Abstract
Polychlorinated biphenyls (PCBs) are a group of 209 persistent organic pollutants, whose documented carcinogenic, neurological, and respiratory toxicities are expansive and growing. However, PCB inhalation exposure assessments have been lacking for North American ambient conditions and lower-chlorinated congeners. We assessed congener-specific inhalation and dietary exposure for 78 adolescent children and their mothers (n = 68) in the Airborne Exposure to Semi-volatile Organic Pollutants (AESOP) Study. Congener-specific PCB inhalation exposure was modeled using 293 measurements of indoor and outdoor airborne PCB concentrations at homes and schools, analyzed via tandem quadrupole GS-MS/MS, combined with questionnaire data from the AESOP Study. Dietary exposure was modeled using Canadian Total Diet Survey PCB concentrations and National Health and Nutrition Examination Survey (NHANES) food ingestion rates. For ∑PCB, dietary exposure dominates. For individual lower-chlorinated congeners (e.g., PCBs 40+41+71, 52), inhalation exposure was as high as one-third of the total (dietary+inhalation) exposure. ∑PCB inhalation (geometric mean (SE)) was greater for urban mothers (7.1 (1.2) μg yr–1) and children (12.0 (1.2) μg yr–1) than for rural mothers (2.4 (0.4) μg yr–1) and children (8.9 (0.3) μg yr–1). Schools attended by AESOP Study children had higher indoor PCB concentrations than did homes, and account for the majority of children’s inhalation exposure.
Introduction
Polychlorinated biphenyls (PCBs) are a ubiquitous group of persistent organic pollutants that are carcinogenic and neurotoxic, with potential endocrine disrupting and immune-suppressing activity.1−5 Toxicological studies demonstrating the dioxin-like properties of PCBs,6 and industrial accidents in Japan and Taiwan7 led to bans on the commercial production and sale of PCB mixtures in many countries. Despite these actions, PCBs are a persistent public health threat in indoor environments, because they were purposefully added to household sealants, paint plasticizers, wood finishes, flame retardants, light ballasts, and electrical capacitors in appliances.8,9 Inadvertent production of PCBs is an additional, emerging concern. PCBs are present in modern pigments used in household paint and many consumer products.10,11 These sources of PCBs are often called “non-Aroclors”, because they are unrelated to the commercial mixtures banned from production in the 1970s. PCBs are also present in outdoor environments due to contributions from legacy, industrial sites (e.g., ref (12)), diffuse, contemporary urban sources,13,14 and, to a lesser amount, volatilization from soil and water bodies.15−17
PCBs are highly lipophilic; bioaccumulate in fats, lipids, and waxes; bioconcentrate in food chains; and are semivolatile. They are both present in the gas phase and associated with solids at ambient temperatures. Because of high PCB concentrations in some animals, studies of dietary PCB exposure have historically taken precedence over dermal and inhalation exposure. However, airborne emissions from newly produced PCBs and legacy sources may lead to inhalation exposure at levels comparable to, and sometimes higher than, dietary exposure.18−20 There is also rising evidence that many lower-MW congeners are mutagenic and tumor promoting (PCB 3, 15, 52, 77)2,21 endocrine disrupting,5 and more strongly agonistic toward thyroid receptors.22 But these congeners are often neglected in inhalation exposure estimates.
∑PCB inhalation exposure has been estimated on a limited basis for residential, school, and other public environments, via indoor air19,23 and sera PCB concentrations (e.g., ref (21)), but these estimates are lacking for North American urban environments.20 Non-North American estimates are often limited to World Health Organization (WHO) indicator congeners (PCB 28, 52, 101, 138, 153, 180) and dioxin-like PCBs. All mono- and dichlorinated congeners, and some other abundant congeners (e.g., PCB 99) have been excluded from these prior estimates, despite their greater likelihood of occurring in the gas phase.
Here we estimate inhalation exposure in urban and rural environments for 201 PCB congeners represented in 156 chromatographic peaks via indoor and outdoor air concentrations at schools and homes. We also estimate dietary exposure to 40 PCB congeners using the most comprehensive market basket survey for PCBs in North America25 and National Health and Nutrition Examination Survey (NHANES) food ingestion rates.26 This congener-specific approach, and the breadth of sampling performed under the AESOP Study (Airborne Exposure to Semi-volatile Organic Pollutants), allow an expansive view of PCB exposure for urban and rural cohorts in East Chicago, Indiana (EC) and the region in and around Columbus Junction, Iowa (CJ).
With these data, we aim to determine: (1) the congener-specific and ∑PCB inhalation and dietary exposure rates for individuals in both locales; (2) the relative importance of school, home, and outdoor environments to inhalation exposure; and (3) the role of generational and gender differences on exposure. We hypothesize that individuals in EC will have greater inhalation exposure than individuals in CJ, because of the proximity of EC homes to the Chicago airshed and the heavily contaminated Indiana Harbor and Ship Canal (IHSC).
Materials and Methods
AESOP Study Design
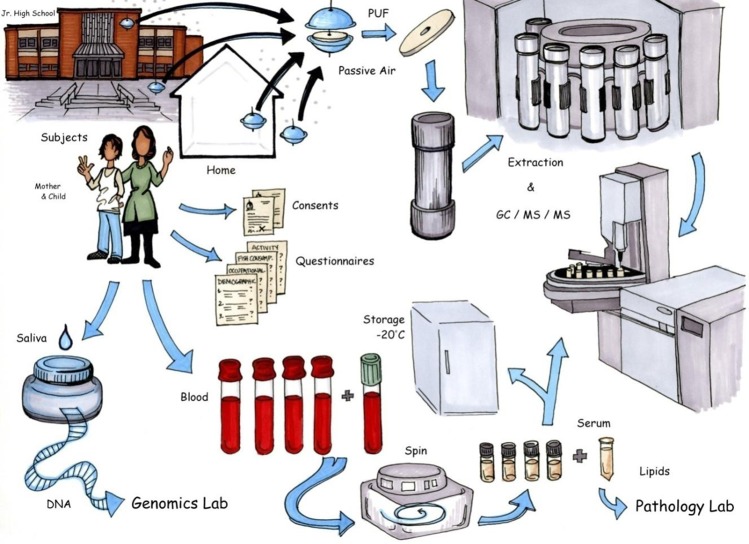
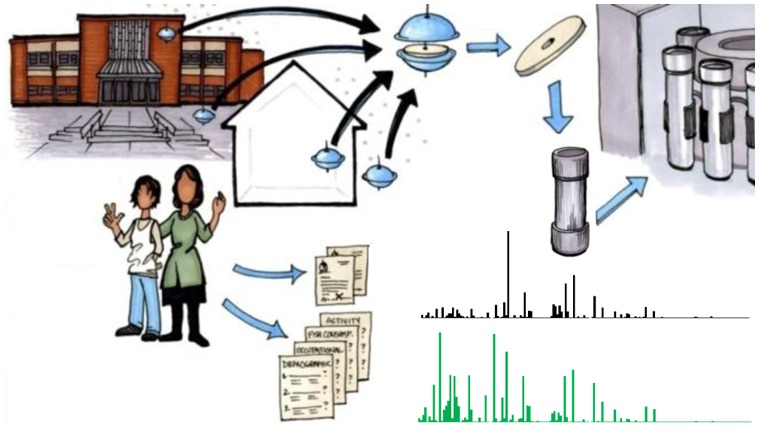
All inhalation exposure data are derived from the AESOP Study (Figure 1), the purpose of which is to evaluate population-level exposure to PCBs in urban (EC) and rural (CJ) cohorts. The region surrounding CJ has no known significant PCB sources and a population of less than 5000 individuals. In contrast, EC is an impoverished community in the Greater Chicago Metropolitan Area whose industrial sites, including refineries, steel mills, and the IHSC, have created potentially strong sources of PCBs. Both communities are multiethnic and predominantly Hispanic.
