Abstract
Osteoarthritis (OA) is a common and disabling joint disorder affecting millions of people worldwide. In OA, pathological changes are seen in all of the joint tissues including bone. Although both cross-sectional and longitudinal epidemiological studies have consistently demonstrated an association between higher bone mineral density (BMD) and OA, suggesting that increased BMD is a risk factor for OA, the mechanisms underlying this observation remain unclear. Recently, novel approaches to examining the BMD-OA relationship have included studying the disease in individuals with extreme high bone mass, and analyses searching for genetic variants associated with both BMD variation and OA, suggesting possible pleiotropic effects on bone mass and OA risk. These studies have yielded valuable insights into potentially relevant pathways that might one day be exploited therapeutically. Although animal models have suggested that drugs reducing bone turnover (antiresorptives) may retard OA progression, it remains to be seen whether this approach will prove to be useful in human OA. Identifying individuals with a phenotype of OA predominantly driven by increased bone formation could help improve the overall response to these treatments. This review aims to summarise current knowledge regarding the complex relationship between BMD and OA.
Introduction
The relationship between bone mineral density (BMD) and osteoarthritis (OA) has long been a subject of debate in the literature. Understanding this relationship has the potential to illuminate the role played by bone in the pathogenesis of OA, and may have important therapeutic implications. The aim of this review is to summarise the evidence to date supporting a positive association between systemic BMD and OA, including recent insights from studies using novel approaches, and to offer a perspective for the future.
BMD and OA: the epidemiological evidence for an association
As far back as the 1960s, it had been observed that features of OA were generally absent in femoral heads excised after hip fracture.1 In 1972, Foss and Byers2 studied a series of femoral heads excised either in the surgical treatment of hip fracture or total hip replacement for OA; they observed very few pathological changes of OA in the fracture specimens, whereas bone density appeared to be increased in the OA patients, as assessed on radiographs of the second metacarpal. Since then, numerous studies have been published examining the relationship between systemic BMD and OA, and several authors have reviewed this topic.1,3,4
OA is recognised to be a heterogeneous disease that can be defined in different ways (for example, in terms of clinical symptoms, radiographic change or a combination of these features).5 Despite this, epidemiological studies of the relationship between BMD and OA have almost exclusively defined OA radiographically, usually using a summary grading system such as the Kellgren–Lawrence grade.6 Earlier studies were cross-sectional in design, comparing BMD (generally measured at the spine and/or hip) in OA cases with that in unaffected controls (Table 1). The hip and knee joints have been most studied, with several groups finding evidence of higher systemic BMD in those with OA at these joint sites in a variety of populations.7,8,9,10,11 Higher BMD has also been reported in association with OA of the spine10,12 and hand.9,10,13
Table 1. Cross-sectional studies examining the association between BMD and OA using dual X-ray absorptiometry (DXA).
| Reference | Population | Joint site (OA) | OA definition | Site of BMD measurement | Conclusions |
|---|---|---|---|---|---|
| Hart et al.10 | Women from the UK Chingford study (n=979 hands and knees, n=579 lumbar spine) | Knee, hand (1st CMCJ, DIPJs), lumbar spine | Radiographic (K&L grade ⩾2) | Femoral neck and lumbar spine (L1–L4) | Lumbar spine BMD higher in OA cases vs controls (all joint sites). Femoral neck BMD higher in OA cases vs controls at CMC joint, knee, lumbar spine. Associations persisted on adjusting for spinal osteophytes. |
| Nevitt et al.7 | 4090 Caucasian women from the US Study of Osteoporotic fractures, mean age 71 years | Hip | Radiographic (definite osteophytes or narrowing, plus cysts or sclerosis) | Hip (femoral neck, Ward's triangle, trochanter, intertrochanteric), lumbar spine | Increased BMD at all sites in subjects with moderate–severe OA of either hip, increased BMD at femoral neck and lumbar spine in subjects with milder hip OA. Associations persisted on adjusting for vertebral body osteophytes/subchondral sclerosis. OA hips with osteophytes, but not isolated JSN, associated with increased BMD. |
| Peel et al.12 | 375 women aged 40–85 years from a UK primary care population | Spine | Radiographic (K&L grade ⩾2) | Lumbar spine, femoral neck and total body | BMD increased at all sites in the OA group. |
| Burger et al.8 | 2745 men and women from the Rotterdam Study (Netherlands), mean age 69 years | Knee and hip | Radiographic (K&L grade ⩾2) | Femoral neck | BMD 3–8% higher in the group with OA (not significant for knee OA in men, P=0.07). In general, BMD increased according to the number of joint sites affected and increasing OA severity (K&L grade). |
| Sowers et al.9 | 573 Caucasian women from the Michigan bone health study, aged 24–45 years | Hand and knee | Radiographic (K&L grade ⩾2) | Proximal femur, lumbar spine and total body | Total body BMD positively associated with highest OA grade at both hand and knee. Total body BMD associated with knee OA (K&L grade ⩾2). |
| Chaganti et al.11 | 3929 men from the US MrOS study | Hip | Radiographic (summary grade 0–4, OA defined as grade ⩾2) | Lumbar spine, total hip, femoral neck, trochanteric | Higher DXA BMD at all sites in moderate/severe OA group vs mild/no OA. Volumetric BMD elevated at hip and L1 vertebra in the severe OA group. |
Abbreviations: BMD, bone mineral density; CMCJ, carpometacarpal joint; DIPJ, distal interphalangeal joint; JSN, joint space narrowing; IRF, individual radiographic feature (of OA); K&L, Kellgren & Lawrence grade (summary grade for OA); OA, Osteoarthritis.
This represents some key studies as selected by the authors, and is not exhaustive.
