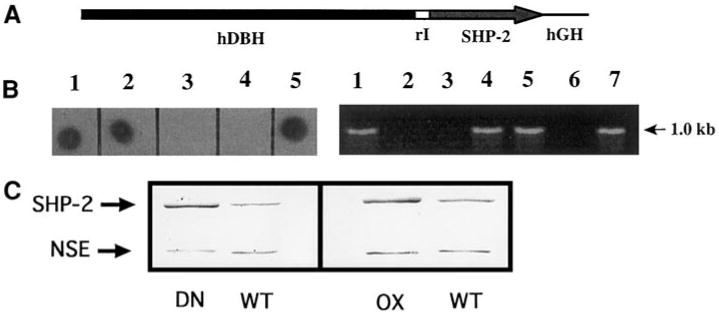
Production of SHP-2 transgenic mice. (A) DNA construct used to generate SHP-2 transgenic mice. A 5.8-kb regulatory sequence from the human dopamine β-hydroxylase gene (hDBH) drives expression of a 1.9-kb SHP-2 wild type or Cys→Ser mutant cDNA. rI, 171-bp segment spanning the first intron of rat insulin II gene; hGH, 799-bp portion of the human growth hormone gene. (B) The SHP-2 transgene detected by dot blot of mouse tail DNA with the hGH sequences or by genomic PCR using a primer pair corresponding to the SHP-2 cDNA and the hGH tag, respectively. (Left) 3/5 mice in a litter carry the transgene (lanes 1, 2, 5). (Right) 4/7 mice in a litter were identified as transgenic by genomic PCR (lanes 1, 4, 5, 7). Lanes 3, 4 (left) and 2, 3, 6 (right) contain DNA from wt littermates. (C) SHP-2 protein is overexpressed in superior cervical ganglia (SCGs) from transgenic animals. (Left) 8 μg of protein from a pair of wt or DN SCGs was run on SDS PAGE and detected on a Western blot with anti-SHP-2 antibody. (Right) 5 μg of protein from a pair of wt or OX SCGs was run on SDS PAGE and detected on a Western blot with anti-SHP-2 antibody. After quantification of these bands, blots was reprobed with anti-neuron-specific enolase (NSE) for normalization. In each case, quantitative analysis showed that the transgenic ganglia had a SHP-2/NSE ratio of roughly 2.5. Quantification of blots (n ≥ 3 for each line), using both this normalization method and the slope method (see Materials and Methods) showed that SCGs from both DN and OX lines consistently contained 2-2.5 times normal levels of immunoreactive SHP-2.
Immunohistochemical procedures
For whole mount studies of initial axon growth, mouse embryos (E10.5–E11.5) were collected (yolk sacs were genotyped) and fixed in freshly prepared 2% paraformaldehyde. Endogenous peroxidase activity was blocked by incubation in 5% H2O2/methanol for 5–8 h at room temperature, depending on embryo size. Rehydrated embryos were incubated in blocking solution (0.5% Triton X-100, 2% nonfat dry milk in PBS) followed by a 1:100 dilution of the monoclonal anti-neurofilament antibody (Sigma, N5264) in blocking solution, overnight at 4°C. After repeated washes, antibody was detected by HRP-conjugated goat anti-mouse IgG (Promega) diluted 1:800. Fresh submaxillary glands from adult mice were fixed in 3% paraformaldehyde and embedded in OCT. Tissue sections (15 μm) were incubated overnight in a 1:100 dilution of polyclonal anti-TH antibody (Pel Freez P40101-0), and bound antibody was detected by goat anti-rabbit IgG Alexa488 diluted 1:200. We examined TH staining in three wt mice, three DN SHP-2 mice, and two OX SHP-2 mice.
Histology of sympathetic ganglia
SCGs were dissected from 6-month-old mice, fixed in 10% neutral-buffered formalin overnight, dehydrated, and equilibrated with xylene. Paraffin-embedded tissue sections (6 μm) were defatted, rehydrated, and stained with hematoxylin (Sigma MHS-16) and eosin Y (Sigma HT110-1-16). Photomicrographs were analyzed by using NIH Image software. Neuron density was obtained by calculating the ratio of neuron nuclei to the cross-sectional area of a selected field. Ganglion size was estimated from the surface areas of sections from the midpoint (largest diameter) of each ganglion.
Catecholamine histofluorescence
Submaxillary glands and pineal glands were removed from adult female mice, with or without intraperitoneal injection of 100 μg of α-methyl norepinephrine in 1 mM ascorbic acid 1 h before dissection. Fresh tissue was embedded in OCT and frozen in liquid nitrogen. Tissue sections (15 μm) were cut and mounted onto slides, and catecholamine histofluorescence was produced as described (De la Torre, 1980). Sections were air dried and heated in oil to 100°C, then coverslipped and viewed by epifluorescence. Fluorescence images were quantified by using NIH Image. Overall, we examined catecholamine histofluorescence in submaxillary glands from 12 wt mice, 14 DN SHP-2 mice, and 9 OX SHP-2 mice; and pineal glands from 2 wt mice and 2 DN SHP-2 mice. Results in all cases were consistent with the data reported.
Mouse SCG culture and neurite outgrowth assay
Neonatal (P0) mouse SCG were dissected, partially dissociated in 0.35% trypsin solution, and cultured on coverslips precoated with poly-D-lysine and laminin as described (Perron and Bixby, 1999). L15 culture medium (Life Technologies), containing 5% rat serum and various vitamins, was supplemented with 100 ng/ml NGF-β (Sigma, N2513) or with NGF plus 100 ng/ml each of BDNF and NT-3 (Peprotech). Neurite lengths were measured from video images by using SigmaScan (Jandel Scientific) or NIH Image software.
Phospho-ERK immunocytochemistry and Western blotting
P0 SCG neurons were isolated and cultured as described above for 6 days. NGF was removed, and the cultures were incubated a further 12 h prior to readdition of 100 ng/ml NGF-β (or no addition in controls). Cultures were fixed 3 h later, then incubated overnight in a 1:100 dilution of phosphospecific ERK1/ERK2 antibody (New England Biolabs 9101S) using Rhodamine-conjugated goat-anti-rabbit IgG (Sigma) as a secondary antibody. ERK staining was quantified by scoring each neuron identified under phase-contrast optics as having fluorescent ERK staining either clearly greater than controls (which had no NGF readdition) or indistinguishable from controls. At least 70 neurons were scored for each condition in each experiment. For Western blots, protein extracts prepared from cultures treated as above were subjected to SDS-PAGE and transferred to nitrocellulose. The membranes were probed with the phosphospecific ERK antibody prior to stripping and reprobing with a pan-ERK antibody.
Results
Creation of SHP-2 Transgenic Mice
To study the biological function of SHP-2 in sympathetic neurons in vivo, a SHP-2 transgene was overexpressed in sympathetic neurons by using a promoter region of the human dopamine-β-hydroxylase (hDBH) gene (Fig. 1A). The hDBH promoter has previously been shown to direct expression to sympathetic neurons in transgenic mice (Kapur et al., 1991; Mercer et al., 1991). Two types of SHP-2 transgene were introduced into the mouse genome: a cDNA encoding wild-type SHP-2 (wtSHP-2) and one encoding a catalytically inactive (DN) SHP-2 in which an essential cysteine (C459) residue is mutated to serine. Overexpression of the catalytically inactive mutant, in vitro, has been shown to inhibit endogenous SHP-2 function (Guan and Dixon, 1991; Hadari et al., 1998; Noguchi et al., 1994; Wright et al., 1997). Overexpression of wt SHP-2 was used as a control to distinguish between phenotypes resulting from inhibition of SHP-2 function and those resulting from aberrant increases in SHP-2 protein (potentially mediated by nonphosphatase domains). The presence of the SHP-2 transgene was detected by using dot blots of total nucleic acid from tail biopsies or by PCR (Fig. 1B). Three independent SHP-2 transgenic lines were established for the SHP-2 mutant (referred to as DN lines) as well as for the wtSHP-2 (referred to as overexpresser or OX lines). Western blots of adult SCG protein extracts revealed increased levels of SHP-2 protein in all transgenic lines (Fig. 1C, and data not shown). Quantitative analysis (see Materials and Methods) demonstrated that SHP-2 protein in SCG isolated from either DN or OX lines was 2-2.5 times that in nontransgenic littermates. Because the antibody used could not distinguish between endogenous SHP-2 and the transgenic protein, it can only be assumed that levels of transgene-generated SHP-2 were 100–150% those of the endogenous protein. This assumption is reasonable, because in PC12 cells, two- to threefold overexpression of SHP-2 does not influence expression of endogenous SHP-2 (Wright et al., 1997).
Sympathetic Neurons Expressing DN SHP-2 Survive and Differentiate
Expression of transgenes using the hDBH promoter commences before E9.5 and is constitutive in later embryos and adults (Kapur et al., 1991; Mercer et al., 1991). To investigate the consequence of inhibiting SHP-2 activity during the development of sympathetic neurons, we first examined the morphology of SCGs from wt and DN SHP-2 adult mice. The overall appearance of the SCG was similar in wt and DN animals (Fig. 2). No differences with respect to either neuronal size or density were observed between SHP-2 DN and wt SCG. Both wt and DN ganglia contained large ovoid neurons of equivalent size, with an average long axis diameter of 19.08 ± 0.91 μm (mean ± SEM) for the wt and 18.77 ± 0.56 μm for the DN neurons, and an average short axis diameter of 14.01 ± 0.60 μm for the wt and 13.46 ± 0.47 μm for the DN (>200 neurons measured per condition). Small round neurons (longest axis <15 μm), usually near the perimeter of the ganglia, had equivalent diameters averaging 11.99 ± 0.36 μm for the wt and 12.31 ± 0.34 μm for the DN (>200 neurons per condition). Neuron densities (see Materials and Methods) measured using medial transverse sections showed no significant differences between ganglia from wt and DN mice [12.57 ± 1.44 arbitrary units (AU), wt; 12.92 ± 1.19 AU, DN; >600 neurons per condition]. As the average sizes of the medial transverse sections from DN SCGs were indistinguishable from wt (not shown), the number of neurons per SCG was not significantly altered by expression of DN SHP-2. Therefore, expression of DN SHP-2 did not appear to interfere with proliferation, morphological maturation, or survival of neurons in the SCG. As we did not obtain exact counts of neurons, small differences in neuron number might not be discovered by our techniques.
Fig. 2.
