Abstract
Objective
Interleukin-8 (IL-8, CXCL8) plays important roles in immune responses at mucosal sites including in the lower genital tract. Since several types of bacteria produce proteases that cleave IL-8 and many types of bacteria can be present in lower genital tract microbiota, we assessed genital fluids for IL-8 cleavage/alteration.
Study Design
Genital fluids collected by lavage from 200 women (23 HIV-seronegative and 177 HIV-seropositive) were tested for IL-8 cleavage/alteration by ELISA.
Results
IL-8 cleaving/altering activity was observed in fluids from both HIV-positive (28%) and HIV-negative women (35%). There was no clear relationship between the activity and the types of bacteria present in the lower genital tract as determined by high-throughput sequencing of the 16S rRNA gene. Protease inhibitors specific for matrix metalloproteinases (MMPs) reduced the activity and a multiplex assay that detects both inactive and active MMPs showed the presence of multiple MMPs, including MMP-1, -3, -7, -8, -9, -10 and -12 in genital secretions from many of the women. The IL-8-cleaving/altering activity significantly correlated with active MMP-9 as well as with cleavage of a substrate that is acted on by several active MMPs.
Conclusions
These studies show that multiple MMPs are present in the genital tract of women and strongly suggest that MMP-9 in genital secretions can cleave IL-8 at this mucosal site. These studies suggest that MMP-mediated cleavage of IL-8 can modulate inflammatory responses in the lower genital tract.
Introduction
The chemokine Interleukin-8 (IL-8, CXCL8) is a member of the CXC chemokine family that plays a number of important roles in immunity including activation and attraction of neutrophils [1,2]. In vitro, IL-8 is produced by neutrophils, macrophages, monocytes and epithelial cells when exposed to either microbial products derived from commensal bacteria or organisms that cause sexually transmitted infections (STI) [3–5]. IL-8 levels in lower genital tract secretions are increased in women with STIs [5–7] and also increased in response to non-STI alterations in lower genital tract microbiota [7–11]. IL-8 elevations in genital secretions and in cultures of epithelial cells have been used as a biomarker of inflammation in clinical trials of microbicides [12,13].
There are several proteases that have been reported to act on IL-8. A protease made by some strains of Streptococcus pyogenes has been shown to cleave the C-terminal alpha helix of IL-8 resulting in IL-8 inactivation [14]. In infected patients, disease severity correlated with the IL-8 protease activity expressed by the S. pyogenes isolates [15]. Porphyromonas gingivalis, an oral bacterium implicated in periodontitis, also produces a protease that can cleave at the amino terminal end of IL-8 [16,17]. The host-derived protease matrix metalloproteinase-9 (MMP-9) has also been reported to cleave IL-8 [18]. MMP-9 removes six amino acids from the N-terminal portion of IL-8, but instead of inactivation, this cleavage results in dramatically enhanced IL-8 signaling through CXCR1.
The bacterial microbiota in the female lower genital tract of different women can be comprised of many different genera of bacteria including Lactobacillus, Prevotella, Megasphaera, Gardnerella, Mobliluncus, Atopobium, Parvimonas, and Sneathia [19–21]. Strains of Streptococcus and Porphyromonas are also present in the genital tract of some women. We hypothesized that some of these genital bacteria could express proteases that cleave IL-8. To explore this hypothesis, we used ELISA to assess a reduction of IL-8 detection after incubation with genital tract fluids collected from 200 different women. Since a reduction of reactivity in the ELISA does not necessarily show cleavage of IL-8, we used the terms “cleavage/alteration” and “cleaving/altering” throughout the paper to indicate reduction in ELISA activity.
Material and Methods
Subjects
Genital samples were obtained from women in the Rwanda Women’s Inter-association Study and Assessment (RWISA). RWISA is an observational prospective cohort study investigating the effectiveness and toxicity of antiretroviral therapy (ART) and comorbidities in HIV-infected Rwandan women.
