Abstract
We have developed a cell culture of guinea pig gallbladder epithelial cells with which to study ion transport. When grown on permeable supports, the cultured epithelia developed a transepithelial resistance (Rt) of ∼500 Ω·cm2. The epithelial cell origin of the cell culture was further confirmed by immunocytochemical localization of cytokeratin. Ionomycin and forskolin increased transepithelial voltage and short-circuit current (Isc) and decreased Rt. The response to ionomycin was transient, whereas that to forskolin was sustained. Both were attenuated by replacement of Cl− and/or . Mucosal addition of the anion transport inhibitors DIDS or diphenylamine-2-carboxylic acid (DPC) blocked the response to ionomycin. The response to forskolin was blocked by DPC but not by DIDS. Ionomycin, but not forskolin, increased intracellular Ca2+ concentration in fura 2-loaded cells. PGE2, histamine, vasoactive intestinal polypeptide, and secretin elicited a sustained increase in Isc. Responses to ATP and CCK were transient. Thus cultured guinea pig gallbladder epithelia display the range of responses observed in the native tissue and are an appropriate model for studies of ion transport in gallbladder and intestinal epithelia.
Keywords: cultured cells; histamine; ATP; vasoactive intestinal polypeptide; prostaglandin E2; secretin; cholecystokinin; anion secretion; diphenylamine-2-carboxylic acid; 4,4′-diisothiocyanostilbene-2,2′-disulfonic acid; ionomycin; intracellular Ca2+; forskolin; cAMP
Cell Culture Systems Provide a homogeneous population of cells that can be grown and studied under defined conditions and manipulated to express foreign proteins. As such, the availability of tissue culture cells has enhanced the ability of investigators to study a wide range of biological and biochemical processes. In gastrointestinal epithelia, cell cultures have been used to study ion transport processes and their regulation by second-messenger pathways. Many of the cell culture systems widely used to study intestinal ion transport, such as T84 (15), Caco-2 (20, 21), and HT-29 (63), are derived from colonic carcinomas. Although these cells display many of the transport characteristics of the normal colonic tissue type from which they were derived, some investigators have suggested caution in their use because of their tumor origin (17).
Obtaining established cell lines or primary cultures of cells derived from the small intestine that display the transport characteristics of more proximal regions of the intestine has been more problematic. For example, the established non-tumor-derived cell line IEC-6 forms a confluent monolayer but maintains many characteristics of poorly differentiated immature cells (45). The development of transepithelial electric parameters typical of epithelial tissues has not been demonstrated for IEC-6 or the closely related cell line IEC-18 (32, 45).
The gallbladder is an absorptive epithelium that elaborates and stores concentrated bile subsequent to isosmotic absorption of Na+ and Cl−. The gallbladder shares many transport characteristics with those observed in the small intestine (5, 49). Both the small intestine and gallbladder are “leaky” epithelia, having a low transepithelial resistance (Rt) and voltage (Vt). Na+ and Cl− absorption involves either a neutral Na-Cl carrier and/or dual exchange proteins for Na+/H+ and operating in parallel (11, 13, 19, 27, 36). Agents that elevate intracellular cAMP [e.g., theophylline, prostaglandin, and possibly vasoactive intestinal polypeptide (VIP) and secretin] and in some cases intracellular Ca2+ (e.g., ionomycin and ATP) stimulate anion secretion (8, 41–43, 50).
The goal of the present study was to develop a primary culture of guinea pig gallbladder epithelial cells to facilitate studies of the regulation of ion transport by this tissue. Other groups have developed primary cultures of gallbladder epithelia from several species (6, 8, 28, 29, 31, 44), including guinea pig (39). However, to our knowledge there are no previous reports of a systematic study in which these cell cultures have been shown to both develop transepithelial electric parameters and respond to hormones characteristic of the native tissue. The results of this study demonstrate that we have successfully developed a primary culture of guinea pig gallbladder cells that can be used for the systematic investigation of the effects of secretagogues on ion transport in gallbladder epithelia. Given the similarities between the transport processes of leaky epithelia such as gallbladder and small intestine, this cell culture may serve as a useful model with which to study transport processes common to these epithelial cell types.
Materials and Methods
Preparation of guinea pig gallbladder epithelial cell cultures
Excised gallbladders from male Hartley guinea pigs (200–300 g, Harlan Sprague-Dawley, Indianapolis, IN) were stripped of underlying muscle and minced. The tissue was incubated in an isolation solution containing 0.005% pronase and 0.1% BSA at 37°C, and the solution was triturated at intervals to facilitate cell isolation. After 1 h, the isolated cells were decanted and washed in three changes of sterile DMEM-Ham's F-12 media containing 20% FBS (Life Technologies, Rockville, MD), ITS media supplement (5 μg/ml insulin, 5 μg/ml transferrin, 5 ng/ml sodium selenium), 0.05 mg/ml hydrocortisone, 2 mM glutamine (Fisher Scientific, Atlanta, GA), and 0.1% penicillin-streptomycin (Life Technologies) (47). Cell viability following isolation as assessed by trypan blue exclusion was >95%. Before seeding on glass coverslips or permeable Transwell supports (Costar, Cambridge, MA), the isolated cells were briefly placed in plastic culture flasks to decrease fibroblast contamination. Transwells (0.78 cm2; Costar 3407) were coated with the basement membrane extract Matrigel (Becton Dickinson Labware, Bedford, MA) before seeding. Cells isolated from the gallbladders of two guinea pigs were pooled and yielded a sufficient number of cells to seed 18 Transwells at a density of 3.0 × 105 cells/Transwell in 200 μl of supplemented DMEM-Ham's F-12 medium.
Immunocytochemistry
Cells grown on glass coverslips were fixed and permeabilized with ice-cold methanol. Cells were first incubated with a primary antibody that recognizes cytokeratin (mouse anti-keratin monoclonal AE1/AE3; Boehringer Mannheim, Indianapolis, IN). The secondary antibody (anti-mouse IgG, Fab-specific TRITC conjugate) was then applied. Cells to which only the secondary antibody was applied without prior addition of primary antibody served as controls. Fluorescence localization of cytokeratin was observed using a Molecular Dynamics Multiprobe 2001 inverted confocal laser microscope with the appropriate setting for Cy3 fluorescence. Laser intensity was adjusted to the lowest attenuation that permitted a strong specific signal and minimal background without significant photobleaching of the dye. The images were digitized, stored, and analyzed using a Silicon Graphics Iris Indigo workstation and printed using a Mitsubishi color video copy processor.
Transepithelial electric parameters of cultured monolayers
Gallbladder epithelial cell cultures grown on Transwells were mounted into Lucite Ussing chambers (Costar) and bathed by a physiological Ringer solution. The Vt and short-circuit current (Isc) were monitored by means of an automatic voltage clamp device (Model VCC-600; Physiologic Instruments, San Diego, CA) in contact with the bathing solutions via Ag-AgCl electrodes inserted into KCl- or Ringer-agar bridges. Experiments were conducted under open-circuit [transepithelial current (It) = 0] or short-circuit conditions (Vt = 0) except for brief periods during which Vt or It was clamped to ±5 mV or 5 μA, respectively. Under both conditions, reported values of Vt and Isc are measured rather than calculated values. Rt was calculated from deflections in Vt produced by bipolar Vt/It pulses using the relation Rt = ΔVt/ΔIt. Secretagogues were added directly to both half-chambers from stock solutions to give appropriate final concentrations. Anion transport inhibitors were added to the apical/mucosal bathing solution. When secretagogues or inhibitors were dissolved in DMSO or ethanol, an equal volume of solvent was added to control cultures. Cultures were equilibrated in the chamber for 20–30 min before the addition of secretagogues or inhibitors. Normal Ringer solution contained (in mM) 140 Na+, 5 K+, 25 , 1 Ca2+, 1 Mg2+, and 124 Cl−. -free solutions contained (in mM) 140 Na+, 5 K+, 1 Ca 2+, 1 Mg 2+, 124 Cl−, and 10 HEPES. For low-Cl− solutions, gluconate replaced Cl− to a final Cl− concentration of 4 mM. -containing solutions were gassed with 95% O2-5% CO2. -free solutions were gassed with 100% O2. Experiments were conducted at 37°C (pH = 7.4).
