Summary
Background
Preclinical and early clinical data support the use of Vascular Epithelial Growth Factor (VEGF)-targeted therapy with trastuzumab in Human Epidermal Receptor 2 (HER2) positive breast cancer. Adding bevacizumab to a taxane (docetaxel or paclitaxel) improves progression free survival (PFS) of metastatic breast cancer (MBC) patients.
Objectives
We evaluated the efficacy and feasibility of combining bevacizumab with trastuzumab and docetaxel in patients with HER2- positive MBC who received 0–1 prior chemotherapy regimens for metastatic disease. The primary end point was PFS.
Materials and Methods
Eligible patients received bevacizumab (15 mg/kg), trastuzumab (8 mg/kg loading dose followed by 6 mg/kg), and docetaxel (100 mg/m2 initially, later amended to 75 mg/m2) every three weeks for six cycles and then were allowed to receive bevacizumab and trastuzumab alone.
Results
Thirteen (50 %) of 26 patients enrolled completed all 6 cycles of bevacizumab, trastuzumab and docetaxel and went on to receive bevacizumab and trastuzumab alone (median: 11 cycles). The most common grade 3 or 4 toxicities include: neutropenia (8 %), septic death (4 %), infection not associated with neutropenia (15 %), fatigue (27 %), mylagia and/or arthraligia (20 %), and hand-foot syndrome (8 %). One patient (4 %) and six patients (23 %) developed grade 3 and grade 2 hypertension, respectively. Two (8 %) patients had transient grade 2 drop in Left Ventricular Ejection Fraction (LVEF) with full recovery later. The median progression free survival (PFS) was 14.3 months (95 % CI: 9.3–35 months), the objective response rate (ORR), defined as the best response of complete response (CR) or partial response (PR) was (12/26) 46 %. The clinical benefit rate (CBR), defined as the best response of CR or PR or stable disease (SD) for at least 24 weeks, was (18/26) 69 % (95 % CI: 48–86 %).
Conclusion
The combination of bevacizumab, trastuzumab and docetaxel is well tolerated and is clinically active in patients with HER2-positive MBC, with response rate and PFS comparable to previous reports utilizing higher dose of docetaxel (100 mg/m2). Recent randomized trials did not demonstrate additional overall survival (OS) benefit of adding bevacizumab to trastuzumab and docetaxel despite an improvement in PFS. Identification of predictive biomarkers and careful patient selection should be incorporated in further investigation of anti-VEGF in breast cancer.
Keywords: Bevacizumab, Trastuzumab, Docetaxel, Metastatic breast cancer, HER2
Introduction
Overexpression of human epidermal growth factor receptor 2 (HER2, erbB2) is a consequence of HER2/neu oncogene amplification and is present in approximately 20–25 % of breast cancers. The use of humanized monoclonal anti-HER2 antibody, trastuzumab, and/or other HER-2/neu targeted agents in combination with chemotherapy, is the current standard of care in patients with HER2-positive early and advanced stage metastatic breast cancer (MBC), with improvements in both progression- free survival (PFS) and overall survival (OS) [1–4].
Angiogenesis is critical for tumor growth and metastasis. HER2 overexpression has been linked with upregulation of vascular endothelial growth factor (VEGF) in cancer cells in vitro and in vivo [5, 6]. It has been suggested that increased angiogenesis contributes to the poor prognosis and aggressive phenotype of HER2-overexpressing breast cancer [7]. These data provide a biological rationale for concurrent targeting of both HER2 and VEGF pathways in HER2-positive breast cancer. Bevacizumab is a humanized monoclonal antibody that targets VEGF-A. In a prior publication from our group, the results of the phase II trial with bevacizumab and docetaxel in MBC patients demonstrated limited toxicity and clinical activity [8]. Adding bevacizumab to a taxane (docetaxel or paclitaxel) improves progression free survival (PFS) of HER2-negative metastatic breast cancer (MBC) patients [9–12]. These studies led us to design a phase II study to evaluate the efficacy and feasibility of combining docetaxel, bevacizumab, and trastuzumab in patients with HER2-positive MBC. We also measured circulating tumor cells (CTCs) prior to and after one cycle of treatment. CTCs enumeration by the CellSearch technique at baseline and during treatment was shown to be an independent prognostic factor for both PFS and OS [13–15]. Therefore, the enumeration of CTCs during treatment for MBC provides a tool to predict progression of disease earlier than standard timing of anatomical assessment using conventional radiological tests.