Written informed consent was obtained in accordance with protocols approved by the Rwanda National Ethics Committee and the Institutional Review Board of Montefiore Medical Center, Bronx NY. Genital samples were collected by cervicovaginal lavage (CVL) performed by irrigation of the cervix with 10 ml of nonbacteriostatic sterile saline, followed by aspiration from the posterior fornix. CVL were transported from the study site to the lab within two hours of collection, aliquoted and frozen.
Measurement of IL-8 cleavage/alteration
IL-8 cleavage/alteration was measured similarly to previously reports [14]. CVL were clarified by centrifugation and diluted 1:4 with RPMI-1640 medium buffered with HEPES (Sigma, St. Louis, MO, added as a source of cations) and 0.1 ml of diluted fluid was added to 0.1 ml (2 ng/ml) of carrier-free recombinant human IL-8 (rhCXCL-8/IL-8, R&D Minneapolis, MN, USA). The mixtures were incubated for 20 h at 4°C or 37°C. Afterward, ELISA was used to determine the concentration of IL-8 (BD Bioscience, San Diego, CA USA). The cutoff for determining if samples were positive for IL-8 cleavage/alteration was set by calculating the standard deviation of negative controls in multiple runs and multiplying by 3. In some experiments, protease inhibitors were added to the incubations; either General Protease Inhibitor (1:25 final concentration) (Sigma), EDTA (2 mmol final concentration), Marimastat (13 nM final concentration, Tocris, Bristol, UK) or CP471474 (16 nM final concentration, Tocris). A culture supernatant from Group A Streptococcus was used as a positive control in all experiments (a kind gift from Paul Sumby, Center for Molecular and Translational Human Infectious Diseases Research, Huston, Texas 77030).
Pyrosequencing of the 16S rRNA gene and identification of bacteria
Bacteria in CVL were pelleted by centrifugation and DNA was isolated using the Fast DNA Spin Kit for Soil (MP Biomedicals, Solon, OH USA). Multitag Pyrosequencing, as described previously [22,23], was performed using 12 bar-coded primer sets each containing the 27F and 355R 16S rRNA gene primers. The Bayesian Classifier provided by the Ribosomal Database II Project (RDP 10) using forward reads only was applied to identify bacteria.
Assays for MMPs
Total MMPs in CVL (both active and latent forms) were measured by a multiplex Luminex assay (R&D Systems Inc. Minneapolis, MN). The plate was read on a Luminex analyzer (LuminexH-Bio-PlexH 200, BIO-Rad Hercules, CA ). The sensitivity of detection of the different MMPs was as follows; MMP-1, 1.1 pg/ml; MMP-7, 7.3 pg/ml; MMP-7, 6.6 pg/ml; MMP-8, 16.6 pg/ml; MMP-9, 13.7 pg/ml; MMP-10, 3.2 pg/ml; and MMP-12, 0.7 pg/ml. A fluorogenic substrate that was susceptible to cleavage by multiple MMPs was used to measure active MMPs in CVL (OmniMMP, Enzolifesciences, Farmingdale, NY). CVL was assayed at 1:5 dilutions with 20 μl of OmniMMP substrate (final concentration 10 uM). The plate was incubated at 37°C for 1 h, and analyzed with excitation of 328 nm and detection of 393 nm. To measure activated MMP-9, an immunocapture system was used (R&D Systems Inc. Minneapolis, MN). The minimum detectable dose (MDD) of active MMP-9 was 0.1 ng/ml.
Statistical Analysis
Statistical analysis was performed using the Instat statistical software package (GraphPad Software). Nonparametric and parametric methods were used for comparisons and to assess correlations, as indicated in the text and figure legends. A p-value of 0.05 or lower was considered significant.
Results
Cleavage/alteration of Recombinant human IL-8 by genital mucosal fluid
An assay similar to one previously reported [14] was used to determine if proteases that could cleave IL-8 were present in the lower genital tract fluid collected from women. Recombinant human IL-8 was incubated for 20 h at either 4°C or 37°C with either saline (negative control), a supernatant from cultures of group A Streptococcus (positive control) or genital mucosal fluid collected by lavage from women. The positive control resulted in 95–100% reduction in ELISA detection (not shown) while incubation with the negative control had no effect on IL-8 (Fig. 1A). Samples from 200 women were tested; 23 from HIV-seronegative women and 177 from HIV-seropositive women. Reduction in IL-8 detection greater than 3% was observed in 57 (29%) of the 200 samples. Fig. 1A shows examples of several of the positive samples including ones from HIV+ subjects with the most reduction (subjects 26 and 56) and three from HIV+ subjects that exhibited no reduction (83, 90 and 100). Note that the genital fluid samples from the subjects had higher levels of IL-8 after 4°C incubation than the saline control because genital fluid from most women contains some endogenous IL-8 [7].