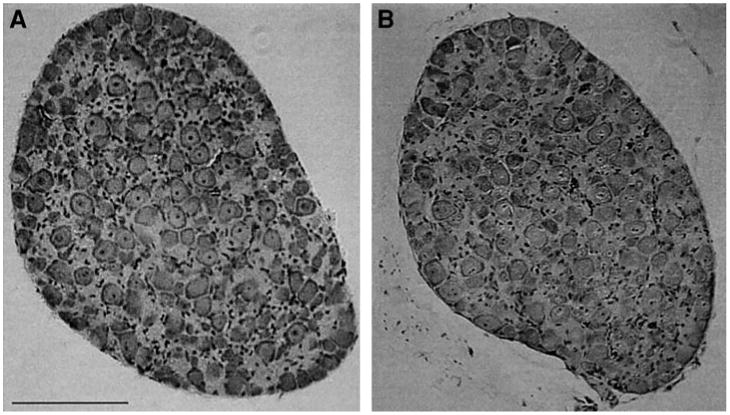
SCGs from SHP-2 DN mice have a normal morphology. SCGs from wild type (A) and SHP-2 DN (B) mice were fixed, sectioned at 6 μm, and stained with hematoxylin/eosin. The SHP-2 DN SCG is similar to the wild type control in the number, size, and density of neurons. Similar results were seen in several ganglia. Scale bar, 100 μm.
We also examined initial axon outgrowth from DN SHP-2 sympathetic neurons using neurofilament staining of whole-mount embryos. In these experiments, we found no differences in the pattern of axon outgrowth between wt and DN SHP embryos when embryos of E10.5-E11.5 were examined (n = 2 embryos wt; 2 embryos, DN SHP-2; data not shown). This age represents the period of axon outgrowth from sympathetic neurons during embryogenesis. Therefore, it is unlikely that overexpression of a DN mutant form of SHP-2 hindered survival, differentiation, or initial axon outgrowth of sympathetic neurons. Although the caliber of axon tracts did not appear different between DN SHP-2 and wt, small effects on numbers of axons in sympathetic nerves would not be seen by our methods.
Expression of DN SHP-2 Leads to Increased Sympathetic Innervation in Submaxillary Glands
Our hypothesis that SHP-2 is required for normal axon growth and guidance predicts that DN SHP-2 expression will lead to defects in sympathetic target innervation. To investigate this possibility, we visualized the distribution of sympathetic fibers in target tissues from adult wt and SHP-2 DN mice using tyrosine hydroxylase (TH) immunostaining. TH-positive fibers were seen around the periphery of individual acini, as expected (Figs. 3A, 3E, and 3F). Surprisingly, the density of TH-immunoreactive fibers in DN submaxillary glands was considerably more intense than in the wt glands (Fig. 3B), suggesting either an increase in the density of ramifying sympathetic fibers or a higher level of TH protein in the DN glands. At higher magnification, it appeared that the DN glands had increased numbers of sympathetic fibers (Figs. 3C and 3D), though this impression was not quantified.
Fig. 3.
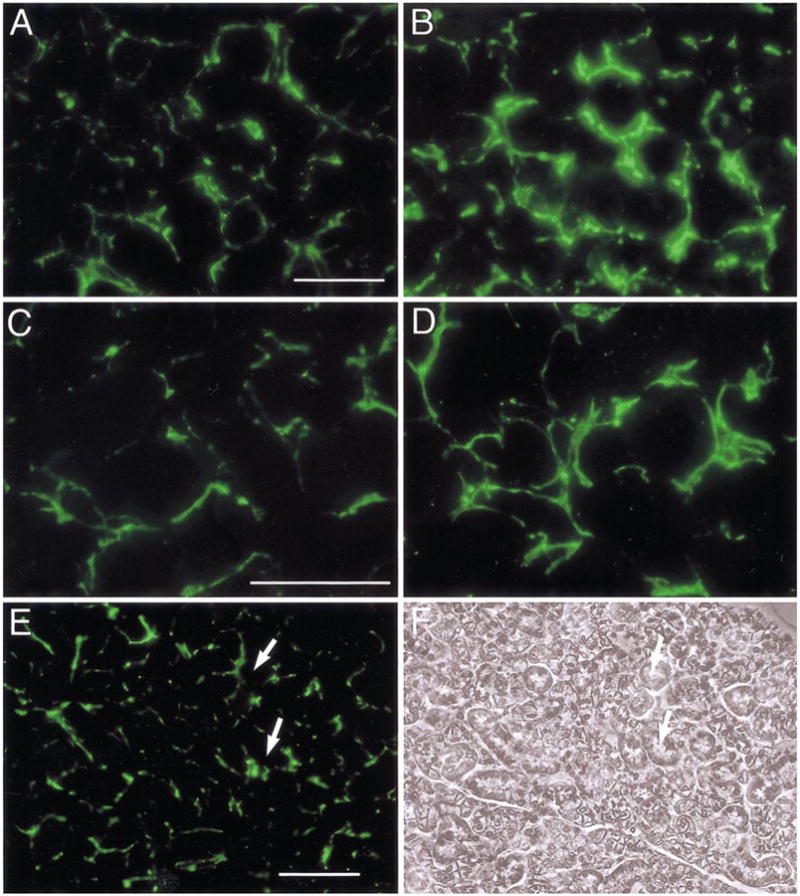
Increased sympathetic innervation of submaxillary glands in SHP-2 DN mice. Sympathetic innervation of submaxillary glands was examined by using antibody to tyrosine hydroxlase (TH), detected with secondary antibodies labeled with Alexa488. (A) In wt mice, individual acini from submaxillary glands are surrounded by TH-positive nerve fibers. (B) In SHP-2 DN glands, staining is noticeably brighter throughout the tissue. (C, D) At higher magnification, an increased number of TH-positive nerve fibers can be seen in the SHP-DN tissue (D), compared with the wt (C). (E) and (F) are TH immunostaining and phase contrast views, respectively, of a DN SHP-2 section at low power. White arrows point to corresponding points in the two micrographs. Scale bar, 30 μm (A, C); 100 μm (E). In this and subsequent figures showing fluorescent images, different tissues were processed together, photographs were taken at the same time under identical illumination conditions, and these were processed identically to facilitate comparison. Representative sections are shown.
To confirm the apparent increase in sympathetic innervation density in SHP-2 DN glands, we examined the distribution of sympathetic fibers in adult mice using catecholamine histofluorescence. Although this technique was slightly less sensitive than TH immunostaining in our hands, we found a similar increase in fiber-related fluorescence in the DN glands, with fluorescent fibers both more intense and more widely distributed than in wt glands (Figs. 4A and 4D). No difference in fluorescence was seen in glands from SHP-2 OX mice compared with controls (data not shown), indicating that this effect was due to inhibition of SHP-2 catalytic function rather than overexpression of other SHP-2 domains.
Fig. 4.
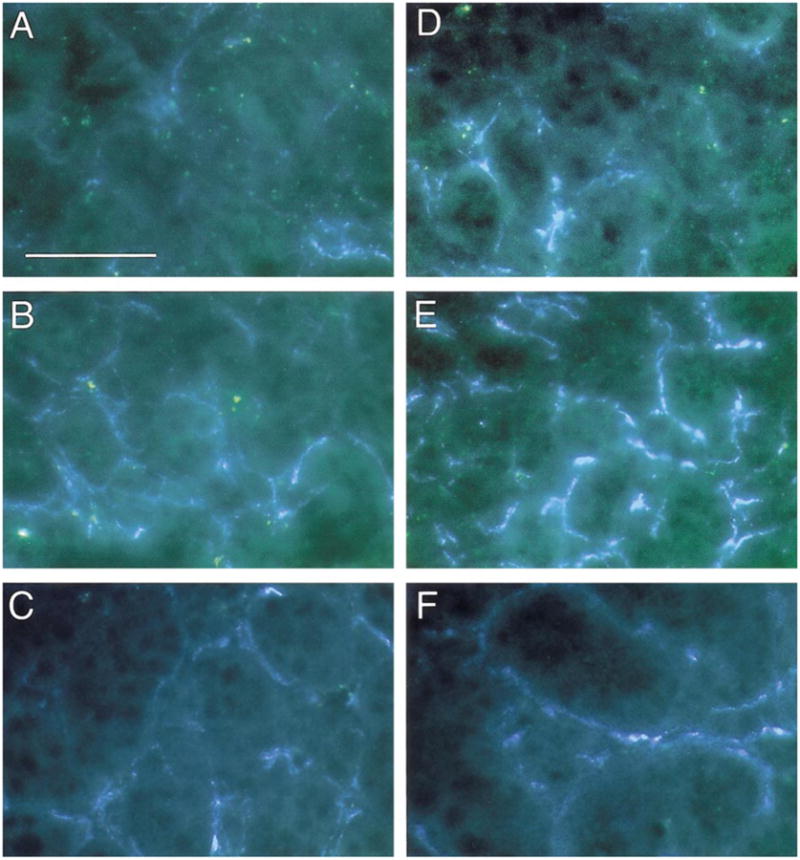
Increased sympathetic innervation of submaxillary glands in SHP-2 DN mice, but not in SHP-2 OX mice. Sympathetic innervation of submaxillary glands was examined by using the glyoxylic acid method for catecholamine histofluorescence. (A, D) Wt (A) and SHP-2 DN (D) gland sections in control conditions. There is increased catecholamine fluorescence in the DN tissue. (B, E) Wt (B) and SHP-2 DN (E) gland sections following injection of α-methyl norepinephrine to saturate catecholamine levels. The increased catecholamine fluorescence in the DN is even more evident, suggesting that the increased levels are due to more sympathetic fibers, rather than increased NE levels in the fibers. Examples shown are representative of multiple experiments (n ≥ 4) on each of three SHP-2 DN lines. (C, F) Wt (C) and SHP-2 OX (F) gland sections following injection of α-methyl norepinephrine to saturate catecholamine levels. Levels of catecholamine fluorescence in the OX gland is similar to that seen in the wt gland. Examples shown are representative of multiple experiments (n ≥ 4) on each of 2 SHP-2 OX lines. Scale bar, 30 μm.