Measurement of intracellular Ca2+
Cultured gallbladder cells plated on coverslips were incubated with fura 2-AM (Molecular Probes, Eugene, OR) for 30 min at 37°C. In some cases, the loading solution contained 5 mM Probenecid to enhance dye uptake. The cells were washed once with HEPES-based Ringer and placed on the stage of a digital-imaging microscope. The dual-wavelength ratiometric technique (23, 55) was used to measure the intracellular Ca2+ concentration ([Ca2+]i). Cellular images were acquired on a Zeiss IM-35 microscope (Carl Zeiss Instruments, Norcross, GA) using a Nikon 40× objective (Southern Micro Instruments, Atlanta, GA) at excitation wavelengths of 340 and 380 nm and an emission wavelength of 510 nm. The images were collected with a Dage ISIT 66 video camera (Dage, Michigan City, IN) and analyzed using Inovison IC300 software (Inovision, Research Triangle, NC) running on a SPARCstation2 workstation (Sun Microsystems, Mountain View, CA). Digitized images were corrected for background fluorescence. The dye within the guinea pig gallbladder cells appeared to be fully Ca2+ sensitive. Thus [Ca2+]i was calculated by extrapolation from a standard curve that had been generated from the measurement of the fluorescent ratio of aliquots of fura 2-free acid over a range of Ca2+ concentrations. Fluorescent ratios of the aliquots were acquired at the same gain setting on the camera that was used for the acquisition of cellular fluorescent ratios.
Reagents
All were purchased from Sigma-Aldrich Chemical (St. Louis, MO) unless otherwise specified.
Statistics
Results are given as means ± SE. The statistical significance of differences was determined from paired or unpaired Student's t-test or ANOVA as appropriate. Values of P < 0.05 were considered statistically significant. In all cases, n represents the number of experiments.
Results
When cultured under appropriate conditions, isolated guinea pig gallbladder epithelial cells developed a confluent monolayer. Contamination by nonepithelial cells was minimized by mechanically stripping the mucosal layer of underlying musculature and preplating cells on plastic to remove fibroblasts. Cell proliferation required the presence of serum in the culture media because cells cultured in a similar defined serum-free medium supplemented with epidermal growth factor remained viable for several days but failed to proliferate. With the inclusion of 20% FBS in the culture medium cell, nidi (5–10 cells) appeared in 1 day that subsequently became confluent in 4–5 days.
The epithelial origin of the resulting cell culture was confirmed from the localization of the epithelial cell marker cytokeratin in the cultured cells by immunocytochemistry using a fluorescently labeled antibody and confocal microscopy. Figure 1 shows the filamentous pattern of intracellular cytokeratin typical of epithelial cells. No fluorescence was observed in control cells, to which only the secondary antibody was applied (data not shown).
Fig. 1.
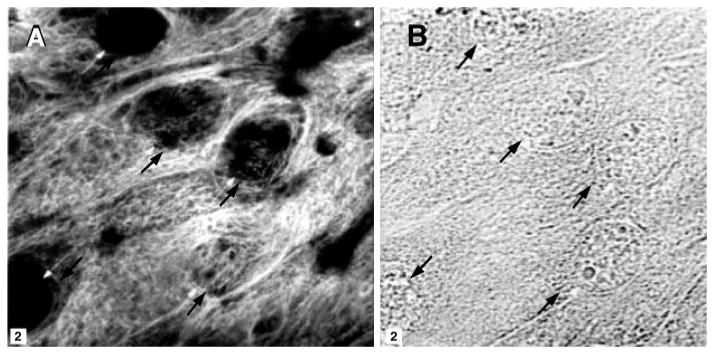
Localization of cytokeratin in cultured guinea pig gallbladder cells. A: fluorescence image. B: corresponding bright-field image. The box marked on each image is 2 μm2. The arrows mark the locations of individual nuclei for the alignment of the 2 images.
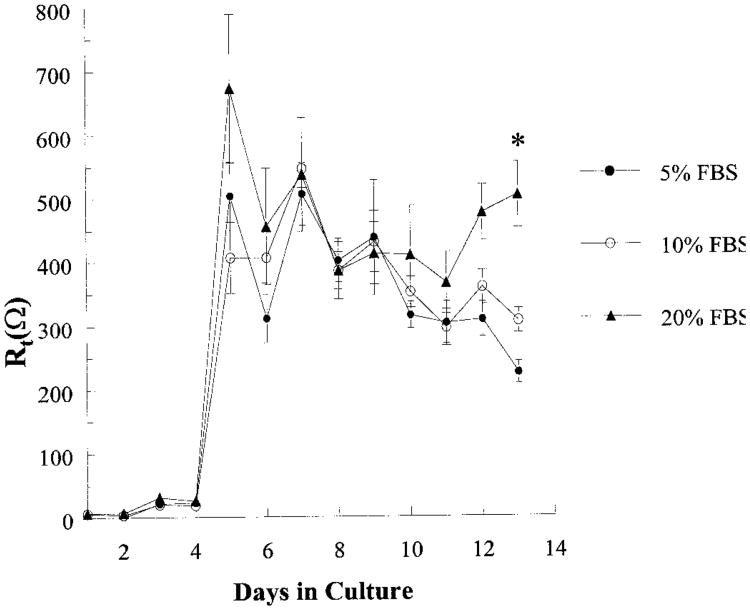
To evaluate functional similarities between cultured epithelia and the native tissue, cells were grown on Transwells for assessment of transepithelial electric characteristics. Rt increased with time in culture, reaching a maximal resistance of 650 ± 99 Ω in 4 days, corresponding to the time of confluence (Fig. 2). Given a Transwell surface area of 0.78 cm2, this corresponds to a Rt of ∼500 Ω·cm2, a value higher than that reported for the native tissue (24, 27). Rt remained stable up to day 14 and then declined. The time course of changes in Rt was also used to evaluate the effect of serum concentration on proliferation and confluence. Although there was no effect of serum concentration in the range of 5–20% FBS on the time at which the cultures reached the maximal Rt, Rt was significantly higher at day 14 for cells grown in 20 vs. 5% FBS (P < 0.05).
Fig. 2.
Development of transepithelial resistance (Rt) for guinea pig gallbladder epithelial cells grown in 5, 10, or 20% FBS with respect to days in culture. Cells from the same preparation were cultured in triplicate either in 5, 10, or 20% FBS, and the Rt was measured daily for 14 days. Each point represents the mean ± SE of 4 experiments (separate preparations). *Significant difference between 5 and 20% FBS.