Figure 1. Cleavage of IL-8 in genital mucosal fluids.
A. Recombinant IL-8 was added to either saline (negative control) or genital fluids (diluted 1:4) from 10 HIV-seropositive subjects and incubated at either 4°C or 37°C for 20 h. Levels of IL-8 were then measured by ELISA. The mean ± SD are shown. B. Recombinant IL-8 was added to genital fluid from subject 26 diluted 1:4 or 1:40 and incubated for 4 or 24 h before detection by ELISA.
Cleavage/alteration was observed in 28% of the samples from the HIV-seropositive women and 35% from the HIV-seronegative women (p>0.05, Chi-square analysis). For the samples from the HIV+ women, we determined if there was a relationship between peripheral blood CD4 number (assessed by flow cytometry) and IL-8 cleaving/altering activity. Surprisingly, we found that the CD4 number was significantly higher in women whose samples were positive (>3% cleavage) for activity (median CD4 of 277 cells/microliter) than in those negative for IL-8 cleavage/alteration (<3% cleavage, median CD4 of 176 cells/microliter, p = 0.004, Mann-Whitney test). In contrast, the plasma HIV levels were not significantly different between the two groups.
The fact that IL-8 cleavage/alteration occurred at 37°C when compared with 4°C suggested that an enzyme was responsible. To determine the heat stability of the activity, a portion of one genital fluid sample was heated at 80°C for 45 min before testing its activity. While the unheated genital fluid reduced IL-8 detection by 55%, heating at 80°C completely eliminated the activity (0% cleavage) indicating the activity was heat labile (data not shown).
The time and concentration dependence of the IL-8 cleaving/altering activity in the sample from subject 26 was also tested. Substantial IL-8 cleavage/alteration was observed by 4 hours when the genital sample was diluted 1:40 with even more cleavage from the more concentrated sample (Fig. 1B). Incubation for 24 hours increased the amount of cleavage/alteration.
Relationship between genital microbiota and IL-8 cleavage
Since previous studies had shown that IL-8 could be cleaved by proteases produced by the bacteria Porphyromonas and Streptococcus [14,16], we investigated a possible bacterial source for the activity. DNA was isolated from the bacterial pellets from nine genital fluid samples with activity and from three with no activity and the bacteria in the samples were identified by high-throughput sequencing of the V1/V2 region of the 16S rRNA gene.
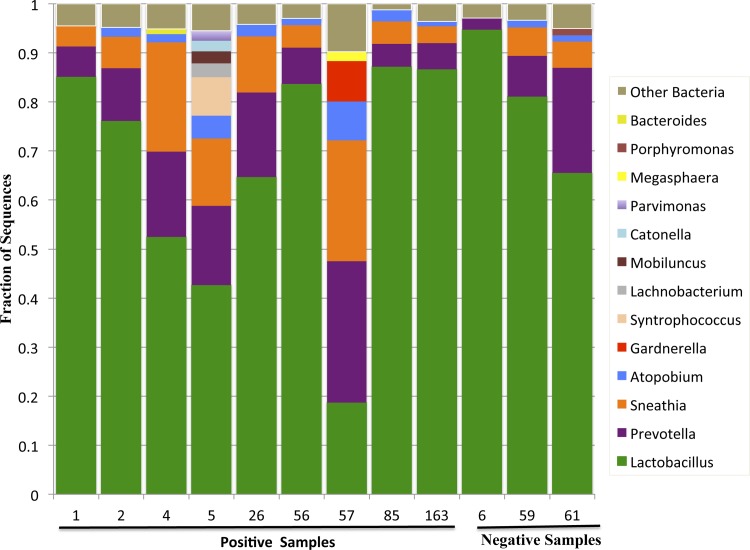
Sequences corresponding to Lactobacillus were found in all samples and accounted for >50% of the sequences in 7/9 of the IL-8 cleavage/altering samples and 3/3 of the samples with no activity (Fig. 2). Sequences corresponding to Prevotella and Sneathia were detected in 12/12 samples and 11/12 samples respectively. Sequences corresponding to Streptococcus were not found in any of the samples. Porphyromonas sequences were found in one of the active samples (subject 5). A few genera, including Gardnerella, Megasphaera and Syntrophococcus were present only in samples positive for the activity, but were only found in only one or two of the positive samples. Thus, overall there was no bacterial genus that correlated closely with the IL-8 cleaving/altering activity and the bacterial profiles of the samples with the highest activity (26 and 56) were similar to the profiles of samples with no activity (Fig. 2).