Figure 1.
Sampling and analysis scheme for AESOP Study. Artist: Jeanne DeWall. Air sampling, questionnaires, subject enrollment, and GC-MS/MS analysis are described in this paper. Collection and analysis of saliva and blood complete the AESOP Study data set, but are described elsewhere.27
Cohorts of 120 mothers and 138 children who breathe air from these environments have been recruited to the AESOP Study through middle schools. Prior to enrollment, subjects provided informed consent or assent in either English or Spanish. All aspects of the AESOP Study have been approved by the Institutional Review Board at the University of Iowa. Air sampling at participants’ homes and schools has occurred on a quarterly (homes) and bi-quarterly (schools) basis (i.e., 45 days). Demographic, activity, occupational, and dietary questionnaires have been administered in English or Spanish on a yearly basis by bilingual, trained field interviewers. The sampling and analysis scheme for the study is illustrated in Figure 1. Analysis of saliva and blood serum samples, although not addressed herein, provides complementary data sets discussed elsewhere.27
PCB Air Sampling
We used passive air samplers (PAS) to measure PCB concentrations indoors and outdoors at schools and homes. PAS collect PCBs on polyurethane foam disks (PUF) from gas and particulate phases, at a rate dependent upon the molecular diffusivity of airborne species, chemical phase equilibria, and deposition kinetics.28,29 We determined the effective sampling rates for our PAS using a mass transfer model that predicts uptake of PCBs by advection and diffusion as a function of meteorological parameters. The method is detailed elsewhere.30 It improves upon the use of depuration compounds to determine sampling rate,31 by incorporating spatial-, temporal-, and congener-specific data, and thereby increases accuracy in exposure calculations.
The resulting sampling rates generally increased with molecular weight, varying from 2.5 m3d–1 (PCB 4) to 3.4 m3d–1 (PCB 205) for indoor environments and from 4.1 m3d–1 (PCB 4) to 7.3 m3d–1 for outdoor environments (PCB 169). Sampling rates are roughly 2-fold greater for outdoor samples than for indoor samples, due to higher wind speeds and advective mass transfer outdoors.32
Sample Preparation, Extraction, and Analysis
Prior to deployment, PUF were cleaned (24 h, Soxhlet apparatus) with a 1:1 (v/v) hexane/acetone mixture and wrapped in aluminum foil within ZipLok bags for storage and transport. Those PUFs ready for deployment were installed in PAS housing and subsequently retrieved by trained field staff. Resulting PUF samples were spiked with surrogate standards (50 ng each of PCB 14, deuterated-65, and 166), extracted with the same hexane/acetone mixture (Accelerated Solvent Extractor, Dionex ASE-300), cleaned through a column of silicic acid, and concentrated, as described by Persoon and Hornbuckle.31 Laboratory blanks and field blanks were included at a 10% rate and treated the same as samples. Finally, after addition of internal standards (20 ng each of deuterated-PCB 30 and 204), samples were analyzed by gas chromatography with tandem mass spectrometry (GC-MS/MS, Agilent 6890N Quattro Micro GC, Waters Micromass MS Technologies) in multiple reaction monitoring mode, using a method derived from EPA Method 1668c, but substantially modified for analysis with the aforementioned instruments.33 Transition ions were selected for each homologue group allowing greater separation of congeners from each other and from background noise than possible using electron capture detection. We used a 60m Supelco SPB-Octyl capillary column for chromatographic separation to isolate most of the dioxin-like congeners. We were able to quantify 201 congeners as a set of 156 individual or coeluting chromatographic peaks. Further details of this method are described elsewhere (Supporting Information (SI)).31,33 Deuterated standards were obtained from CDN Isotopes (Quebec, Canada), and nondeuterated standards from AccuStandard (New Haven, USA).
Quality Assurance and Quality Control
Air samples were
bar coded and transferred with chain of custody documentation. Recoveries
of surrogate standards (arithmetic mean ± SE) averaged 83 ±
2% (PCB 14), 92 ± 2% (deuterated-PCB 65), and 87 ± 2% (PCB
166). Congener-specific limits of quantification (LOQs) were calculated
as the 95% confidence interval 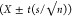 of the
field blank mass per sample. Most
of the congener LOQs were below 0.05 ng (Supporting
Information (SI), Table S1). Infrequently detected congeners
PCB 23 (LOQ = 0.014 ng sample–1), 126 (0.0047 ng
sample–1), 159 (1.0 ng sample–1), and 209 (0.050 ng sample–1) had LOQs higher
than many sample concentrations, and were disregarded in exposure
assessments and other analyses presented here-in.
of the
field blank mass per sample. Most
of the congener LOQs were below 0.05 ng (Supporting
Information (SI), Table S1). Infrequently detected congeners
PCB 23 (LOQ = 0.014 ng sample–1), 126 (0.0047 ng
sample–1), 159 (1.0 ng sample–1), and 209 (0.050 ng sample–1) had LOQs higher
than many sample concentrations, and were disregarded in exposure
assessments and other analyses presented here-in.
Inhalation Exposure
Inhalation exposure for each subject was estimated as the time-integrated product of their airborne PCB congener concentrations and subject-specific inhalation rates:
| eq 1 |
Ti is time spent in location i (hr d–1); Q is the calculated weight-, gender-, and age-specific inhalation rate (m3d–1);34 [PCB]j is the airborne concentration of the jth PCB congener (ng m–3); and f is a conversion factor, 1.52 × 10–2 μg ng–1d2 hr–1 yr–1. The time (Ti) and inhalation rate (Q) terms were derived from questionnaire data, while [PCBj] was derived from PCB measurements. Seasonal activity data (Ti) was matched with corresponding seasonal PCB concentrations, but significant seasonal differences were only observed for indoor EC home samples (summer vs nonsummer) (p=0.009). Modeled locations include (i = 1) inside homes, (i = 2) inside schools, (i = 3) outdoors, and (i = 4) all other environments (e.g., workplace).