A limitation of cross-sectional studies is that the direction of causality cannot be formally assessed. Features of OA such as osteophytes and subchondral sclerosis, if present within the dual X-ray absorptiometry (DXA) field, might artefactually elevate BMD. The site of BMD assessment is important, as it is recognised that osteophytosis affects BMD measured at the spine to a much greater degree than at the hip.14,15 It is therefore worth noting that strong positive associations between hip BMD and large joint OA have been observed in many studies,8,10,11 and that the positive association between lumbar spine BMD and OA at both the hip and knee has been shown to persist after adjusting for the presence of spinal osteophytes.7,10
More recently, the temporal relationship between BMD and OA has been clarified in prospective studies (Table 2). Several longitudinal studies have shown higher BMD to be associated with a greater risk of developing subsequent radiographic knee OA,16,17,18,19,20 although findings have not always been conclusive.17 Although the populations studied have been predominantly female, there is also evidence of a positive association between BMD and incident knee OA in male populations.19,20 A similar association between higher BMD and incident radiographic hip OA has been reported in postmenopausal women,21 whereas Sowers et al.16 found no evidence of a longitudinal association with hand OA in a population of pre- and peri-menopausal women.
Table 2. Longitudinal studies examining the association between BMD and OA.
| Reference | Population | Follow-up period | Joint site (OA) | Incident OA definition | Site of BMD measurement | Conclusions |
|---|---|---|---|---|---|---|
| Sowers et al.16 | 482 women from the US Michigan Bone Health study, mean age 37.4 years | 3 years | Knee and hand | Radiographic (K&L grade ⩾2, from <2 at baseline) | Femoral neck, lumbar spine and total body | BMD (Z-scores) greater at all three sites in women with incident knee OA, and no differences in baseline BMD in women with incident hand OA vs controls. |
| Zhang et al.17 | 473 women from the Framingham study, mean age 71 years | 8 years | Knee | Radiographic (K&L grade ⩾2, from <2 at baseline) | Femoral neck | Trend towards increased incidence knee OA with increasing BMD, mainly via increased osteophytes. Inverse association between baseline BMD and knee OA progression, mainly via reduced risk of progressive JSN. |
| Hart et al.18 | 830 women from the Chingford cohort, mean age 54 years | 48 months | Knee | Radiographic (grade ⩾1 osteophytes or JSN, from grade 0 at baseline) | Lumbar spine and femoral neck | BMD significantly higher at both sites in group with incident osteophytes, and trend towards higher BMD in group with incident JSN. Weak trend towards lower hip BMD in group with progressive osteophytes/JSN. |
| Hochberg et al.21 | 5242 women from the Study of Osteoporotic Fractures, mean age 71 years | 8 years | Hip | Radiographic (minimum JSW ⩽1.5 mm, definite osteophyte or summary grade ⩾2, where feature absent at baseline) | Forearm and total hip | Dose–response relationship between quartile of baseline BMD and incidence of radiographic hip OA (defined by osteophyte or Croft grade ⩾2). No association between BMD and incident hip OA defined by JSN alone. |
| Bergink et al.20 | 1403 men and women from the Rotterdam study, aged >55 years | 6 years | Knee | Radiographic (K&L grade ⩾2 in either knee, vs <2 at baseline) | Femoral neck and lumbar spine | Odds of incident knee OA significantly higher in highest vs lowest quartiles of both femoral neck and lumbar spine BMD. Trend towards increased odds of knee OA progression with higher lumbar but not femoral BMD. |
| Nevitt et al.19 | 1754 men and women from the multicentre osteoarthritis study (MOST), mean age 63 years | 30 months | Knee | Radiographic (K&L grade ⩾2, from 0–1 at baseline) | Femoral neck and total body | Risk of incident knee OA increased with higher BMD in both genders. Higher femoral neck/total body BMD associated with increased risk of incident JSN and osteophytosis. No association between BMD and OA progression observed. |
Abbreviations: BMD, bone mineral density; JSN, joint space narrowing; JSW, joint space width; K&L, Kellgren & Lawrence grade; OA, Osteoarthritis.
This represents some key studies as selected by the authors, and is not exhaustive.
The Kellgren–Lawrence grading system for radiographic OA has been criticised for an overemphasis on the osteophyte,22 whereas in fact distinct radiographic OA phenotypes, with varying degrees of osteophytosis relative to other features such as joint space narrowing (JSN, an indirect measurement of cartilage loss), can be delineated.23,24 In studies in which these individual radiographic features of OA have been quantified separately, increased BMD has generally been reported to be more strongly associated with osteophytosis than JSN.7,17,18,21 Indeed, only one longitudinal study to date has convincingly demonstrated a positive association between BMD and incident JSN at the knee.19 Several possible explanations for this observation, both methodological and biological, can be postulated. A stronger association with osteophytes in this type of study may arise because radiographs are insensitive for the detection of JSN. Alternatively, it may be that individuals with high BMD are prone to develop either osteophytes alone (the clinical relevance of which is uncertain) or a hypertrophic phenotype of OA characterised by vigorous osteophytosis; the term ‘bone-formers' has been coined to describe this.3,7,25 The former hypothesis that osteophytes and JSN may have different relationships with BMD is made plausible by the observation that these two features appear to be associated with distinct genetic variants.26,27 Interestingly, several recent studies using magnetic resonance imaging have actually reported a positive association between BMD and cartilage thickness/volume at the knee in healthy subjects,28,29,30 raising the possibility that measurable cartilage loss could appear later on in the OA disease trajectory in individuals with higher BMD.
When interpreting the results of these studies, it is important to recognise the limited concordance between radiographic and symptomatic OA.31 Lower prevalence estimates for symptomatic compared with radiographic OA in the general population reflect the fact that radiographic OA is not always accompanied by clinical disease.32 Relatively few studies have examined the relationship between BMD and clinically relevant OA, although it has been reported that individuals with radiographic OA have similar degrees of BMD elevation independent of the presence of joint symptoms.7,10
Controversies and inconsistencies
Despite the weight of evidence that systemic BMD and radiographic OA are positively associated, some inconsistencies and areas of controversy remain. Cross-sectional investigations into the relationship between OA and bone turnover, which is generally inversely related to BMD owing to its role in bone loss,33 have yielded conflicting findings. Levels of serum or urine bone turnover markers have been reported as both increased34 and decreased12,16 in individuals with radiographic OA of different joints compared with controls, with other studies reporting no association between bone turnover markers and radiographic OA severity.35 Conversely, prospective studies suggest that higher rates of bone turnover may be related to more rapid OA progression. In the Chingford study population, higher bone resorption markers were noted in postmenopausal women with progressive, but not stable, radiographic knee OA.36 Similarly, Dieppe et al.37 found increased uptake on bone scintigraphy (indicating increased bone turnover) to be associated with greater subsequent progression of JSN in a mixed-gender population with symptomatic knee OA.