Similar to the situation for TH staining, increased catecholamine histofluorescence in the DN SHP-2 submaxillary glands might be due to a change in catecholamine metabolism of sympathetic fibers, rather than a change in the density of sympathetic nerve growth. To address this issue, we injected a norepinephrine analog (α-methyl norepinephrine) into the peritoneal cavity of mice 1 h before dissection. This procedure loads sympathetic axons and terminals with maximum concentrations of catecholamine (ElShamy et al., 1996), allowing for direct comparisons of sympathetic fiber densities in wt and DN SHP-2 glands. Injection of α-methyl norepinephrine increased catecholamine histofluorescence in both DN and wt submaxillary glands, as expected. However, the increased distribution of fluorescence in the DN SHP-2 compared with the control glands was even more evident (Figs. 3B and 3E). Examination of SHP-2 OX submaxillary glands after injection of α-methyl norepinephrine again revealed no differences between OX and wt glands (Figs. 3C and 3F). Thus, the increased catecholamine fluorescence in DN SHP-2 glands likely reflects an increased level of sympathetic innervation due to inhibition of SHP-2 activity in the sympathetic neurons.
Quantification of fluorescence levels in wt and transgenic submaxillary glands (after α-methyl norepinephrine treatment) confirmed these qualitative impressions. While the average fluorescence level above background in DN SHP-2 glands was 5.1 ± 1.4-fold that in wt littermates (n = 2 animals), the average level in OX SHP-2 glands was 0.99 ± 0.05-fold that of wt (n = 2 animals). Therefore, using two separate measures, inhibition of SHP-2 activity in sympathetic neurons led to increased densities of sympathetic innervation in the submaxillary gland. Thus, SHP-2 activity appears to be required in a signaling pathway that regulates sympathetic innervation of the submaxillary gland.
Expression of DN SHP-2 Leads to Increased Innervation of Pineal Glands
To determine whether increased sympathetic innervation of target organs in DN SHP-2 mice was limited to the submaxillary gland, we used catecholamine histofluorescence to examine sympathetic innervation of pineal glands, which receive predominant innervation from the SCG (Karasek, 1983). To obviate differences due to changing levels of norepinephrine, wt and DN SHP-2 mice were injected intraperitoneally with α-methyl norepinephrine 1 h before removing the glands. A clear and reproducible increase in catecholamine fluorescence in DN SHP-2 pineal glands compared with wt was observed (Fig. 5, and data not shown). Therefore, inhibition of SHP-2 function leads to increased terminal innervation of pineal as well as submaxillary glands. These results demonstrate that the DN effects on target innervation are not limited to a single target organ, but may be general among sympathetic targets. Thus, inhibiting SHP-2 activity in sympathetic neurons does not affect neuronal survival, initial axon outgrowth, or the ability to reach target organs, but leads to abnormally dense innervation of target organs. These results are consistent with the idea that SHP-2 activity is required for regulation of axon growth by NGF, as innervation within targets, but not initial axon growth to targets, appears to depend on an NGF gradient (Hoyle et al., 1993).
Fig. 5.
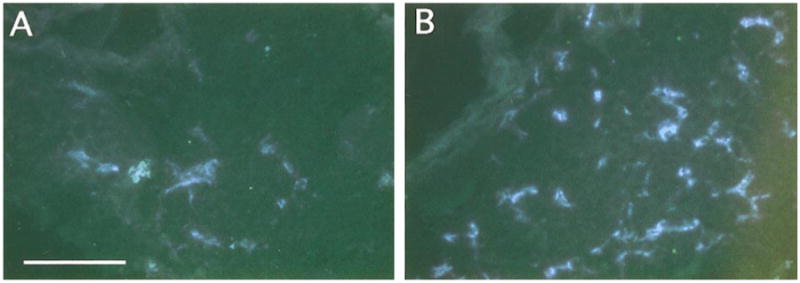
Increased sympathetic innervation of pineal glands in SHP-2 DN mice. Sympathetic innervation of pineal glands was examined as for submaxillary glands. Animals were injected intraperitoneally with α-methyl norepinephrine prior to dissection to saturate catecholamine levels. (A) Representative section illustrating fluorescence levels in the wt pineal. (B) Representative section illustrating fluorescence levels in the SHP-2 DN pineal. Fluroescence levels are clearly higher in the SHP-2 DN gland. Similar results were obtained in three independent experiments. Scale bar, 100 μm.
DN SHP-2 Inhibits NGF-Dependent Neurite Growth from Cultured SCG Neurons
Interference with SHP-2 function in PC-12 cells, through overexpression of DN SHP-2, inhibits NGF-induced neuronal differentiation of PC12 cells, as assessed by reduced neurite outgrowth (Wright et al., 1997). As PC12 cells are thought to be a model for sympathetic neurons (Greene and Tischler, 1976), this result suggests that expression of DN SHP-2 should inhibit neurite growth from sympathetic neurons, which is NGF-dependent in vitro (Coughlin et al., 1977; Coughlin and Collins, 1985). To test this idea, we examined NGF-induced axon outgrowth from wt and SHP-2 transgenic sympathetic neurons in vitro. Neonatal SCGs were dissected, partially dissociated, and cultured on poly-d-lysine/laminin substrates in medium supplemented with 100 ng/ml NGF, a concentration demonstrated to be supramaximal for survival and differentiation of sympathetic neurons in vitro (Coughlin and Collins, 1985; Mizel and Bamburg, 1976). Dissociated wt SCG neurons grew numerous long neurites after 24 h; some neurites intertwined to form a meshwork (Fig. 6A). In contrast, DN SHP-2 SCG neurons rarely extended neurites, and those neurites that formed were extremely short (Fig. 6E). Inhibition of neurite growth by DN SHP-2 was also clearly observed in SCG explants, in which both cell–cell interactions and neurite fasciculation were maintained. DN SHP-2 SCG explants had many fewer and shorter neurites compared with wt explants of similar area (Figs. 6B and 6F). Despite these differences in neurite growth, the appearance of dissociated neurons, as well as the size and appearance of explants, were comparable in wt and DN SHP-2 cultures.
Fig. 6.
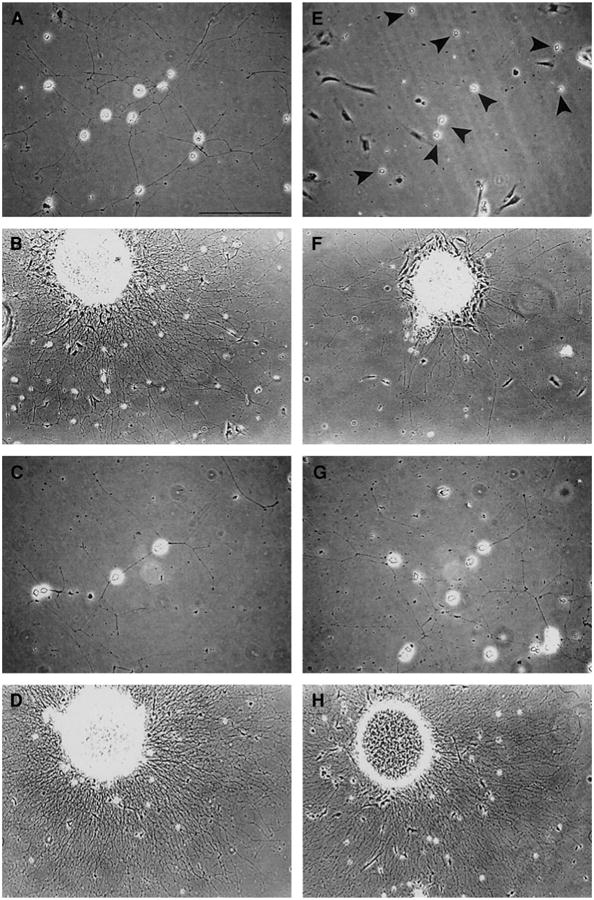
NGF-stimulated neurite outgrowth from cultured sympathetic neurons. SCGs from neonatal mice were partially dissociated and cultured on poly-d-lysine/laminin substrates for 24 h in medium supplemented with 100 ng/ml NGF prior to examination. (A) Dissociated SCG neurons from a wt mouse grow extensive networks of neurites under these conditions. (E) Similar numbers of dissociated neurons (black arrowheads) are present in a culture from a SHP-2 DN littermate, but neurites are essentially absent. (B) A ganglionic explant from a wt mouse exhibits a dense halo of long neurites. (F) A ganglionic explant from a SHP-2 DN littermate shows sparse neurite growth, even though the size of the explant is similar. (C) Dissociated SCG neurons from a second wt mouse also grow extensive networks of neurites. (G) Dissociated neurons from a SHP-2 OX littermate exhibit a similar degree of neurite growth. (D) A ganglionic explant from a second wt mouse exhibits a dense halo of long neurites. (H) A ganglionic explant from a SHP-2 OX littermate exhibits similar neuritic growth. Thus, SHP-2 DN SCGs, but not SCGs overexpressing wt SHP-2, are deficient in NGF-dependent neurite outgrowth. Similar results were observed in 19 experiments on 3 different SHP-2 DN lines, and in 11 experiments on 3 SHP-2 OX lines. Scale bar, 100 μm.
Inhibition of neurite growth from SCG neurons might in principle be due to overexpression of SHP-2 per se, rather than a dominant negative effect. To examine this possibility, neurite growth from SHP-2 OX sympathetic neurons was examined under the same culture conditions. No clear differences in neurite outgrowth between OX SHP-2 and wt controls could be detected. This was true whether we examined dissociated neurons (Figs. 6C and 6G) or ganglionic explants (Figs. 6D and 6H). These results indicate that the robust inhibition of neurite formation seen in DN SHP-2 sympathetic neuron cultures is due to a dominant-negative inhibition of SHP-2 phosphatase activity.
To quantify the inhibition of neurite growth, we measured the lengths of neurites from SCG neurons in dissociated cell cultures. Wt SCG neurons grew long neurites (>150 μm; Fig. 7A), while neurite outgrowth from DN SHP-2 neurons was minimal. The average neurite length of DN SHP-2 neurons was only 5% of that observed for wt neurons (Fig. 7B).
Fig. 7.