Inhalation exposure at home is derived from PAS measurements at each subject’s household (typically two indoor samples), whereas inhalation exposure outdoors and at schools is estimated from mean concentrations at each locale (outdoors: CJ n = 41, EC n = 48; schools: CJ n = 11, EC n = 13). Inhaled concentrations in other environments (i = 4) (e.g., offices, stores, churches) are imputed using mean indoor home concentrations in the respective locales as a proxy (CJ n = 35, EC n = 34).
Dietary Exposure
Dietary exposure to PCBs was calculated as the product of U.S. average food consumption rates (NHANES, 26) and PCB concentrations in fish, dairy, meat, oils, and eggs (Canadian Total Diet Survey (TDS), Health Canada).53 Age- and gender-specific NHANES food ingestion rates were used, creating differences between subgroups’ dietary exposure. This TDS includes 40 abundant PCB congeners and is the most comprehensive dietary data set in North America. Data from Toronto (collected in 1996) and Winnipeg (1994) were used as the geographically closest proxies to EC and CJ, respectively. CJ is approximately equidistant from Toronto and Winnipeg. However, Winnipeg was chosen as a proxy for CJ because of its geographical context (i.e., Great Plains rather than Great Lakes) and more agriculture-based economy relative to Toronto. Oil, margarine, butter, and poultry data were unavailable for Winnipeg, and a 10-year national TDS average was used as a substitute for these food groups.
The acquisition, extraction, clean up, analysis and QA/QC measures for the Canadian TDS samples are as described in Newsome et al.25 and SI. In brief, TDS foods were collected from supermarkets, prepared as for consumption, composited into categories, and quantified by GC/MS. The resulting data were published in a limited form in print25 and online.35 Here we use congener-, location-, and food-specific concentrations from the same data set, but which were previously unpublished in this form.32
Statistical Analysis
Student’s t test (two-sided, unequal variances) was used to evaluate statistical differences between cohorts and air samples. All sample distributions of home air measurements met assumptions of log-normality and were analyzed after log transformation, whereas sample distributions of school air measurements were normally distributed and were not log-transformed. A paired Student’s t test (two-sided) was used to evaluate children’s vs mothers’ exposure in each locale. The use of Grubbs’ Test revealed four households which had consistently high ∑PCB concentrations. Additional samples from these households were quantified and validated these extreme values, which were excluded from population estimates. All statistical analyses were performed using Minitab.36
Results
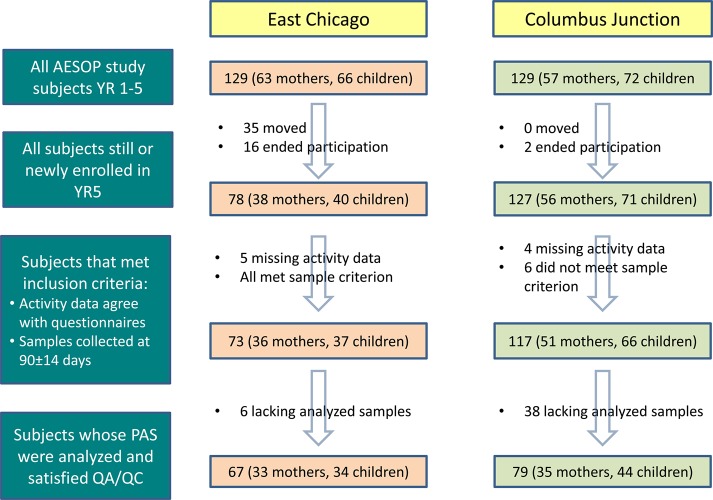
Cohorts for this paper were selected from the pool of AESOP Study participants on the basis of enrollment in the most recent study year (2012–2013), logical consistency of their activity data with other questionnaire responses, deployment duration of corresponding air samples (i.e., if within 90 ± 14 days), and fulfillment of quality assurance and quality control (QA/QC) criteria (Figure 2). Logical consistency included, for example, similarity between a subject’s work history (e.g., 40 h/week) and their activity data (e.g., > 40 h/week in “other” inhalation category). The resulting EC cohort (33 households) had lower incomes (p < 0.001), poverty income ratios (PIR) (p = 0.001), and years of education (p = 0.01) than the CJ cohort (35 households) (Table 1). Hispanic individuals represent a large component of participants in both locations (71% in EC and 53% in CJ) (Table 1).
Figure 2.
Screening and selection process for modeled cohort. QA/QC criteria include recovery of surrogate standards between 40 and 150% and concurrent collection of field blanks.
Table 1. Cohort and Community Demographic Dataa.
| Scale | Demographic Parameter | East Chicago | Columbus Junction |
|---|---|---|---|
| community | population size | 29 698b | 4350c |
| year middle school built | 1976, 1968 | 1918 | |
| year high school built | 1986 | 1961 | |
| median house value | $86,000b | $100,200c | |
| residents foreign born | 14.7%, 91% Latinob | 20.9%, 97% Latinob | |
| cohort | cohort size (children) | 68 (33) | 80 (45) |
| median years mother lived in home (SE) | 5.25 (1.2) | 11.5 (1.4) | |
| mothers’ ethnicity/race | |||
| Hispanic | 71% | 53% | |
| white (non-Hispanic) | 9% | 44% | |
| African American | 21% | 0% | |
| multirace/other | 0% | 3% | |
| homes with smokers | 9% | 11% | |
| mother median age in years (SE) | 40.7 (1.1) | 47.0 (0.8) | |
| children median age in years (SE) | 17.2 (0.3) | 17.3 (0.2) | |
| median household incomed | $21,250 | $50,000 | |
| PIR: | |||
| income <1.0 × FPL | 50% | 22% | |
| 1.0 × FPL to 1.5 × FPL | 32% | 13% | |
| 1.5 × FPL to 2.0 × FPL | 12% | 30% | |
| 2.0 × FPL to 5.0 × FPL | 6% | 35% | |
| income >5.0 × FPL | 0% | 0% | |
| mothers’ educational attainment | |||
| less than high school | 41% | 31% | |
| high school/GED | 35% | 22% | |
| some college | 15% | 17% | |
| B.A./B.S. or higher | 9% | 14% | |
| grad/prof. degree | 0% | 17% | |
PIR, Poverty income ratio: ratio of household income to FPL. FPL, Federal poverty level guideline, by size of household.