Studies examining the relationship between OA and rates of bone loss, also inversely related to BMD, have reached similarly opposing conclusions, with bone loss in individuals with OA found to be reduced,16 increased,8,38 or variable depending upon the site of assessment of both OA and BMD.39 In a longitudinal study, more rapid bone loss over an 8-year period was associated with an increased risk of progressive OA at the knee.17 Findings from prospective studies that greater bone loss and turnover may be associated with more rapid OA progression would seem at odds with those discussed earlier, suggesting that higher, rather than lower, BMD is a risk factor for OA. One potential explanation is that OA develops over time in a phasic manner, with periods of active bone turnover corresponding with radiographic progression interspersed with quiescent phases in which bone turnover may be normal or reduced.23 Furthermore, BMD changes occurring in different subchondral bone regions in OA are not uniform. In particular, the cancellous bone underlying the thickened subchondral bone plate may become osteopaenic in OA,40 as observed in studies reporting a decrease in DXA BMD in subchondral bone regions of osteoarthritic knees compared with controls,41 a phenomenon attributed to ‘stress-shielding'.42 Another possibility is that the relationship of BMD with incident versus progressive OA may differ;17 this concept will be discussed further below.
In addition, differences in bone size could confound the association between BMD and OA.43 DXA scans measure areal BMD, which is affected by bone size.44,45 Larger bones have increased volume in relation to their area; therefore, dividing bone mineral content by bone area to obtain apparent areal BMD will lead to an overestimation of BMD proportional to size. This is relevant, because several studies have observed increases in bone size in individuals with OA, both local to44,45,46 and distant from47 the affected joint. Measurement of volumetric BMD (vBMD) using quantitative computed tomography (QCT) avoids this problem. Abdin-Mohamed et al.48 in 2009 used peripheral QCT to compare tibial vBMD in men and women from the Hertfordshire cohort study with and without radiographic knee OA. An increase in tibial cross-sectional area at the 38% slice and tibial cortical area at the 14% slice was found in men but not in women with OA; however, no difference in vBMD between the groups was found. In contrast, a much larger study by Chaganti et al.11 including 2384 men from the US-based MrOS study found evidence of increased vBMD using QCT at the hip and lumbar spine in men with severe radiographic hip OA compared with controls. As such techniques become more widespread, it seems likely that more data on the relationship between OA and vBMD will emerge.
A positive association between BMD and OA would imply that individuals with OA should have a reduced risk of fracture; however, several studies have instead observed either no difference in fracture incidence between OA affected individuals and controls39,49 or an increase in fracture risk,50 although it should be noted that the type of OA definition used in these studies has varied. It has long been speculated that this may relate to an increased risk of falls in OA cases,50 and indeed a recent large prospective study supported this hypothesis, showing that an association between (self-reported) OA and fracture was largely attenuated by adjusting for incident falls.51
Finally, and intriguingly, longitudinal studies have suggested that, in contrast to incident OA, progression of pre-existing OA may be inversely related to BMD. This finding was particularly striking in a paper by Zhang et al.17 studying the Framingham population, in whom a clear association between increasing age-specific femoral neck BMD quartiles and reduced risk of knee OA progression over the 8-year study period was seen. Hart et al.18 also observed a trend towards lower hip BMD in those with progressive knee OA versus non-progressors. In contrast, Nevitt et al.19 failed to find any association between BMD at the femoral neck or whole body and knee OA progression. If an inverse association between BMD and OA progression does hold true, it may provide important insights into the role of bone in the pathogenesis of OA at different stages of the disease. However, it has also been proposed that this observation, rather than reflecting a true difference in risk factors for OA incidence and progression, represents an epidemiological artefact resulting from aspects of the design of longitudinal studies of OA progression (see Zhang et al.52 for a detailed discussion of this issue).
OA in high bone mass individuals: a novel approach
To date, the evidence for an association between BMD and OA is derived largely from studies in the general population; however, several conditions exist in which BMD is markedly elevated from relatively early in life. Studying OA in these individuals could potentially provide valuable insights into the BMD-OA relationship, particularly as it is clearer in this group that increases in BMD precede the onset of OA, consistent with a causal relationship. Until recently, data on OA in these high bone mass (HBM) conditions have been limited to case reports and case series. For example, early-onset OA has been reported in association with autosomal dominant osteopetrosis.53,54 In contrast, sclerosteosis does not appear to be associated with degenerative arthritis,55 and no increased risk of OA in association with activating mutations of LRP5 has thus far been reported; however, the extreme rarity of these monogenic HBM conditions, coupled with the high prevalence of OA within the general population, may make any such association difficult to detect.
The UK-based HBM study represents the largest collection of individuals with extremely high BMD studied to date.56 HBM index cases were initially identified through systematic screening of NHS DXA databases for BMD Z- and/or T-scores ⩾+4, excluding scans with artefactual causes of BMD elevation, as previously described.56 Further HBM cases were subsequently identified through DXA screening of first-degree relatives of index cases, resulting in recruitment of just over 350 cases with unexplained HBM.56 We recently studied the prevalence and phenotype of OA in this HBM population. HBM individuals were found to have a higher prevalence of self-reported joint replacement and use of nonsteroidal anti-inflammatory drugs compared with unaffected family controls, implying an increased risk of OA as ascertained by clinical, as opposed to radiographic, end points.57
Recently, this work was extended to examine the relationship between HBM and radiographic OA phenotypes. An increased prevalence of radiographic hip OA was seen in HBM cases compared with a control group comprising both unaffected family members and general population controls. In line with findings in the general population with respect to BMD, the hip OA phenotype in HBM was characterised by bony features such as osteophytosis and subchondral sclerosis.58 In contrast, when the individual radiographic features of OA were analysed separately, there was little evidence that HBM is associated with JSN. As HBM is associated with an increased prevalence of clinical OA (as demonstrated by our finding of higher rates of joint replacement58), this may imply an association between isolated osteophytosis and clinical symptoms such as pain in this population. However, accurate quantification of joint space width using plain radiographs is hampered by a number of methodological issues, and to what extent HBM leads to OA through osteophytosis independently of cartilage loss and JSN remains to be established.