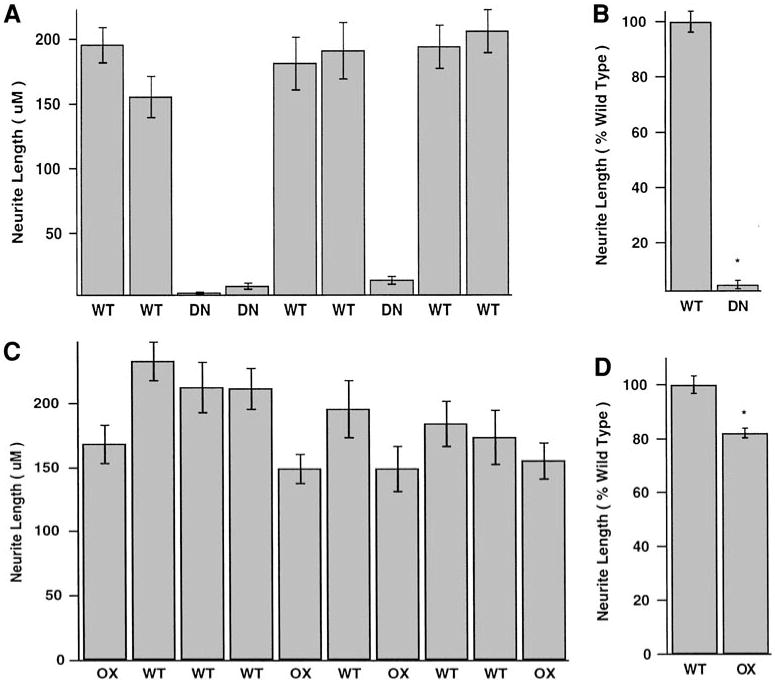
Quantification of NGF-induced neurite outgrowth from dissociated SCG neurons. (A) Paired SCGs from individual neonatal mice in a single litter (6 wt and 3 SHP-2 DN mice) were cultured for 24 h. Average neurite lengths were measured for at least 20 randomly selected neurons in each culture, and the data were plotted as mean ± SEM. The presence of the SHP-2 DN transgene inhibits neurite growth almost completely. (B) Normalized data from 3 independent experiments (total of 12 wt, 8 DN cultures) performed as in (A). Overexpression of DN SHP-2 resulted in a 95% inhibition of neurite growth. (C) Paired SCGs from individual neonatal mice in a single litter (6 wt and 4 SHP-2 OX mice) were cultured for 24 h. Average neurite lengths were measured for at least 20 randomly selected neurons in each culture, and the data were plotted as mean ± SEM. Neurite lengths in SHP-2 OX cultures are roughly comparable to those in controls (D) Normalized data from 2 independent experiments (total of 9 wt, 10 OX cultures) performed as in (C). The data reveal a small (19%) but statistically significant inhibition of neurite growth by overexpression of wt SHP-2. *, P < 0.001.
Quantitative analysis of OX SHP-2 neurons confirmed that neurite growth was near normal, but revealed a subtle effect of SHP-2 overexpression on neurite growth (Figs. 7C and 7D). On average OX SHP-2 neurite lengths were 82% of those for wt neurons (Fig. 7D). These data demonstrate that SHP-2 activity is required for NGF-dependent neurite growth from sympathetic neurons in vitro, and also suggest that increasing SHP-2 protein levels above normal produces suboptimal signals for neurite formation.
DN SHP-2 Neurons Are NGF-Responsive, and SHP-2 Is Not Absolutely Required in the NGF Survival Pathway
Both survival and neurite growth of neonatal sympathetic neurons in vitro depend on NGF (Campenot, 1977; Coughlin et al., 1977; Coughlin and Collins, 1985; Hendry, 1977; Levi-Montalcini, 1976). In our SCG culture experiments, numerous DN SHP-2 neurons survived for more than 7 days, suggesting that the neurons remained NGF-responsive, and that NGF survival pathways were not inhibited by a decrease in SHP-2 activity. However, quantitative assessment revealed that surviving neurons in DN SHP-2 cultures averaged 33 ± 11% (mean ± SEM) of wt neuronal numbers after 3 days in vitro. Therefore, inhibition of SHP-2 function had an effect on NGF-dependent neuronal survival in vitro. In principle, neurons that remained in DN SHP-2 cultures could somehow become independent of NGF for survival. To test this possibility, we cultured neonatal SCG neurons (wt and DN SHP-2) in NGF-supplemented medium for 72 h, then NGF was withdrawn for 96 h. During the 3-day culture in NGF, we observed an expected survival of both wt and SHP-2 DN neurons (Figs. 8A and 8C). Wt neurons formed a dense network of neurites (Fig. 8A), while DN SHP-2 neurons displayed sparse neurite outgrowth (Fig. 8C). Similarly, both wt and SHP-2 DN neurons survived well in SCG explants, but the number and the length of neurites from DN SHP-2 explants were greatly reduced compared with wt explants of comparable area (data not shown). In all cases, removal of NGF caused progressive neuronal death and the associated beading and eventual disappearance of neurites. After 4 days in the absence of NGF, almost all of the dissociated neurons died, but glial cells (whose survival is NGF-independent) remained (Figs. 8B and 8D). Neuron death induced by NGF withdrawal was also evident in SCG explants (not shown). Thus, the survival of DN SHP-2 neurons is NGF-dependent, implying that an NGF-dependent survival pathway is functional in a substantial fraction of these neurons. Thus, about one-third of DN SHP-2 neurons can survive in an NGF-dependent manner, while these same neurons are almost completely inhibited with respect to neurite growth. These data suggest that the signaling pathway underlying NGF-induced neurite outgrowth requires SHP-2, while the pathway underlying NGF-dependent survival is much less dependent on SHP-2, at least for some SCG neurons.
Fig. 8.
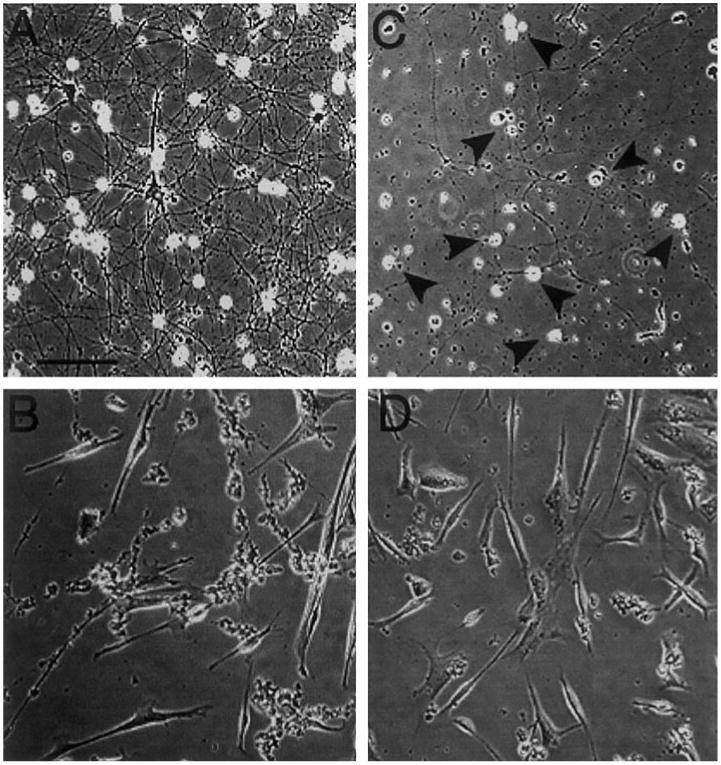
Sympathetic neurons overexpressing DN SHP-2 are dependent on NGF for survival in vitro. SCG neurons were cultured in the presence of 100 ng/ml NGF for 72 h; NGF was then removed prior to culture for an additional 96 h. Phase-contrast microphotographs of identical fields were taken immediately before and 96 h after NGF withdrawal. (A) Control (wt) dissociated neurons exhibit a dense network of processes prior to NGF withdrawal. (B) This same field is devoid of neurons 96 h after NGF withdrawal. (C) SHP-2 DN neurons have normal, phase-bright cell bodies after 72 h (black arrowheads), but process outgrowth is sparse (compare with A). (D) The neurons have disappeared 96 h after NGF withdrawal. Scale bar, 40 μm.
Our in vivo data show that DN SHP-2 SCG neurons exhibit normal survival, but NGF-dependent survival in vitro is significantly decreased. One explanation for this discrepancy is the presence in vivo of other growth factors, such as NT-3, which is known to mediate survival of a fraction of SCG neurons and to potentiate survival in the presence of limiting NGF concentrations (Farinas, 1999; Belliveau et al., 1997; see also Kobayashi and Matsuoka, 2000). To test this idea, we cultured wt and SHP-2 DN neurons for 24 h in the presence of NGF, NT-3, and BDNF (100 ng/ml each). This cocktail of neurotrophins resulted in near-normal survival of DN SHP-2 neurons (80 ± 8% of wt), suggesting that survival of DN SHP-2 neurons in vivo may result from the presence of multiple neurotrophins with survival activity on these cells. Interestingly, DN SHP-2 neurons cultured in the neurotrophin cocktail were still largely inhibited with respect to neurite extension (data not shown).
SHP-2 Protein Is a Positive Regulator of NGF-Stimulated ERK Activation
Our results, both in vivo and in vitro, are consistent with the view that survival and induction of neurite growth result from distinct pathways, both activated by NGF (see Zhang et al., 2000), and indicate that SHP-2 is a critical component of a neurite outgrowth pathway. NGF and other neurotrophins activate ERKs in neuronal cells (Kaplan and Miller, 2000; Segal and Greenberg, 1996; Watson et al., 2001), and activation of ERKs 1 and 2 (ERK1/2) is thought to be important in the induction of neurite outgrowth (Dimitropoulou and Bixby, 2000; Fukuda et al., 1995; Perron and Bixby, 1999; Schmid et al., 1999). Moreover, for many polypeptide growth factors (FGF, EGF, PDGF, insulin), there is evidence that SHP-2 function is required for ERK1/2 activation (Bennett et al., 1994, 1996; Hadari et al., 1998; Milarski and Saltiel, 1994; Noguchi et al., 1994; Rivard et al., 1995; Tang et al., 1995; Yamauchi et al., 1995; Zhao et al., 1995). Taken together, the data suggest that ERK activation is a part of a SHP-2 mediated neurite outgrowth pathway triggered by NGF. As one test of this idea, we examined whether inhibition of SHP-2 activity, resulting from DN SHP-2, could inhibit ERK1/2 activation by NGF in SCG neurons. When NGF was withdrawn from both wt and DN SHP-2-cultured SCG neurons for 12 h, ERK activation was low, as demonstrated by immunocytochemistry using an antibody specific for phosphorylated (activated) ERK (Fig. 9D). Acute readdition of NGF to cultures of wt neurons led to a sharp increase in phospho-ERK staining, such that intense fluorescence was visible in both cell bodies and neurites (Fig. 9E). When NGF was added to DN SHP-2 neuron cultures, however, the abundance of immunoreactive phospho-ERK was similar to that in control DN cultures with no NGF addition (Fig. 9F). More than 92% of wt SCG neurons exhibited strong phospho-ERK staining upon acute readdition of NGF (n = 183 neurons, 2 experiments), while only 20% of SHP-2 DN neurons exhibited staining levels above those of non-NGF-treated controls (n = 143 neurons, 2 experiments).
