Abstract
Patients with type 1 diabetes (T1D) suffer excessive morbidity and mortality following myocardial infarction (MI) that is not fully explained by the metabolic effects of diabetes. Acute MI is known to trigger a profound innate inflammatory response with influx of mononuclear cells and production of proinflammatory cytokines that are crucial for cardiac repair. We hypothesized that these same pathways might exert ‘adjuvant effects’ and induce pathological responses in autoimmune-prone T1D hosts. Here we show that experimental MI in nonobese diabetic (NOD) mice - but not in control C57BL/6 mice - results in a severe post-infarction autoimmune (PIA) syndrome characterized by destructive lymphocytic infiltrates in the myocardium, infarct expansion, sustained cardiac IgG autoantibody production and Th1 effector cell responses against cardiac (α-)myosin. PIA was prevented by inducing tolerance to α-myosin, demonstrating that immune responses to cardiac myosin are required for this disease process. Extending these findings to humans, we developed a panel of immunoassays for cardiac autoantibody detection and found autoantibody positivity in 83% post-MI T1D patients. We further identified shared cardiac myosin autoantibody signatures between post-MI T1D patients and non-diabetic patients with myocarditis – that were absent in post-MI type 2 diabetic patients - and confirmed the presence of myocarditis in T1D by cardiac magnetic resonance imaging techniques. These data provide experimental and clinical evidence for a distinct post-MI autoimmune syndrome in T1D. Our findings suggest that PIA may contribute to worsened post-MI outcomes in T1D, and highlight a role for antigen-specific immunointervention to selectively block this pathway.
INTRODUCTION
Over past decades, new knowledge about basic mechanisms underlying the pathogenesis of cardiovascular disease (CVD) has led to aggressive pharmacological and interventional therapies, resulting in a major decline in mortality from myocardial infarction (MI) in the general population (1). Despite this progress, CVD accounts for 65–70% of deaths in individuals with type 1 diabetes (T1D) (2, 3) and incurs a ~13-fold increase in age-adjusted mortality rates in T1D patients compared to the non-diabetic population (3). This excess mortality has shown essentially no improvement over the past 30 years, despite improved outcomes from other diabetes complications, in particular, renal failure (4) which has long been considered the primary driver of CVD mortality in T1D (5). While chronic hyperglycemia has been established as a key mediator of CVD risk in T1D (6), the mechanisms accounting for the excessive post-MI mortality are poorly understood. Although numerous factors related to diabetes have been implicated, none have been unique to T1D (2).
Type 1 diabetes is an autoimmune disorder caused by T lymphocyte-mediated destruction of the pancreatic β cells (“insulitis”). Once established, insulitis can be detected indirectly by screening serum for autoantibodies to islet antigens. The α-cell specificity of this autoimmune attack has been attributed to specific alleles of major histocompatibility complex (MHC) class II, most notably HLA-DQ8 (hereafter, DQ8). However, non-MHC genes that are associated with more broad spectrum defects in immunological tolerance are also required and are thought to underlie the clustering of other autoimmune disorders in individuals with T1D (7). Environmental factors are also critical for the development of T1D, and it has been assumed that in genetically susceptible individuals, an inflammatory trigger – presumably, an infection or other cause of β-cell injury – is required for disease expression.
Inflammation plays a crucial role in the early stages of tissue repair following MI (8, 9). After acute MI, signals are generated that trigger the influx of neutrophils, macrophages and dendritic cells into the infarct zone (10, 11), along with the release of proinflammatory cytokines such as tumor necrosis factor-α (TNF-α), interleukin (IL)-1β and IL-6 (9). While these innate immune responses are crucial for repairing the damaged heart, these same cytokines and signals from necrotic cells are known to be particularly potent maturation factors for dendritic cells, transforming them into highly immunogenic antigen-presenting cells capable of activating adaptive immune responses (12, 13). However, there has been substantial debate about whether endogenous (‘danger‘) signals generated by tissue damage can by themselves – in the absence of adjuvant or microbial stimuli – fully activate adaptive immune responses (14, 15). It has been postulated that in tissue injury settings, the released self-antigens should not be recognized as ‘foreign’ because high-avidity T cells specific for these self-antigens would normally have been deleted during thymic negative selection, a major barrier against autoimmunity.
We and others have shown that ‘humanized’ transgenic nonobese diabetic (NOD) mice expressing DQ8, instead of endogenous murine I-Ag7, develop early mortality due to spontaneous autoimmune myocarditis (16, 17), raising the possibility that myocarditis is part of the spectrum of organ-specific autoimmune disorders in T1D. More recently, we demonstrated that myocarditis in DQ8+NOD mice is caused by cardiac α-myosin heavy chain (MyHC)-specific CD4+ T cells that escape thymic negative selection. We further showed that due to impaired central tolerance mechanisms, the normal human T-cell repertoire is also highly enriched in reactivities to α-MyHC (18), potentially placing the heart at risk for autoimmune attack after MI. Indeed, transient autoimmune reactions to the heart are commonly observed in the general population following MI (19, 20). Here, we tested the hypothesis that in T1D-prone hosts, these post-infarction reactions would persist and become amplified, with pathological consequences. Previous reports have shown that MI outcomes are worsened in mice with pre-existing immunization-induced autoimmune myocarditis (21, 22).
In this study, we show that experimental MI triggers a de novo myocarditis-like syndrome in NOD mice with progressive lymphocytic infiltration of the myocardium and infarct expansion, and we further demonstrate that this disease process can be prevented by inducing tolerance to α-MyHC. Using a panel of newly established cardiac autoantibody assays, we show a high prevalence of cardiac myosin autoantibodies in T1D patients with ischemic heart disease, and confirm the presence of myocardial inflammation in an autoantibody-positive patient by cardiac magnetic resonance imaging. Taken together, these data suggest a role for autoimmunity in the poor CVD outcomes of T1D.
RESULTS
Progressive lymphocytic infiltration of the myocardium and impaired infarct healing in NOD mice
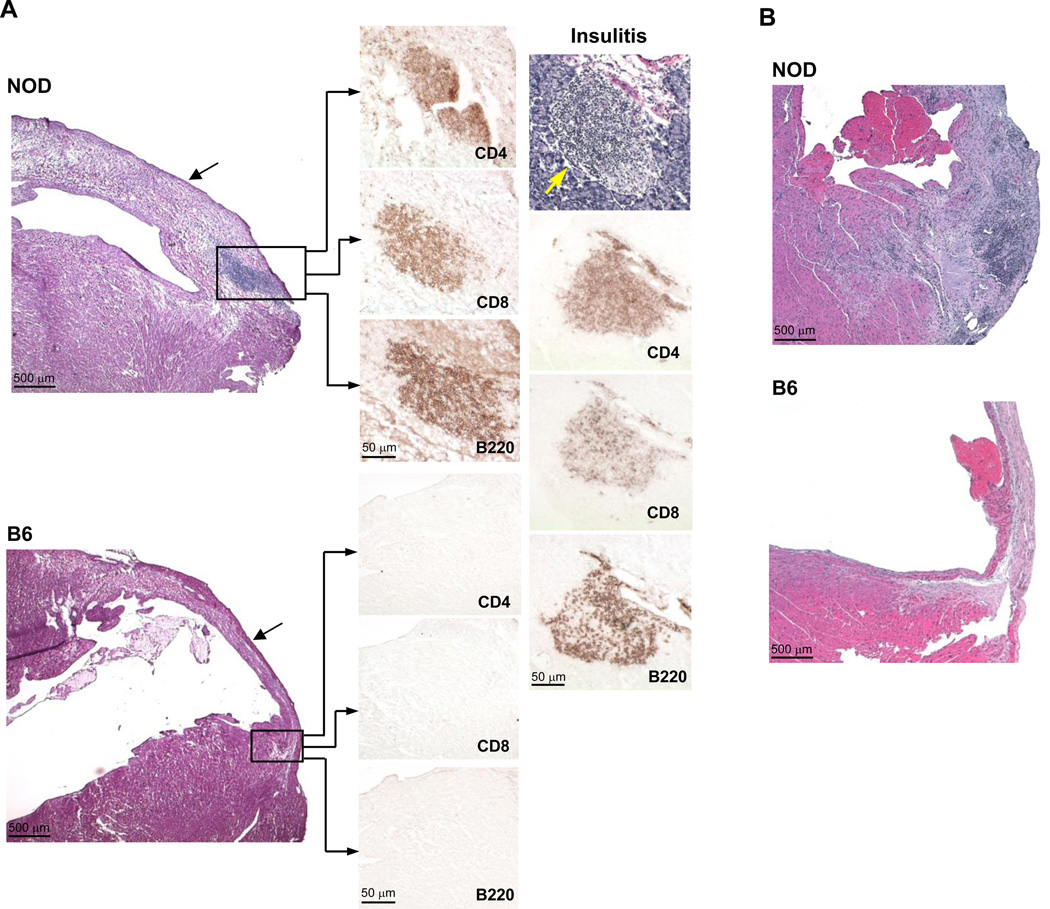
Although the outcomes of MI have been previously examined in diabetic mouse models, they have not, to our knowledge, been reported in a model of autoimmune T1D. We, therefore, experimentally induced MI by occlusion of the left anterior descending coronary artery, in young (7– to 8-wk-old), normoglycemic NOD and control C57BL/6 (hereafter, B6) mice, and followed the mice for up to 8 wk post-MI. Histological analyses of the NOD hearts 21 d after infarction was notable in showing dense lymphocytic infiltrates that were most pronounced in the peri-infarct border zone (Figure 1A, upper left panel). Immunohistochemical analysis further revealed that the cellular composition of the myocardial infiltrates (Figure 1A, middle panel) closely resembled that found in the spontaneously arising pancreatic islet infiltrates in NOD mice (‘insulitis’, Figure 1A, right panel), with B220+ B lymphocytes, CD4+ and CD8+ T lymphocytes comprising the most abundant cell types present. In addition, the infarct appeared markedly edematous with poor scar formation (Figure 1A, upper left panel, arrrow), suggesting impaired infarct healing. These histopathological changes became more pronounced over time: by 8 wk post-MI, the infiltrates extended into the non-infarcted myocardium with further swelling and expansion of the infarct zone (Figure 1B). As expected, the control post-infarcted B6 hearts were devoid of lymphocytic infiltrates and showed normal infarct healing and scar formation (Figure 1A, lower left panel; Figure 1B).