Figure 2. Lower genital tract microbiota in samples with or without IL-8-cleaving activity.
The microbiota in the lower genital tract of women with or without IL-8-cleaving activity was identified by pyrosequencing of the 16S rRNA gene. The 13 most predominant bacterial genera are graphed.
MMPs and IL-8 cleavage/alteration
Since no clear relationship between the bacterial microbiota and IL-8 cleavage/alteration was observed, the type of protease responsible for the activity was next investigated. Samples with high activity (samples 26 and 56) were tested in the presence of several protease inhibitors. PMSF, a serine protease inhibitor, had no effect on activity (not shown). However, both EDTA and a protease inhibitor mixture (general protease inhibitor, GPI) reduced IL-8 cleavage/alteration with similar efficacy (Fig. 3A). Since EDTA is known to inhibit matrix metalloproteinases (MMPs), we next tested the effect of two MMP inhibitors, Marimastat and C471474, on IL-8 cleavage. Both of the MMP inhibitors reduced IL-8 cleavage/altering compared to the DMSO control, although Marimastat was more effective (Fig. 3B).
Figure 3. The effect of protease inhibitors on the IL-8-cleaving activity in genital tract fluid.
A. Two samples positive for IL-8 cleavage (samples 26 and 56) were incubated with recombinant IL-8 for 20 h at 37°C in the presence or absence (No I) of EDTA or a cocktail of protease inhibitors (general protease inhibitor, GPI). B. MMP inhibitors Marimastat and C471474 were tested. For both A and B, control is IL-8 incubated without genital fluid or inhibitors. Samples were analyzed at 20 h by IL-8 ELISA. Shown are the mean values ± SD. * p<0.05, **p<0.1, t test compared to no inhibitor.
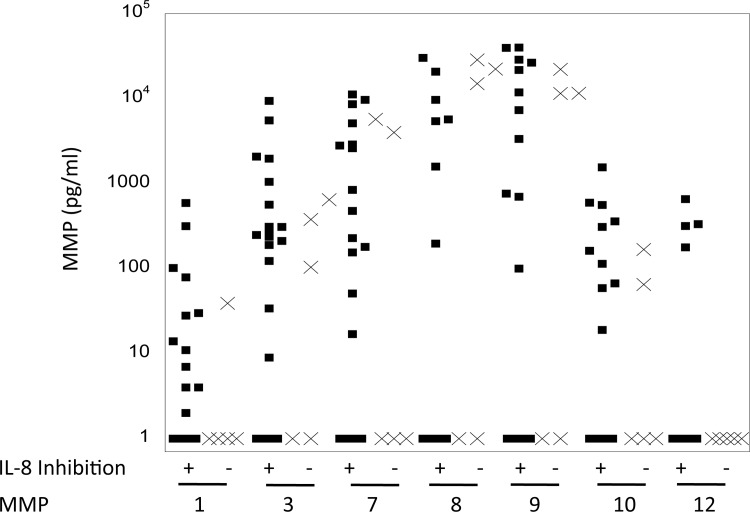
Since the inhibitor experiments suggested MMPs could be involved in IL-8 cleavage/alteration, we next used a multiplex assay to measure the total amount (both active and inactive) of MMPs 1, 3, 7, 8, 9, 10 and 12 in samples that were positive (n = 22) or negative (n = 5) for activity (Fig. 4). All of the MMPs could be detected in at least some of the samples. When comparing IL-8-cleavaging/altering-positive and –negative samples, none of the MMPs were significantly different between the two groups.