Data derived from census reports for the surrounding community: East Chicago, Indiana and Louisa County, Iowa (United States Census Bureau (USCB) (2014), State Data Center of Iowa Statistics, available at http://www.iowadatacenter.org/).
Data for Columbus Community School District; population values are estimated from city census data plus a percentage of the nonmunicipal Louisa County population (USCB 2014).
Mean of the midpoint values for data collected as an income range.
AESOP communities were chosen to minimize differences between the cohorts. However, differences in cohort demographics directly or indirectly effect PCB concentrations described here-in. For example, income disparities may help explain differences in PCB concentrations in EC v CJ, because the use, identification, and removal of PCBs requires economic decisions. We do not assess these possibilities here, but rather characterize the cohort in a manner consistent with epidemiological norms.
Airborne PCB Concentrations
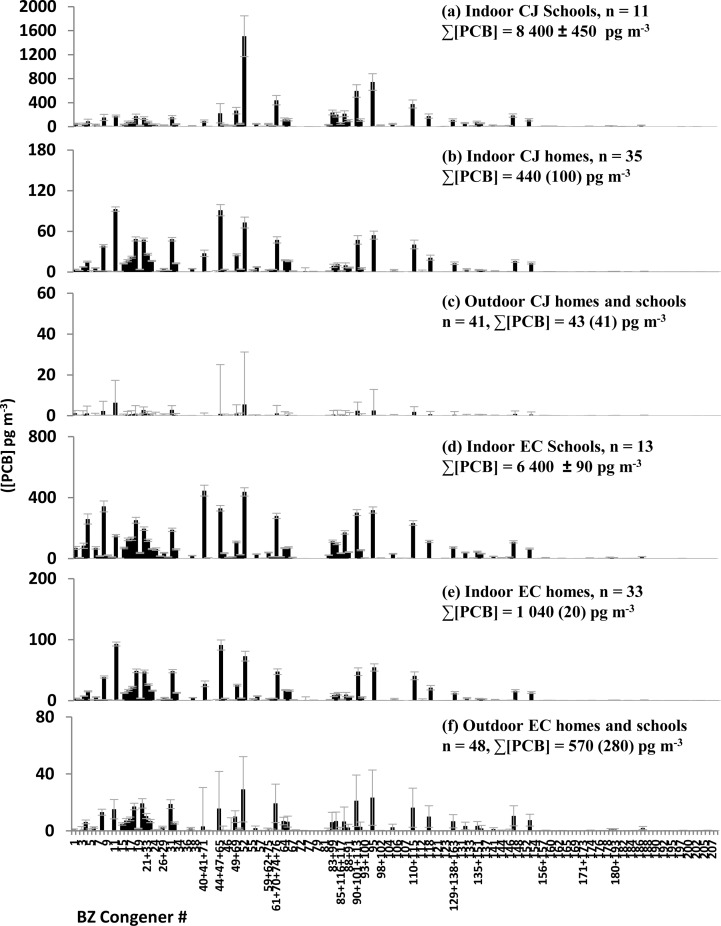
Geometric mean (SE) ∑PCB indoor air concentrations for EC homes (1.0 (0.02) ng m–3) were significantly higher (p < 0.001) than geometric mean ∑PCB indoor air concentrations for CJ homes (0.44 (0.1) ng m–3). One outlier home in EC had ∑PCB indoor air concentrations of 74 ± 40 ng m–3 (n = 3 samples). Three (3) outlier homes in CJ, had arithmetic mean ∑PCB indoor air concentrations (± SE) ranging from 7.2 ± 4.1 ng m–3 to 17.3 ± 1.7 ng m–3 (n = 3). Arithmetic mean ∑PCB indoor air concentration at EC schools (6.4 ± 0.1 ng m–3, n = 13) was higher than arithmetic mean ∑PCB indoor air concentrations at EC homes (p = 0.013), likewise for CJ schools (8.4 ± 0.4) ng m–3, n = 11) and homes (p < 0.001). CJ Schools also appear to be enriched in higher-MW congeners as compared to CJ homes (Figure 3). EC middle schools were built in 1968 and 1976, while the CJ middle school was built in 1918 (Table 1). Outdoor samples in both locations had about 10-fold lower ∑PCB concentrations than was measured inside homes or schools, but congener profiles were very similar inside and outside homes in the same locale (Figure 3).
Figure 3.
Mean airborne PCB concentrations, derived from Harner passive air samplers deployed for ∼90 days (homes) or ∼45 days (schools). PCB masses were measured via tandem quadrupole GC-MS/MS. Different y-axis scales are used. Error bars are standard error. Indoor school samples were normally distributed and are thus presented as an arithmetic mean ± SE. Other samples were log-normally distributed and are presented as a geometric mean (SE).
Dietary PCB Concentrations
Sum (∑) PCB concentrations in sampled foods range from below detection limits (e.g., skim milk) to 6.7 ng g–1 wet weight (i.e., fresh marine fish sampled in Toronto).32 For both locales, fish had the highest ∑PCB concentrations (e.g., CJ: 4.5 ± 1.3 ng g–1) followed by eggs, lipids and oils (1.0 ± 0.2) ng g–1), meat (0.55 ± 0.03) ng g–1) and dairy (0.37 ± 0.12) ng g–1). Five hexa- and pentachlorinated congeners (PCB 99, 110, 118, 138, and 153) accounted for 42% and 49% of the PCBs measured in Toronto, and Winnipeg foods, respectively. The remaining 35 PCBs were present at concentrations of 375 pg g–1 wet weight or less.