Figure 1. Acute myocardial infarction (MI) induces progressive lymphocytic infiltration of the heart with impaired infarct healing in NOD mice.
(A) Serial sections through the infarct zone of representative NOD and B6 hearts 3 wk post-MI. H&E staining (left upper panel) showing lymphocytic infiltrates in the peri-infarct zone (box) along with infarct swelling (arrow) in a post-MI NOD heart. Immunohistochemical staining (middle panel) showing that the myocardial infiltrates consist mostly of B220+ B cells, CD4+ and CD8+ T cells, mirroring the cellular composition to the native pancreatic ‘insulitis’ lesions (right panel, yellow arrow). Post-MI B6 mice did not develop lymphocytic infiltrates and showed normal infarct scar formation (arrow). (B) H&E staining of NOD and B6 hearts 8 wk following MI showing extension of the infiltrates into the non-infarcted myocardium and further infarct expansion in NOD mice.
Sustained production of circulating autoantibodies to MyHC and the Z-disk protein, α-actinin-2
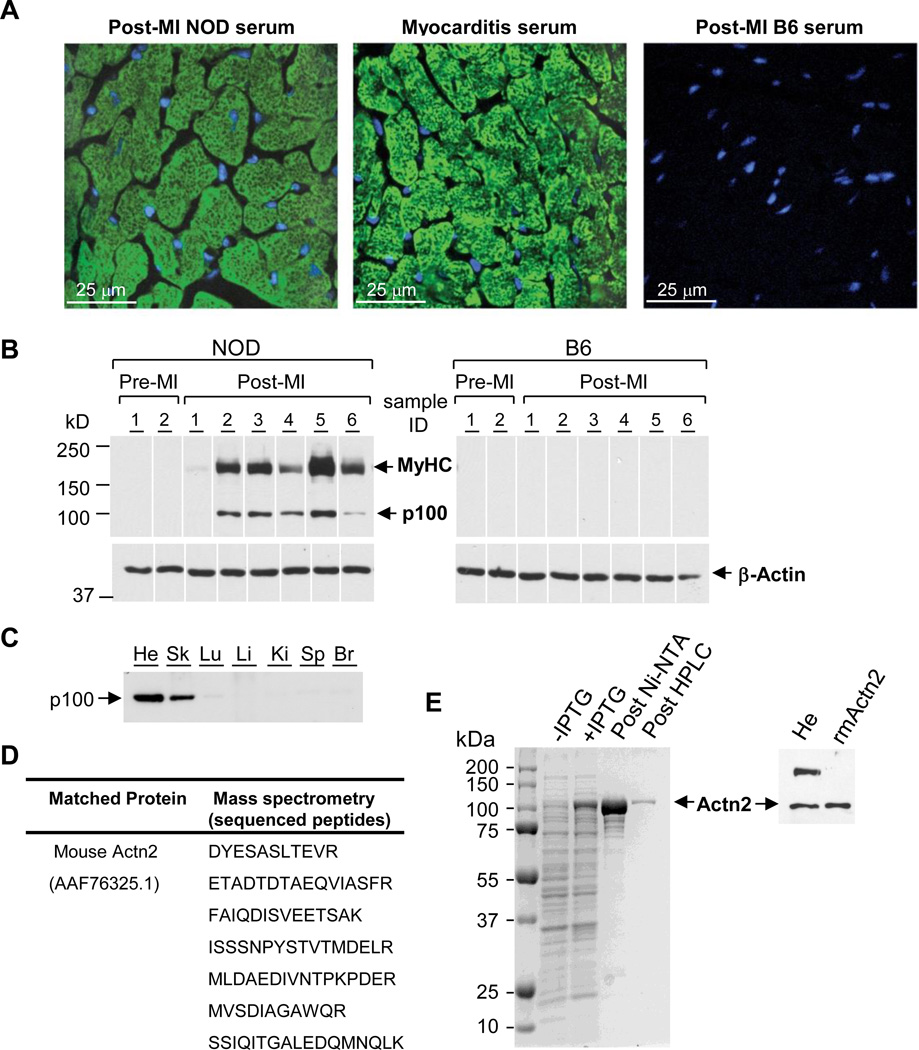
Consistent with our previous studies, none of the NOD mice tested positive for cardiac autoantibodies at baseline, prior to infarction (17, 18). However, as early as 2 wk after MI, NOD – but not B6 – mice developed high-titers of circulating IgG antibodies which, by indirect immunofluorescence and confocal microscopy, produced a distinct myofibrillar pattern on normal heart tissue sections, similar to that produced by serum from DQ8+NOD mice with myocarditis (“Myocarditis serum”, Figure 2A). Western blotting revealed that circulating autoantibodies from post-MI NOD mice not only recognized cardiac myosin heavy chain (MyHC) but also, unexpectedly, a second ~100 kDa protein which was detectable only in detergent (SDS)-containing lysates (“p100”, Figure 2B). The tissue distribution of p100 was notable for abundant expression in heart and skeletal muscle, and absence in lung, liver, kidney, spleen and brain (Figure 2C). Immunoprecipitation of the 100 kDa band from heart extracts with serum from post-MI NOD mice, followed by excision and enzymatic digestion of the band from the gel and analysis by tandem mass spectrometry, revealed that its peptide sequences were identical to that of the 103,854 kDa actin-binding protein, α-actinin-2 (hereafter, Actn2; Figure 2D), a major structural component of the sarcomeric Z-disk. To further confirm that Actn2 was the 100 kDa antigen recognized by post-MI NOD autoantibodies, we cloned Actn2 cDNA from mouse heart mRNA using RT-PCR, produced recombinant Actn2 as a polyhistidine-tagged fusion protein in E. coli (Figure 2E, left panel), and purified the Actn2 protein on a Ni2+charged Sepharose affinity column, followed by a size-exclusion chromatography. Subsequent immunoblot assays demonstrated that post-MI NOD serum recognized the recombinant mouse Actn2 protein identical to the natively produced Actn2 protein in mouse heart lysates (Figure 2E, right panel).
Figure 2. Acute MI triggers the production of autoantibodies to myosin heavy chain (MyHC) and α-actinin-2 (Actn2) in NOD mice.
(A) Representative indirect immunofluorescence staining on normal mouse heart sections using sera from the indicated mice. Green indicates serum staining; blue indicates nuclear staining (DAPI). (B) Immunoblots containing normal heart extracts probed with sera from individual NOD and B6 mice before MI (Pre-MI) and 3 wk post-MI, or a β-actin mAb as a loading control. Data are representative of 5 experiments. (C) Western blot probed with post-MI NOD serum showing tissue distribution of p100. (He: heart; Sk: skeletal muscle; Lu: lung; Li: liver; Ki: kidney; Sp: spleen; Br: brain). (D) Identification of p100 protein as Actn2. The sequenced peptides shown have 100% identity to mouse Actn2. (E) Expression and purification (left panel) of recombinant mouse Actn2 (rmActn2); Western blot confirming that post-MI sera recognized rmActn2 identical to the Actn2 produced in heart lysates (right panel).
Analysis of the prevalence of autoantibodies to cardiac myosin and Actn2 by ELISA and Western blotting techniques revealed positivity in the majority (72/81 = 89%) of post-MI NOD mice, with autoantibody persistence for at least 8 wk after MI (Figure S1A). In contrast, cardiac autoantibodies were absent or barely detectable in the post-infarcted control B6 mice (n=56), even when followed up to 12 wk post-MI (Figure S1B). Importantly, NOD mice developed autoantibody responses to cardiac myosin and Acnt2 after smaller infarctions (‘microinfarctions’) produced by placing a suture around the left coronary artery, without permanent ligation (Figure S1C). These manipulations resulted in focal areas of myocardial fibrosis by Masson’s trichrome staining (Figure S2), rather than the widespread necrosis characteristic of permanent ligation. However, the frequency of autoimmune responses after microinfarction was much less than after full-scale myocardial infarction, with 40% of microinfarcted NOD mice (6/15) exhibiting positive cardiac autoantibody titers (Figure S1C). These findings suggested that the severity of post-MI autoimmunity correlated with the magnitude of the initial cardiac injury. Importantly, sham-MI NOD mice (n=10) that received open-chest thoracotomy alone, but no coronary occlusion, did not develop autoantibodies or cardiac infiltrates (Figures S1C and S3). Thus, the development of autoimmunity was strictly due to myocardial injury and did not result from the nonspecific effects of open-chest surgical trauma or anesthesia.