Figure 4. Total MMP levels in mucosal fluid samples.
The concentration of MMPs 1, 3, 7, 8, 9, 10 and 12 were measured by Luminex immunoassay in mucosal fluid samples. 22 samples positive for IL-8 cleavage (square symbols) and 5 samples negative for IL-8 cleavage (x symbols) were evaluated.
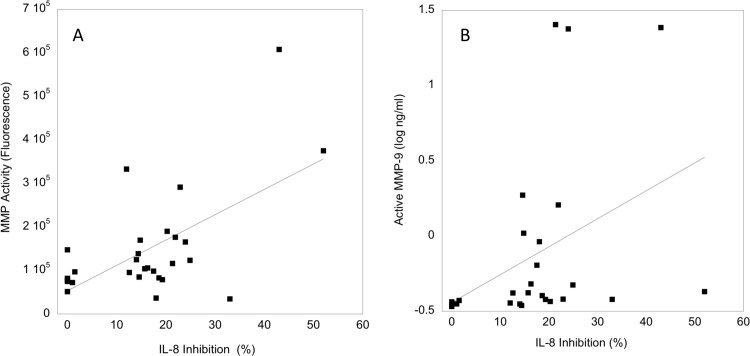
Since the total MMPs measured above contained both active and inactive MMPs, we analyzed active MMPs using a fluorogenic substrate that is cleaved by multiple MMPs. There was a significant correlation between IL-8 cleavage/alteration and the activity defined by the broad MMP substrate (Fig. 5A, Spearman r = 0.606, p = 0.02). Also, since MMP-9 has been previously associated with cleavage of IL-8, we compared IL-8 cleavage/alteration with active MMP-9. There was also a significant correlation between the activity and active MMP-9 levels (Fig. 5B, Spearman r = 0.690, p = 0.0001).
Figure 5. Relationship of active MMPs and IL-8 cleavage.
A. The amount of MMP activity in genital fluids 22 samples positive for IL-8 cleavage and 5 negative for IL-8 cleavage was measured using a fluorogenic substrate and plotted against the % cleavage of IL-8 for each sample. B. The amount of active MMP-9 in the 27 samples was plotted against the % cleavage of IL-8 for each sample.
Discussion
This study shows that an activity that can act on IL-8 is present in the lower genital tract fluid of some women. Inhibitor studies of samples collected from the two women with the highest activity strongly suggested that MMPs were causing IL-8 cleavage. Further, a strong positive correlation was observed between the IL-8 cleaving/altering activity and active MMP-9 levels as well as between the activity and cleavage of a substrate that can detect multiple active MMPs. Together these data, along with previous studies showing that MMP-9 can act on IL-8 [18], indicate that in most of the samples, MMPs and specifically MMP-9 was responsible for the IL-8 cleaving/altering activity. However, several of the samples that had relatively high levels of IL-8 cleavage/alteration had relatively low MMP-9 activity (Fig. 5B). Some of the samples with low MMP-9 activity had relatively high activity with the substrate that can be cleaved by multiple MMPs (Fig. 5A). Thus, our results suggest that in some of the samples, MMPs other than MMP-9 might participate in cleavage of IL-8. Several other MMPs have been reported to act on other chemokines [24]. For example, MMP-3 can cleave MCP-1 and MMP-8 can cleave CXCL5, although other MMPs have not been reported to act on IL-8 [24]. Alternatively, it is possible that other proteases, possibly either host- or bacterially-derived, could be present and act on IL-8 in the lower genital tract samples. A previous study of stability of IL-8 in lavage specimens showed that pooling lavages and incubating at 37°C for 24 h decreased IL-8 detection by about 50% [12]. However, in that study it is not clear that an enzymatic activity was responsible for the decreases in IL-8 and MMPs were not assessed.