Patients and methods
Patients
The trial was approved by the Ohio State University and University of Pittsburgh institutional review boards. Eligible patients were required to have a histologically confirmed, HER2-positive MBC, as demonstrated by 3+staining by immunohistochemistry and/or gene amplification [ratio of HER2:centromere 17≥2.0] by fluorescence in situ hybridization; Eastern Cooperative Oncology Group performance status (ECOG) (PS)≤2; absolute neutrophil count≥1,000/mm3, platelets ≥ 100,000/mm3 and hemoglobin ≥ 9 g/dl; institutionally defined normal renal and hepatic function tests, a urine protein/creatinine ratio≤1 and left ventricular ejection fraction (LVEF) of at least 50 % as determined by Multigated Acquisition Scan (MUGA) or echocardiogram. Patients with stable or previously treated brain metastases were eligible. Prior trastuzumab and up to one prior chemotherapy regimen in the metastatic setting was permitted, provided that study entry was at least 6 months after prior taxane. Patients were required to have evaluable disease. Select exclusion criteria included: inadequately controlled hypertension, myocardial infarction, or unstable angina within 6 months prior to study enrollment, The New York Heart Association (NYHA) class II or higher congestive heart failure (CHF), evidence of coagulopathy or bleeding diathesis, major surgical procedure within 28 days or minor surgical procedure within 7 days prior to study enrollment.
Study treatment
Patients received trastuzumab (loading dose of 8 mg/kg on day 1 of the first cycle, followed by a maintenance dose of 6 mg/kg on day 1 of each subsequent cycle, administered prior to bevacizumab); bevacizumab (15 mg/kg, prior to chemotherapy) and docetaxel (100 mg/m2 initially, later amended to 75 mg/m2) intravenously on day 1 of every 21 day cycles. Since two of the first four patients treated with 100 mg/m2 of docetaxel had developed grade 3 fatigue, the study was amended to a reduced dose of docetaxel at 75 mg/m2. Six treatment cycles with all three agents were planned unless there was disease progression, unacceptable toxicity, and voluntary patient withdrawal of consent or treating physician’s discretion. The decision to stop docetaxel after 6 cycles was up to the discretion of the treating physician. Patients, who were deriving benefit on protocol, could continue on trastuzumab and bevacizumab alone after six cycles.
Patients did not receive granulocyte colony-stimulating factor (G-CSF) support for primary prophylaxis. However, occurrence of a febrile neutropenia in any treatment cycle prompted filgrastim or peg-filgrastim in the subsequent cycles. Dose modification (interruptions, adjustments, or discontinuations) of docetaxel was permitted for toxicity according to protocol pre-specified criteria. Indications for docetaxel dose reduction included grade 4 neutropenia or febrile neutropenia or non-hematologic grade 3 or 4 toxicities. Up to 2 dose reductions of docetaxel, 60 mg/m2 and 50 mg/m2, respectively, were permitted. Administration of trastuzumab or bevacizumab could be delayed for up to 60 days, but no dose modifications for toxicity were allowed.
Trastuzumab was discontinued permanently if grade 3/4 LVEF drop occurred. Trastuzumab was held for patients with asymptomatic drop in LVEF by>10 % from baseline or for LVEF decrease to below 50 %. Trastuzumab was restarted if a LVEF improved to the normal range after 4 weeks. Patients with two consecutive or three intermittent delays of trastuzumab discontinued the agent permanently. Bevacizumab was held for grade 3 LVEF drop or proteinuria (≥3.5 g proteinuria/24 h), and discontinued permanently for any related grade 4 events, as well as uncontrolled hypertension despite oral antihypertensive drugs, pulmonary or CNS hemorrhage, arterial thromboembolic event, GI perforation or fistula/wound dehiscence. Patients with venous thrombosis requiring full-dose anticoagulation were allowed to continue bevacizumab if they were properly anticoagulated on warfarin (or other anticoagulants) and had no grade 3 or 4 hemorrhagic event.