Subsequent characterisation of knee OA in this group revealed a similar, osteophyte-predominant, radiographic OA phenotype.59 One caveat is that HBM individuals tend to have a greater BMI,56 which is well recognised as a risk factor for OA at a number of sites, particularly the knee.60 However, although greater BMI appeared to contribute to the association between HBM and knee OA, this association persisted after BMI adjustment,59 and the relationship between HBM and hip OA appeared to be independent of BMI.58 It should also be noted that although the HBM cases within this population displayed several clinical features suggestive of a mild skeletal dysplasia, no evidence was found of any significant gait abnormality compared with controls.56
We speculate that HBM individuals manifest a ‘bone-forming' tendency, which contributes to their risk of OA. This is supported by the additional observation that HBM individuals also have a greater prevalence and severity of radiographic pelvic enthesophytes (bony spurs at tendon and ligament insertions).61 Although the genetic basis for increased BMD in the majority of these HBM cases remains to be determined, and is the subject of ongoing studies, a genome-wide association analysis has shown overrepresentation of single-nucleotide polymorphisms (SNPs) that were previously shown to be associated with BMD variation in the general population.62 It is hoped that whole-exome sequencing in this unique group, currently underway, could identify novel pathways with a role in both OA and bone mass regulation.
Mechanisms, including recent insights from genetic studies
Although the existence of an association between BMD and OA is now generally accepted, the mechanisms underlying this observation remain elusive. Many investigators have focussed on the role of subchondral bone in the cartilage loss that characterises most OA (see40 for a recent review). In the 1970s, Radin and Rose63 proposed that increased stiffness of subchondral bone might lead to articular cartilage degeneration through the mechanical effects of increased shear stress. However, subsequent studies using a variety of techniques at different stages of OA progression have revealed complex and opposing changes in apparent density, material density, and stiffness in osteoarthritic subchondral bone,64,65,66 suggesting that this explanation is too simplistic.40,67 Thinning of the articular cartilage from below, owing to reactivation of endochondral ossification at the bone–cartilage interface in OA joints resulting in tidemark duplication and advancement, may represent another important mechanism by which increased bone formation could drive the OA process.40,67 The action of soluble mediators released from bone on the articular cartilage (and vice versa) has also been of interest (reviewed by Lories and Luyten67). Potentially important signalling pathways include the Wnt and bone morphogenetic protein pathways, and transforming growth factor -β.67
Another potential mechanism that could underpin the observed association between BMD and OA is genetic pleiotropy—that is, the existence of genetic variants that contribute to both BMD variation and OA risk.26 A recent paper by Yerges-Armstrong et al.69 analysed associations between BMD SNPs, identified in a recent genome-wide association study meta-analysis,68 and knee OA in two population cohorts. Knee OA was defined either radiographically as Kellgren–Lawrence grade ⩾2 (definite osteophytes) or by the presence of a knee replacement. Four BMD-associated SNPs were found to be also associated with the presence of knee OA, although none reached conventional genome-wide significance levels. Two of these variants are located near wnt signalling pathway genes, including one SNP (rs3736228) close to LRP5. The association of each of these SNPs with knee OA was in the hypothesised direction, with the higher BMD allele associated with increased knee OA risk. Earlier this year, an abstract by the same authors reported a similar analysis for radiographic hip OA, identifying only one variant with a nominal association with radiographic hip OA (P=0.03); this SNP (rs7217932) is located near the SOX9 gene that codes for part of the endochondral ossification pathway.70 Similarly, as recently reviewed by Reynard and Loughlin,71 some OA susceptibility genes have been shown to be associated with variation in BMD, and the functional annotations of OA susceptibility genes identified to date implicate bone-centred pathways such as skeletal development and morphogenesis, as well as osteoblast development/differentiation as playing a role in OA.
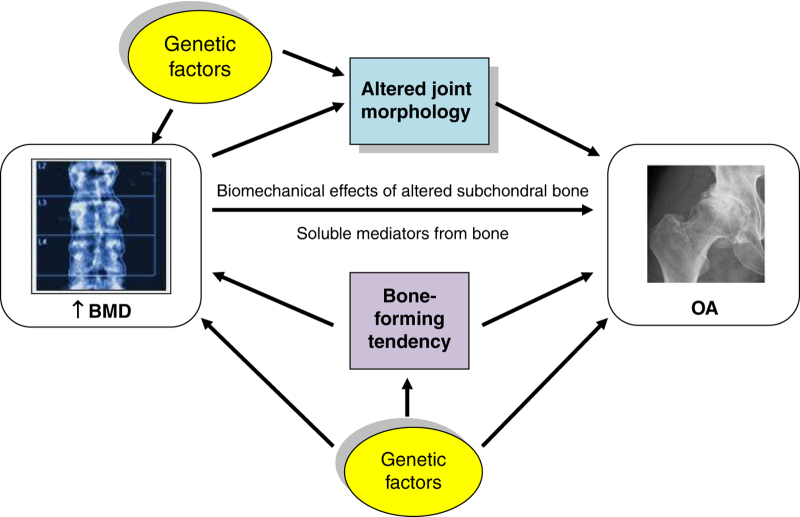
There are a number of potential mechanisms by which genetic variants associated with both BMD and OA could modify OA risk. The simplest of these is via increased BMD itself, termed ‘mediated' pleiotropy.72 Alternatively, BMD genes could directly influence other phenotypes that in turn increase the risk of developing OA (‘biological' pleiotropy72), such as cartilage thickness or joint shape;73 these characteristics have been termed ‘endophenotypes', and their study could help clarify relevant mechanisms.74 For example, a variant allele in the DOT1L gene has been associated with increased cartilage thickness (measured as joint space width) and a decreased risk of hip OA.75 Another wnt pathway SNP has been found to influence hip morphology in women, and to modify the association between a particular morphological variant and radiographic hip OA.76 Finally, bone-active OA susceptibility genes might be expected to directly increase the risk of developing phenotypes of OA characterised by bony features as a result of their effects on osteophyte formation and subchondral sclerosis. Potential mechanisms underlying the BMD-OA association are illustrated in Figure 1.