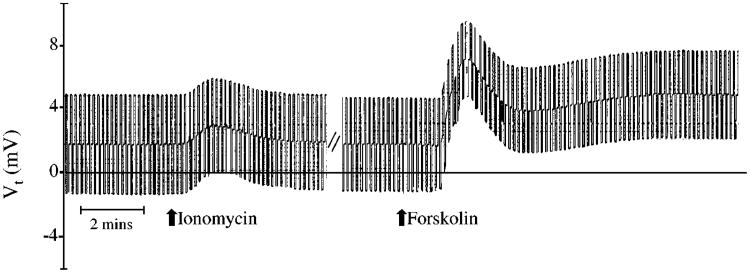
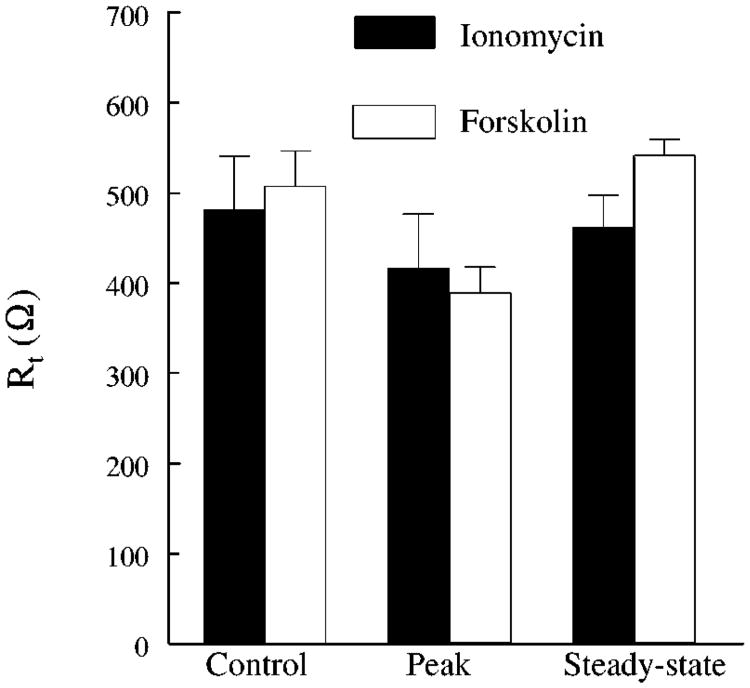
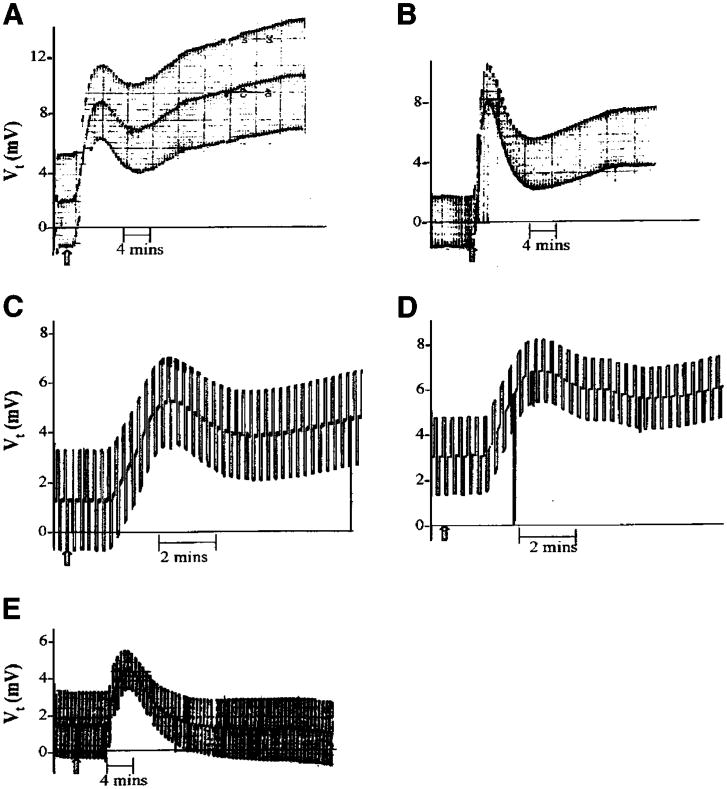
Cultures grown on Transwells having a Rt > 300 Ω were placed in modified Ussing chambers, and the responses to either 1 × 10−6 M ionomycin or 5 × 10−5 M forskolin (which elevate intracellular Ca2+ and cAMP, respectively) were evaluated. Figure 3 and Table 1 show that ionomycin produced transient changes in Vt and in Isc, respectively, whereas forskolin produced a biphasic, sustained increase. In both cases, Rt tended to decrease at the peak of the changes in Isc and then returned toward normal (Fig. 4). These changes are similar to the changes observed in native tissue that result from a stimulation of anion secretion (26, 42, 51).
Fig. 3.
Response of cultured guinea pig gallbladder epithelial monolayers to 1 × 10−6 M ionomycin and 5 × 10−5 M forskolin. The deflections in transepithelial voltage (Vt) result from bipolar current pulses used to measure Rt. The steady-state Vt is represented by the midpoint of the deflections. Drugs were added as indicated at the arrows. Results are shown for a typical experiment.
Table 1. Effect of gastrointestinal secretagogues on Vt and Isc of cultured guinea pig gallbladder epithelial cells.
| Basal | Peak | Steady State | |||||
|---|---|---|---|---|---|---|---|
|
|
|
|
|||||
| Vt | Isc | Vt | Isc | Vt | Isc | n | |
| Forskolin | 1.4 ± 0.5 | 3.2 ± 1.4 | 8.4 ± 1.1* | 28.1 ±3.8* | 6.7 ± 1.4* | 18.4 ±3.6* | 6 |
| PGE2 | 2.0 ± 0.9 | 6.5 ± 2.1 | 6.0 ± 2.2 | 16.9 ± 2.2* | 7.5 ± 2.6 | 16.5 ± 1.7* | 5 |
| Histamine | 1.8 ± 0.2 | 6.4 ± 1.7 | 4.8 ± 0.7* | 20.1 ± 4.3* | 3.8 ± 0.4* | 15.7 ± 3.4* | 7 |
| VIP | 3.7 ± 0.3 | 12.0 ± 2.4 | 7.3 ± 2.5 | 21.2 ± 3.0* | 7.8 ± 3.0 | 21.5 ± 3.8* | 4 |
| Secretin | 3.6 ± 1.1 | 5.6 ± 1.9 | 7.7 ± 1.4* | 28.2 ± 3.4* | 6.9 ± 2.0 | 16.3 ± 3.3 | 5 |
| Ionomycin | 2.1 ± 0.7 | 5.8 ± 0.4 | 2.8 ± 0.6 | 8.4 ± 0.6* | 1.8 ± 0.5 | 6.3 ± 0.6 | 6 |
| ATP | 2.8 ± 0.3 | 4.2 ± 0.3 | 4.9 ± 0.5* | 9.8 ± 0.7* | 3.5 ± 0.6 | 5.4 ± 0.6 | 4 |
| CCK | 1.2 ± 0.1 | 6.1 ± 1.4 | 1.9 ± 0.2* | 8.5 ± 1.3* | 1.4 ± 0.2* | 6.7 ± 1.4 | 3 |
Values are means ± SE; n = no. of experiments. Secretagogues were added to both half-chambers. Transepithelial voltage (Vt) and short circuit current (Isc) are measured as described in MATERIALS AND METHODS. Ionomycin, ATP, and CCK elicited transient increases in Vt and Isc. Responses to forskolin, PGE2, histamine, vasoactive intestinal polypeptide (VIP), and secretin were sustained.
P < 0.05 vs. basal Vt or Isc. In each secretin experiment, the steady-state Isc exceeded the basal Isc, although the difference was not statistically significant.
Fig. 4.
Rt before (control) and after (peak and steady-state) the addition of 1 × 10−6 M ionomycin and 5 × 10−5 M forskolin. Each bar represents the mean ± SE of 7 Transwells from 3 different preparations.