Inhalation Exposure
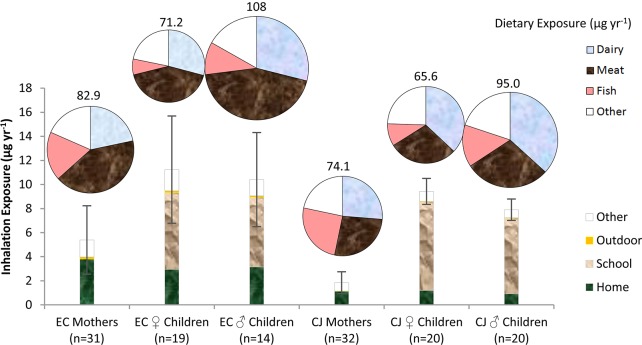
For both locales, mean inhalation exposure was greater for children than for mothers (Figure 4) (p < 0.001). This significant difference is driven by greater airborne ∑PCB concentrations in schools than homes (Figure 3), and the greater amount of time that children spend in schools than do mothers (Table 2). For AESOP children, about half of PCB inhalation exposure occurred inside schools, while mothers received about two-thirds of their inhalation exposure inside homes. Outdoor environments represented <5% of inhalation exposure (Figure 4), due to lower PCB concentrations and less time spent outside (< 3.5 h day–1, Table 2). Mean inhalation exposure was also greater for children and mothers in urban environments (EC) than for children (p = 0.001) and mothers (p < 0.001) in rural environments (CJ) (Figure 4). These differences between inhalation exposure for EC and CJ residents are more pronounced for pentachlorinated congeners, such as PCB 118, which are about 5-fold more concentrated inside EC than CJ homes (Figure 3). In both locales, mono- to penta-chlorinated congeners represent 91–93% of total PCB inhalation exposure (SI, Table S3). Coeluting congeners PCBs 40+41+71 and 44+47+65, the WHO indicator congener PCB 52, and the non-Aroclor PCB 11 were inhaled in the greatest amounts by mothers, whereas congeners PCBs 61+70+74+76, PCB 95, and PCBs 90+101+113 (in addition to the above) were inhaled at high rates by children (∼0.60 μg yr–1 congener–1) (SI, Table S3). Notwithstanding PCB 11, cosine theta statistical comparisons (0.60–0.78 on a scale of 0 to 1, with 1 representing identical profiles) show fair agreement between Aroclor 1248 and inhalation exposure profiles.37
Figure 4.
Inhalation (bar) and dietary (pie) exposure by location and food group excluding houses with extreme values. “Other” dietary sources include butter, fats and oils, margarine, and eggs. “Other” inhalation sources include time spent at locations not measured directly, such as churches, places of work, and other public areas.
Table 2. Time-Activity Summary for AESOP Study Participants (hr d–1) (Arithmetic Mean ± SE).
| location | EC mothers (n = 33) | EC children (n = 34) | CJ mothers (n = 35) | CJ children (n = 44) |
|---|---|---|---|---|
| home | 14.3 ± 0.9 | 11.8 ± 0.6 | 13.2 ± 0.6 | 11.2 ± 1.1 |
| schoola | 0.1 ± 0.1 | 5.5 ± 0.2 | 0.6 ± 0.3 | 6.3 ± 1.1 |
| outside | 3.6 ± 0.7 | 3.1 ± 0.6 | 2.7 ± 0.2 | 2.8 ± 0.2 |
| other | 6.0 ± 0.6 | 3.6 ± 0.3 | 7.5 ± 0.6 | 3.8 ± 1.3 |
Children’s time spent in schools, as used for exposure modeling, was determined by school calendars, not questionnaires (this table).
Dietary Exposure
Sum (∑) PCB dietary exposure was greater than ∑PCB inhalation exposure for most individuals (Figure 4), except for the EC household with highest airborne PCB concentrations (74 ± 40 ng m–3). Dairy and meat contributed the greatest amount to ingested ∑PCB, depending on the age, location, and sex of the subjects (Figure 4). Meats contributed the greatest amount of PCBs to dietary exposure in EC, whereas dairy contributed the greatest amount of PCBs to dietary exposure in CJ (Figure 4). Male children appear to ingest more PCBs than female children or mothers (Figure 4, SI, Table S3). These differences arise from different food ingestion rates for males vs females and for mothers vs children.26
Discussion
We have estimated inhalation (∑201PCB) and dietary (∑40PCB) exposures for mother/child cohorts in East Chicago, Indiana, and Columbus Junction, Iowa. The scope of air sampling performed here, via the AESOP Study, includes Aroclor and non-Aroclor, lower-chlorinated congeners that have rarely been measured in exposure assessments. The non-Aroclor PCB 11, for example, was present at about 100 ng m–3 in many indoor environments. Likewise PCB 8 was present at concentrations ranging from 10 ng m–3 to more than 400 ng m–3. Neither of these congeners have been measured in prior inhalation exposure studies (Table 3).The ∑PCB inhalation rates for our subjects (2.43 (0.38) to 12.0 (1.18)μg yr–1) are similar to those for individuals living in urban areas in the United Kingdom19 and China,23 but are less than those estimated for individuals in contaminated environments (Table 3). Previous estimates have been based on 40 or fewer congeners and, among the lower-chlorinated congeners (mono- to pentachloro), often contain only the WHO indicators: PCB 28, 52, and 101 (Table 3). In this study, other lower-chlorinated congeners represent 70–80% of mean ∑PCB inhalation exposure, including the potentially neurotoxic congeners PCB 11, 40, 51, and 95.3
Table 3. Review of Inhalation Exposure Estimates for Contaminated Environments and Ambient Aira.
| study | population/location | country | mean/medianb inhalation (μg yr–1) | [∑PCB] range indoor air (ng m–3) | (no.) congeners estimated |
|---|---|---|---|---|---|
| AESOP Study | EC children | U.S. | 15.0 | 0.2–15 | (201) All congeners not used as standards and with mean concentrations > LOQ |
| CJ children | 9.8 | 0.4–160 | |||
| EC mothers | 8.6 | 0.2–15 | |||
| CJ mothers | 3.3 | 0.4–160 | |||
| Gabrio et al. 200041 | teachers, contaminated school buildings | Germany | 10 000b | 1,587–10,655 | (6) WHO indicatorsc |
| Liebl et al. 200442 | contaminated school | Germany | 2800b | 690–20,800 | (6) WHO indicators |
| Meyer et al. 201324 | contaminated flats | Denmark | 1100b | 43.3–1,060 | (24) WHO + Dioxin-like + 6 others |
| Schettgen et al. 201243 | contaminated office building | Germany | 2400b | 0–4,280 | (18) WHO indicators, 12 others in sera |
| Schwenk et al. 200244 | contaminated school | Germany | 36 000b | 1,000–25,000 | (6) WHO indicators |
| Currado and Harrad 199818 | ambient exposure | U.K. | 40.2 | 1.109–68.608 | (36) WHO indicators + 30 others |
| Harrad et al. 200619 | ambient exposure | U.K. | 54.8 | 0.487–101.762 | (36) WHO indicators + 30 others |
| Xing et al. 201123 | workers, electronic recycling facility | China | 59.2 | 16.6d | (37) WHO + Dioxin-like + 19 others |
| residents near electronics recycling facility | 24.5 | 8.51d | |||
LOQ, limit of quantification; n.a., nonapplicable; WHO, World Health Organization; no., number.
Median statistics for [PCB] are provided for these studies, and exposure is estimated here as [PCB](μg m–3) × 16 m3 d–1 × 365 d yr–1 and multiplied by 0.667(assuming 16 h d–1 at home) or 0.333 (assuming 8 h d–1 at schools/offices).
WHO indicator congeners include PCB 28, 52, 101, 138, 153, and 180.