Fig. 9.
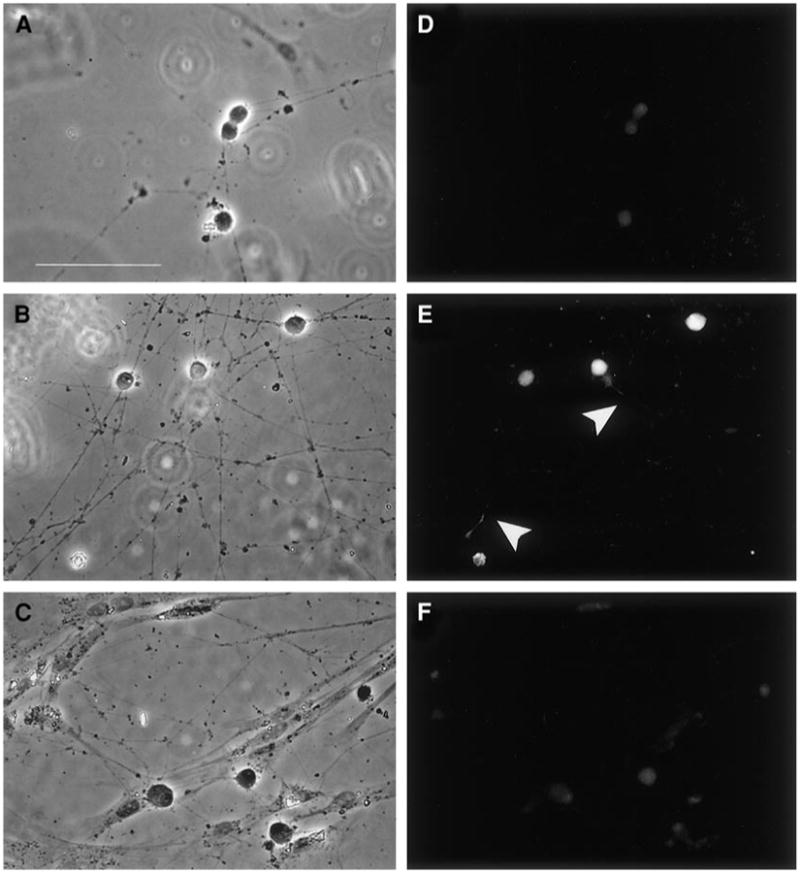
DN SHP-2 inhibits NGF-stimulated ERK activation in sympathetic neurons. Neonatal SCG neurons were cultured in the presence of 100 ng/ml NGF for 6 days, withdrawn from NGF for 12 h, and acutely stimulated by addition of 100 ng/ml NGF 3 h prior to fixation and staining. ERK activation was monitored by immunofluorescence using a phospho-specific antibody to ERK1/2 Left panels (A–C) are phase-contrast microphotographs; (D–F) Corresponding fluorescence images showing phospho-ERK staining. (A, D) Controls in which SHP-2 DN SCG neurons were withdrawn from NGF without acute readdition. (B, E) Wt neurons show strongly stained cell bodies as well as stained processes (white arrowheads). (C, F) Levels of staining in SHP-2 DN neurons after NGF addition are comparable with those seen in controls (compare D and F). Similar results were seen in 3 independent experiments. Scale bar, 100 μm.
To test ERK activation more quantitatively, we measured ERK activation by Western blotting with phospho-ERK antibodies, using total ERK levels as a normalization control. Both wt and OX SHP-2 neurons showed robust induction of ERK phosphorylation 3 h after acute NGF addition, while ERK phosphorylation remained at baseline levels in DN SHP-2 neurons (Fig. 10). Normalized phospho-ERK levels were four-fold higher for wt or for OX SHP-2 neurons after NGF addition, while DN SHP-2 neurons showed phospho-ERK levels indistinguishable from baseline (Table 1). Thus, SHP-2 activity is required in sympathetic neurons for ERK activation by NGF. Our findings in primary neurons are in accord with earlier studies in the PC12 cell line (Wright et al., 1997). Together with our findings that SHP-2 activity is required for NGF-stimulated neurite growth in sympathetic neurons, these data suggest that a neurite outgrowth signaling pathway activated by NGF requires both SHP-2 activity and phosphorylation of ERK1/2. In contrast, survival pathways are less dependent on both SHP-2 activity and ERK1/2 activation.
Fig. 10.
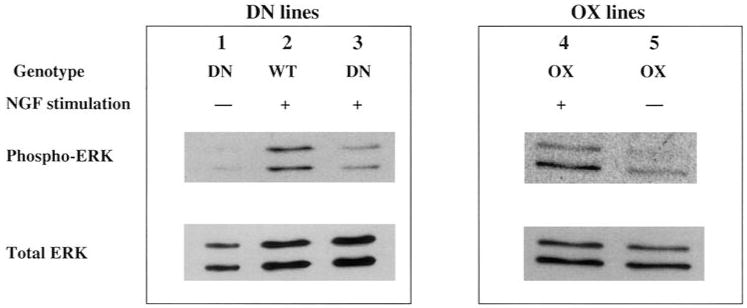
DN SHP-2, but not OX SHP-2, inhibits NGF activation of ERK1/2 in sympathetic neurons. SCG neurons, either wt, DN SHP-2 (DN), or OX SHP-2 (OX), were cultured and stimulated with NGF as described in Fig. 9. Neuronal extracts were subjected to SDS-PAGE and Western blotting. Membranes were probed with a phosphospecific ERK antibody (phospho-ERK), then stripped and reprobed with a pan-ERK1/2 antibody (total ERK) as a normalization control. Robust stimulation of both p44 ERK1 and p42 ERK2 is apparent in the wt and OX lanes after NGF stimulation, while the DN lane is similar to the –NGF control.
TABLE 1. Quantification of Phospho-ERK Activation by NGF.
| Neurons (condition) | pERK/tERK | Fold stimulation |
|---|---|---|
| (−NGF) | 4.8 ± 1.3 | NA |
| wt (+NGF) | 20 ± 3.3 | 4.0 |
| DN SHP-2 (+NGF) | 5 ± 0.8 | 1.0 |
| OX SHP-2 (+NGF) | 21 | 4.3 |
Note. SCG culture and NGF stimulation were performed on cultured neurons as described in Fig. 9. Protein extracts from untreated or NGF-treated cultures were subjected to SDS-PAGE and Western blotting with phospho-ERK antibodies, followed by stripping of the membrane and reprobing with pan-ERK antibodies as a normalization standard. ERK bands were quantified by using NIH Image, and the ratio of phospho-ERK (pERK) to total ERK (tERK) was calculated. N = 3 (−NGF), or 2 (wt, DN SHP-2; +NGF); the OX SHP-2 data are from a single experiment.
Discussion
Using a dominant negative SHP-2 mutant in transgenic mice, we have begun to define pathways in which SHP-2 is normally involved during the development of sympathetic neurons. Inhibition of endogenous SHP-2 function did not alter survival of sympathetic neurons in vivo. However, inhibition of SHP-2 reduced NGF-dependent survival of these neurons in vitro, and SHP-2 function was required in at least two phases of axon growth. In vitro, expression of a DN-SHP2 mutant inhibited NGF-dependent neurite outgrowth and the activation of ERK in NGF-dependent sympathetic neurons, though not the initial sprouting of neurites. In vivo, interference with normal SHP-2 signaling resulted in increased axon density within the targets of sympathetic innervation. These data are consistent with a mechanism in which NGF-induced axon extension and termination within targets requires signaling pathways that include SHP-2, whereas NGF-induced survival is achieved by an intracellular signaling pathway that is less dependent on SHP-2.
Interpretation of our results depends on the degree to which transgene expression achieves specific inhibition of SHP-2 function. Several lines of evidence suggest that the mutation we introduced to mice specifically inhibited endogenous SHP-2 activity. First, the C459S mutant has been shown to inhibit SHP-2 function as a dominant negative in a number of independent systems, in vitro (Bennett et al., 1994, 1996; Milarski and Saltiel, 1994; Noguchi et al., 1994; Rivard et al., 1995; Yamauchi et al., 1995; Zhao et al., 1995), and does not appear to nonspecifically influence other signaling pathways in cultured mammalian cells. (Araki et al., 2000; Hadari et al., 1998). In PC12 cells, an in vitro model of sympathetic neurons, DN SHP-2, but not DN SHP-1 (a closely related SH2-containing PTP) inhibits NGF-induced neuronal differentiation, even though both SHP-1 and SHP-2 are phosphorylated in response to NGF. Importantly, this inhibition is seen with overexpression to a level similar to that reached in our transgenic animals (Wright et al., 1997). In addition, overexpression of DN SHP-2 did not result in reduced viability of sympathetic neurons or discernable defects in vivo, other than those noted, as might be expected if the DN protein interacted nonspecifically with incorrect signaling proteins or pathways. Indeed, because sympathetic neurons in which SHP-2 was overexpressed behaved for the most part like wt neurons, it appears that the abnormal aspects of axon and neurite outgrowth observed in DN SHP-2 neurons were largely a consequence of a reduced activity of the SHP-2 phosphatase domain.