Induction of T helper type 1 (Th1) effector responses to cardiac myosin
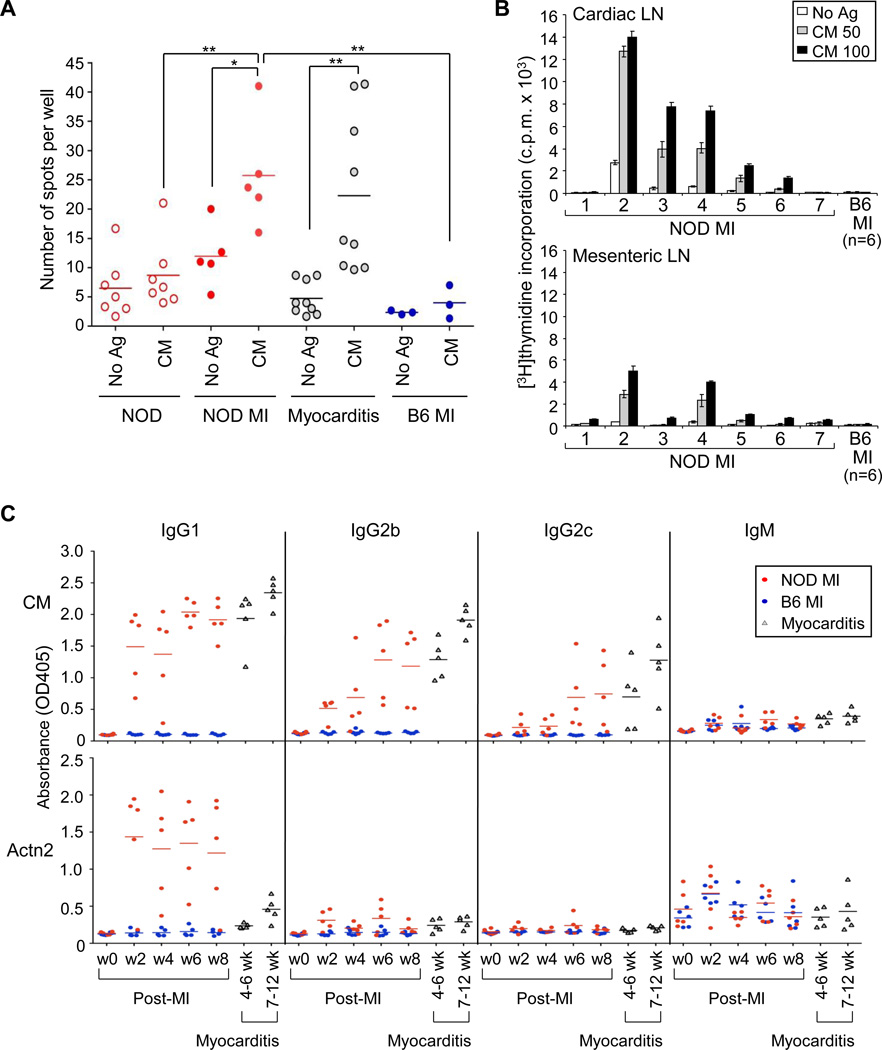
The development of lymphocytic infiltrates in the myocardium and circulating IgG autoantibodies to cardiac myosin and Actn2 suggested that signals from ischemic heart injury alone – in the absence of microbial triggers – induced adaptive immune responses in NOD mice. To examine whether cardiac myosin and Actn2 were also targets of Th1 cells that are the main mediators of destruction in spontaneous myocarditis (18), we performed interferon (IFN)-γ enzyme-linked immunospot (ELISPOT) assays on splenocytes from 21 d post-MI NOD mice and B6 mice. These studies revealed that MI induced robust Th1 effector responses to cardiac myosin that were similar in magnitude to the responses found in DQ8+NOD mice with myocarditis (Figure 3A). We also found strong dose-dependent T cell responses to cardiac myosin in cardiac-draining lymph nodes (Figure 3B, upper panel), which were markedly enlarged by 8 wk post-MI. These responses were substantially greater than those found in mesenteric lymph nodes (Figure 3B, lower panel). As expected, none of post-MI B6 control mice showed enlarged cardiac lymph nodes or increased T cell responses to cardiac myosin (Figure3A and 3B).
Figure 3. T and B cell responses in post-MI NOD mice and post-MI B6 mice.
(A) Splenocytes from 3 wk post-MI NOD and B6 mice were stimulated in vitro with 25 µg/ml of cardiac myosin (CM) and IFN-γ-producing T cells were detected by ELISPOT assays. Splenocytes from unmanipulated NOD mice (‘NOD’) and DQ8+NOD mice with spontaneous myocarditis (‘Myocarditis’) were examined for comparison. *P < 0.05; **P < 0.001. (B) Cardiac-draining lymph node (LN, upper panel) and mesenteric LN (lower panel) cells from 7 NOD and 6 B6 (pooled) mice 8 wk after MI were stimulated with the indicated amounts of CM, and T cell responses were detected by 3[H]thymidine incorporation. (C) Dynamics of anti-CM and anti-Actn2 immunoglobulin (Ig) isotype switching was determined in bi-weekly serial sera from post-MI NOD and B6 mice (n=5 mice/group). w0=pre-MI bleed; w=wk post-MI. Serial sera from the same DQ8+NOD mice (n=5) taken between ages 4–6 wk and then at 7–12 wk, were analyzed side-by-side for comparison.
Surprisingly, we were unable to detect augmented T-cell responses to Actn2 in post-MI NOD mice, regardless of whether we used IFN-γ ELISPOT or proliferation assays (Figure S4). To further understand the basis for these differential T cell reactivities, we measured the isotypes of autoantibodies specific to cardiac myosin and Actn2 in serial serum samples collected bi-weekly from post-MI NOD and B6 mice (Figure 3C). As shown in Figure 3C, modest increases of cardiac myosin-specific IgM antibodies were observed by 2 wk post-MI in both NOD and B6 mice, suggesting comparable T-cell independent immune responses in the two strains. However, cardiac myosin-specific IgG1, IgG2b, and IgG2c autoantibodies were found only in NOD mice, with the peak titer to IgG1 occurring earlier than that to IgG2b and IgG2c (Figure 3C, upper panel). In contrast, cardiac myosin-specific IgG3 and IgA responses were not detectable (Figure S5). These studies suggested that both Th1 and Th2 cells contributed to the cardiac myosin-specific IgG antibody switch. Interestingly, the post-MI cardiac myosin-specific autoantibody isotype profiles were almost identical to those found in DQ8+NOD mice with spontaneous myocarditis (Figure 3C).
In contrast to cardiac myosin, the Actn2-specific autoantibodies were almost exclusively IgG1, with minimal IgG2b and no IgG2c production (Figure 3C, lower panel), suggesting a Th2 cell-dominated response and providing an explanation for our inability to detect Actn2-specific Th1 responses in post-MI NOD mice. Thus, experimental MI triggered the sustained breakdown of T and B cell tolerance to cardiac myosin in NOD mice, with the development of chronic lymphocytic infiltration in the myocardium and impaired infarct healing. We denote this constellation of pathological and immunological features ‘post-myocardial infarction autoimmunity’ (hereafter, PIA).
No evidence of myositis after acute skeletal muscle injury in NOD mice
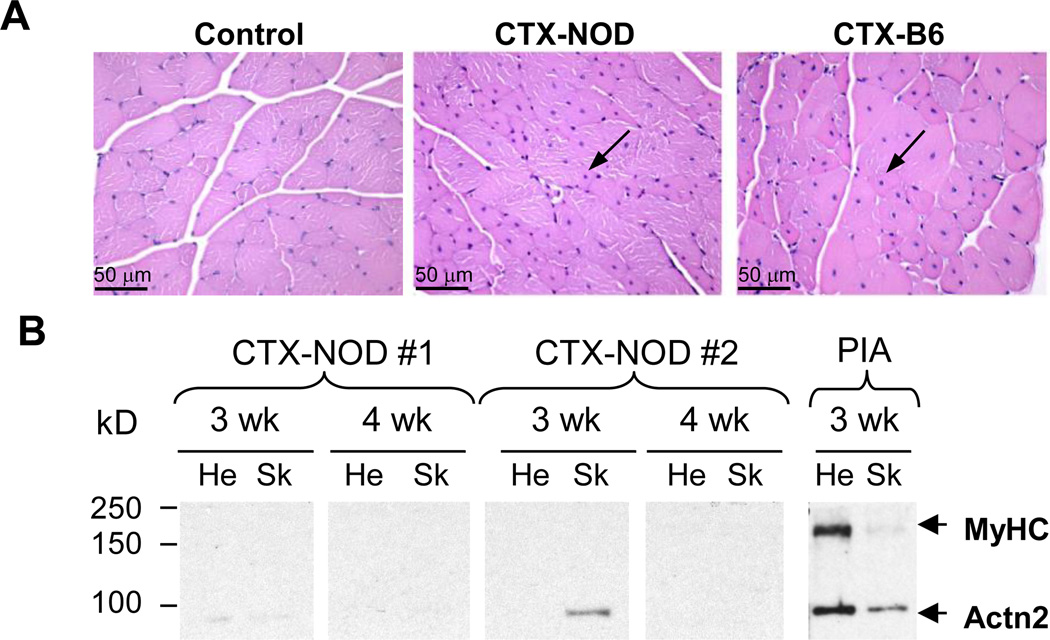
One mechanism by which autoantibodies are postulated to arise after necrotic heart injury is via the release of normally sequestered intracellular proteins to the immune system (23). Since Actn2 is expressed at similar levels in skeletal and cardiac muscle (Figure 2C) but α-MyHC is only expressed in cardiac muscle, we next tested whether autoimmunity could also be elicited by skeletal muscle injury. To this end, we subjected the 4 hindlimb muscles (tibialis anterior and triceps surae on each leg) of NOD and B6 mice to one of three different forms of acute necrotic skeletal muscle injuries: cold injury (dry ice), mechanical injury (crush injury), and chemically induced injury (cardiotoxin [CTX] injection] (24). Cardiotoxin was of particular interest because it has been shown to elicit strong proinflammatory responses, with recruitment of ~10-fold increased frequency of inflammatory cells to the damaged muscle and induction of markedly enhanced local cytokine and chemokine gene expression (24). Following each type of muscle injury, mice (n = 4 female NOD and B6 mice per group except for CTX, n = 10 female mice of each strain) were followed with weekly screening of sera for cardiac and skeletal muscle autoantibodies. In contrast to acute MI, none of these three forms of skeletal muscle injuries elicited histological evidence of myositis, and completely normal muscle regeneration was observed 4 wk after injury, i.e., transverse muscle sections showed myocytes of varying diameters, containing centrally located nuclei characteristic of newly regenerated fibers (Figure 4A, arrows), identical in appearance to the regenerated B6 muscle fibers. In addition, none of the mice developed sustained autoantibodies to skeletal myosin or Actn2, although low-titer responses were sometimes observed by Western blot analysis (Figure 4B). Thus, the development of PIA was not due to a generalized predisposition to tissue injury-induced autoimmunity or to intrinsic defects in tissue-repair responses in NOD mice. These findings suggested that the activation of T cells specific for released cardiac myosin might be required for the induction of PIA.