No range reported.
Congener profiles for inhalation exposure resemble Aroclor 1248 with additional contributions from Aroclor 1254 and the non-Aroclor PCB 11. The latter congener is produced as a byproduct of paint pigment manufacturing11 and is thus likely ubiquitous in residential and commercial buildings, especially those with green, yellow, or other organic paint pigments. Given the presence of paint in virtually all indoor environments, we expect these results to be generalizable within the U.S.
Aroclor PCBs 40+41+71, and 52 were also abundant in AESOP Study homes. PCB 40 reduces cell dopamine content,38 but confirmation of other health effects is scarce. Biological effects of PCB 52 include potential tumor promotion, granule neuronal cell death, and immune suppression.20,39,40 This PCB is, by far, the most abundant congener in the air of Columbus Junction schools (Figure 3) and is inhaled at the highest rates by CJ children (SI, Table S3).
Dietary PCB exposure is often greater than inhalation exposure, but this difference may be less pronounced for lower-chlorinated congeners which have higher volatility and less potential for bioaccumulation. Our subjects have inhalation-to-diet exposure ratios that increase with decreasing PCB chlorination. This ratio approaches 1:2 for the tetrachlorinated PCBs 40+41+71 and 44+47+65, and 52 (SI, Table S3), and may be greater than 1:1 for di- and trichlorinated congeners not measured in diet (e.g., PCB 8, 11, 18 + 30). Dietary ∑PCB exposure estimated for residents of the United Kingdom (340 μg yr−1), Finland (438 μg yr−1), and South Korea (198 μg yr−1) are similar in scale to those reported here, but result from greater contributions of grains and vegetables (South Korea) and fish (Finland, U.K.).45−47
Here we calculate higher ambient inhalation exposure in an urban (EC) compared to a rural (CJ) environment, based on differences in airborne ∑PCB concentrations. Elsewhere we show that EC children have blood enriched with lower-molecular weight PCBs relative to other congeners, a finding that may denote increased inhalation exposure for this demographic.27 However, we did not see a significant difference between serum ∑PCB concentrations for EC and CJ subjects.27 This finding is likely driven by greater lifetime dietary exposure, as compared to inhalation exposure, and higher rates of removal for lower congeners (often inhaled) as compared to higher congeners (often ingested).48,49
Schools and other buildings of masonry construction are prone to PCB contamination, due to the presence of PCB-laden caulking and sealants.21,50,51 Schools in the AESOP Study have lower PCB concentrations than those reported for contaminated schools and apartments in North America and Europe (Table 3). However, AESOP study schools still account for 46–53% of inhalation exposure for children, and they demonstrate significantly higher airborne PCB concentrations than do homes. As school districts in the U.S. consider remedial options,52 it is important to understand the scope of contamination. Our results demonstrate that both new and old school buildings have higher indoor air PCB concentrations than do homes regardless of year of construction or locale.
A potentially important limitation of this analysis includes the use of imputed values for a portion (∼24%) of inhalation exposure. There may be some mothers, in particular, who work in buildings with elevated PCB concentrations. We evaluated this possibility by documenting subjects’ entire work history: none have worked in industries with known PCB risks. Other sources of uncertainty include pulmonary absorption and inhalation rates, time-activity data, and PAS methodology. Inhalation rates used here are well-described for physiologically diverse U.S. populations,34 and are age-, gender-, and weight-specific for each subject. However, especially active individuals, for example, those that spend 4-fold greater time at moderate activity levels, may have 15–20% greater inhalation. Our assessment also assumes complete pulmonary absorption. We think this is reasonable: pulmonary absorption should increase with KOA, and even the low-KOA congener PCB 11 has exhibited pulmonary absorption rates of 99.8%.49 Also unaddressed in our model is uncertainty in time activity data, which may alter inhalation exposure by 10–15% (i.e., if an individual living in a PCB-contaminated home spends 5 fewer hours at home than reported). Finally, as with all PAS-based studies, uncertainty in sampling rates affects estimated PCB concentrations. Fortunately, our indoor sampling rates have low variability and agree well with values derived from depuration compounds. Furthermore, sampling rate uncertainty for more variable, outdoor environments is close to 10% for PAS.30
Uncertainty in dietary exposure includes the use of older TDS data sets (1994–1996), which may have greater concentrations than current food supplies, due to slight decadal decreases in environmental PCB concentrations since the 1990s. (53) Additionally, our dietary estimates are limited to 40 congeners and may miss some relevant exposure to less abundant congeners.
The AESOP Study provides the first congener-specific PCB inhalation exposure estimates for ambient environments in North America. Here we present inhalation exposure estimates for 201 congeners, the majority of which have been excluded from previous inhalation exposure studies, including the abundant congeners PCB 8 and 11. The results of 293 indoor and outdoor PAS measurements, analyzed across 201 congeners at schools and homes, indicate higher ∑PCB concentrations in urban than in rural AESOP Study homes. The potentially neurotoxic congeners PCBs 40+41+71 and 44+47+65, and 52 were inhaled at the greatest rates. In both study locales, ∑PCB inhalation exposure was greater for children than for mothers, due to 5- to 10-fold greater ∑PCB concentrations in schools than in homes. Estimated ∑PCB dietary exposure was greater than ∑PCB inhalation exposure for all AESOP Study participants except a mother and a child in a contaminated home in the urban locale. Bloodborne PCB measurements of the same cohorts27 provide the future opportunity to evaluate relationships between dietary and inhalation exposure and individual body burden.
Acknowledgments
This research is a project of the Iowa Superfund Research Program and is funded by NIH Grant No. P42 ES013661. Nick Herkert, Bailey Hadnott, Sean Nichols, and Dingfei Hu performed sample preparation and quantification. Nancy Morales and Barbara Mendenhall performed field sampling and cohort management as embedded field staff. Scott Spak generated model results and reviewed methods text for the PAS modeling. We thank our AESOP Study subjects and our Community Advisory Boards in both communities.
Supporting Information Available
Supporting Information for this article includes additional methods description as well as congener-specific exposure estimates (Table S3), activity level assumptions (Table S2), and summary statistics for LOQs (Table S1). Details on modeled sampling rates, PAS placement and handling, lab blank results, use of questionnaire data, and dietary measurements are contained therein. This material is available free of charge via the Internet at http://pubs.acs.org.
The authors declare no competing financial interest.