Our results, both in vitro and in vivo, provide evidence that NGF activates at least two independent signaling pathways in sympathetic neurons: a SHP-2-dependent signaling pathway that influences axon outgrowth and a separate survival signaling pathway that is less dependent on SHP-2. The existence of independent NGF-activated signaling pathways is consistent with several previous lines of evidence. Pharmacological experiments with sympathetic neurons in vitro (Greene et al., 1990) suggest that distinct mechanisms underlie the respective abilities of NGF to mediate survival and to promote neurite growth. In PC12 cells, deletion of the conserved juxtamembrane domain of trkA impairs NGF-mediated neurite outgrowth, but not survival (Peng et al., 1995). In addition, trkA receptors on PC12 cells mediate NGF-induced survival through a PI3kinase/Akt module, while internalized receptors regulate NGF-induced differentiation by a PI3 kinase/ Akt-independent mechanism (Zhang et al., 2000). Studies of the signaling mechanisms in sympathetic neurons have suggested that NGF-dependent neurite extension, but not neuron survival, requires the activation of ERKs (Creedon et al., 1996; Virdee and Tolkovsky, 1996). In our in vitro experiments, NGF-dependent ERK1/2 phosphorylation, and NGF-dependent neurite extension were both severely impaired in neurons expressing DN SHP-2. The reduced phosphorylation, and thus reduced activation of ERK suggests that SHP-2 acts upstream of ERK in at least one NGF-activated signaling pathway present in sympathetic neurons. SHP-2 may also be upstream of MEK/ERK activation in a NGF-activated signaling pathway leading to differentiation of PC12 cells (Wright et al., 1997). Thus, we propose that NGF-stimulated neurite extension from sympathetic neurons, in vitro, requires SHP-2-mediated activation of ERK. This hypothesis is in accord with mounting evidence that ERK activation is a critical step in the induction of neurite extension by varying stimuli; these include cell adhesion molecules and RPTPs, as well as soluble growth factors and neurotrophins (Dimitropoulou and Bixby, 2000; Drosopoulos et al., 1999; Perron and Bixby, 1999; Schmid et al., 1999). Sympathetic neuron survival, in contrast, is unaffected by DN SHP-2 expression in vivo, and in vitro is less dependent on SHP-2 function and ERK activation. Therefore, our results suggest that NGF signaling pathways underlying neuron survival diverge from a SHP-2-dependent pathway that mediates axon extension. Although the levels of DN SHP-2 expression we achieved were strongly inhibitory in this and similar studies, it is also possible that the SHP-2-dependent survival pathway(s) requires only low levels of SHP-2 function compared with neurite growth pathways. It is not clear, however, why only a fraction (33%) of DN SHP-2 sympathetic neurons survived in vitro in an NGF-dependent manner. There appear to be multiple NGF-mediated survival pathways in these neurons (Kuruvilla et al., 2000); perhaps only one is SHP-2-dependent.
The ability of NGF to initiate axon extension from sympathetic neurons in vitro was severely compromised by expression of DN SHP-2. Therefore, the seemingly normal initial axon growth from DN SHP-2 sympathetic neurons in vivo would suggest that NGF is not critical for axon extension from sympathetic neurons, in situ. This latter conclusion is consistent with other in vivo observations. NGF is primarily synthesized in the targets of sympathetic innervation, but not until around the time of target innervation (Korsching and Thoenen, 1988), suggesting that axon outgrowth to sympathetic targets is largely NGF-independent. Branching of sympathetic axons, however, occurs mainly within NGF-rich target tissues, rather than during the extension of sympathetic axons from ganglia to target tissues (Rubin, 1985). In vivo, therefore, NGF may control axon branching and termination within targets, but not outgrowth from sympathetic neurons per se.
Because NGF-initiated signals are required for the survival of most wt sympathetic neurons in vivo, information regarding the importance these signals may have during axon extension is difficult to ascertain from the analysis of NGF-, TrkA-, or p75- (low affinity NGF receptor) null mouse mutants. Sympathetic neurons in trkA-deficient mice fail to innervate the submandibular gland, even though they innervate more proximal targets. This could reflect the loss of a NGF-activated process in sympathetic axons growing to distal targets, but may rather reflect the survival-promoting effects of NGF (and possibly NT-3 acting through trkA), such that neurons are not viable long enough to innervate distal targets (Fagan et al., 1996; Francis and Landis, 1999). Indeed, normal extension of axons to target tissues may depend on the activation of trkA receptors by other neurotrophins, such as NT-3, rather than NGF (Belliveau et al., 1997; White, 1998; Francis et al., 1999). NT-3 is more effective at stimulating sympathetic neuron neurite outgrowth than survival (Belliveau et al., 1997). In the case of sensory neurons, analysis of mice lacking both trkA and the proapoptotic protein Bax suggest that trkA activation is required for growth of peripheral axon branches (Patel et al., 2000). We have preliminary evidence that NT-3 (together with BDNF) increased survival of DN SHP-2 neurons in vitro, without a major effect on neurite growth. These results are consistent with the idea that trkA-dependent survival signaling is less dependent on SHP-2 than the neurite growth pathway.
Although other neurotrophins may influence axon growth, innervation within targets by sympathetic neurons is clearly NGF-dependent. Sympathetic neuron innervation densities within target tissues correlate directly with the local concentrations of NGF (Korsching and Thoenen, 1983; Shelton and Reichardt, 1984). Moreover, overproduction of NGF in target tissues leads to increased densities of sympathetic innervation (Albers et al., 1994; Edwards et al., 1989; Hassankhani et al., 1995). NGF also promotes terminal branching of sensory axons (Diamond et al., 1987; Gallo and Letourneau, 1998; Owen et al., 1989). Interestingly, overexpression of NGF in sympathetic neurons in mice leads to hypertrophy of sympathetic ganglia, an increase in the total number of sympathetic axons, but a marked decrease of innervation density in target tissues. The decreased innervation of targets in mice can be overcome by simultaneous overexpression of NGF in target tissues (Hoyle et al., 1993). This was interpreted by the authors to suggest that a gradient of NGF (high levels in targets, low levels in nerve) is required for appropriate innervation of target tissues. NGF expression in nerve fibers would disrupt the gradient, but a gradient could be partially restored by overexpression of NGF in targets. This idea can help explain the results of DN SHP-2 expression in sympathetic neurons. We hypothesize that after reaching the target, neurons grow into the target and branch influenced by an NGF gradient, and presumably also stimulated by growth signals from other neurotrophins (see below). Terminal branches would stop growing when they detect an appropriate level of NGF within the gradient. In DN SHP-2 sympathetic neurons, although appropriate levels of NGF would be “seen,” downstream signaling that elicits axon termination would be greatly inhibited.
In vitro studies suggest that the control of sympathetic axon outgrowth by neurotrophins is complex. Antagonistic interactions between the TrkA and p75 neurotrophin receptors regulate sympathetic neuron axon extension and target innervation (Kohn et al., 1999). In addition, a number of growth factors and neurotrophins induce neurite outgrowth from sympathetic neurons. These include IGFs, insulin, NT-3, and BDNF (Recio-Pinto et al., 1986; Zackenfeld et al., 1995; Belliveau et al., 1997; Botchkarev et al., 1998). Any one or all of these may provide influence to extending axons in vivo. In addition, cellular adhesion molecules, extracellular matrix proteins, and RPTPs are known to influence axon extension from neurons, at least in vitro. Hence, any number of environmental cues may influence axon outgrowth at any one time. In vitro experiments will not satisfactorily mimic all the influences at work on extending axons in vivo. Our results stress that interpretation of in vivo function from in vitro assays is not always straightforward. DN SHP-2 expression inhibited sympathetic neurite growth in vitro, but unexpectedly increased sympathetic innervation levels in vivo.
The mechanism through which DN-SHP2 inhibited NGF-stimulated neurite outgrowth in vitro, yet led to excessive axon growth in sympathetic targets in vivo, is not clear. At least three mechanisms likely contribute to this finding. First, whether a guidance cue such as NGF is attractive or repulsive to growth cones depends on the other signals impinging on this growth cone (Hopker et al., 1999). As noted above, these signals are extraordinarily complex for sympathetic neurons. Second, exposure of the entire neuron to a constant concentration of NGF is very different from exposure of the axon terminals to a gradient of this protein. Recent evidence makes clear, for example, that signals from NGF arising in the terminal differ from those arising in the cell body (Kuruvilla et al., 2000; Watson et al., 2001). Finally, overgrowth of sympathetic fibers in vivo may involve NGF-independent, SHP-2-dependent pathways in addition to the NGF pathway.
In sum, our data indicate that SHP-2 participates in signaling pathways, initiated by NGF, that regulate axon extension and termination of axons within targets of sympathetic innervation. The SHP-2-dependent pathway that mediates neurite outgrowth also leads to activation of ERK1/2, and is distinct from NGF-activated survival signaling pathways in sympathetic neurons. Using an in vivo approach, we have demonstrated that SHP-2 is also required to respond to target-generated factors, presumably including NGF, which provide a “cessation signal” to branching axons extending from sympathetic neurons. Thus, it would appear that SHP-2 is involved in multiple steps in the growth of sympathetic axons. We propose that sympathetic neurons during development use a SHP-2/ERK signaling pathway to interpret their spatial position within NGF gradients. Further experiments will be required to test this model and identify possible interactions with other signaling pathways that modulate the response of sympathetic axons to NGF.
Acknowledgments
We thank Dr. Cong Wang for the cloning and sequencing of the SHP-2 cDNA. This work was supported by grants to J.L.B. from the NIH and the NSF. L.H-O. was supported by an NSF Minority Postdoctoral Fellowship.