Figure 1.
Schematic diagram illustrating some potential mechanisms underlying the association between BMD and OA.
Targeting bone turnover in the treatment of OA
Extensive research has been performed with the aim of identifying agents that prevent structural progression in OA. In addition to directly targeting articular cartilage, which has yielded largely negative results, strategies have been developed to target adjacent tissues including bone. As reviewed by Roux and Richette,77 antiresorptive agents, including bisphosphonates and cathepsin-K inhibitors, have demonstrated beneficial effects on structural progression in experimental animal models of OA, suggesting that treatments targeting bone may have a role in managing the disease. However, in humans, two randomised controlled trials of risedronate for knee OA failed to demonstrate a significant reduction in JSN over time,78,79 and similarly a recent systematic review concluded that there was limited evidence that bisphosphonates are an effective treatment option for pain in OA.80
Following these negative studies, there has been a recent resurgence of interest in manipulating bone turnover for the treatment of OA following the publication of the SEKOIA trial of strontium ranelate for the treatment of knee OA.81 This mixed-gender, multicentre study demonstrated a reduction in the progression of JSN in the treatment group over a 3-year period,81 and as such it has been heralded as the first positive disease-modifying OA drug trial. However, in contrast to the radiographic findings, a small beneficial effect on symptoms in this trial was confined to the group taking the higher (2 g per day) dose.81 As discussed by Lafeber and van Laar82 in their accompanying editorial, a number of questions remain to be addressed before strontium can be adopted clinically as a treatment for OA, and recent concerns regarding the cardiovascular safety of this drug may prove prohibitive in the patient group most in need;83 nevertheless, these results lend further support to the concept of using pharmacotherapies targeting bone to treat OA.
A possible reason for the largely disappointing results of OA drug trials to date is failure to stratify potential participants by OA phenotype. As stated earlier, it is recognised that in any given patient with OA changes in different joint tissues (including cartilage, bone and synovium) may predominate.84 Phenotyping OA, for example into hypertrophic versus atrophic variants based on the extent of bony change and osteophytosis visible radiographically,24,85 attempts to identify these pathogenic subgroups. Many commentators now believe that the key to success in OA treatment may be rational targeting of treatments with a specific mode of action to patients with a relevant subtype of the disease.82,84,86 However, at what point during the OA disease trajectory such treatments would prove most useful remains unclear.
Conclusions
Novel approaches have recently shed new light on the relationship between BMD and OA. Genetic studies, and observations regarding OA in individuals with extreme HBM, have helped to highlight mechanisms that may be relevant in explaining the BMD-OA association. Such insights could potentially provide new avenues to be explored therapeutically. In addition, identifying individuals in whom OA is predominantly driven by changes in bone might improve response to treatment by providing a basis for therapeutic stratification.
Acknowledgments
Dr Sarah Hardcastle and Dr Celia Gregson would like to acknowledge ongoing funding support provided by Arthritis Research UK.
Footnotes
The authors declare no conflict of interest.
References
- Dequeker J, Aerssens J, Luyten FP. Osteoarthritis and osteoporosis: clinical and research evidence of inverse relationship. Aging Clin Exp Res 2003;15:426–439. [DOI] [PubMed] [Google Scholar]
- Foss MV, Byers PD. Bone density, osteoarthrosis of the hip, and fracture of the upper end of the femur. Ann Rheum Dis 1972;31:259–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane NE, Nevitt MC. Osteoarthritis, bone mass, and fractures: how are they related? Arthritis Rheum 2002;46:1–4. [DOI] [PubMed] [Google Scholar]
- Stewart A, Black AJ. Bone mineral density in osteoarthritis. Curr Opin Rheumatol 2000;12:464–467. [DOI] [PubMed] [Google Scholar]
- Arden N, Nevitt MC. Osteoarthritis: epidemiology. Best Pract Res Clin Rheumatol. 2006;20:3–25. [DOI] [PubMed] [Google Scholar]
- Kellgren JK. The Epidemiology of Chronic Rheumatism. Atlas of Standard Radiographs Vol 2: Lawrence JS (ed.) Blackwell Scientific: Oxford, UK, 1963;. [Google Scholar]
- Nevitt MC, Lane NE, Scott JC, Hochberg MC, Pressman AR, Genant HK et al. Radiographic osteoarthritis of the hip and bone mineral density. The Study of Osteoporotic Fractures Research Group. Arthritis Rheum 1995;38:907–916. [DOI] [PubMed] [Google Scholar]
- Burger H, van Daele PL, Odding E, Valkenburg HA, Hofman A, Grobbee DE et al. Association of radiographically evident osteoarthritis with higher bone mineral density and increased bone loss with age. The Rotterdam Study. Arthritis Rheum 1996;39:81–86. [DOI] [PubMed] [Google Scholar]
- Sowers MF, Hochberg M, Crabbe JP, Muhich A, Crutchfield M, Updike S. Association of bone mineral density and sex hormone levels with osteoarthritis of the hand and knee in premenopausal women. Am J Epidemiol 1996;143:38–47. [DOI] [PubMed] [Google Scholar]