To our knowledge this is the first report of active MMPs in the lower genital tract of women that shows the presence of multiple MMPs, although we did not test if all the MMPs were active. All of the tested MMPs including MMP-1, -3, -7, -8, -9, -10 and -12 were found in at least some of the women while none of the MMPs were found in all of the women. A study by Heng et al. [25] measured MMPs 1, -2, -3, -7, -8, -9, -12, and -13 and showed that MMP-7 was increased in labor, although MMP activity was not evaluated in that study. Active MMPs have multiple complex roles in inflammation including inhibition and potentiation [24], so that the active MMPs detected in our study could potentially alter responses to STDs or microbiota in the lower genital tract.
In this study, IL-8 cleaving/altering activity was observed in samples from both HIV-seropositive and –seronegative women. Thus, the presence of the activity does not appear to be associated with HIV-induced immunodeficiency. In fact, in the HIV-seropositive women, CD4 counts were higher in samples with activity than in samples with no activity. This could suggest that the expression of the protease in the genital tract is controlled through immune responses, and that the activity levels are consequently reduced in immunodeficient subjects. In contrast to our results, in a study of crevicular MMP-9 in HIV-infected subjects, a significant negative correlation between MMP-9 and CD4 levels was seen [26]. The IL-8 cleaving/altering activity was also not associated with types of bacterial microbiota. All but two of the nine positive samples had a microbiota that was dominated by Lactobacillus, which in previous studies has been associated with low levels of inflammation as determined by levels of pro-inflammatory cytokines [7,8,10,11,27–29]. However, it is possible that other infectious or non-infectious inflammatory stimuli could have induced the expression of the IL-8 cleaving activity in the women, but the women in this cohort were not tested for these. Neutrophils or macrophages could be the source of MMPs in our studies [24]. Interestingly, a cytolytic factor that can lyse neutrophils has been reported to be produced by Gardnerella [30]. Such a factor produced by genital bacteria could increase the levels of host-derived IL-8 degrading enzymes in the genital tract due to release from neutrophils.
Since IL-8 cleavage in many of the genital samples in this study was likely mediated by MMP-9, cleavage would be expected to substantially (approximately 10×) increase IL-8 biological activity as previously reported [18]. Due to the presence of endogenous IL-8 and other chemokines [7,28], and also potentially cleaved IL-8 in lower genital tract fluids, the bioactivity resulting from IL-8 cleavage could not be assessed in our study. A large increase in IL-8 bioactivity due to cleavage could substantially influence the amount of inflammation that occurs during STIs or other causes of lower genital tract inflammation that may have negative effects such as increasing HIV susceptibility [31]. Further study of the role that MMPs play in modulating genital inflammation are warranted, especially since inhibitors of MMPs are available that could be used to reduce pathogenic effects [32].
Data Availability
All relevant data are within the paper.