Study assessments
Baseline tumor imaging (CT scans of chest, abdomen, bone scan and CT/MRI of brain) was performed within 4 weeks before study entry. Study visits were scheduled every cycle (3 weeks). Adverse effects were assessed according to the National Cancer Institute Common Terminology Criteria for Adverse Events (version 3.0) at each study visit and for approximately 30 days after last treatment. LVEF was monitored with MUGA or echocardiogram at baseline and every three cycles (9 weeks). Urine protein: creatinine ratio and routine blood tests were assessed at baseline and every 3 weeks. Patients were evaluated for efficacy every 3 cycles (9 weeks) by Response Evaluation Criteria in Solid Tumors (RECIST) version 1.0.
CTC (Circulating tumor cells)
CTCs were evaluated at baseline (on day 1 prior to initiating treatment) and after one treatment cycle (day 22 prior to treatment cycle 2). The assays were done using CellSearch® system, the USA Food and Drug Administration (FDA) approved technology for CTC detection and characterization in the Pharma Services Lab (Veridex, LLC, Huntingdon Valley, PA) as previously reported [16]. The CellSearch® technology (Veridex) is the most widely used in clinical studies that demonstrated the prognostic value of CTCs in patients with various forms of solid tumors, including breast, prostate and colon cancer [13, 17, 18].
Statistical design and analysis
This single arm phase II study was designed to estimate PFS and to evaluate safety of the combined trastuzumab, bevacizumab and docetaxel regimen. A sample size of 39 patients was proposed at significance level of 10% and power of 90% to detect if the one year PFS is 70% for the combined treatment based on a one-sided one sample binomial test. This study was terminated prior to the accrual goal of all 39 subjects because of the results of a phase III study of the same treatment combination (AVEREL) showed no significantly increased efficacy, but increased toxicity when bevacizumab was added to docetaxel and trastuzumab [19]. Total of 26 treated patients were enrolled in this study prior to its termination. 22 patients were evaluated for efficacy per Response Evaluation Criteria in Solid Tumors (RECIST) by local radiologist (RECIST version 1.0). Four patients were not evaluable for best response as they came off therapy for toxicity prior to response assessment. PFS was defined as the time from enrollment to the first documented disease progression or death. Data for those without progression was censored at the last follow-up date. PFS curve was estimated using Kaplan-Meier method. The secondary objectives of the study were clinical benefit rate, response rates and change in circulating tumor cells. The clinical benefit rate (CBR) was defined as the sum of partial response (PR) or complete response (CR), or stable disease (SD) for at least 24 weeks.
Results
Patient characteristics
Between September 2007 and November 2012, 26 patients enrolled with the following characteristics (Table 1): median age 54 years (range 38–73 years), median performance status 1 (range 0–2), 18(69 %) were postmenopausal, 14 (54 %) estrogen and/or progesterone receptor positive, 12 (46 %) had visceral involvement, 10(38 %) received adjuvant chemotherapy with 7(27 %) received prior anthracyclines and 8 (31 %) patients received prior taxanes in the adjuvant setting. All 26 patients received this study regimen in first line setting for MBC.
Table 1.
Patient characteristics
| N (%) | |
|---|---|
| Age, years, Median, Range | 54, 38–73 |
| Post menopausal | 18 (69 %) |
| ECOG PS | |
| 0 | 15 (58 %) |
| 1 | 9 (35 %) |
| 2 | 2 (8 %) |
| Hormone receptor Status | |
| ER and/or PR positive | 14 (54 %) |
| ER negative | 12 (46 %) |
| Prior adjuvant Therapy | |
| Taxanes | 8 (31 %) |
| Anthracyclines | 7 (27 %) |
| Metastatic Sites | |
| 1–2 | 25 (96 %) |
| ≥3 | 1 (4 %) |
| Visceral disease present | 12 (46 %) |
Abbreviations: ER: Estrogen Receptor; PR: Progesterone Receptor; ECOG: Eastern Cooperative Oncology Group
Treatment
Twenty six patients received total of 347 treatment cycles. Thirteen (50 %) patients completed all six planned three-drug combination treatment cycles. The median cycles of docetaxel was 6 (range 1–10). After completion of six three-drug combination treatment, all of these thirteen patients went on to receive additional biological therapy with bevacizumab and/or trastuzumab without docetaxel for a median of 6 cycles (range 1–65 cycles for bevacizumab) and 7.5 (range 1–70 cycles for trastuzumab), respectively.