Ion substitution experiments were conducted to determine the ionic dependency of the ionomycin- and forskolin-stimulated Isc. Figure 5 shows that replacing both Cl− and significantly inhibited ionomycin and forskolin responses. The forskolin-stimulated Isc was significantly attenuated when either Cl− or was replaced. These results are expected if anion secretion underlies the changes in Isc.
Fig. 5.
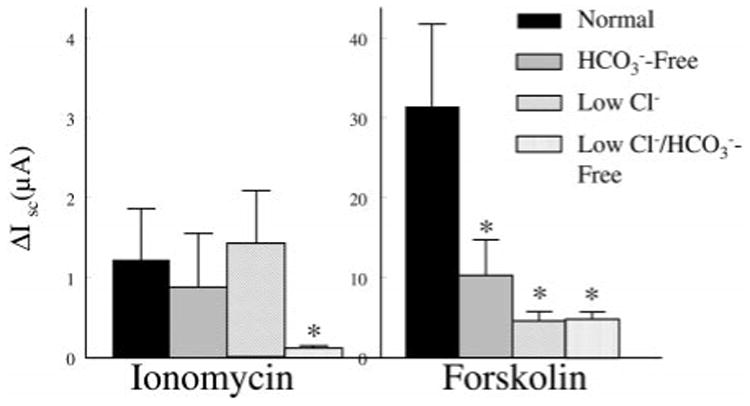
Effect of ion substitution on peak changes in short-circuit current (Isc) following 1 × 10−6 M ionomycin or 5 × 10−5 M forskolin. Each bar is the mean ± SE of 7 Transwells from 3 different preparations. * Significant difference from normal Ringer solution.
To further evaluate the contribution of anion secretion to the ionomycin- and forskolin-stimulated Isc, we determined the effect of anion transport inhibitors (Fig. 6). Anion transport inhibitors were added to the mucosal bathing solution. The response to ionomycin was blocked by DIDS (1 × 10−4 M) and diphenylamine-2-carboxylic acid (DPC; 5 × 10−5 M), whereas the response to forskolin was blocked by DPC but not DIDS. This sensitivity to anion transport inhibitors provides additional evidence that the ionomycin- and forskolin-stimulated Isc represents anion secretion.
Fig. 6.
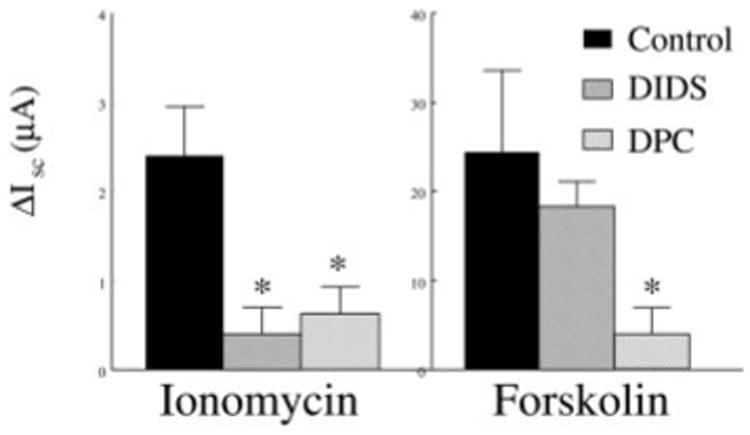
Effect of anion transport inhibitors on ionomycin- and forskolin-stimulated Isc. Blocker concentrations were 1 × 10−5 M for DIDS and 5 × 10−5 M for diphenylamine-2-carboxylic acid (DPC). Each bar is the mean ± SE of 7 Transwells from 3 different preparations. *Significant difference from controls.
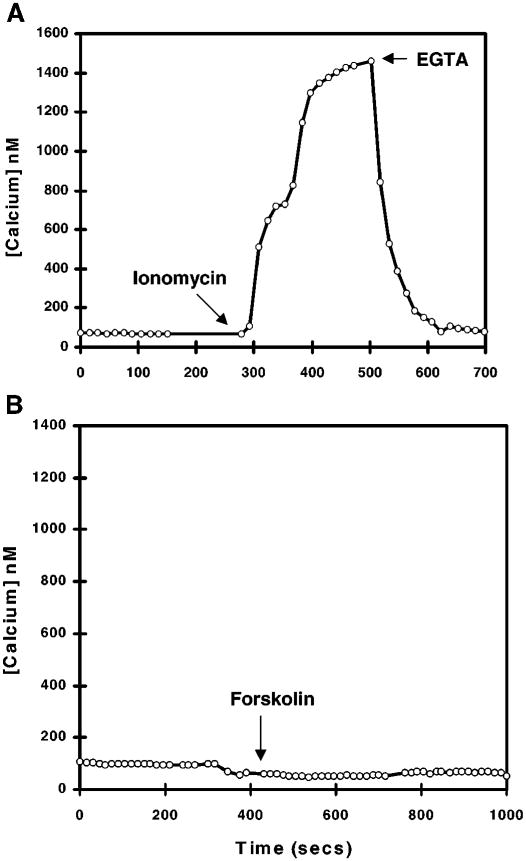
Changes in Isc following the addition of ionomycin and forskolin should reflect independent activation of Ca2+- and cAMP-dependent pathways, respectively. To confirm this, we evaluated the effect of ionomycin and forskolin on [Ca2+]i in cells loaded with the fluorescent Ca2+ indicator fura 2 with the use of fluorescent video microscopy (Fig. 7). Resting [Ca2+]i was 60 nM. Ionomycin caused a sustained increased [Ca2+]i to 1.3 μM, whereas forskolin had no effect.
Fig. 7.
Changes in intracellular Ca2+ concentration ([Calcium]) in fura 2-loaded cultured guinea pig gallbladder epithelial cells following the addition of 1 × 10−6 M ionomycin (A) or 5 × 10−5 M forskolin (B). Ionomycin and the Ca2+ chelator EGTA were added after forskolin to demonstrate that the cells and measuring system were functioning appropriately (data not shown).
Ionomycin and forskolin modulate ion transport by affecting intracellular Ca2+ and cAMP levels, respectively, at intracellular sites downstream of the binding of a secretagogue or transport modulator to its plasma membrane receptor. To evaluate the plasma membrane receptor profile of cultured gallbladder epithelia, we assessed the effect of several modulators of gastrointestinal ion transport on Vt and Isc. Figure 8 shows typical changes in Vt in response to the addition (indicated by the arrows) of PGE2 (10−6 M), histamine (10−4 M), VIP (10−8 M), secretin (10−7 M), and ATP (10−4 M). The effect of these secretagogues on mean Vt and Isc is shown in Table 1. Responses to PGE2, histamine, VIP, and secretin were sustained as was the case for forskolin. ATP and CCK elicited transient responses similar to that observed for ionomycin. Carbachol (10−3 M) and glucagon (10−6 M) had no effect on the transepithelial electric parameters (data not shown).
Fig. 8.
Change in Vt in response to 1 × 10−6 M PGE2 (A), 1 × 10−4 M histamine (B), 1 × 10−8 M vasoactive intestinal polypeptide (C), 1 × 10−6 M secretin (D), and 1 × 10−4 M ATP (E). The deflections in Vt result from bipolar current pulses used to measure Rt. The steady-state Vt is represented by the midpoint of the deflections. Drugs were added as indicated at the arrows. Data are for typical experiments. Changes in Isc are qualitatively similar to changes in Vt. The mean Isc and Vt data are shown in Table 1.