Funding Statement
GTS and AL received National Institutes of Health grant P01 AI08297. ALF received National Institutes of Health grant P30 AI082151. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Roebuck KA (1999) Regulation of interleukin-8 gene expression. J Interferon Cytokine Res 19: 429–438. [DOI] [PubMed] [Google Scholar]
- 2. Mukaida N, Harada A, Matsushima K (1998) Interleukin-8 (IL-8) and monocyte chemotactic and activating factor (MCAF/MCP-1), chemokines essentially involved in inflammatory and immune reactions. Cytokine Growth Factor Rev 9: 9–23. [DOI] [PubMed] [Google Scholar]
- 3. Pivarcsi A, Nagy I, Koreck A, Kis K, Kenderessy-Szabo A, et al. (2005) Microbial compounds induce the expression of pro-inflammatory cytokines, chemokines and human beta-defensin-2 in vaginal epithelial cells. Microbes Infect 7: 1117–1127. [DOI] [PubMed] [Google Scholar]
- 4. Schaefer TM, Fahey JV, Wright JA, Wira CR (2005) Innate immunity in the human female reproductive tract: antiviral response of uterine epithelial cells to the TLR3 agonist poly(I:C). J Immunol 174: 992–1002. [DOI] [PubMed] [Google Scholar]
- 5. Shaio MF, Lin PR, Liu JY, Yang KD (1995) Generation of interleukin-8 from human monocytes in response to Trichomonas vaginalis stimulation. Infect Immun 63: 3864–3870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Simhan HN, Anderson BL, Krohn MA, Heine RP, Martinez de Tejada B, et al. (2007) Host immune consequences of asymptomatic Trichomonas vaginalis infection in pregnancy. Am J Obstet Gynecol 196: 59 e51–55 [DOI] [PubMed] [Google Scholar]
- 7. Spear GT, Kendrick SR, Chen HY, Thomas TT, Bahk M, et al. (2011) Multiplex immunoassay of lower genital tract mucosal fluid from women attending an urban STD clinic shows broadly increased IL1ss and lactoferrin. PLoS One 6: e19560 10.1371/journal.pone.0019560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Spear GT, Zariffard MR, Cohen MH, Sha BE (2008) Vaginal IL-8 levels are positively associated with Candida albicans and inversely with lactobacilli in HIV-infected women. J Reprod Immunol 78: 76–79. 10.1016/j.jri.2007.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Steele C, Fidel PL Jr. (2002) Cytokine and chemokine production by human oral and vaginal epithelial cells in response to Candida albicans. Infect Immun 70: 577–583. 10.1128/IAI.70.2.577-583.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Basso B, Gimenez F, Lopez C (2005) IL-1beta, IL-6 and IL-8 levels in gyneco-obstetric infections. Infect Dis Obstet Gynecol 13: 207–211. 10.1080/10647440500240664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mitchell CM, Balkus J, Agnew KJ, Cohn S, Luque A, et al. (2008) Bacterial vaginosis, not HIV, is primarily responsible for increased vaginal concentrations of proinflammatory cytokines. AIDS Res Hum Retroviruses 24: 667–671. 10.1089/aid.2008.0268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fichorova RN (2004) Guiding the vaginal microbicide trials with biomarkers of inflammation. J Acquir Immune Defic Syndr 37 Suppl 3: S184–193. [PMC free article] [PubMed] [Google Scholar]
- 13. Fichorova RN, Bajpai M, Chandra N, Hsiu JG, Spangler M, et al. (2004) Interleukin (IL)-1, IL-6, and IL-8 predict mucosal toxicity of vaginal microbicidal contraceptives. Biol Reprod 71: 761–769. [DOI] [PubMed] [Google Scholar]
- 14. Edwards RJ, Taylor GW, Ferguson M, Murray S, Rendell N, et al. (2005) Specific C-terminal cleavage and inactivation of interleukin-8 by invasive disease isolates of Streptococcus pyogenes. J Infect Dis 192: 783–790. [DOI] [PubMed] [Google Scholar]
- 15. Turner CE, Kurupati P, Jones MD, Edwards RJ, Sriskandan S (2009) Emerging role of the interleukin-8 cleaving enzyme SpyCEP in clinical Streptococcus pyogenes infection. J Infect Dis 200: 555–563. 10.1086/603541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dias IH, Marshall L, Lambert PA, Chapple IL, Matthews JB, et al. (2008) Gingipains from Porphyromonas gingivalis increase the chemotactic and respiratory burst-priming properties of the 77-amino-acid interleukin-8 variant. Infect Immun 76: 317–323. 10.1128/IAI.00618-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mikolajczyk-Pawlinska J, Travis J, Potempa J (1998) Modulation of interleukin-8 activity by gingipains from Porphyromonas gingivalis: implications for pathogenicity of periodontal disease. FEBS Lett 440: 282–286. [DOI] [PubMed] [Google Scholar]