Funding Statement
National Institutes of Health, United States
Supplementary Material
References
- Cogliano V. J. Assessing the cancer risk from environmental PCBs. Environ. Health Perspect 1998, 1066317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espandiari P.; Glauert H. P.; Lehmler H. J.; Lee E. Y.; Srinivasan C.; Robertson L. W. Polychlorinated biphenyls as initiators in liver carcinogenesis: Resistant hepatocyte model. Toxicol. Appl. Pharmacol. 2003, 186155–62. [DOI] [PubMed] [Google Scholar]
- Hansen L. G. Stepping backward to improve assessment of PCB congener toxicities. Environ. Health Perspect 1998, 106Suppl 1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper N.; Connor K.; Steinberg M.; Safe S.. Immunosuppressive activity of polychlorinated biphenyl mixtures and congeners: Non-additive (antagonistic) interactions. Fundam. Appl. Toxicol. 199527(131). [DOI] [PubMed] [Google Scholar]
- Plíšková M.; Vondráček J.; Canton R. F.; Nera J.; Kočan A.; Petrík J.; et al. Impact of polychlorinated biphenyls contamination on estrogenic activity in human male serum. Environ. Health Perspect 2005, 113101277–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinney J. D.; Chae K.; Gupta B. N.; Moore J. A.; Goldstein J. A. Toxicological assessment of hexachlorobiphenyl isomers and 2, 3, 7, 8-tetrachlorodibenzofuran in chicks: I. Relationship of chemical parameters. Toxicol. Appl. Pharmacol. 1976, 36165–80. [DOI] [PubMed] [Google Scholar]
- Chen Y. C.; Yu M. L.; Rogan W.; Gladen B. C.; Hsu C. C. A 6-year follow-up of behavior and activity disorders in the Taiwan Yu-cheng children. Am. J. Public Health 1994, 843415–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutzinger O.; Safe S.; Zitko Z.. The Chemistry of PCBs; CRC Press: Boca Raton, FL. 1974. [Google Scholar]
- Priha E.; Hellman S.; Sorvari J. PCB contamination from polysulphide sealants in residential areas—Exposure and risk assessment. Chemosphere 2005, 594537–543. [DOI] [PubMed] [Google Scholar]
- Guo J.; Capozzi S. L.; Kraeutler T. M.; Rodenburg L. A. Global distribution and local impacts of inadvertently generated polychlorinated biphenyls in pigments. Environ. Sci. Technol. 2014, 48158573–8580. [DOI] [PubMed] [Google Scholar]
- Hu D.; Hornbuckle K. C. Inadvertent polychlorinated biphenyls in commercial paint pigments. Environ. Sci. Technol. 2009, 4482822–2827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorhees D. J.; Cullen A. C.; Altshul L. M. Exposure to polychlorinated biphenyls in residential indoor air and outdoor air near a Superfund site. Environ. Sci. Technol. 1997, 31123612–3618. [Google Scholar]
- Simcik M. F.; Zhang H.; Eisenreich S. J.; Franz T. P. Urban contamination of the Chicago/coastal Lake Michigan atmosphere by PCBs and PAHs during AEOLOS. Environ. Sci. Technol. 1997, 3172141–2147. [Google Scholar]
- Wethington D. M.; Hornbuckle K. C. Milwaukee, WI, as a source of atmospheric PCBs to Lake Michigan. Environ. Sci. Technol. 2005, 39157–63. [DOI] [PubMed] [Google Scholar]
- Bushart S. P.; Bush B.; Barnard E. L.; Bott A. Volatilization of extensively dechlorinated polychlorinated biphenyls from historically contaminated sediments. Environ. Toxicol. Chem. 1998, 17101927–1933. [Google Scholar]
- Harrad S.; Ren J.; Hazrati S.; Robson M. Chiral signatures of PCB#s 95 and 149 in indoor air, grass, duplicate diets andhuman faeces. Chemosphere 2006, 63, 1368–1376. [DOI] [PubMed] [Google Scholar]
- Robson M.; Harrad S. Chiral PCB signatures in air and soil: Implications for atmospheric source apportionment. Environ. Sci. Technol. 2004, 3861662–1666. [DOI] [PubMed] [Google Scholar]
- Currado G. M.; Harrad S. Comparison of polychlorinated biphenyl concentrations in indoor and outdoor air and the potential significance of inhalation as a human exposure pathway. Environ. Sci. Technol. 1998, 32203043–3047. [Google Scholar]
- Harrad S.; Hazrati S.; Ibarra C. Concentrations of polychlorinated biphenyls in indoor air and polybrominated diphenyl ethers in indoor air and dust in Birmingham, United Kingdom: Implications for human exposure. Environ. Sci. Technol. 2006, 40154633–4638. [DOI] [PubMed] [Google Scholar]
- Norström K.; Czub G.; McLachlan M. S.; Hu D.; Thorne P. S.; Hornbuckle K. C. External exposure and bioaccumulation of PCBs in humans living in a contaminated urban environment. Environ. Int. 2010, 368855–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann L.; Esch H. L.; Kirby P. A.; Robertson L. W.; Ludewig G. 4-Monochlorobiphenyl (PCB3) induces mutations in the livers of transgenic Fisher 344 rats. Carcinogenesis 2006, 282471–478. [DOI] [PubMed] [Google Scholar]
- Cheek A. O.; Kow K.; Chen J.; McLachlan J. A. Potential mechanisms of thyroid disruption in humans: Interaction of organochlorine compounds with thyroid receptor, transthyretin, and thyroid-binding globulin. Environ. Health Perspect. 1999, 1074273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing G. H.; Liang Y.; Chen L. X.; Wu S. C.; Wong M. H. Exposure to PCBs, through inhalation, dermal contact and dust ingestion at Taizhou, China–A major site for recycling transformers. Chemosphere 2011, 834605–611. [DOI] [PubMed] [Google Scholar]
- Meyer H. W.; Frederiksen M.; Göen T.; Ebbehøj N. E.; Gunnarsen L.; Brauer C.; et al. Plasma polychlorinated biphenyls in residents of 91 PCB-contaminated and 108 non-contaminated dwellings—An exposure study. Int. J. Hyg. Environ. Health 2013, 2166755–762. [DOI] [PubMed] [Google Scholar]
- Newsome W. H.; Davies D. J.; Sun W. F. Residues of polychlorinated biphenyls (PCB) in fatty foods of the Canadian diet. Food Addit. Contam. 1998, 15119–29. [DOI] [PubMed] [Google Scholar]
- Bowman S. A.; Martin C. L.; Carlson J. L.; Clemens J. C.; Lin B.-H.; Moshfegh A. J.. Retail Food Commodity Intakes: Mean Amounts of Retail Commodities per Individual, 2007–08. Washington, D.C.; U.S. Department of Agriculture, Agricultural Research Service: Beltsville, MD,2013. [Google Scholar]
- Marek R. F.; Thorne P. S.; Wang K.; DeWall J.; Hornbuckle K. C. PCBs and OH-PCBs in serum from children and mothers in urban and rural U.S. communities. Environ. Sci. Technol. 2013, 4773353–3361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartkow M. E.; Booij K.; Kennedy K. E.; Muller J. F.; Hawker D. W. Passive air sampling theory for semivolatile organic compounds. Chemosphere 2005, 602170–176. [DOI] [PubMed] [Google Scholar]
- Shoeib M.; Harner T. Characterization and comparison of three passive air samplers for persistent organic pollutants. Environ. Sci. Technol. 2002, 36194142–4151. [DOI] [PubMed] [Google Scholar]
- Petrich N. T.; Spak S. N.; Carmichael G. R.; Hu D.; Martinez A.; Hornbuckle K. C. Simulating and explaining passive air sampling rates for semivolatile compounds on polyurethane foam passive samplers. Environ. Sci. Technol. 2013, 47158591–8598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persoon C.; Hornbuckle K. C. Calculation of passive sampling rates from both native PCBs and depuration compounds in indoor and outdoor environments. Chemosphere. 2009, 747917–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UI EHSC (University of Iowa, Environmental Health Sciences Research Center. Online Data Repository for Airborne and Dietary PCB concentrations; http://cph.uiowa.edu/ehsrc/AESOP-Repository2014/index, 2014.