Figure 4. No evidence of myositis with normal muscle regeneration after acute skeletal muscle injury in NOD mice.
(A) Representative H&E stained cross sections of muscles from untreated NOD mice (’control’), and from NOD and B6 mice 4 wk after i.m. injection of cardiotoxin (CTX-NOD, CTX-B6, respectively). Arrows point to centralized nuclei characteristic of newly regenerated skeletal muscle. (B) Western blots containing heart (He) and skeletal muscle (Sk) lysates probed with sera from two representative CXT-NOD mice, and a post-MI NOD mouse (‘PIA’).
Prevention of PIA by induction of central tolerance to α-MyHC
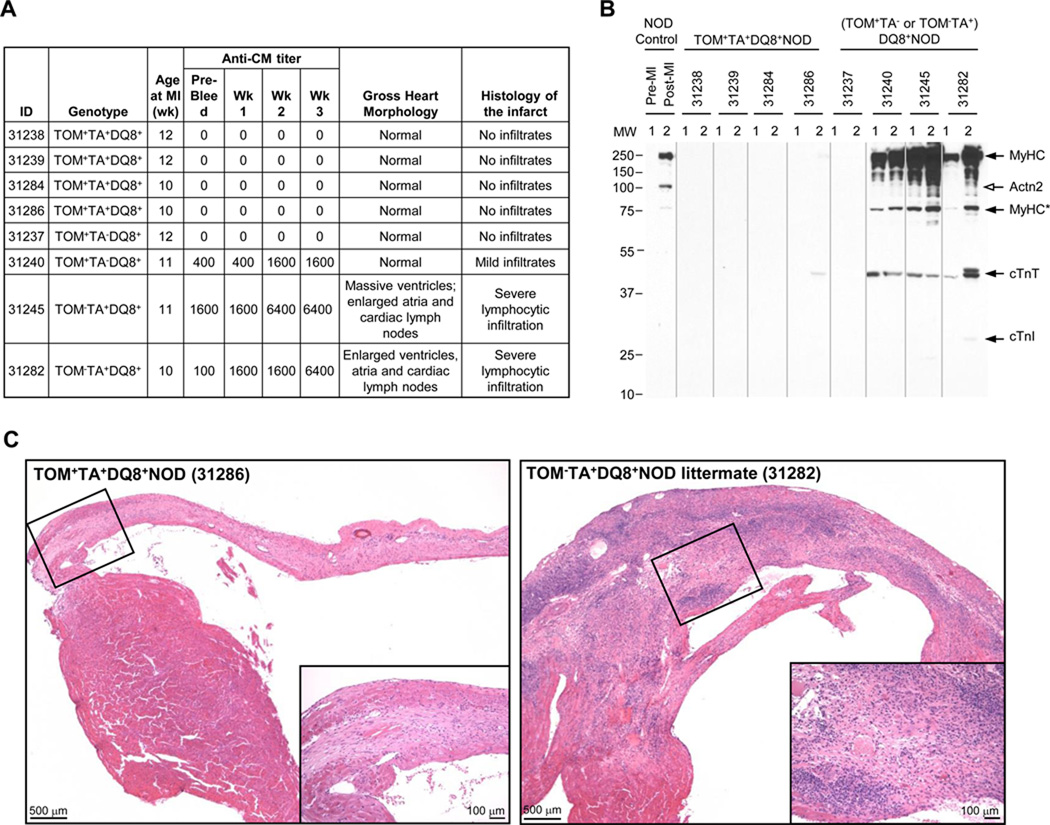
We recently reported that the transgenic expression of α-MyHC in the thymus of DQ8+NOD mice conferred tolerance to cardiac myosin and prevented the development of myocarditis, demonstrating that cardiac myosin is a primary autoantigen in this spontaneous autoimmune disease process (18). To directly test whether immune responses to cardiac myosin are also required for the development of PIA, we induced MI in transgenic thymic α-MyHC-expressing TOM+TA+DQ8+NOD mice (n = 4) and in control non-thymic myosin-expressing (TOM+TA− or TOM−TA+)DQ8+NOD mice (n = 4), with follow-up to 20 days. As expected from our previous studies (18), prior to MI none of the thymic-myosin expressing TOM+TA+DQ8+NOD mice, but 3 of 4 control (TOM+TA− or TOM− TA+)DQ8+NOD mice tested positive for cardiac myosin autoantibodies with titers ranging from 1:100 to 1:1600 (Figure 5A). Following MI, there was a marked increase in cardiac myosin autoantibody titers in 3 of 4 control DQ8+NOD mice, with 2 mice developing titers to 1:6400 by 20 d post-MI (Figure 5B). Western blotting using serum from control mice confirmed these findings but also showed the appearance of autoantibodies to cardiac troponin T (cTnT, Figure 5B) and weaker reactivity to cardiac troponin I (cTnI). In contrast, none of the thymic myosin-expressing TOM+TA+DQ8+NOD mice developed cardiac myosin autoantibody titers by ELISA, although one mouse showed faint reactivity to cTnT by Western blotting (Figure 5B). Necropsy findings correlated with these results with the two highest-titer DQ8+NOD mice demonstrating dilated hearts and massive enlargement of cardiac draining lymph nodes 20 d post-MI, but normal heart sizes in the other mice (Figure 5C). Histology of the post-MI hearts revealed severe pathology in the control TOM−TA+DQ8+NOD mouse hearts, with dense lymphocytic infiltrates throughout the infarct zone, infarct expansion and lymphocytic invasion into non-infarcted myocardium (Figure 5C, right panel), suggesting exacerbated PIA in DQ8+NOD mice compared with WT NOD mice (Figure 1, upper panel). These findings are consistent with previous reports showing impaired infarct healing in mice with pre-existing myocarditis (21). In contrast, none of the post-MI hearts from the α-MyHC-tolerant TOM+TA+DQ8+NOD mice exhibited lymphocytic infiltrates or evidence of impaired infarct healing, and instead showed dense scar formation (Figure 5C, left panel). These findings suggest that the autoimmune response in PIA was antigen-specific and driven by the loss of tolerance to a single autoantigen, α-MyHC.
Figure 5. PIA is augmented in DQ8+NOD mice and prevented by inducing thymic tolerance to α-MyHC.
(A) Features of the pre- and post-MI (TOM+TA− or TOM−TA+) DQ8+NOD and TOM+TA+DQ8+NOD mice. (B) Immunoblots containing normal mouse heart extracts probed with the indicated sera. NOD WT control, Pre-MI = pre-bleed; Post-MI = 21 d post-MI; For (TOM+TA+ or TOM+TA− or TOM−TA+) DQ8+NOD mice sera, 1 = pre-MI bleed; 2 = 20 d post-MI bleed. Cardiac antigens recognized by sera are indicated on the right. The open arrow indicates the position of Actn2, which was detected in serum from the post-MI NOD control, but not in the (TOM+TA− or TOM−TA+) DQ8+NOD mice. MyHC* is a MyHC degradation product. (C) H&E staining of 20 d post-MI hearts of the control TOM−TA+DQ8+NOD mouse (31282) and the thymic α-MyHC-expressing TOM+TA+DQ8+NOD littermate (31286).
Discovery of a PIA syndrome in human T1D patients
The development of PIA in NOD mice and its augmentation in DQ8+NOD mice raised the possibility that T1D human patients might also develop PIA following MI. Because circulating cardiac autoantibodies were detected in NOD mice soon after MI and remained persistently elevated (Figure 3C), it appeared that the exact timing of the sample collection relative to the date of the coronary event would not be critical.
Although indirect immunofluorescence serum staining of heart sections (Figure 2A), Western blotting (Figure 2B) and ELISA techniques (Figure 3C) were all reliable for cardiac myosin autoantibody detection in mice, we found that human sera performed poorly in these assays with a high prevalence of cardiac myosin autoreactivity in healthy control sera (Figure S6). Moreover, since full-length human α- and β-MyHC proteins could not be efficiently produced in E. coli, we were reliant on using cadaveric human heart tissues as a source of antigen, which prevented assay standardization. We therefore investigated whether fluid-phase radioimmunoprecipitation assays (RIAs), that are widely used to diagnose T1D and other organ-specific autoimmune diseases (25), might also be useful for identifying autoimmune heart disease. RIAs were thus developed using in vitro transcribed and translated complementary DNAs (cDNAs) encoding human α-MyHC (MYH6), a major T cell autoantigen in human myocarditis (18), and human β-MyHC (MYH7) which is 93% identical to α-MyHC and is the predominant myosin expressed in adult human ventricle. As positive controls for validating these assays, we examined serum reactivities from 18 consecutively recruited patients with myocarditis established on clinical, pathological (endomyocardial biopsy), or cardiac magnetic resonance imaging (MRI) criteria (26). Negative controls consisted of 78 consecutively recruited healthy subjects. With these new assays, we found that 2/78 (3%) of healthy control subjects, but 5/18 (28%) patients with myocarditis tested positive for α- or β-MyHC autoantibodies (Table 1). While the prevalence of MyHC autoantibodies in myocarditis patients was relatively low, the patterns of reactivity were striking with positivity to both human α- and β-MyHC (Table 1, red circles), similar to the dual isoform reactivity of serum from DQ8+NOD mice with myocarditis (18) but distinctive from the two healthy control subjects in which reactivity was only to a single MyHC isoform. Interestingly, the duration of symptoms was relatively short (mean = 7 d; range, 4–11d) in the full-length α- and β MyHC autoantibody-positive myocarditis patients (Table 1), suggesting an acute disease process.