- Hart DJ, Mootoosamy I, Doyle DV, Spector TD. The relationship between osteoarthritis and osteoporosis in the general population: the Chingford Study. Ann Rheum Dis 1994;53:158–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaganti RK, Parimi N, Lang T, Orwoll E, Stefanick ML, Nevitt M et al. Bone mineral density and prevalent osteoarthritis of the hip in older men for the Osteoporotic Fractures in Men (MrOS) Study Group. Osteoporos Int 2010;21:1307–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peel NF, Barrington NA, Blumsohn A, Colwell A, Hannon R, Eastell R. Bone mineral density and bone turnover in spinal osteoarthrosis. Ann Rheum Dis 1995;54:867–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haugen IK, Slatkowsky-Christensen B, Orstavik R, Kvien TK. Bone mineral density in patients with hand osteoarthritis compared to population controls and patients with rheumatoid arthritis. Ann Rheum Dis 2007;66:1594–1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G, Peacock M, Eilam O, Dorulla G, Braunstein E, Johnston CC. Effect of osteoarthritis in the lumbar spine and hip on bone mineral density and diagnosis of osteoporosis in elderly men and women. Osteoporos Int 1997;7:564–569. [DOI] [PubMed] [Google Scholar]
- Kanis JA, Gluer CC. An update on the diagnosis and assessment of osteoporosis with densitometry. Committee of Scientific Advisors, International Osteoporosis Foundation. Osteoporos Int 2000;11:192–202. [DOI] [PubMed] [Google Scholar]
- Sowers M, Lachance L, Jamadar D, Hochberg MC, Hollis B, Crutchfield M et al. The associations of bone mineral density and bone turnover markers with osteoarthritis of the hand and knee in pre- and perimenopausal women. Arthritis Rheum 1999;42:483–489. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Hannan MT, Chaisson CE, McAlindon TE, Evans SR, Aliabadi P et al. Bone mineral density and risk of incident and progressive radiographic knee osteoarthritis in women: the Framingham Study. J Rheumatol 2000;27:1032–1037. [PubMed] [Google Scholar]
- Hart DJ, Cronin C, Daniels M, Worthy T, Doyle DV, Spector TD. The relationship of bone density and fracture to incident and progressive radiographic osteoarthritis of the knee: the Chingford Study. Arthritis Rheum 2002;46:92–99. [DOI] [PubMed] [Google Scholar]
- Nevitt MC, Zhang Y, Javaid MK, Neogi T, Curtis JR, Niu J et al. High systemic bone mineral density increases the risk of incident knee OA and joint space narrowing, but not radiographic progression of existing knee OA: the MOST study. Ann Rheum Dis 2010;69:163–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergink AP, Uitterlinden AG, Van Leeuwen JP, Hofman A, Verhaar JA, Pols HA. Bone mineral density and vertebral fracture history are associated with incident and progressive radiographic knee osteoarthritis in elderly men and women: the Rotterdam Study. Bone 2005;37:446–456. [DOI] [PubMed] [Google Scholar]
- Hochberg MC. Do risk factors for incident hip osteoarthritis (OA) differ from those for progression of hip OA? J Rheumatol Suppl 2004;70:6–9. [PubMed] [Google Scholar]
- Spector TD, Cooper C. Radiographic assessment of osteoarthritis in population studies: whither Kellgren and Lawrence? Osteoarthritis Cartilage 1993;1:203–206. [DOI] [PubMed] [Google Scholar]
- Dieppe P. Subchondral bone should be the main target for the treatment of pain and disease progression in osteoarthritis. Osteoarthritis Cartilage 1999;7:325–326. [DOI] [PubMed] [Google Scholar]
- Ledingham J, Dawson S, Preston B, Milligan G, Doherty M. Radiographic patterns and associations of osteoarthritis of the hip. Ann Rheum Dis 1992;51:1111–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers J, Shepstone L, Dieppe P. Bone formers: osteophyte and enthesophyte formation are positively associated. Ann Rheum Dis 1997;56:85–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector TD, MacGregor AJ. Risk factors for osteoarthritis: genetics. Osteoarthritis Cartilage 2004;12:S39–S44. [DOI] [PubMed] [Google Scholar]
- Uitterlinden AG, Burger H, van Duijn CM, Huang Q, Hofman A, Birkenhager JC et al. Adjacent genes, for COL2A1 and the vitamin D receptor, are associated with separate features of radiographic osteoarthritis of the knee. Arthritis Rheum 2000;43:1456–1464. [DOI] [PubMed] [Google Scholar]
- Cicuttini F, Wluka A, Davis S, Strauss BJ, Yeung S, Ebeling PR. Association between knee cartilage volume and bone mineral density in older adults without osteoarthritis. Rheumatology (Oxford) 2004;43:765–769. [DOI] [PubMed] [Google Scholar]
- Berry PA, Wluka AE, Davies-Tuck ML, Wang Y, Strauss BJ, Dixon JB et al. Sex differences in the relationship between bone mineral density and tibial cartilage volume. Rheumatology (Oxford) 2011;50:563–568. [DOI] [PubMed] [Google Scholar]
- Brennan SL, Pasco JA, Cicuttini FM, Henry MJ, Kotowicz MA, Nicholson GC et al. Bone mineral density is cross sectionally associated with cartilage volume in healthy, asymptomatic adult females: Geelong Osteoporosis Study. Bone 2011;49:839–844. [DOI] [PubMed] [Google Scholar]
- Bedson J, Croft PR. The discordance between clinical and radiographic knee osteoarthritis: a systematic search and summary of the literature. BMC Musculoskelet Disord 2008;9:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neogi T, Zhang Y. Epidemiology of osteoarthritis. Rheum Dis Clin North Am 2013;39:1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melton LJ 3rd, Khosla S, Atkinson EJ, O'Fallon WM, Riggs BL. Relationship of bone turnover to bone density and fractures. J Bone Miner Res 1997;12:1083–1091. [DOI] [PubMed] [Google Scholar]
- Stewart A, Black A, Robins SP, Reid DM. Bone density and bone turnover in patients with osteoarthritis and osteoporosis. J Rheumatol 1999;26:622–626. [PubMed] [Google Scholar]
- Jordan KM, Syddall HE, Garnero P, Gineyts E, Dennison EM, Sayer AA et al. Urinary CTX-II and glucosyl-galactosyl-pyridinoline are associated with the presence and severity of radiographic knee osteoarthritis in men. Ann Rheum Dis 2006;65:871–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettica P, Cline G, Hart DJ, Meyer J, Spector TD. Evidence for increased bone resorption in patients with progressive knee osteoarthritis: longitudinal results from the Chingford study. Arthritis Rheum 2002;46:3178–3184. [DOI] [PubMed] [Google Scholar]