- USEPA (United States Environmental Protection Agency). Method 1668C: Chlorinated biphenyl congeners in water, soil, sediment, biosolids, and tissue by HRGC/HRMS, 2010; http://water.epa.gov/scitech/methods/cwa/upload/M1668C_11June10-PCB_Congeners.pdf.
- USEPA. Exposure Factors Handbook. National Center for Environmental Assessment, EPA/600/R-09/052F; National Center for Environmental Assessment, Office of Research and Development: Arlington, VA, 2011; http://cfpub.epa.gov/ncea/risk/recordisplay.cfm?deid=236252. [Google Scholar]
- Health Canada. Total Diet Survey; http://www.hc-sc.gc.ca/fn-an/surveill/total-diet/index-eng.php.
- Minitab 17 Statistical Software, [Computer software]; Minitab, Inc, State College, PA, 2010; www.minitab.com. [Google Scholar]
- Davis J. C.Statistics and Data Analysis in Geology; John Wiley & Sons, Inc: New York, NY, 1986. [Google Scholar]
- Shain W.; Bush B.; Seegal R. Neurotoxicity of polychlorinated biphenyls: Structure-activity relationship of individual congeners. Toxicol. Appl. Pharmacol. 1991, 111133–42. [DOI] [PubMed] [Google Scholar]
- Tan Y.; Chen C. H.; Lawrence D.; Carpenter D. O. Ortho-substituted PCBs kill cells by altering membrane structure. Toxicol. Sci. 2004, 80154–59. [DOI] [PubMed] [Google Scholar]
- Yilmaz B.; Sandal S.; Chen C. H.; Carpenter D. O. Effects of PCB 52 and PCB 77 on cell viability, [Ca2+](i) levels and membrane fluidity in mouse thymocytes. Toxicology 2006, 2172184–193. [DOI] [PubMed] [Google Scholar]
- Gabrio T.; Piechotowski I.; Wallenhorst T.; Klett M.; Cott L.; Friebel P.; et al. PCB-blood levels in teachers, working in PCB-contaminated schools. Chemosphere 2000, 4091055–1062. [DOI] [PubMed] [Google Scholar]
- Liebl B.; Schettgen T.; Kerscher G.; Broding H. C.; Otto A.; Angerer J.; Drexler H. Evidence for increased internal exposure to lower chlorinated polychlorinated biphenyls (PCB) in pupils attending a contaminated school. Int. J. Hyg. Environ. Health 2004, 2074315–324. [DOI] [PubMed] [Google Scholar]
- Schettgen T.; Alt A.; Preim D.; Keller D.; Kraus T. Biological monitoring of indoor-exposure to dioxin-like and non-dioxin-like polychlorinated biphenyls (PCB) in a public building. Toxicol. lett. 2012, 2131116–121. [DOI] [PubMed] [Google Scholar]
- Schwenk M.; Gabrio T.; Päpke O.; Wallenhorst T. Human biomonitoring of polychlorinated biphenyls and polychlorinated dibenzodioxins and dibenzofuranes in teachers working in a PCB-contaminated school. Chemosphere 2002, 472229–233. [DOI] [PubMed] [Google Scholar]
- Harrison N.; Wearne S.; Gem M. D. M.; Gleadle A.; Starting J.; Thorpe S.; et al. Time trends in human dietary exposure to PCDDS, PCDDS and PCBS in the UK. Chemosphere 1998, 3791657–1670. [DOI] [PubMed] [Google Scholar]
- Kiviranta H.; Ovaskainen M. L.; Vartiainen T. Market basket study on dietary intake of PCDD/Fs, PCBs, and PBDEs in Finland. Environ. Int. 2004, 307923–932. [DOI] [PubMed] [Google Scholar]
- Son M. H.; Kim J. T.; Park H.; Kim M.; Paek O. J.; Chang Y. S. Assessment of the daily intake of 62 polychlorinated biphenyls from dietary exposure in South Korea. Chemosphere 2012, 898957–963. [DOI] [PubMed] [Google Scholar]
- Hu X.; Lehmler H. J.; Adamcakova-Dodd A.; Thorne P. S. Elimination of inhaled 3, 3′-dichlorobiphenyl and the formation of the 4-hydroxylated metabolite. Environ. Sci. Technol. 2013, 4794743–4751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X.; Adamcakova-Dodd A.; Thorne P. S. The fate of inhaled 14C-labeled PCB11 and its metabolites in vivo. Environ. Int. 2014, 63, 92–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas K., Jianping Xue R. W., & Whitaker P. J. D. United States Environmental Protection Agency EPA/600/R-12/051, September 30, 2012; Polychlorinated Biphenyls. www.epa.gov/ord.
- Herrick R. F.; McClean M. D.; Meeker J. D.; Baxter L. K.; Weymouth G. A. An unrecognized source of PCB contamination in schools and other buildings. Environ. Health Perspect 2004, 112101051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrick R. F. PCBs in school-persistent chemicals, persistent problems. New Solut. 2010, 201115–26. [DOI] [PubMed] [Google Scholar]
- Salamova A.; Pagano J. J.; Holsen T. M.; Hites R. A. Post-1990 temporal trends of PCBs and organochlorine pesticides in the atmosphere and in fish from Lakes Erie, Michigan, and Superior. Environ. Sci. Technol. 2013, 47169109–9114. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Health Canada. Total Diet Survey; http://www.hc-sc.gc.ca/fn-an/surveill/total-diet/index-eng.php.