Table 1.
Characteristics of the cardiac autoantibody-positive patients
| Diagnosis | Patient ID |
Age (y) | Gender | Timing of sample from MI (y) |
α-MyHC FL |
α-MyHC Fragments | β-MyHC FL |
Actn2 | cTnI | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| S1 | S2 | LMM | |||||||||
| T1D+MI+ | TID-1 | 68 | F | u | − | − | − | − | − | + | − |
| TID-2 | 56 | F | 7 | − | + | + | − | − | − | − | |
| TID-5 | 39 | F | 2 | − | − | − | + | − | |||
| TID-6 | 59 | M | 4 | + | − | − | − | − | |||
| TID-7 | 67 | F | 5 | − | + | − | − | − | − | − | |
| TID-8 | 61 | M | 7 | + | − | − | − | + | |||
| TID-9 | 69 | M | 8 | − | + | − | − | − | − | − | |
| TID-10 | 57 | F | 5 | − | + | − | − | − | − | − | |
| TID-12 | 52 | M | u | − | + | − | − | − | − | − | |
| TID-13 | 19 | M | 5 | − | − | − | + | − | − | − | |
| TID-14 | 46 | F | 8 | − | + | − | − | − | − | − | |
| TID-15 | 66 | F | u | − | − | − | − | − | + | − | |
| TID-16 | 59 | M | 3 | + | − | − | − | − | |||
| TID-17 | 55 | M | 3 | − | − | − | − | − | − | + | |
| TID-18 | 54 | F | 0.5 | − | + | − | − | − | − | + | |
| T2D+MI+ | T2D-4 | 63 | M | 10 | − | − | − | + | − | − | − |
| T2D-5 | 57 | M | 0.9 | − | − | − | − | + | − | − | |
| T2D-20 | 59 | F | 11 | − | + | − | − | − | − | + | |
| Duration of symptoms (d) | |||||||||||
| Myocarditis (Non-diabetic) |
M-1 | 36 | M | 6 | − | + | + | − | − | ||
| M-2 | 51 | F | 15 | − | − | − | − | − | − | + | |
| M-3 | 50 | F | 137 | − | + | + | − | − | − | − | |
| M-4 | 21 | M | 196 | − | − | − | + | − | − | − | |
| M-5 | 33 | M | 4 | − | + | + | − | − | |||
| M-6 | 26 | M | 866 | − | + | − | − | − | − | − | |
| M-7 | 21 | M | 8 | + | − | − | − | − | |||
| M-8 | 19 | M | 13 | − | + | − | + | − | + | − | |
| M-9 | 20 | F | 4 | − | − | + | − | − | − | − | |
| M-10 | 55 | M | 5 | + | − | − | − | − | |||
| M-11 | 37 | F | 11 | + | − | − | − | − | |||
| M-12 | 20 | M | 1 | − | − | − | − | − | + | − | |
Autoantibody reactivity was analyzed by RIA to the following human cardiac proteins: full-length (FL) α-MyHC; S1, S2, and LMM fragments of α-MyHC; FL β-MyHC; α-actinin-2 (Actn2); and cardiac troponin I (cTnI). −, negative or +, positive for antibodies as defined in Figure 6B; u, timing of MI unknown. Patient T1D-13 has T1D and familial hypercholesterolemia. Red circles indicate dual positivity to FL α- and β-MyHC.
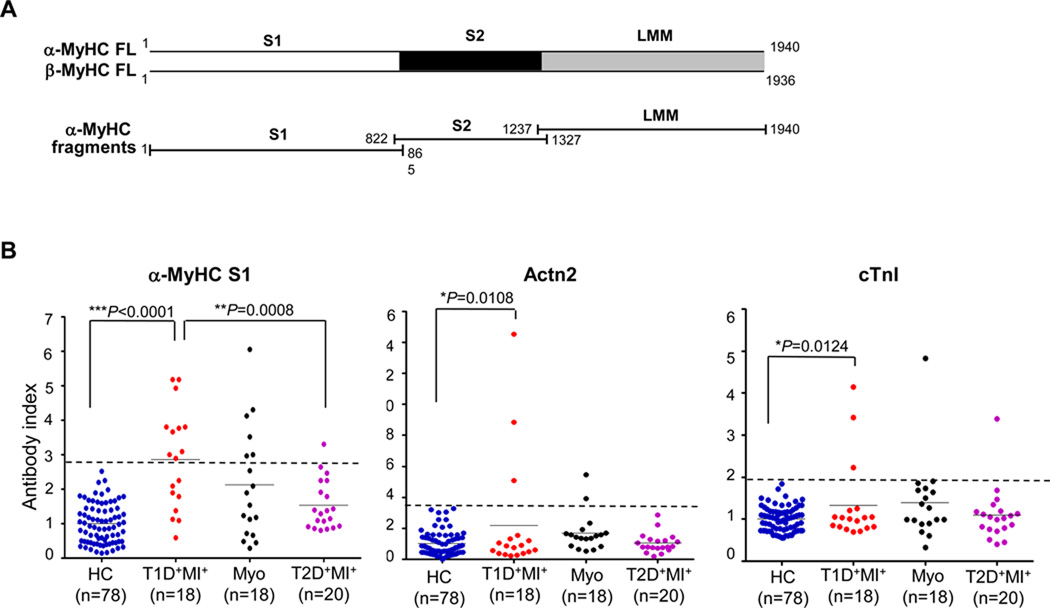
To further refine these assays, we developed RIAs using overlapping cDNA fragments corresponding to the three major functional domains of α-MyHC (S1, S2 and LMM) (Figure 6A). With these fragment assays, the sensitivity of cardiac myosin autoantibody detection almost doubled with 10/18 (56%) of myocarditis patients testing positive for one or more fragments (Table 1), compared to 3/78 healthy controls (4%, P < 0.0001). Based on these results, we developed RIAs for the detection of autoantibodies to human Actn2 and human cardiac troponin I (cTnI). Cardiac troponin I was of special interest because it has been implicated as an autoantigen in mouse models of autoimmune cardiomyopathy (21, 27) and autoantibodies to cTnI have been reported in a subset of human patients immediately post-MI (28).
Figure 6. Evidence for a PIA syndrome in human T1D patients.
(A) Scheme of constructs used in cardiac myosin autoantibody radioimmunoprecipitation assays. FL, full length; fragments corresponding to the S1, S2 and LMM domains of human α-MyHC. (B) Prevalence of autoantibodies to the S1 domain of human α-MyHC (α-MyHC S1), human α-Actinin-2 (Actn2), and human cardiac troponin I (cTnI) in healthy control (HC), post-MI T1D (T1D+MI+), myocarditis (Myo), and post-MI type 2 diabetic (T2D+MI+) subjects. All assays were performed in duplicate as described in Materials and Methods. The dashed lines indicate the upper limit of the normal range, defined as the mean of the antibody index values obtained from the healthy controls plus 3 SD.
The ischemic heart disease cohort consisted of 18 post-MI T1D patients, 56% females (10/18), mean age of 55 ± 12 y with mean time interval from MI to autoantibody testing of 4.5 y (range, 0.3–8 y) and 20 consecutively recruited post-MI type 2 diabetes (T2D) patients, 70% males (14/20), mean age of 60 ± 10 y with mean time interval from MI to antibody testing of 8.1 y (range, 0.1–20 y) (Supplementary Table 1). The diagnosis of T1D was made by clinical history and confirmed by high resolution HLA-DQB genotyping, with 16/18 subjects (89%) testing positive for the high T1D-risk DQB1*0201 or DQB1*0302 genotypes (Supplementary Table 1).
Our results showed that 15/18 (83%) of post-MI T1D patients tested positive to autoantibodies to one or more cardiac antigens (α-MyHC, β-MyHC, cTnI or Actn2) or fragments of α-MyHC (S1, S2, or LMM), in contrast to 3/20 (15%) post-MI T2D patients and 3/78 (4%) healthy control subjects (T1D post-MI versus T2D post-MI patients, P = 0.0001; T1D post-MI versus healthy control subjects, P < 0.0001; T2D post-MI versus healthy control subjects, P = 0.1834) (Table 1). Furthermore, despite substantial differences in the etiologies and clinical presentations between the post-MI T1D and myocarditis patients, the prevalence and specificities of the cardiac autoantibodies in the two conditions was similar (Figure 6 and Table 1), with predominant targeting of cardiac myosin (Table 1). In particular, we found that 4 of 18 (22%) post-MI T1D subjects tested positive for autoantibodies to both α- and β-MyHC, which was also a feature of myocarditis serum, but was absent in serum from post-MI T2D patients and healthy control subjects (Table 1).