- Dieppe P, Cushnaghan J, Young P, Kirwan J. Prediction of the progression of joint space narrowing in osteoarthritis of the knee by bone scintigraphy. Ann Rheum Dis 1993;52:557–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding C, Cicuttini F, Boon C, Boon P, Srikanth V, Cooley H et al. Knee and hip radiographic osteoarthritis predict total hip bone loss in older adults: a prospective study. J Bone Miner Res 2010;25:858–865. [DOI] [PubMed] [Google Scholar]
- Arden NK, Nevitt MC, Lane NE, Gore LR, Hochberg MC, Scott JC et al. Osteoarthritis and risk of falls, rates of bone loss, and osteoporotic fractures. Study of Osteoporotic Fractures Research Group. Arthritis Rheum 1999;42:1378–1385. [DOI] [PubMed] [Google Scholar]
- Burr DB, Gallant MA. Bone remodelling in osteoarthritis. Nat Rev Rheumatol 2012;8:665–673. [DOI] [PubMed] [Google Scholar]
- Karvonen RL, Miller PR, Nelson DA, Granda JL, Fernandez-Madrid F. Periarticular osteoporosis in osteoarthritis of the knee. J Rheumatol 1998;25:2187–2194. [PubMed] [Google Scholar]
- Buckland-Wright C. Subchondral bone changes in hand and knee osteoarthritis detected by radiography. Osteoarthritis Cartilage 2004;12:S10–S19. [DOI] [PubMed] [Google Scholar]
- Javaid MK, Arden NK. Bone and osteoarthritis: what is the relationship? Arthritis Rheum 2013;65:1418–1420. [DOI] [PubMed] [Google Scholar]
- Javaid MK, Lane NE, Mackey DC, Lui LY, Arden NK, Beck TJ et al. Changes in proximal femoral mineral geometry precede the onset of radiographic hip osteoarthritis: The study of osteoporotic fractures. Arthritis Rheum 2009;60:2028–2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arokoski JP, Arokoski MH, Jurvelin JS, Helminen HJ, Niemitukia LH, Kroger H. Increased bone mineral content and bone size in the femoral neck of men with hip osteoarthritis. Ann Rheum Dis 2002;61:145–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wluka AE, Wang Y, Davis SR, Cicuttini FM. Tibial plateau size is related to grade of joint space narrowing and osteophytes in healthy women and in women with osteoarthritis. Ann Rheum Dis 2005;64:1033–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochberg MC, Lethbridge-Cejku M, Scott WW Jr., Reichle R, Plato CC, Tobin JD. Upper extremity bone mass and osteoarthritis of the knees: data from the Baltimore Longitudinal Study of Aging. J Bone Miner Res 1995;10:432–438. [DOI] [PubMed] [Google Scholar]
- Abdin-Mohamed M, Jameson K, Dennison EM, Cooper C, Arden NK. Volumetric bone mineral density of the tibia is not increased in subjects with radiographic knee osteoarthritis. Osteoarthritis Cartilage 2009;17:174–177. [DOI] [PubMed] [Google Scholar]
- Jones G, Nguyen T, Sambrook PN, Lord SR, Kelly PJ, Eisman JA. Osteoarthritis, bone density, postural stability, and osteoporotic fractures: a population based study. J Rheumatol 1995;22:921–925. [PubMed] [Google Scholar]
- Arden NK, Griffiths GO, Hart DJ, Doyle DV, Spector TD. The association between osteoarthritis and osteoporotic fracture: the Chingford Study. Br J Rheumatol 1996;35:1299–1304. [DOI] [PubMed] [Google Scholar]
- Prieto-Alhambra D, Nogues X, Javaid MK, Wyman A, Arden NK, Azagra R et al. An increased rate of falling leads to a rise in fracture risk in postmenopausal women with self-reported osteoarthritis: a prospective multinational cohort study (GLOW). Ann Rheum Dis 2013;72:911–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Niu J, Felson DT, Choi HK, Nevitt M, Neogi T. Methodologic challenges in studying risk factors for progression of knee osteoarthritis. Arthritis Care Res (Hoboken) 2010;62:1527–1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casden AM, Jaffe FF, Kastenbaum DM, Bonar SF. Osteoarthritis associated with osteopetrosis treated by total knee arthroplasty. Report of a case. Clin Orthop Relat Res 1989; (247): 202–207. [PubMed] [Google Scholar]
- Benichou OD, Laredo JD, de Vernejoul MC. Type II autosomal dominant osteopetrosis (Albers-Schonberg disease): clinical and radiological manifestations in 42 patients. Bone 2000;26:87–93. [DOI] [PubMed] [Google Scholar]
- Hamersma H, Gardner J, Beighton P. The natural history of sclerosteosis. Clin Genet 2003;63:192–197. [DOI] [PubMed] [Google Scholar]
- Gregson CL, Steel SA, O'Rourke KP, Allan K, Ayuk J, Bhalla A et al. 'Sink or swim': an evaluation of the clinical characteristics of individuals with high bone mass. Osteoporos Int 2012;23:643–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardcastle SA, Gregson CL, Deere KC, Davey Smith G, Dieppe P, Tobias JH. High bone mass is associated with an increased prevalence of joint replacement: a case-control study. Rheumatology (Oxford) 2013;52:1042–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardcastle SA, Dieppe P, Gregson CL, Hunter D, Thomas GER, Arden NK, Spector TD et al. Prevalence of radiographic hip osteoarthritis is increased in high bone mass. Osteoarthritis Cartilage 2014;22:1120–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardcastle SA, Dieppe P, Gregson CL, Arden NK, Spector TD, Hart DJ, Edwards MH et al. Individuals with high bone mass have an increased prevalence of radiographic knee osteoarthritis. Bone 2015;71:171–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felson DT, Lawrence RC, Dieppe PA, Hirsch R, Helmick CG, Jordan JM et al. Osteoarthritis: new insights. Part 1: the disease and its risk factors. AnnIntern Med 2000;133:635–646. [DOI] [PubMed] [Google Scholar]
- Hardcastle SA, Dieppe P, Gregson CL, Arden NK, Spector TD, Hart DJ, Edwards MH et al. Osteophytes, enthesophyte and high bone mass; a bone-forming triad with relevance for osteoarthritis? Arthritis Rheumatol 2014;66:2429–2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregson C, Leo PJ, Clark GR, Davey Smith G, Brown MA, Tobias JH et al. A GWAS in an extreme high bone mass population shows excess signal from genes associated with BMD in the normal population (abstract). European Calcified Tissue Society Congress 2013; Lisbon, Portugal: Bone Abstracts 2013 PP31.