Discussion
The goal of this study was to develop a primary culture of guinea pig gallbladder epithelial cells with which to study the regulation of ion transport in mammalian gallbladder. Our criteria for achieving this goal included demonstrating that the cell culture 1) develops a confluent monolayer of epithelial cells that is stable over time, 2) develops a Rt, Vt, and Isc, 3) lends itself to the application of several different experimental techniques, and, importantly, 4) displays the range of responses associated with the native tissue. The data support our success in developing a primary cell culture that meets these criteria.
First, immunocytochemistry demonstrated an intracellular distribution of cytokeratin in cultured gallbladder cells typical of epithelial cells. The localization of cytokeratin is widely used as a marker for epithelial cell origin (18, 39, 40). The cell culture reaches confluency in 4 days and is stable for 5–7 days. Second, cell cultures grown on permeable supports develop transepithelial electric parameters Vt, Rt, and Isc. Thus the effects of various transport modulators on ion transport can be assessed from changes in these parameters. Third, we have demonstrated that the primary culture can be used in a number of techniques required for studies of the regulation of ion transport. This includes the Ussing Isc technique and the use of fluorescence indicator dyes that offer a convenient measure of intracellular ion concentrations such as Ca2+. Although not shown in this study, we have also successfully initiated patch clamp studies in both whole cell and cell-attached configurations to further characterize cells derived from cultured monolayers.
Fourth, and importantly, the primary cultures of guinea pig gallbladder epithelial cells display responses to transport modulators observed for the native tissue. For example, agents that elevate intracellular cAMP (e.g., theophylline, forskolin, prostaglandin, and possibly VIP and secretin) and in some cases Ca2+ (e.g., ionomycin and ATP) stimulate anion secretion by Necturus, guinea pig, porcine, and human gallbladder (8, 28, 41–43). Mechanisms underlying secretion are typical of secretory epithelia in that Ca2+ and cAMP open anion-conductive pathways in the apical membrane (28, 42, 43, 50, 51). Our results clearly demonstrate appropriate responses of cultured monolayers to agents that elevate Ca2+ and cAMP, ionomycin, and forskolin, respectively. These agents increased Vt and Isc and reduced Rt. The ionomycin- and forskolin-stimulated Isc were attenuated by replacement of bath Cl− and/or and blocked by anion transport inhibitors, consistent with stimulation of anion secretion. The ionomycin- and forskolin-stimulated responses differed in anion transport inhibitor sensitivity, ability to elevate [Ca2+]i, and time course, suggesting activation of distinct pathways. In the absence of measurements of ion fluxes, we cannot exclude alternative interpretations for the results of anion substitution and blocker experiments (e.g., effects on cation fluxes subsequent to changes in intracellular pH). However, activation of anion secretion is the most plausible explanation consistent with our data and the literature (51).
The different sensitivity of ionomycin- and forskolin-stimulated responses to the anion transport inhibitors DPC and DIDS observed in this study suggests similarities between Cl− efflux pathways in human and guinea pig gallbladder epithelia. In our studies, DPC and DIDS blocked the ionomycin-stimulated increase in Isc. Although the forskolin-stimulated response was blocked by DPC, DIDS was ineffective. Inhibition by DPC of cAMP-stimulated secretion in the native gallbladder epithelia has been attributed to an effect on apical exchange (42). In human gallbladder epithelia (8), differential sensitivity of Ca2+- and cAMP-dependent Cl− secretion to anion transport inhibitors was suggested to reflect activation of separate Cl− efflux pathways. This appears to be the case in a number of secretory epithelia, in which DIDS has been observed to block Ca 2+-dependent Cl− currents and channels (1, 2) but has no effect on cAMP-dependent Cl− currents and channels (1, 2, 4, 7, 9, 14, 16). On the other hand, DPC blocks both Ca2+- and cAMP-dependent Cl− currents and channels (1, 4, 7). The molecular species underlying DIDS-sensitive currents are unclear; however, DPC-sensitive, cAMP-dependent currents appear to be associated with the cystic fibrosis transmembrane conductance regulator CFTR (4, 12, 48). In Necturus gallbladder, anion transport inhibitors (28, 30, 53) did not block cAMP-dependent Cl− currents. Thus cultured guinea pig gallbladder epithelial cells closely resemble cultured human gallbladder epithelial cells (8).
Given that anion secretion appears to underlie both ionomycin- and forskolin-stimulated Isc, the observed differences in the time course of changes in Isc would not be expected. Forskolin elicited a biphasic, sustained increase in Vt and Isc and a fall in Rt. Ionomycin elicited similar but transient changes in transepithelial electric parameters. In bovine pancreatic duct cells and T84 cells, agents that increase [Ca2+]i elicit a transient and rapid increase in Isc, whereas the response to agents that elevate cAMP is delayed but sustained (1, 14, 16). The mechanisms underlying these differences are unclear. One possible explanation is that K+ efflux occurring concomitantly with anion secretion blunts the change in Isc related to anion secretion. In support of this, ionomycin-stimulated K+ efflux is five times that elicited by PGE2 in native guinea pig gallbladder (26). An alternative explanation for the transient nature of the Isc response to ionomycin is that some inhibitory pathway activated by Ca turns off secretion, as suggested by Barrett (3) and Wang et al. (60) for T84 cells.
To evaluate the plasma membrane receptor profile of cultured gallbladder epithelia, we assessed the effect of several modulators of gallbladder ion transport on Vt and Isc. PGE2, histamine, VIP, secretin, and ATP stimulated Isc at concentrations observed to be effective on native guinea pig gallbladder epithelia and other anion secreting epithelia (33–35, 41, 43, 46, 51, 52, 58). CCK also stimulated the Isc, but carbachol and glucagon had no effect on the transepithelial electric parameters. To our knowledge, carbachol and glucagon have not been reported to stimulate Isc in native guinea pig gallbladder. ATP and CCK elicited a transient response similar to that observed for ionomycin. Responses to PGE2, histamine, VIP, and secretin were sustained, as was that for forskolin. These similarities suggest that PGE2, histamine, VIP, and secretin activate cAMP-dependent pathways, whereas ATP and CCK activate Ca2+-dependent pathways in cultured guinea pig gallbladder epithelial cells. Elevation of cAMP by PGE2, VIP, and secretin has been observed for gallbladder epithelial cells from several species (41, 43). Likewise, elevation of [Ca2+]i by ATP was observed for several anion secretory epithelia in which ATP acts at plasma membrane purinergic receptors (8, 59, 64). In Necturus gallbladder, ATP increased apical and basolateral K+ conductance and increased apical Cl− permeability (10). Chinet and colleagues (8) recently demonstrated the presence of P2Y2 purinoceptors on cultured human gallbladder cells, the activation of which elicits anion secretion through activation of Ca2+-dependent pathways. In preliminary studies, we have observed increased [Ca2+]i following ATP addition to fura 2-loaded cells (25).
The response of cultured gallbladder epithelial cells to histamine and CCK also resembles that of forskolin and ionomycin, respectively. However, the second-messenger pathways activated by histamine and CCK in gallbladder epithelial cells are less clear. In some epithelial cell types, histamine elicits anion secretion by binding to H1 receptors and activating Ca2+-dependent signaling pathways (46, 52), whereas in others both Ca2+- and cAMP-dependent pathways are activated (33, 54, 61). The increase in cAMP was shown to result from eicosanoid production, which then stimulated ion transport. Although the effect of CCK on anion secretion in gallbladder epithelial cells has not been reported, in pancreatic acinar cells CCK stimulates secretion via activation a Ca2+-dependent pathway (62). Together, these results demonstrate a physiological role for these agents in regulating cultured gallbladder epithelial ion transport and, importantly, that the cell culture is an appropriate model system for the native tissue.