In addition, the α-MyHC S1 domain was recognized by serum in 10/12 (83%) post-MI T1D patients who tested positive for cardiac myosin autoantibodies, either in the full-length or fragment assays (Table 1). Reactivity also clustered in the S1 domain of serum samples from myocarditis patients, albeit at lower prevalence (6/10 cardiac myosin autoantibody-positive subjects), likely reflecting the greater clinical heterogeneity of this group (26). Importantly, since the serum samples from the post-MI T1D subjects were obtained many years after the cardiac event, the cardiac autoantibodies appeared to be persistent. Indeed, in a limited longitudinal follow-up study of two post-MI T1D patients (T1D-2 and T1D-8, Table 1), the subjects tested positive for cardiac myosin autoantibodies approximately 1 y after the initial serum sample collection, confirming autoantibody persistence.
Confirmation of myocarditis by noninvasive cardiac magnetic resonance imaging (MRI) techniques in a post-MI T1D patient with presumed PIA
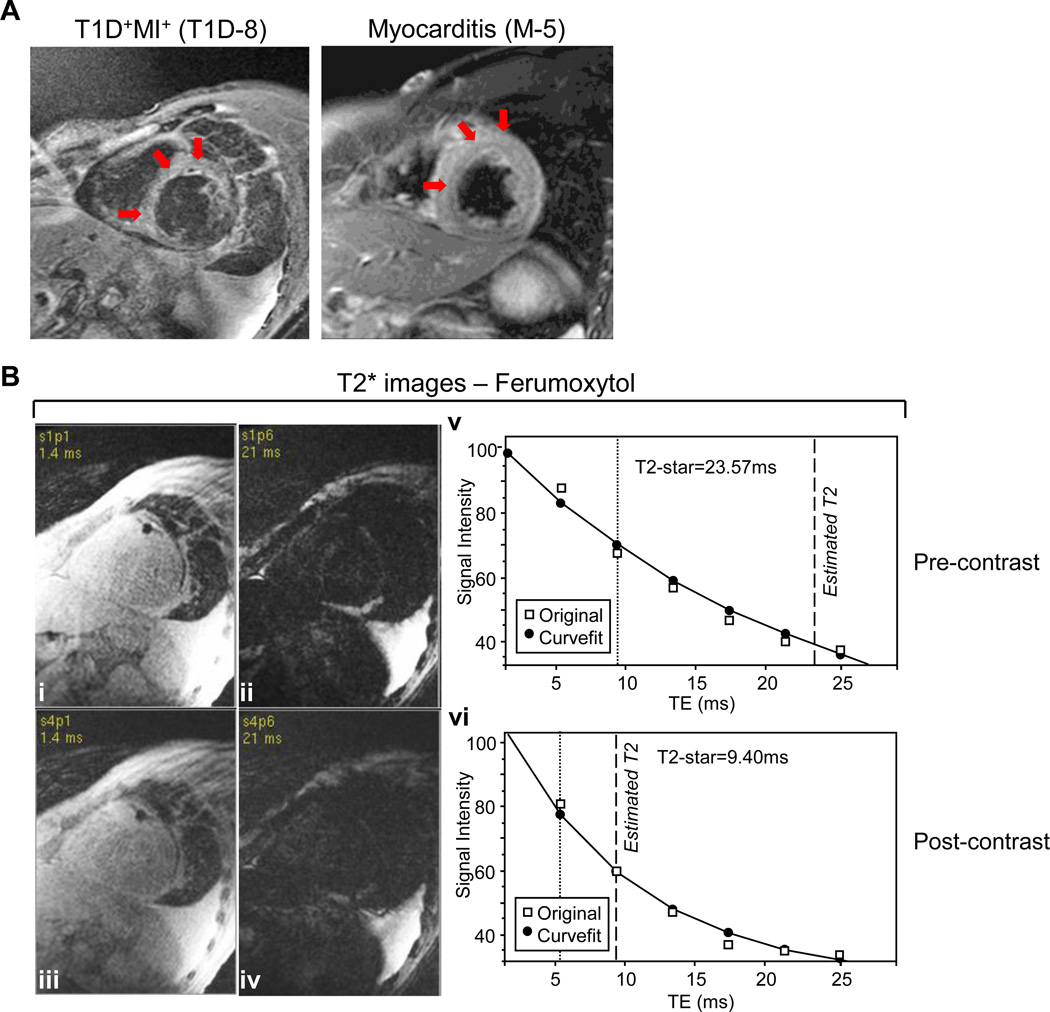
While these autoantibody profiles were suggestive of a chronic myocardial autoimmune process in post-MI T1D patients, we sought to obtain additional evidence of myocarditis. Cardiac MRI has recently emerged as a leading non-invasive tool to diagnose myocarditis as well as to follow its course in patients (29). We therefore tested the feasibility of using MRI to detect myocarditis in post-MI patient T1D-8, a 61-y-old male with longstanding T1D who tested positive for multiple cardiac autoantibodies including α-MyHC, β-MyHC, α-MyHC S1, and cTnI (Table 1, Figure 7). The patient’s cardiac history was notable for an inferior MI approximately 6 y prior to entering our study, with initial preserved left ventricular function (normal ejection fraction of 65%), but subsequent development of global biventricular dysfunction and a steady decline in ejection fraction that was not explained by the extent of his coronary artery disease. Cardiac MRI with conventional contrast agents on T2-weighted black-blood short-tau inversion recovery (STIR) technique revealed evidence of diffuse myocardial inflammation (Figure 7A). The T2-signal intensity was markedly elevated, consistent with myocardial edema from myocarditis, and was similar in severity to subject M-5 (Table 1, Figure 7A), a 33-y-old male with no significant past medical history who presented with acute myocarditis. Further evidence of myocardial inflammation in patient T1D-8 was demonstrated by a complementary approach using the superparamagnetic iron oxide-based contrast agent, Ferumoxytol, which is avidly taken up by macrophages (Figure 7B). MRI with similar iron oxide contrast agents has been used to image early post-MI inflammatory repair processes in mice (30) and pancreatic insulitis lesions in human T1D patients (31). T2* by cardiac MRI is a decay constant which is inversely proportional to uptake of the iron-oxide contrast media by myocardial tissue. Comparing the T2* measurement before contrast injection to myocardial T2* by cardiac MRI at 24 h after injection of Ferumoxytol showed markedly reduced T2* values (from 24 msec immediately pre-contrast to 9 msec 24 h post-contrast) as reflected by the rapid decay curve (Figure 7B, vi). This was strongly indicative of increased uptake of iron-contrast by an ongoing inflammatory reaction in this patient’s myocardium. Taken together, this patient’s clinical, immunological, and cardiac MRI findings suggest that a PIA syndrome also develops in human T1D.
Figure 7. Cardiac magnetic resonance imaging (MRI) confirmation of myocarditis in a post-MI T1D patient with presumed PIA.
(A) Cardiac MRI of post-MI T1D patient TID-8 (left panel) with progressive heart failure (LVEF = 18%) and dilated cardiomyopathy and as a positive control, myocarditis patient M-5 (right panel) with a moderately reduced LVEF (42%) but a normal LV size.(B) Myocardial inflammation was also demonstrated in patient TID-8 by comparing T2* images before (i and ii, at echo times of 1.4 and 21 msec, respectively) and after (iii and iv, at echo times of 1.4 and 21 msec, respectively) injection of the iron-oxide contrast agent, Ferumoxytol. At 24 hours after injection, there was evidence of accumulation of iron-oxide in the myocardium, indicated by the decay constants of T2* (decay curves shown in v and vi, 24 msec from before vs. 9.4 msec after iron-oxide injection). Patient TID-8 experienced sudden cardiac death less than 2 years later.
DISCUSSION
Although until now, the poor CVD outcomes in T1D have been mainly attributed to the metabolic effects of diabetes (6, 32) the data presented here point to an alternative or additional explanation for why T1D patients are at increased risk for adverse events following MI. In particular, we demonstrate for the first time that experimental MI in normoglycemic NOD mice triggers the development of a severe post-infarction autoimmune (“PIA”) syndrome characterized by the presence of persistent cardiac autoantibodies, proinflammatory Th1 effector responses to cardiac myosin, destructive lymphocytic infiltrates in the myocardium and impaired infarct healing with pathological remodeling. We further demonstrate that although acute necrotic injury from MI might have been expected to result in the loss of tolerance to multiple autoantigens, PIA is primarily driven by T-cell responses against a single autoantigen, α-MyHC, resembling spontaneous autoimmune disease (18). Using a novel panel of sensitive and specific RIAs, we then show that 83% of post-MI T1D patients have positivity for cardiac autoantibodies. We further demonstrate that the autoantibodies from post-MI T1D patients predominantly target the S1 domain of human α-MyHC, and identify cardiac myosin autoantibody profiles that are shared between post-MI T1D and non-diabetic patients with acute myocarditis, but are absent in post-MI T2D patients and healthy controls. Detailed cardiac MRI studies in one such autoantibody-positive post-MI T1D patient demonstrated the presence of diffuse myocardial inflammation. Collectively, these studies indicate that MI triggers PIA in both NOD mice and T1D patients, raising the possibility that this disease process contributes to poor post-MI outcomes in T1D.
We propose that PIA is mediated by several mechanisms that work independently and in combination to break self-tolerance and promote autoimmunity. First, absence of α-MyHC expression in the thymus results in the escape of high avidity cardiac myosin-specific autoreactive T cells into the peripheral blood of both humans and mice (18). Second, tissue necrosis from MI releases massive amounts of cardiac myosin, providing a potent antigenic stimulus (‘Signal 1’) to promote clonal expansion of T cells already enriched in cardiac myosin specificities. Third, the innate post-MI inflammatory repair response - characterized by the influx of macrophages and dendritic cells that are required to remove necrotic tissue, along with local production of high concentrations of proinflammatory cytokines and chemokines - would be expected to provide the requisite co-stimulatory signals (‘Signal 2’) to promote maturation and migration of dendritic cells to cardiac-draining lymph nodes. Collectively, these three factors likely explain why the risk of generating autoimmune responses to the heart is so high in the general population following MI; however, these reactions are typically mild and resolve with tissue healing (19, 20). Our findings indicate that in the setting of the immunoregulatory defects of T1D - and potentially in other autoimmune diseases - these responses persist and expand, leading to uncontrolled (‘runaway’) myocardial autoimmunity and impaired infarct healing (Figure 1, Figure 5). Thus, in many respects, PIA resembles other chronic inflammatory disorders in which tissue destruction results from a vicious circle maintained by an uncontrolled local immune response (33).