- Radin EL, Paul IL, Rose RM. Role of mechanical factors in pathogenesis of primary osteoarthritis. Lancet 1972;1:519–522. [DOI] [PubMed] [Google Scholar]
- Li B, Aspden RM. Composition and mechanical properties of cancellous bone from the femoral head of patients with osteoporosis or osteoarthritis. J Bone Miner Res 1997;12:641–651. [DOI] [PubMed] [Google Scholar]
- Li ZC, Dai LY, Jiang LS, Qiu S. Difference in subchondral cancellous bone between postmenopausal women with hip osteoarthritis and osteoporotic fracture: implication for fatigue microdamage, bone microarchitecture, and biomechanical properties. Arthritis Rheum 2012;64:3955–3962. [DOI] [PubMed] [Google Scholar]
- Day JS, Ding M, van der Linden JC, Hvid I, Sumner DR, Weinans H. A decreased subchondral trabecular bone tissue elastic modulus is associated with pre-arthritic cartilage damage. J Orthop Res 2001;19:914–918. [DOI] [PubMed] [Google Scholar]
- Lories RJ, Luyten FP. The bone-cartilage unit in osteoarthritis. Nat Rev Rheumatol 2011;7:43–49. [DOI] [PubMed] [Google Scholar]
- Estrada K, Styrkarsdottir U, Evangelou E, Hsu YH, Duncan EL, Ntzani EE et al. Genome-wide meta-analysis identifies 56 bone mineral density loci and reveals 14 loci associated with risk of fracture. Nat Genet 2012;44:491–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yerges-Armstrong LM, Yau MS, Liu Y, Krishnan S, Renner JB, Eaton CB et al. Association Analysis of BMD-associated SNPs with Knee Osteoarthritis. J Bone Miner Res 2014;29:1373–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yerges-Armstrong L. Genetic Association Analysis of Radiographic Hip Osteoarthritis with Established Loci for Bone Mineral Density: Data from the Osteoarthritis Initiative. OARSI 2014; Paris, France: Osteoarthritis and Cartilage 2014, S416.
- Reynard LN, Loughlin J. Insights from human genetic studies into the pathways involved in osteoarthritis. Nat Rev Rheumatol 2013;9:573–583. [DOI] [PubMed] [Google Scholar]
- Davey Smith G, Hemani G. Mendelian randomisation: genetic anchors for causal inference in epidemiological studies. Hum Mol Genet 2014;23:R89–R98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker-LePain JC, Lane NE. Relationship between joint shape and the development of osteoarthritis. Curr Opin Rheumatol 2010;22:538–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobias JH. Wnt signalling and the genetics of osteoarthritis. IBMS BoneKey 2012;9:X. [Google Scholar]
- Castano Betancourt MC, Cailotto F, Kerkhof HJ, Cornelis FM, Doherty SA, Hart DJ et al. Genome-wide association and functional studies identify the DOT1L gene to be involved in cartilage thickness and hip osteoarthritis. Proc Natl Acad Sci USA 2012;109:8218–8223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker-Lepain JC, Lynch JA, Parimi N, McCulloch CE, Nevitt MC, Corr M et al. Variant alleles of the Wnt antagonist FRZB are determinants of hip shape and modify the relationship between hip shape and osteoarthritis. Arthritis Rheum 2012;64:1457–1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux C, Richette P. Impact of treatments for osteoporosis on osteoarthritis progression. Osteoporos Int 2012;23:S881–S883. [DOI] [PubMed] [Google Scholar]
- Spector TD, Conaghan PG, Buckland-Wright JC, Garnero P, Cline GA, Beary JF et al. Effect of risedronate on joint structure and symptoms of knee osteoarthritis: results of the BRISK randomized, controlled trial [ISRCTN01928173]. Arthritis Res Ther 2005;7:R625–R633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bingham CO 3rd, Buckland-Wright JC, Garnero P, Cohen SB, Dougados M, Adami S et al. Risedronate decreases biochemical markers of cartilage degradation but does not decrease symptoms or slow radiographic progression in patients with medial compartment osteoarthritis of the knee: results of the two-year multinational knee osteoarthritis structural arthritis study. Arthritis Rheum 2006;54:3494–3507. [DOI] [PubMed] [Google Scholar]
- Davis AJ, Smith TO, Hing CB, Sofat N. Are bisphosphonates effective in the treatment of osteoarthritis pain? A meta-analysis and systematic review. PloS one 2013;8:e72714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reginster JY, Badurski J, Bellamy N, Bensen W, Chapurlat R, Chevalier X et al. Efficacy and safety of strontium ranelate in the treatment of knee osteoarthritis: results of a double-blind, randomised placebo-controlled trial. Ann Rheum Dis 2013;72:179–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafeber FP, van Laar JM. Strontium ranelate: ready for clinical use as disease-modifying osteoarthritis drug? Ann Rheum Dis 2013;72:157–161. [DOI] [PubMed] [Google Scholar]
- Medicines and Healthcare Products Regulatory Agency. Strontium ranelate: cardiovascular risk-restricted indication and new monitoring requirements. MHRA Drug Safety Update [Internet] 2014;7:S1. Available from: http://www.mhra.gov.uk/home/groups/dsu/documents/publication/con392897.pdf.
- Bijlsma JW, Berenbaum F, Lafeber FP. Osteoarthritis: an update with relevance for clinical practice. Lancet 2011;377:2115–2126. [DOI] [PubMed] [Google Scholar]
- Kerkhof HJ, Meulenbelt I, Akune T, Arden NK, Aromaa A, Bierma-Zeinstra SM et al. Recommendations for standardization and phenotype definitions in genetic studies of osteoarthritis: the TREAT-OA consortium. Osteoarthritis Cartilage 2011;19:254–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roubille C, Pelletier JP, Martel-Pelletier J. New and emerging treatments for osteoarthritis management: will the dream come true with personalized medicine? Expert Opin Pharmacother 2013;14:2059–2077. [DOI] [PubMed] [Google Scholar]