Although the responses of cultured cells to secretagogues were similar to those of the native tissue, the cultured guinea pig gallbladder epithelia differed from the native tissue with respect to Rt. Rt was considerably higher in cultured epithelia, at 500 vs. 50 Ω·cm2 (24, 51). Presently, we do not have an explanation for this observation. However, a comparison of the resistance of cultures grown on filters to resistance of native tissue is complicated by the elaboration of the surface area of native tissues by the presence of folds and ridges. This would lead to an underestimate of true surface area and, therefore, resistance in native tissue. We also cannot discount the possibility that our culture conditions (e.g., matrix, growth factors, etc.) select for a specific cell type or change some of the monolayer characteristics. Alternatively, the difference in Rt may reflect the absence of a factor that normally regulates junctional permeability. It is important to note, however, that the response of the Rt to secretagogues is identical to that of native gallbladder epithelia.
To our knowledge, this is the first systematic study in which a gallbladder epithelial cell culture has been shown to develop transepithelial electric parameters and to respond to hormones characteristic of the native tissue. Other groups have developed primary cultures of gallbladder epithelia from several species for use in a variety of different studies (6, 8, 28, 29, 31, 39, 44). Most pertinent to the present study, O'Brien et al. (39) demonstrated that epithelioid outgrowths from guinea pig gallbladder explants expressing cytokeratin could be maintained for 10–14 days in culture. These cells failed to form confluent monolayers and were not characterized beyond preliminary single-channel measurements. Details concerning the culture conditions were not given; thus we cannot speculate as to differences in our culture conditions.
Many cell culture systems presently used to study regulation of intestinal epithelial ion transport are of colonic tumor origin (17). In addition, small intestinal cell lines derived from fetal tissue such as IEC-6 and IEC-18 cells display immature phenotypes (32, 45). Gallbladder and small intestinal epithelial cells have a number of similarities with respect to ion transport mechanisms and regulation. Like guinea pig gallbladder, the mammalian small intestine absorbs Na+ by an electroneutral process, secretes , and responds similarly to intestinal hormones and transport modulators such as VIP, secretin, histamine, and PGE2 (reviewed in Refs. 5 and 49). Given this, the primary gallbladder epithelial cell culture described in this study may provide a new model with which to examine mechanisms and regulation of ion transport common to these epithelia.
In summary, our laboratory has developed a primary culture of guinea pig gallbladder cell that displays transport processes and hormonal responses typical of the native tissue. The cultured guinea pig gallbladder cells can be studied using the wide range of techniques currently available to study epithelial ion transport and its regulation. As such, the preparation provides a useful model system with which to study transport in gallbladder and intestinal epithelial cells.
Acknowledgments
We thank Dr. Catherine S. Chew for helpful discussions, Andrew Shaw for assistance with confocal microscopy, and Erin Booker for technical assistance.
This work was supported by grant GM-08241–13 and grant RR-11598 from the National Institutes of Health. L. Hammonds-Odie was supported by NASA cooperative agreement NCC8-179.
References
- 1.Al-Nakkash L, Cotton CU. Bovine pancreatic duct cells express cAMP- and Ca2+-activated apical membrane Cl− conductances. Am J Physiol Gastrointest Liver Physiol. 1997;273:G204–G216. doi: 10.1152/ajpgi.1997.273.1.G204. [DOI] [PubMed] [Google Scholar]
- 2.Anderson MP, Welsh MJ. Calcium and cAMP activate different chloride channels in the apical membrane of normal and cystic fibrosis epithelia. Proc Natl Acad Sci USA. 1991;88:6003–6007. doi: 10.1073/pnas.88.14.6003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barrett K. Positive and negative regulation of chloride secretion in T84 cells. Am J Physiol Cell Physiol. 1993;265:C859–C868. doi: 10.1152/ajpcell.1993.265.4.C859. [DOI] [PubMed] [Google Scholar]
- 4.Chan LN, Chung YW, Leung PS, Liu CQ, Chan HC. Activation of an adenosine 3′,5′-cyclic monophosphate-dependent Cl− conductance in response to neurohormonal stimuli in mouse endometrial epithelial cells: the role of cystic fibrosis transmembrane conductance regulator. Biol Reprod. 1999;60:374–380. doi: 10.1095/biolreprod60.2.374. [DOI] [PubMed] [Google Scholar]
- 5.Chang EB, Rao MC. Intestinal water and electrolyte transport. In: Johnson LR, Alpers DH, Christensen J, Jacobson ED, Walsh JH, editors. Physiology of the Gastrointestinal Tract. New York: Raven; 1994. pp. 2027–2081. [Google Scholar]
- 6.Chapman WC, Fisk J, Schol D, Debelak JP, Washington MK, Bluth RF, Pierce D, Williams LF., Jr Establishment and characterization of primary gallbladder epithelial cell cultures in the prairie dog. J Surg Res. 1998;80:35–43. doi: 10.1006/jsre.1998.5401. [DOI] [PubMed] [Google Scholar]
- 7.Cheng HS, Leung PV, Chew SB, Leung PS, Lam SY, Wong WS, Wang ZD, Chan HC. Concurrent and independent and Cl− secretion in a human pancreatic duct cell line (CAPAN-1) J Membr Biol. 1998;164:155–167. doi: 10.1007/s002329900401. [DOI] [PubMed] [Google Scholar]
- 8.Chinet T, Fouassier L, Dray-Charier N, Imam-Ghali M, Morel H, Mergey M, Dousset B, Parc R, Paul A, Housset C. Regulation of electrogenic anion secretion in normal and cystic fibrosis gallbladder mucosa. Hepatology. 1999;29:5–13. doi: 10.1002/hep.510290142. [DOI] [PubMed] [Google Scholar]
- 9.Cliff WH, Schoumacher RA, Frizzell RA. CAMP-activated Cl− channels in CFTR-transfected cystic fibrosis pancreatic epithelial cells. Am J Physiol Cell Physiol. 1992;262:C1154–C1160. doi: 10.1152/ajpcell.1992.262.5.C1154. [DOI] [PubMed] [Google Scholar]
- 10.Cotton CU, Reuss L. Electrophysiological effects of extracellular ATP on Necturus gallbladder epithelium. J Gen Physiol. 1991;97:949–971. doi: 10.1085/jgp.97.5.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cremaschi D, Porta C, Botta G, Meyer G. Nature of the neutral Na+-Cl− coupled entry at the apical membrane of rabbit gallbladder epithelium. IV. Na+/H+, double exchange, hydrochlorothiazide-sensitive Na+-Cl− symport and Na+-K+-2Cl−-cotransport are all involved. J Membr Biol. 1992;129:221–235. doi: 10.1007/BF00232905. [DOI] [PubMed] [Google Scholar]