Although the role of inflammation has been most intensively studied with respect to the development of atherosclerosis and acute plaque rupture (34), inflammatory pathways also play a crucial role in the early stages of cardiac repair after MI. However, timely resolution of the inflammatory infiltrate is essential for optimal infarct healing, and inflammation around the zone of hypoxic necrosis makes a major contribution to the final size of the infarct and the clinical outcome (8, 9). Multiple “points of control” exist to ensure that the inflammatory response is contained both topographically and temporally. These suppressive signals are critical for healing because they prevent a persistent, expanding inflammatory response (9). Although the cellular and molecular events responsible for downregulation and containment of the post-MI inflammatory cascade remain unknown, our data suggest that the development of PIA interferes with infarct healing such that the dominant effect is chronic inflammation, adverse left-ventricular remodeling and ongoing myocardial damage (Figure 1, Figure 5).
Previous studies have shown that myocarditis can be induced in genetically susceptible strains of mice by administration of dendritic cells loaded with dead cardiomyocytes or α-MyHC peptides, but only provided that the dendritic cells were first activated by microbial or viral adjuvants (35). In contrast, our studies show that in autoimmune-prone NOD hosts, MI functions as an “endogenous adjuvant” and induces the full development of adaptive immune responses (‘adaptive autoimmunity’) (14), in the absence of microbial stimuli. In addition, while it has been debated whether immune responses from necrotic cell death are primarily stimulated by the release of endogenous ‘danger’ signals (such the high-mobility group box 1 protein), or by the release of intracellular self-antigens (13), the prevention of PIA in α-MyHC tolerant mice (Figure 5) suggests that the activation of autoreactive T cells by the release of cardiac myosin is essential for this disease process. The cardiac tissue-specificity of PIA was also underscored by the lack of histological evidence of enhanced insulitis, thyroiditis or sialitis in NOD MI mice compared to NOD sham-MI mice (Figure S7).
Although PIA was characterized by the development of autoantibodies to Actn2, this was not accompanied by the induction of Th1-type responses that drive myocarditis in DQ8+NOD mice (18), with Actn2-specific isotype profiles showing predominantly humoral responses (Figure 3). In searching public array databases, we found that Actn2 expression was absent or barely at detection levels in both mouse thymic medullary epithelial cells and extra-thymic Aire-expressing cells, resembling the absent expression of α-MyHC in these cell types (Supplementary Table 2). These findings indicate that the presence or absence of antigen in thymus and peripheral lymphoid organs cannot be the only criterion that defines a pathogenic autoantigen. Recent studies have emphasized the influence of quantitative events in determining the differentiation and function of helper T cells, with high antigen doses favoring the development of Th1 responses, and low antigen doses favoring the development of Th2 responses (36). Along this line, previous studies have shown that Acnt2 is highly susceptible to hypoxic damage and is preferentially released from myofilaments, even after mild ischemia (37), a property that is postulated to underlie the rapid (minutes to hours) unraveling of the Z-disk after ischemic injury (38). Furthermore, while Actn2 is a major component of the Z-disk, it accounts for less than 20% of the Z-disk weight (39), raising the possibility that by ~7 days post-MI when the influx of macrophages and dendritic cells into the myocardium is maximum (11, 30), there may be insufficient amounts of Actn2 remaining to prime Th1 responses. Thus, the Actn2 effector class might also be influenced by the peripheral target organ responses to tissue injury. Regardless of the mechanisms involved, it should be noted that post-MI DQ8+NOD mice did not develop autoantibodies to Actn2 (Figure 5), suggesting an additional role for MHC genes in the generation of autoimmunity to Actn2.
The present study has some limitations. First, while we could clearly establish a cause-and-effect relationship between MI and induction of cardiac autoimmunity in NOD mice, our human studies were cross-sectional. It will be important in future studies to measure the temporal appearance and specificity of autoantibody production in prospectively collected pre- and post-myocardial infarction samples from T1D patients. Second, although our patients were carefully phenotyped and immunologically characterized with multiple autoantibody assays, our sample size was relatively small. In particular, we were only able to perform MRI studies on a single T1D patient. However, we found clear evidence of myocardial inflammation using two complementary MRI techniques. These proof-of-concept studies open up the possibility for a more focused analysis of larger numbers of patients. Finally, given recent evidence suggesting that human myocarditis is T-cell mediated (18), it will be important to determine whether the presence of cardiac autoantibodies in post-MI patients correlates with increased frequencies of cardiac myosin-specific T cells in peripheral blood.
The development of transient autoimmune reactions to the heart following MI was first suggested over one-half century ago (19). More recent studies have shown post-mortem evidence of myocardial inflammation in patients who died shortly after MI, but the antigen-specificity of these immune responses was not defined (40). Our studies establish an immunological basis for such responses. Furthermore, applying methods that are widely used for the diagnosis of other organ-specific autoimmune diseases but that have never been used before for detecting autoimmune heart disease, we demonstrate that T1D patients frequently develop persistent cardiac autoimmune responses following MI. In addition, we identify autoantibody signatures that may indicate ongoing “active” myocarditis, a condition that is linked to the progression to chronic dilated cardiomyopathy (29). The potential relevance of our findings is underscored by recent studies showing that heart failure is a major complication of T1D, occurring in one of 30 patients with T1D aged 41–45 years (32). Our data raise the possibility that PIA contributes to poor post-MI outcomes in T1D patients, and point to a role for antigen-specific T-cell tolerance therapies to selectively block this pathogenic process.
Supplementary Material
Footnotes
SUPPLEMENTARY MATERIALS
Materials and Methods
Fig. S1. Cardiac autoantibody production in NOD MI, NOD micro-MI, NOD sham-MI and B6 MI mice.
Fig. S2. Histology of NOD and B6 mouse hearts after microinfarction.
Fig. S3. Histology of NOD and B6 mouse hearts after sham-infarction.
Fig. S4. Analysis of T-cell responses to Actn2 in post-MI NOD mice.
Fig. S5. Analysis of IgA and IgG3 autoantibodies to CM and Actn2 in post-MI NOD and B6 mice.
Fig. S6. Analysis of cardiac autoantibodies in humans by Western blotting.
Fig. S7. Insulitis, sialitis and thyroiditis in post-MI versus sham-MI NOD mice.
Table S1. Characteristics of the T1D and T2D ischemic heart disease cohorts.
Table S2. Microarray data of expression of Actn2, Myh6, and Myh7 genes in mTECs and eTACs from Aire+/+and Aire−/− mice.