- 18. Van den Steen PE, Proost P, Wuyts A, Van Damme J, Opdenakker G (2000) Neutrophil gelatinase B potentiates interleukin-8 tenfold by aminoterminal processing, whereas it degrades CTAP-III, PF-4, and GRO-alpha and leaves RANTES and MCP-2 intact. Blood 96: 2673–2681. [PubMed] [Google Scholar]
- 19. Fredricks DN, Fiedler TL, Marrazzo JM (2005) Molecular identification of bacteria associated with bacterial vaginosis. N Engl J Med 353: 1899–1911. [DOI] [PubMed] [Google Scholar]
- 20. Gajer P, Brotman RM, Bai G, Sakamoto J, Schutte UM, et al. (2012) Temporal dynamics of the human vaginal microbiota. Sci Transl Med 4: 132ra152 10.1126/scitranslmed.3003605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Spear GT, Sikaroodi M, Zariffard MR, Landay AL, French AL, et al. (2008) Comparison of the Diversity of the Vaginal Microbiota in HIV-Infected and HIV-Uninfected Women with or without Bacterial Vaginosis. J Infect Dis 198: 1131–1140. 10.1086/591942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Spear G, Rothaeulser K, Fritts L, Gillevet PM, Miller CJ (2012) In captive rhesus macaques, cervicovaginal inflammation is common but not associated with the stable polymicrobial microbiome. PLoS One 7: e52992 10.1371/journal.pone.0052992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spear GT, Kersh E, Guenthner P, Vishwanathan SA, Gilbert D, et al. (2012) Longitudinal Assessment of Pigtailed Macaque Lower Genital Tract Microbiota by Pyrosequencing Reveals Dissimilarity to the Genital Microbiota of Healthy Humans. AIDS Res Hum Retroviruses. [DOI] [PMC free article] [PubMed]
- 24.Nissinen L, Kahari VM (2014) Matrix metalloproteinases in inflammation. Biochim Biophys Acta. [DOI] [PubMed]
- 25. Heng YJ, Di Quinzio MK, Liong S, Permezel M, Rice GE, et al. (2012) Temporal investigation of matrix metalloproteinases and their inhibitors in human cervicovaginal fluid in late pregnancy and labor. Reprod Sci 19: 55–63. 10.1177/1933719111413299 [DOI] [PubMed] [Google Scholar]
- 26. Alpagot T, Suzara V, Bhattacharyya M (2006) The associations between gingival crevice fluid matrix metalloproteinase-9, tissue inhibitor of metalloproteinase-1 and periodontitis in human immunodeficiency virus-positive patients. J Periodontal Res 41: 491–497. [DOI] [PubMed] [Google Scholar]
- 27. Cauci S, Driussi S, Guaschino S, Isola M, Quadrifoglio F (2002) Correlation of local interleukin-1beta levels with specific IgA response against Gardnerella vaginalis cytolysin in women with bacterial vaginosis. Am J Reprod Immunol 47: 257–264. [DOI] [PubMed] [Google Scholar]
- 28. Cummins JE, Christensen L, Lennox JL, Bush TJ, Wu Z, et al. (2006) Mucosal innate immune factors in the female genital tract are associated with vaginal HIV-1 shedding independent of plasma viral load. AIDS Res Hum Retroviruses 22: 788–795. [DOI] [PubMed] [Google Scholar]
- 29. Yudin MH, Landers DV, Meyn L, Hillier SL (2003) Clinical and cervical cytokine response to treatment with oral or vaginal metronidazole for bacterial vaginosis during pregnancy: a randomized trial. Obstet Gynecol 102: 527–534. [DOI] [PubMed] [Google Scholar]
- 30. Rottini G, Dobrina A, Forgiarini O, Nardon E, Amirante GA, et al. (1990) Identification and partial characterization of a cytolytic toxin produced by Gardnerella vaginalis. Infect Immun 58: 3751–3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Roberts L, Liebenberg L, Barnabas S, Passmore JA (2012) Vaginal microbicides to prevent human immunodeficiency virus infection in women: perspectives on the female genital tract, sexual maturity and mucosal inflammation. Best Pract Res Clin Obstet Gynaecol 26: 441–449. 10.1016/j.bpobgyn.2012.02.002 [DOI] [PubMed] [Google Scholar]
- 32. Khokha R, Murthy A, Weiss A (2013) Metalloproteinases and their natural inhibitors in inflammation and immunity. Nat Rev Immunol 13: 649–665. 10.1038/nri3499 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.