- 12.Cunningham SA, Worrell RT, Benos DJ, Frizzell RA. cAMP-stimulated ion currents in Xenopus oocytes expressing CFTR cRNA. Am J Physiol Cell Physiol. 1992;262:C783–C788. doi: 10.1152/ajpcell.1992.262.3.C783. [DOI] [PubMed] [Google Scholar]
- 13.Dausch R, Spring KR. Regulation of NaCl entry into Necturus gallbladder epithelium by protein kinase C. Am J Physiol Cell Physiol. 1994;266:C531–C535. doi: 10.1152/ajpcell.1994.266.2.C531. [DOI] [PubMed] [Google Scholar]
- 14.Dharmsathaphorn K, Cohn J, Beuerlein G. Multiple calcium-mediated effector mechanisms regulated chloride secretory responses in T84 cells. Am J Physiol Cell Physiol. 1989;256:C1224–C1230. doi: 10.1152/ajpcell.1989.256.6.C1224. [DOI] [PubMed] [Google Scholar]
- 15.Dharmsathaphorn K, McRoberts JA, Mandel KG, Tisdale L, Masui D. A human colonic tumor cell line that maintains vectorial electrolyte transport. Am J Physiol Gastrointest Liver Physiol. 1984;246:G204–G208. doi: 10.1152/ajpgi.1984.246.2.G204. [DOI] [PubMed] [Google Scholar]
- 16.Dharmsathaphorn K, Pandol SJ. Mechanisms of chloride secretion induced by carbachol in a colonic epithelial cell line. J Clin Invest. 1986;77:348–354. doi: 10.1172/JCI112311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Evans GS, Flint N, Potten CS. Primary cultures for studies of cell regulation and physiology in intestinal epithelium. Annu Rev Physiol. 1994;56:399–417. doi: 10.1146/annurev.ph.56.030194.002151. [DOI] [PubMed] [Google Scholar]
- 18.Fouassier L, Chinet T, Tober B, Caryon A, Balladur P, Mergey M, Paul A, Poupon AR, Capeau J, Barbu V, Housset C. Endothelin-1 is synthesized and inhibits cyclic adenosine monophosphate dependent anion secretion by an autocrine/paracrine mechanism in gallbladder epithelial cells. J Clin Invest. 1998;101:2881–2888. doi: 10.1172/JCI2821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garvin JL, Spring KR. Regulation of apical membrane ion transport in Necturus gallbladder. Am J Physiol Cell Physiol. 1992;263:C187–C193. doi: 10.1152/ajpcell.1992.263.1.C187. [DOI] [PubMed] [Google Scholar]
- 20.Grassett E, Bernabeu J, Pinto M. Epithelial properties of human colonic carcinoma cell line Caco-2: effect of secretagogues. Am J Physiol Cell Physiol. 1985;248:C410–C418. doi: 10.1152/ajpcell.1985.248.5.C410. [DOI] [PubMed] [Google Scholar]
- 21.Grassett E, Pinto M, Dussaulx E, Zweibaum A, Desjeux JF. Epithelial properties of human colonic carcinoma cell line Caco-2: electrical parameters. Am J Physiol Cell Physiol. 1984;247:C260–C267. doi: 10.1152/ajpcell.1984.247.3.C260. [DOI] [PubMed] [Google Scholar]
- 22.Gray MA, Pollard CE, Harris A, Coleman L, Greenwell JR, Argent BE. Anion selectivity and block of the small-conductance chloride channel on pancreatic duct cells. Am J Physiol Cell Physiol. 1990;259:C752–C761. doi: 10.1152/ajpcell.1990.259.5.C752. [DOI] [PubMed] [Google Scholar]
- 23.Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca 2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- 24.Gunter-Smith PJ. Apical membrane potassium conductance in guinea pig gallbladder epithelial cells. Am J Physiol Cell Physiol. 1988;255:C808–C815. doi: 10.1152/ajpcell.1988.255.6.C808. [DOI] [PubMed] [Google Scholar]
- 25.Gunter-Smith PJ, Abdulkadir O, Terrell R, London D. ATP affects guinea pig gallbladder epithelial ion transport via P2y receptors (Abstract) FASEB J. 1999;13:A727. [Google Scholar]
- 26.Gunter-Smith PJ, Sample KD. Two distinct potassium efflux pathways in guinea pig gallbladder epithelial cells (Abstract) Physiologist. 1992;35:A5. [Google Scholar]
- 27.Heintze K, Petersen KU, Oiles P, Saverymuttu SH, Wood JR. Effects of bicarbonate on fluid and electrolyte transport by the guinea pig gallbladder: a bicarbonate-chloride exchange. J Membr Biol. 1979;45:43–59. doi: 10.1007/BF01869294. [DOI] [PubMed] [Google Scholar]
- 28.Heming TA, Copello J, Reuss L. Regulation of cAMP-activated apical membrane chloride conductance in gallbladder epithelium. J Gen Physiol. 1994;103:1–18. doi: 10.1085/jgp.103.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Housset C, Caroayon A, Housset B, Legendre C, Hannonun L, Poupon R. Endothelin-1 secretion by human gallbladder epithelial cells in primary culture. Lab Invest. 1993;69:750–755. [PubMed] [Google Scholar]
- 30.Kottra G. Calcium is not involved in the cAMP-mediated stimulation of Cl− conductance in the apical membrane of Necturus gallbladder epithelium. Pflügers Arch. 1995;429:647–658. doi: 10.1007/BF00373985. [DOI] [PubMed] [Google Scholar]
- 31.Kuver R, Savard C, Nguyen TD, Osborne WR, Lee SP. Isolation and long-term culture of gallbladder epithelial cells from wild-type and CF mice. In Vitro Cell Dev Biol Anim. 1997;33:104–109. doi: 10.1007/s11626-997-0030-5. [DOI] [PubMed] [Google Scholar]
- 32.Ma TY, Hollander D, Bhalla D, Nguyen H, Krugliak P. IEC-18, a nontransformed small intestinal cell line for studying epithelial permeability. J Lab Clin Med. 1992;120:329–341. [PubMed] [Google Scholar]
- 33.McCabe RD, Smith PL. Effects of histamine receptor antagonists on ion transport in rabbit descending colon. Am J Physiol Gastrointest Liver Physiol. 1984;247:G411–G418. doi: 10.1152/ajpgi.1984.247.4.G411. [DOI] [PubMed] [Google Scholar]
- 34.McMillian MJ, Soltoff SP, Cantley LC, Rudel RA, Talamo BR. Extracellular ATP increases free cytosolic calcium in rat parotid acinar cell. Biochem J. 1988;255:291–300. [PMC free article] [PubMed] [Google Scholar]
- 35.McMillian MK, Soltoff SP, Cantley LC, Rudel RA, Talamo BR. Two distinct cytosolic calcium responses to extracellular ATP in rat parotid acinar cells. Br J Pharmacol. 1993;108:453–461. doi: 10.1111/j.1476-5381.1993.tb12825.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meyer G, Bot G, Rosette C, Cremaschi D. The nature of the neutral Na+-Cl− coupled entry at the apical membrane of rabbit gallbladder. III. An analysis of transport on membrane vesicles. J Membr Biol. 1990;118:107–120. doi: 10.1007/BF01868468. [DOI] [PubMed] [Google Scholar]
- 37.Morris AP, Frizzell RA. Ca2+-dependent Cl− channels in undifferentiated human colonic cells (HT-29). I. Single-channel properties. Am J Physiol Cell Physiol. 1993;264:C968–C976. doi: 10.1152/ajpcell.1993.264.4.C968. [DOI] [PubMed] [Google Scholar]
- 38.Morris AP, Frizzell RA. Ca2+-dependent Cl− channels in undifferentiated human colonic cells (HT-29). II. Regulation and rundown. Am J Physiol Cell Physiol. 1993;264:C977–C985. doi: 10.1152/ajpcell.1993.264.4.C977. [DOI] [PubMed] [Google Scholar]