Supplementary references
REFERENCES
- 1.Brown JR, O'Connor GT. Coronary heart disease and prevention in the United States. N Engl J Med. 2010;362:2150. doi: 10.1056/NEJMp1003880. [DOI] [PubMed] [Google Scholar]
- 2.Libby P, Nathan DM, Abraham K, Brunzell JD, Fradkin JE, Haffner SM, Hsueh W, Rewers M, Roberts BT, Savage PJ, Skarlatos S, Wassef M, Rabadan-Diehl C. Report of the National Heart, Lung, and Blood Institute-National Institute of Diabetes and Digestive and Kidney Diseases Working Group on Cardiovascular Complications of Type 1 Diabetes Mellitus. Circulation. 2005;111:3489. doi: 10.1161/CIRCULATIONAHA.104.529651. [DOI] [PubMed] [Google Scholar]
- 3.Secrest AM, Becker DJ, Kelsey SF, Laporte RE, Orchard TJ. Cause-specific mortality trends in a large population-based cohort with long-standing childhood-onset type 1 diabetes. Diabetes. 2010;59:3216. doi: 10.2337/db10-0862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pambianco G, Costacou T, Ellis D, Becker DJ, Klein R, Orchard TJ. The 30-year natural history of type 1 diabetes complications: the Pittsburgh Epidemiology of Diabetes Complications Study experience. Diabetes. 2006;55:1463. doi: 10.2337/db05-1423. [DOI] [PubMed] [Google Scholar]
- 5.Krolewski AS, Kosinski EJ, Warram JH, Leland OS, Busick EJ, Asmal AC, Rand LI, Christlieb AR, Bradley RF, Kahn CR. Magnitude and determinants of coronary artery disease in juvenile-onset, insulin-dependent diabetes mellitus. Am J Cardiol. 1987;59:750. doi: 10.1016/0002-9149(87)91086-1. [DOI] [PubMed] [Google Scholar]
- 6.Nathan DM, Cleary PA, Backlund JY, Genuth SM, Lachin JM, Orchard TJ, Raskin P, Zinman B. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 2005;353:2643. doi: 10.1056/NEJMoa052187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Todd JA. Etiology of type 1 diabetes. Immunity. 2010;32:457. doi: 10.1016/j.immuni.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 8.Entman ML, Michael L, Rossen RD, Dreyer WJ, Anderson DC, Taylor AA, Smith CW. Inflammation in the course of early myocardial ischemia. FASEB J. 1991;5:2529. doi: 10.1096/fasebj.5.11.1868978. [DOI] [PubMed] [Google Scholar]
- 9.Frangogiannis NG. The immune system and cardiac repair. Pharmacol Res. 2008;58:88. doi: 10.1016/j.phrs.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nahrendorf M, Pittet MJ, Swirski FK. Monocytes: protagonists of infarct inflammation and repair after myocardial infarction. Circulation. 2010;121:2437. doi: 10.1161/CIRCULATIONAHA.109.916346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang J, Yu ZX, Fujita S, Yamaguchi ML, Ferrans VJ. Interstitial dendritic cells of the rat heart. Quantitative and ultrastructural changes in experimental myocardial infarction. Circulation. 1993;87:909. doi: 10.1161/01.cir.87.3.909. [DOI] [PubMed] [Google Scholar]
- 12.Gallucci S, Lolkema M, Matzinger P. Natural adjuvants: endogenous activators of dendritic cells. Nat Med. 1999;5:1249. doi: 10.1038/15200. [DOI] [PubMed] [Google Scholar]
- 13.Kono H, Rock KL. How dying cells alert the immune system to danger. Nat Rev Immunol. 2008;8:279. doi: 10.1038/nri2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chervonsky AV. Influence of microbial environment on autoimmunity. Nat Immunol. 2010;11:28. doi: 10.1038/ni.1801. [DOI] [PubMed] [Google Scholar]
- 15.Iwasaki A, Medzhitov R. Regulation of adaptive immunity by the innate immune system. Science. 2010;327:291. doi: 10.1126/science.1183021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Elliott JF, Liu J, Yuan ZN, Bautista-Lopez N, Wallbank SL, Suzuki K, Rayner D, Nation P, Robertson MA, Liu G, Kavanagh KM. Autoimmune cardiomyopathy and heart block develop spontaneously in HLA-DQ8 transgenic IAbeta knockout NOD mice. Proc Natl Acad Sci U S A. 2003;100:13447. doi: 10.1073/pnas.2235552100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Taylor JA, Havari E, McInerney MF, Bronson R, Wucherpfennig KW, Lipes MA. A spontaneous model for autoimmune myocarditis using the human MHC molecule HLA-DQ8. J Immunol. 2004;172:2651. doi: 10.4049/jimmunol.172.4.2651. [DOI] [PubMed] [Google Scholar]
- 18.Lv H, Havari E, Pinto S, Gottumukkala RV, Cornivelli L, Raddassi K, Matsui T, Rosenzweig A, Bronson RT, Smith R, Fletcher AL, Turley SJ, Wucherpfennig K, Kyewski B, Lipes MA. Impaired thymic tolerance to alpha-myosin directs autoimmunity to the heart in mice and humans. J Clin Invest. 2011;121:1561. doi: 10.1172/JCI44583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dressler W. A post-myocardial infarction syndrome; preliminary report of a complication resembling idiopathic, recurrent, benign pericarditis. J Am Med Assoc. 1956;160:1379. doi: 10.1001/jama.1956.02960510005002. [DOI] [PubMed] [Google Scholar]
- 20.Moraru M, Roth A, Keren G, George J. Cellular autoimmunity to cardiac myosin in patients with a recent myocardial infarction. International Journal of Cardiology. 2006;107:61. doi: 10.1016/j.ijcard.2005.02.036. [DOI] [PubMed] [Google Scholar]
- 21.Goser S, Andrassy M, Buss SJ, Leuschner F, Volz CH, Ottl R, Zittrich S, Blaudeck N, Hardt SE, Pfitzer G, Rose NR, Katus HA, Kaya Z. Cardiac troponin I but not cardiac troponin T induces severe autoimmune inflammation in the myocardium. Circulation. 2006;114:1693. doi: 10.1161/CIRCULATIONAHA.106.635664. [DOI] [PubMed] [Google Scholar]
- 22.Volz HC, Buss SJ, Li J, Goser S, Andrassy M, Ottl R, Pfitzer G, Katus HA, Kaya Z. Autoimmunity against cardiac troponin I in ischaemia reperfusion injury. European Journal of Heart Failure. 2011;13:1052. doi: 10.1093/eurjhf/hfr098. [DOI] [PubMed] [Google Scholar]
- 23.Nussinovitch U, Shoenfeld Y. The Clinical and Diagnostic Significance of Anti-myosin Autoantibodies in Cardiac Disease. Clinical Reviews in Allergy & Immunology. 2011 doi: 10.1007/s12016-010-8229-8. [DOI] [PubMed] [Google Scholar]
- 24.Sherwood RI, Christensen JL, Conboy IM, Conboy MJ, Rando TA, Weissman IL, Wagers AJ. Isolation of adult mouse myogenic progenitors: functional heterogeneity of cells within and engrafting skeletal muscle. Cell. 2004;119:543. doi: 10.1016/j.cell.2004.10.021. [DOI] [PubMed] [Google Scholar]
- 25.Bingley PJ, Williams AJ. Validation of autoantibody assays in type 1 diabetes: workshop programme. Autoimmunity. 2004;37:257. doi: 10.1080/08916930410001710677. [DOI] [PubMed] [Google Scholar]
- 26.Stewart GC, Lopez-Molina J, Gottumukkala RV, Rosner GF, Anello MS, Hecht JL, Winters GL, Padera RF, Baughman KL, Lipes MA. Myocardial parvovirus B19 persistence: lack of association with clinicopathologic phenotype in adults with heart failure. Circ Heart Fail. 2011;4:71. doi: 10.1161/CIRCHEARTFAILURE.110.958249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Okazaki T, Tanaka Y, Nishio R, Mitsuiye T, Mizoguchi A, Wang J, Ishida M, Hiai H, Matsumori A, Minato N, Honjo T. Autoantibodies against cardiac troponin I are responsible for dilated cardiomyopathy in PD-1-deficient mice. Nat Med. 2003;9:1477. doi: 10.1038/nm955. [DOI] [PubMed] [Google Scholar]
- 28.Eriksson S, Hellman J, Pettersson K. Autoantibodies against cardiac troponins. N Engl J Med. 2005;352:98. doi: 10.1056/NEJM200501063520123. [DOI] [PubMed] [Google Scholar]
- 29.Mahrholdt H, Sechtem U. Noninvasive differentiation between active and healed myocarditis by cardiac magnetic resonance: are we there yet? JACC Cardiovasc Imaging. 2009;2:139. doi: 10.1016/j.jcmg.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 30.Sosnovik DE, Nahrendorf M, Deliolanis N, Novikov M, Aikawa E, Josephson L, Rosenzweig A, Weissleder R, Ntziachristos V. Fluorescence tomography and magnetic resonance imaging of myocardial macrophage infiltration in infarcted myocardium in vivo. Circulation. 2007;115:1384. doi: 10.1161/CIRCULATIONAHA.106.663351. [DOI] [PubMed] [Google Scholar]
- 31.Gaglia JL, Guimaraes AR, Harisinghani M, Turvey SE, Jackson R, Benoist C, Mathis D, Weissleder R. Noninvasive imaging of pancreatic islet inflammation in type 1A diabetes patients. J Clin Invest. 2011;121:442. doi: 10.1172/JCI44339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lind M, Bounias I, Olsson M, Gudbjornsdottir S, Svensson AM, Rosengren A. Glycaemic control and incidence of heart failure in 20,985 patients with type 1 diabetes: an observational study. Lancet. 2011;378:140. doi: 10.1016/S0140-6736(11)60471-6. [DOI] [PubMed] [Google Scholar]
- 33.Thaunat O, Field AC, Dai J, Louedec L, Patey N, Bloch MF, Mandet C, Belair MF, Bruneval P, Meilhac O, Bellon B, Joly E, Michel JB, Nicoletti A. Lymphoid neogenesis in chronic rejection: evidence for a local humoral alloimmune response. Proc Natl Acad Sci U S A. 2005;102:14723. doi: 10.1073/pnas.0507223102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 35.Eriksson U, Ricci R, Hunziker L, Kurrer MO, Oudit GY, Watts TH, Sonderegger I, Bachmaier K, Kopf M, Penninger JM. Dendritic cell-induced autoimmune heart failure requires cooperation between adaptive and innate immunity. Nat Med. 2003;9:1484. doi: 10.1038/nm960. [DOI] [PubMed] [Google Scholar]
- 36.O'Garra A, Gabrysova L, Spits H. Quantitative events determine the differentiation and function of helper T cells. Nat Immunol. 2011;12:288. doi: 10.1038/ni.2003. [DOI] [PubMed] [Google Scholar]
- 37.Van Eyk JE, Powers F, Law W, Larue C, Hodges RS, Solaro RJ. Breakdown and release of myofilament proteins during ischemia and ischemia/reperfusion in rat hearts: identification of degradation products and effects on the pCa-force relation. Circ Res. 1998;82:261. doi: 10.1161/01.res.82.2.261. [DOI] [PubMed] [Google Scholar]
- 38.Ganote CE, Vander Heide RS. Cytoskeletal lesions in anoxic myocardial injury. A conventional and high-voltage electron-microscopic and immunofluorescence study. Am J Pathol. 1987;129:327. [PMC free article] [PubMed] [Google Scholar]
- 39.Frank D, Kuhn C, Katus HA, Frey N. The sarcomeric Z-disc: a nodal point in signalling and disease. J Mol Med (Berl) 2006;84:446. doi: 10.1007/s00109-005-0033-1. [DOI] [PubMed] [Google Scholar]
- 40.Abbate A, Bonanno E, Mauriello A, Bussani R, Biondi-Zoccai GG, Liuzzo G, Leone AM, Silvestri F, Dobrina A, Baldi F, Pandolfi F, Biasucci LM, Baldi A, Spagnoli LG, Crea F. Widespread myocardial inflammation and infarct-related artery patency. Circulation. 2004;110:46. doi: 10.1161/01.CIR.0000133316.92316.81. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.