References
- Ahmad S, Banville D, Zhao Z, Fischer EH, Shen SH. A widely expressed human protein-tyrosine phosphatase containing src homology 2 domains. Proc Natl Acad Sci USA. 1993;90:2197–2201. doi: 10.1073/pnas.90.6.2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albers KM, Wright DE, Davis BM. Overexpression of nerve growth factor in epidermis of transgenic mice causes hypertrophy of the peripheral nervous system. J Neurosci. 1994;14:1422–1432. doi: 10.1523/JNEUROSCI.14-03-01422.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araki T, Yamada M, Ohnishi H, Sano S, Uetsuki T, Hatanaka H. Shp-2 specifically regulates several tyrosine-phosphorylated proteins in brain-derived neurotrophic factor signaling in cultured cerebral cortical neurons. J Neurochem. 2000;74:659–668. doi: 10.1046/j.1471-4159.2000.740659.x. [DOI] [PubMed] [Google Scholar]
- Belliveau DJ, Krivko I, Kohn J, Lachance C, Pozniak C, Rusakov D, Kaplan D, Miller FD. NGF and neurotrophin-3 both activate TrkA on sympathetic neurons but differentially regulate survival and neuritogenesis. J Cell Biol. 1997;136:375–388. doi: 10.1083/jcb.136.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett AM, Hausdorff SF, O'Reilly AM, Freeman RM, Neel BG. Multiple requirements for SHPTP2 in epidermal growth factor-mediated cell cycle progression. Mol Cell Biol. 1996;16:1189–1202. doi: 10.1128/mcb.16.3.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett AM, Tang TL, Sugimoto S, Walsh CT, Neel BG. Protein-tyrosine-phosphatase SHPTP2 couples platelet-derived growth factor receptor beta to Ras. Proc Natl Acad Sci USA. 1994;91:7335–7339. doi: 10.1073/pnas.91.15.7335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bixby JL. Receptor tyrosine phosphatases in axon growth and guidance. Neuroreport. 2000;11:R5–R10. doi: 10.1097/00001756-200007140-00001. [DOI] [PubMed] [Google Scholar]
- Brinster RL, Chen HY, Trumbauer M, Senear AW, Warren R, Palmiter RD. Somatic expression of herpes thymidine kinase in mice following injection of a fusion gene into eggs. Cell. 1981;27:223–231. doi: 10.1016/0092-8674(81)90376-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campenot RB. Local control of neurite development by nerve growth factor. Proc Natl Acad Sci USA. 1977;74:4516–4519. doi: 10.1073/pnas.74.10.4516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chisholm A, Tessier-Lavigne M. Conservation and divergence of axon guidance mechanisms. Curr Opin Neurobiol. 1999;9:603–615. doi: 10.1016/S0959-4388(99)00021-5. [DOI] [PubMed] [Google Scholar]
- Coughlin MD, Boyer DM, Black IB. Embryologic development of a mouse sympathetic ganglion in vivo and in vitro. Proc Natl Acad Sci USA. 1977;74:3438–3442. doi: 10.1073/pnas.74.8.3438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coughlin MD, Collins MB. Nerve growth factor-independent development of embryonic mouse sympathetic neurons in dissociated cell culture. Dev Biol. 1985;110:392–401. doi: 10.1016/0012-1606(85)90098-3. [DOI] [PubMed] [Google Scholar]
- Cowley S, Paterson H, Kemp P, Marshall CJ. Activation of MAP kinase kinase is necessary and sufficient for PC12 cell differentiation and for transformation of NIH 3T3 cells. Cell. 1994;77:841–852. doi: 10.1016/0092-8674(94)90133-3. [DOI] [PubMed] [Google Scholar]
- Creedon DJ, Johnson EM, Lawrence JC. Mitogen-activated protein kinase-independent pathways mediate the effects of nerve growth factor and cAMP on neuronal survival. J Biol Chem. 1996;271:20713–20718. doi: 10.1074/jbc.271.34.20713. [DOI] [PubMed] [Google Scholar]
- De la Torre JC. An improved approach to histofluorescence using the SPG method for tissue monoamines. J Neurosci Methods. 1980;3:1–5. doi: 10.1016/0165-0270(80)90029-1. [DOI] [PubMed] [Google Scholar]
- Desai CJ, Sun Q, Zinn K. Tyrosine phosphorylation and axon guidance: Of mice and flies. Curr Opin Neurobiol. 1997;7:70–74. doi: 10.1016/s0959-4388(97)80122-5. [DOI] [PubMed] [Google Scholar]
- Diamond J, Coughlin M, Macintyre L, Holmes M, Visheau B. Evidence that endogenous beta nerve growth factor is responsible for the collateral sprouting, but not the regeneration, of nociceptive axons in adult rats. Proc Natl Acad Sci USA. 1987;84:6596–6600. doi: 10.1073/pnas.84.18.6596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitropoulou A, Bixby JL. Regulation of retinal neurite growth by alterations in MAPK/ERK kinase (MEK) activity. Brain Res. 2000;858:205–214. doi: 10.1016/s0006-8993(00)01946-6. [DOI] [PubMed] [Google Scholar]
- Drosopoulos NE, Walsh FS, Doherty P. A soluble version of the receptor-like protein tyrosine phosphatase kappa stimulates neurite outgrowth via a Grb2/MEK1-dependent signaling cascade. Mol Cell Neurosci. 1999;13:441–449. doi: 10.1006/mcne.1999.0758. [DOI] [PubMed] [Google Scholar]
- Edwards RH, Rutter WJ, Hanahan D. Directed expression of NGF to pancreatic beta cells in transgenic mice leads to selective hyperinnervation of the islets. Cell. 1989;58:161–170. doi: 10.1016/0092-8674(89)90412-1. [DOI] [PubMed] [Google Scholar]
- ElShamy WM, Linnarsson S, Lee KF, Jaenisch R, Ernfors P. Prenatal and postnatal requirements of NT-3 for sympathetic neuroblast survival and innervation of specific targets. Development. 1996;122:491–500. doi: 10.1242/dev.122.2.491. [DOI] [PubMed] [Google Scholar]
- Fagan AM, Zhang H, Landis S, Smeyne RJ, Silos-Santiago I, Barbacid M. TrkA, but not TrkC, receptors are essential for survival of sympathetic neurons in vivo. J Neurosci. 1996;16:6208–6218. doi: 10.1523/JNEUROSCI.16-19-06208.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farinas I. Neurotrophin actions during the development the peripheral nervous system. Microsc Res Tech. 1999;45:233–242. doi: 10.1002/(SICI)1097-0029(19990515/01)45:4/5<233::AID-JEMT7>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Feng GS. Shp-2 tyrosine phosphatase: signaling one cell or many. Exp Cell Res. 1999;253:47–54. doi: 10.1006/excr.1999.4668. [DOI] [PubMed] [Google Scholar]
- Feng GS, Hui CC, Pawson T. SH2-containing phosphotyrosine phosphatase as a target of protein-tyrosine kinases. Science. 1993;259:1607–1611. doi: 10.1126/science.8096088. [DOI] [PubMed] [Google Scholar]
- Francis N, Landis S. Cellular and molecular determinants of sympathetic neuron development. Annu Rev Neurosci. 1999;22:541–566. doi: 10.1146/annurev.neuro.22.1.541. [DOI] [PubMed] [Google Scholar]
- Francis N, Farinas I, Brennan C, Rivas-Plata K, Backus C, Reichardt L, Landis S. NT-3, like NGF, is required for survival of sympathetic neurons, but not their precursors. Dev Biol. 1999;210:411–427. doi: 10.1006/dbio.1999.9269. [DOI] [PubMed] [Google Scholar]
- Freeman RM, Jr, Plutzky J, Neel BG. Identification of a human src homology 2-containing protein-tyrosine-phosphatase: A putative homolog of Drosophila corkscrew. Proc Natl Acad Sci USA. 1992;89:11239–11243. doi: 10.1073/pnas.89.23.11239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda M, Gotoh Y, Tachibana T, Dell K, Hattori S, Yoneda Y, Nishida E. Induction of neurite outgrowth by MAP kinase in PC12 cells. Oncogene. 1995;11:239–244. [PubMed] [Google Scholar]
- Gallo G, Letourneau PC. Localized sources of neurotrophins initiate axon collateral sprouting. J Neurosci. 1998;18:5403–5414. doi: 10.1523/JNEUROSCI.18-14-05403.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene LA, Tischler AS. Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor. Proc Natl Acad Sci USA. 1976;73:2424–2428. doi: 10.1073/pnas.73.7.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene LA, Volonte C, Chalazonitis A. Purine analogs inhibit nerve growth factor-promoted neurite outgrowth by sympathetic and sensory neurons. J Neurosci. 1990;10:1479–1485. doi: 10.1523/JNEUROSCI.10-05-01479.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan KL, Dixon JE. Evidence for protein-tyrosine-phosphatase catalysis proceeding via a cysteine-phosphate intermediate. J Biol Chem. 1991;266:17026–17030. [PubMed] [Google Scholar]
- Hadari YR, Kouhara H, Lax I, Schlessinger J. Binding of Shp2 tyrosine phosphatase to FRS2 is essential for fibroblast growth factor-induced PC12 cell differentiation. Mol Cell Biol. 1998;18:3966–3973. doi: 10.1128/mcb.18.7.3966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassankhani A, Steinhelper ME, Soonpaa MH, Katz EB, Taylor DA, Andrade-Rozental A, Factor SM, Steinberg JJ, Field LJ, Federoff HJ. Overexpression of NGF within the heart of transgenic mice causes hyperinnervation, cardiac enlargement, and hyperplasia of ectopic cells. Dev Biol. 1995;169:309–321. doi: 10.1006/dbio.1995.1146. [DOI] [PubMed] [Google Scholar]
- Hayashi M, Edgar D, Thoenen H. Nerve growth factor changes the relative levels of neuropeptides in developing sensory and sympathetic ganglia of the chick embryo. Dev Biol. 1985;108:49–55. doi: 10.1016/0012-1606(85)90008-9. [DOI] [PubMed] [Google Scholar]
- Henderson CE. Role of neurotrophic factors in neuronal development. Curr Opin Neurobiol. 1996;6:64–70. doi: 10.1016/s0959-4388(96)80010-9. [DOI] [PubMed] [Google Scholar]
- Hendry IA. Cell division in the developing sympathetic nervous system. J Neurocytol. 1977;6:299–309. doi: 10.1007/BF01175193. [DOI] [PubMed] [Google Scholar]
- Holland SJ, Peles E, Pawson T, Schlessinger J. Cell-contact-dependent signalling in axon growth and guidance: Eph receptor tyrosine kinases and receptor protein tyrosine phosphatase beta. Curr Opin Neurobiol. 1998;8:117–127. doi: 10.1016/s0959-4388(98)80015-9. [DOI] [PubMed] [Google Scholar]
- Hopker VH, Shewan D, Tessier-Lavigne M, Poo M, Holt C. Growth cone attraction to netrin-1 is converted to repulsion by laminin-1. Nature. 1999;401:69–73. doi: 10.1038/43441. [DOI] [PubMed] [Google Scholar]
- Hoyle GW, Mercer EH, Palmiter RD, Brinster RL. Expression of NGF in sympathetic neurons leads to excessive axon outgrowth from ganglia but decreased terminal innervation within tissues. Neuron. 1993;10:1019–1034. doi: 10.1016/0896-6273(93)90051-r. [DOI] [PubMed] [Google Scholar]