- 39.O'Brien JA, James PS, Torre M. Primary cultures of guinea-pig gallbladder epithelial cells suitable for patch-clamp studies. Exp Physiol. 1991;76:807–810. doi: 10.1113/expphysiol.1991.sp003546. [DOI] [PubMed] [Google Scholar]
- 40.Oda D, Lee SP, Hayashi A. Long-term culture and partial characterization of dog gallbladder epithelial cells. Lab Invest. 1991;64:682–692. [PubMed] [Google Scholar]
- 41.O'Grady SM, Wolters PJ, Hiuldebrand K, Brown DR. Regulation of ion transport in porcine gallbladder: effect of VIP and norepinephrine. Am J Physiol Cell Physiol. 1989;257:C52–C57. doi: 10.1152/ajpcell.1989.257.1.C52. [DOI] [PubMed] [Google Scholar]
- 42.Petersen KU, Cormann P, Macherey HJ, Sprakties G, Winterhager JM. Electrogenic and electroneutral -secretion by guinea pig gallbladder epithelium: discriminatory abilities of Cl− channel blockers. JPharamcol Exp Ther. 1993;266:65–73. [PubMed] [Google Scholar]
- 43.Petersen KU, Goergen R, Hofken F, Macherey HJ, Sprakties G. Electrogenic bicarbonate secretion in gallbladder: induction by barium via neuronal, possibly VIP-ergic pathways. Naunyn Schmeidebergs Arch Pharmacol. 1993;348:526–535. doi: 10.1007/BF00173214. [DOI] [PubMed] [Google Scholar]
- 44.Plevris JN, Walker SW, Harrison DJ, Dhariwal A, Hayes PC, Bouchier IA. Primary culture of bovine gallbladder epithelial cells. Gut. 1993;34:1612–1615. doi: 10.1136/gut.34.11.1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Quaroni A, May RJ. Establishment and characterization on intestinal epithelial cell cultures. Methods Cell Biol. 1980;21B:403–427. [PubMed] [Google Scholar]
- 46.Riach RA, Duncan G, Williams MR, Webb SF. Histamine and ATP mobilize calcium by activation of H1 and P2u receptors in human lens epithelial cells. J Physiol (Lond) 1995;486:273–282. doi: 10.1113/jphysiol.1995.sp020810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sahi J, Wiggins MP, Gibori GB, Layden TJ, Rao MC. Calcium regulated chloride permeabilities in primary cultures of rabbit colonocytes. J Cell Physiol. 1996;168:276–283. doi: 10.1002/(SICI)1097-4652(199608)168:2<276::AID-JCP6>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 48.Schwiebert EM, Flotte T, Cutting GR, Guggino WB. Both CFTR and outwardly rectifying chloride channels contribute to cAMP-stimulated whole cell chloride currents. Am J Physiol Cell Physiol. 1994;266:C1464–C1477. doi: 10.1152/ajpcell.1994.266.5.C1464. [DOI] [PubMed] [Google Scholar]
- 49.Sellin J. The pathophysiology of diarrhea. In: Schultz SG, Andreoli TE, Brown AM, Fambrough DM, Hoffman JF, Welsh MJ, editors. Molecular Biology of Membrane Transport Disorders. New York: Plenum; 1996. pp. 541–563. [Google Scholar]
- 50.Sprakties G, Macherey HJ, Petersen KU. Secretin and somatostatin as modulators of electrolyte transport in guinea pig gallbladder epithelium. J Pharmacol Exp Ther. 1993;265:273–280. [PubMed] [Google Scholar]
- 51.Stewart CP, Winterhager JM, Heintze K, Petersen KU. Electrogenic bicarbonate secretion by guinea pig gallbladder epithelium: apical membrane exit. Am J Physiol Cell Physiol. 1989;256:C736–C749. doi: 10.1152/ajpcell.1989.256.4.C736. [DOI] [PubMed] [Google Scholar]
- 52.Tessier GJ, Traynor TR, Kannan MS, O'Grady SM. Mucosal histamine inhibits Na absorption and stimulates Cl secretion across equine tracheal epithelium. Am J Physiol Lung Cell Mol Physiol. 1991;261:L456–L461. doi: 10.1152/ajplung.1991.261.6.L456. [DOI] [PubMed] [Google Scholar]
- 53.Torres RJ, Altenberg GA, Cohn JA, Reuss L. Polarized expression of cAMP-activated chloride channels in isolated epithelial cells. Am J Physiol Cell Physiol. 1996;271:C1574–C1582. doi: 10.1152/ajpcell.1996.271.5.C1574. [DOI] [PubMed] [Google Scholar]
- 54.Traynor TR, Brown DR, O'Grady SM. Effects of inflammatory mediators on electrolyte transport across the porcine distal colon epithelium. J Pharmacol Exp Ther. 1993;264:61–66. [PubMed] [Google Scholar]
- 55.Tsien RY. New calcium indicators and buffers with high selectivity against magnesium and protons: design, synthesis, and properties of prototype structures. Biochemistry. 1980;19:2396–2404. doi: 10.1021/bi00552a018. [DOI] [PubMed] [Google Scholar]
- 56.Uribe JM, Gelbamann CM, Traynor-Kaplan AE, Barrett KE. Epidermal growth factor inhabits Ca2+-dependent Cl transport in T84 human colonic epithelial cells. Am J Physiol Cell Physiol. 1996;271:C914–C922. doi: 10.1152/ajpcell.1996.271.3.C914. [DOI] [PubMed] [Google Scholar]
- 57.Uribe JM, Sanchez de Medina F, Traynor-Kaplan AE, Barrett KE. Inhibition of calcium dependent chloride secretion by carbachol and EGF: possible involvement of phosphatidylinositol 3-kinase (Abstract) Gastroenterology. 1995;108:A334. [Google Scholar]
- 58.Vajanaphanich M, Wasserman M, Buranawuti T, Barrett KE, Traynor-Kaplan AE. Carbachol uncouples chloride secretion from Ca2+: association with tyrosine kinase activity (Abstract) Gastroenterology. 1993;104:A286. [Google Scholar]
- 59.Van Scott MR, Chunet TC, Burnette AD, Paradiso AM. Purinergic regulation of ion transport across nonciliated bronchiolar epithelial (Clara) cells. Am J Physiol Lung Cell Mol Physiol. 1995;269:L30–L37. doi: 10.1152/ajplung.1995.269.1.L30. [DOI] [PubMed] [Google Scholar]
- 60.Wang YZ, Cooke HJ, Su HC, Fertel R. Histamine augments colonic secretion in guinea pig distal colon. Am J Physiol Gastrointest Liver Physiol. 1990;258:G432–G439. doi: 10.1152/ajpgi.1990.258.3.G432. [DOI] [PubMed] [Google Scholar]
- 61.Wasserman SI, Barrett KE, Huott PA, Beuerlein G, Kagnoff MF, Dharmsathaphorn K. Immune-related intestinal Cl− secretion. I. Effect of histamine on the T84 cell line. Am J Physiol Cell Physiol. 1988;254:C53–C62. doi: 10.1152/ajpcell.1988.254.1.C53. [DOI] [PubMed] [Google Scholar]
- 62.Williams JA. Regulation of pancreatic acinar cell function by intracellular calcium. Am J Physiol Gastrointest Liver Physiol. 1980;238:G269–G279. doi: 10.1152/ajpgi.1980.238.4.G269. [DOI] [PubMed] [Google Scholar]
- 63.Wils P, Legrain S, Frenois E, Scherman D. HT-29–18-Cl intestinal cells: a new model for studying the epithelial transport of drugs. Biochim Biophys Acta. 1993;1177:134–138. doi: 10.1016/0167-4889(93)90032-k. [DOI] [PubMed] [Google Scholar]
- 64.Wilson SM, Rakhit S, Murdoch R, Pedian JD, Elder HY, Baines DL, Ko WH, Wong PYD. Activation of apical P2u purine receptors permits inhibition of adrenaline-evoked cyclic AMP accumulation in cultured sweat gland epithelial cells. J Exp Biol. 1996;199:2153–2160. doi: 10.1242/jeb.199.10.2153. [DOI] [PubMed] [Google Scholar]