- Kaplan DR, Miller FD. Neurotrophin signal transduction in the nervous system. Curr Opin Neurobiol. 2000;10:381–391. doi: 10.1016/s0959-4388(00)00092-1. [DOI] [PubMed] [Google Scholar]
- Kapur RP, Hoyle GW, Mercer EH, Brinster RL, Palmiter RD. Some neuronal cell populations express human dopamine beta-hydroxylase-lacZ transgenes transiently during embryonic development. Neuron. 1991;7:717–727. doi: 10.1016/0896-6273(91)90275-5. [DOI] [PubMed] [Google Scholar]
- Karasek M. Ultrastructure of the mammalian pineal gland: Its comparative and functional aspects. In: Reiter RJ, editor. Pineal Research Reviews. Vol. 1. Alan R. Liss; New York: 1983. pp. 1–48. [Google Scholar]
- Kobayashi M, Matsuoka I. Enhancement of sympathetic neuron survival by synergistic action of NT3 and GDNF. Neuroreport. 2000;11:2541–2545. doi: 10.1097/00001756-200008030-00039. [DOI] [PubMed] [Google Scholar]
- Kohn J, Aloyz RS, Toma JG, Haak-Frendscho M, Miller FD. Functionally antagonistic interactions between the TrkA and p75 neurotrophin receptors regulate sympathetic neuron growth and target innervation. J Neurosci. 1999;19:5393–5408. doi: 10.1523/JNEUROSCI.19-13-05393.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korsching S, Thoenen H. Nerve growth factor in sympathetic ganglia and corresponding target organs of the rat: Correlation with density of sympathetic innervation. Proc Natl Acad Sci USA. 1983;80:3513–3516. doi: 10.1073/pnas.80.11.3513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korsching S, Thoenen H. Developmental changes of nerve growth factor levels in sympathetic ganglia and their target organs. Dev Biol. 1988;126:40–46. doi: 10.1016/0012-1606(88)90236-9. [DOI] [PubMed] [Google Scholar]
- Kuruvilla R, Ye H, Ginty DD. Spacially and functionally distinct roles of the PI3-K effector pathway during NGF signaling in sympathetic neurons. Neuron. 2000;27:499–512. doi: 10.1016/s0896-6273(00)00061-1. [DOI] [PubMed] [Google Scholar]
- Levi-Montalcini R. The nerve growth factor: Its role in growth, differentiation and function of the sympathetic adrenergic neuron. Prog Brain Res. 1976;45:235–258. doi: 10.1016/S0079-6123(08)60993-0. [DOI] [PubMed] [Google Scholar]
- Lo DC. Neurotrophic factors and synaptic plasticity. Neuron. 1995;15:979–981. doi: 10.1016/0896-6273(95)90085-3. [DOI] [PubMed] [Google Scholar]
- Mercer EH, Hoyle GW, Kapur RP, Brinster RL, Palmiter RD. The dopamine beta-hydroxylase gene promoter directs expression of E. coli lacZ to sympathetic and other neurons in adult transgenic mice. Neuron. 1991;7:703–716. doi: 10.1016/0896-6273(91)90274-4. [DOI] [PubMed] [Google Scholar]
- Milarski KL, Saltiel AR. Expression of catalytically inactive Syp phosphatase in 3T3 cells blocks stimulation of mitogen-activated protein kinase by insulin. J Biol Chem. 1994;269:21239–21243. [PubMed] [Google Scholar]
- Mizel SB, Bamburg JR. Studies on the action of nerve growth factor, I. Characterization of a simplified in vitro culture system for dorsal root and sympathetic ganglia. Dev Biol. 1976;49:11–19. doi: 10.1016/0012-1606(76)90254-2. [DOI] [PubMed] [Google Scholar]
- Noguchi T, Matozaki T, Horita K, Fujioka Y, Kasuga M. Role of SH-PTP2, a protein-tyrosine phosphatase with Src homology 2 domains, in insulin-stimulated Ras activation. Mol Cell Biol. 1994;14:6674–6682. doi: 10.1128/mcb.14.10.6674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen DJ, Logan A, Robinson PP. A role for nerve growth factor in collateral reinnervation from sensory nerves in the guinea pig. Brain Res. 1989;476:248–255. doi: 10.1016/0006-8993(89)91245-6. [DOI] [PubMed] [Google Scholar]
- Palmiter RD, Sandgren EP, Avarbock MR, Allen DD, Brinster RL. Heterologous introns can enhance expression of transgenes in mice. Proc Natl Acad Sci USA. 1991;88:478–482. doi: 10.1073/pnas.88.2.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel TD, Jackman A, Rice FL, Kucera J, Snider WD. Development of sensory neurons in the absence of NGF/ TrkA signaling in vivo. Neuron. 2000;25:345–357. doi: 10.1016/s0896-6273(00)80899-5. [DOI] [PubMed] [Google Scholar]
- Paves H, Saarma M. Neurotrophins as in vitro growth cone guidance molecules for embryonic sensory neurons. Cell Tissue Res. 1997;290:285–297. doi: 10.1007/s004410050933. [DOI] [PubMed] [Google Scholar]
- Peng X, Greene LA, Kaplan DR, Stephens RM. Deletion of a conserved juxtamembrane sequence in Trk abolishes NGF-promoted neuritogenesis. Neuron. 1995;15:395–406. doi: 10.1016/0896-6273(95)90043-8. [DOI] [PubMed] [Google Scholar]
- Perron JC, Bixby JL. Distinct neurite outgrowth signaling pathways converge on ERK activation. Mol Cell Neurosci. 1999;13:362–378. doi: 10.1006/mcne.1999.0753. [DOI] [PubMed] [Google Scholar]
- Pluskey S, Wandless TJ, Walsh CT, Shoelson SE. Potent stimulation of SH-PTP2 phosphatase activity by simultaneous occupancy of both SH2 domains. J Biol Chem. 1995;270:2897–2900. doi: 10.1074/jbc.270.7.2897. [DOI] [PubMed] [Google Scholar]
- Rivard N, McKenzie FR, Brondello JM, Pouyssegur J. The phosphotyrosine phosphatase PTP1D, but not PTP1C, is an essential mediator of fibroblast proliferation induced by tyrosine kinase and G protein-coupled receptors. J Biol Chem. 1995;270:11017–11024. doi: 10.1074/jbc.270.18.11017. [DOI] [PubMed] [Google Scholar]
- Rubin E. Development of the rat superior cervical ganglion: Ganglion cell maturation. J Neurosci. 1985;5:673–684. doi: 10.1523/JNEUROSCI.05-03-00673.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxton TM, Henkemeyer M, Gasca S, Shen R, Rossi DJ, Shalaby F, Feng GS, Pawson T. Abnormal mesoderm patterning in mouse embryos mutant for the SH2 tyrosine phosphatase Shp-2. EMBO J. 1997;16:2352–2364. doi: 10.1093/emboj/16.9.2352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid RS, Graff RD, Schaller MD, Chen S, Schachner M, Hemperly JJ, Maness PF. NCAM stimulates the Ras-MAPK pathway and CREB phosphorylation in neuronal cells. J Neurobiol. 1999;38:542–558. [PubMed] [Google Scholar]
- Segal RA, Greenberg ME. Intracellular signaling pathways activated by neurotrophic factors. Annu Rev Neurosci. 1996;19:463–489. doi: 10.1146/annurev.ne.19.030196.002335. [DOI] [PubMed] [Google Scholar]
- Shelton DL, Reichardt LF. Expression of the beta-nerve growth factor gene correlates with the density of sympathetic innervation in effector organs. Proc Natl Acad Sci USA. 1984;81:7951–7955. doi: 10.1073/pnas.81.24.7951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snider WD. Functions of the neurotrophins during nervous system development: What the knockouts are teaching us. Cell. 1994;77:627–638. doi: 10.1016/0092-8674(94)90048-5. [DOI] [PubMed] [Google Scholar]
- Sofroniew MV, Howe CL, Mobley WC. Nerve growth factor signaling, neuroprotection, and neural repair. Annu Rev Neurosci. 2001;24:1217–1281. doi: 10.1146/annurev.neuro.24.1.1217. [DOI] [PubMed] [Google Scholar]
- Tang TL, Freeman RM, Jr, O'Reilly AM, Neel BG, Sokol SY. The SH2-containing protein-tyrosine phosphatase SH-PTP2 is required upstream of MAP kinase for early Xenopus development. Cell. 1995;80:473–483. doi: 10.1016/0092-8674(95)90498-0. [DOI] [PubMed] [Google Scholar]
- Thoenen H, Angeletti PU, Levi-Montalcini R, Kettler R. Selective induction by nerve growth factor of tyrosine hydroxylase and dopamine- -hydroxylase in the rat superior cervical ganglia. Proc Natl Acad Sci USA. 1971;68:1598–1602. doi: 10.1073/pnas.68.7.1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker KL, Meyer M, Barde YA. Neurotrophins are required for nerve growth during development. Nat Neurosci. 2001;4:29–37. doi: 10.1038/82868. [DOI] [PubMed] [Google Scholar]
- Virdee K, Tolkovsky AM. Inhibition of p42 and p44 mitogen-activated protein kinase activity by PD98059 does not suppress nerve growth factor-induced survival of sympathetic neurones. J Neurochem. 1996;67:1801–1805. doi: 10.1046/j.1471-4159.1996.67051801.x. [DOI] [PubMed] [Google Scholar]
- Vogel W, Lammers R, Huang J, Ullrich A. Activation of a phosphotyrosine phosphatase by tyrosine phosphorylation. Science. 1993;259:1611–1614. doi: 10.1126/science.7681217. [DOI] [PubMed] [Google Scholar]
- Watson FL, Heerssen HM, Bhattacharyya A, Klesse L, Lin MZ, Segal RA. Neurotrophins use the ERK5 pathway to mediate a retrograde survival response. Nat Neurosci. 2001;4:981–988. doi: 10.1038/nn720. [DOI] [PubMed] [Google Scholar]
- White DM. Contribution of neurotrophin-3 to the neuropeptide Y-induced increase in neurite outgrowth of rat dorsal root ganglion cells. Neuroscience. 1998;86:257–263. doi: 10.1016/s0306-4522(98)00034-7. [DOI] [PubMed] [Google Scholar]
- Wright JH, Drueckes P, Bartoe J, Zhao Z, Shen SH, Krebs EG. A role for the SHP-2 tyrosine phosphatase in nerve growth-induced PC12 cell differentiation. Mol Biol Cell. 1997;8:1575–1585. doi: 10.1091/mbc.8.8.1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamauchi K, Milarski KL, Saltiel AR, Pessin JE. Protein-tyrosine-phosphatase SHPTP2 is a required positive effector for insulin downstream signaling. Proc Natl Acad Sci USA. 1995;92:664–668. doi: 10.1073/pnas.92.3.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Moheban DB, Conway BR, Bhattacharyya A, Segal RA. Cell surface Trk receptors mediate NGF-induced survival while internalized receptors regulate NGF-induced differentiation. J Neurosci. 2000;20:5671–5678. doi: 10.1523/JNEUROSCI.20-15-05671.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z, Tan Z, Wright JH, Diltz CD, Shen SH, Krebs EG, Fischer EH. Altered expression of protein-tyrosine phosphatase 2C in 293 cells affects protein tyrosine phosphorylation and mitogen-activated protein kinase activation. J Biol Chem. 1995;270:11765–11769. doi: 10.1074/jbc.270.20.11765. [DOI] [PubMed] [Google Scholar]