Of the 26 patients, 13(50 %) patients discontinued therapy secondary to side effects for following reasons: 3(12 %)- voluntarily withdrew consent; 1(4 %) each for a wound complication, nephrotic syndrome, neuropathy, fatigue, vertigo, GI toxicities, bacteremia complicated with endocarditis and empyema, decrease in LVEF, persistent epistaxis and bleeding anal ulcer. One patient died of febrile neutropenia eight days after starting treatment. Two (8 %) patients came off study due to requiring surgery for fixation of fracture and hemorrhoidectomy. Eight (31 %) patients discontinued therapy secondary to disease progression. One patient continues on therapy with stable disease after cycle 28.
Toxicity
Most toxicities were≤grade 2. Two of the first four patients treated with 100 mg/m2 of docetaxel developed grade 3 fatigue and the trial was amended to reduce the dose of docetaxel to 75 mg/m2. Most common grade 2, 3 and 4 toxicities are outlined in table 2. Most common grade 3 and 4 toxicities included: fatigue in 7 patients (27 %), mylagia and/or arthraligia in 5 patients (20 %), hand-foot syndrome in 2 patients (8 %), vision changes 2 patients (8 %). Four patients (15 %) had grade 3 and 4 infection not associated with neutropenia, including one patient who was diagnosed with methicillin-sensitive staphylococcus aureus bacteremia who also developed endocarditis and empyema. Two (8 %) patients had a grade 2 drop in LVEF after 3 cycles, One patient had a symptomatic drop in LVEF which recovered after brief discontinuation of trastuzumab, after which patient was able to continue on trastuzumab. One patient had symptomatic decrease in LVEF from 60 to 40 % after 3 cycles, transtuzumab and bevacizumab were held, the patient received docetaxol alone for the following two cycles. She was then taken off study as repeat echocardiogram showed no evidence of LVEF recovery after holding transtuzumab and bevacizumab for two cycles. Her follow-up testing demonstrated full recovery of LVEF four months later.
Table 2.
Summary of adverse events
| Toxicity | Grade 1,2 N (%) |
Grade 3 N (%) |
Grade 4 N (%) |
|---|---|---|---|
| Hematologic | |||
| Anemia | 10 (39 %) | 1 (4 %) | |
| Neutropenia | 2 (8 %) | 2 (8 %) | |
| Thrombocytopenia | 1 (4 %) | ||
| Non hematologic | |||
| Febrile neutropenia | 1 (4 %) | 1 (4 %)* | |
| Fever without neutropenia | 8 (31 %) | ||
| Infection | 20 (77 %) | 4 (15 %) | |
| Constitutional | |||
| Fatigue | 17 (65 %) | 7 (27 %) | |
| Arthralgia | 18 (69 %) | 3 (12 %) | |
| Myalgia | 17 (65 %) | 2 (8 %) | |
| GI | |||
| Abdominal pain | 12 (46 %) | ||
| Constipation | 12 (46 %) | ||
| Diarrhea | 18 (69 %) | 1 (4 %) | |
| Nausea | 17 (65 %) | 1 (4 %) | |
| Vomiting | 13 (50 %) | 1 (4 %) | |
| Skin | |||
| Rash | 11 (42 %) | ||
| hand-foot syndrome | 7 (27 %) | 2 (8 %) | |
| Cardiovascular decrease LVEF |
3 (12 %) | ||
| Hypertension | 6 (23 %) | 1 (4 %) | |
| Atrial fibrillation | 1 (4 %) | ||
| Pulmonary embolism | 1 (4 %) | ||
| Respiratory | |||
| Dyspnea | 15 (58 %) | 1 (4 %) | |
| Pleural effusion | 1 (4 %) | ||
| Neurologic | |||
| Headaches | 14 (54 %) | 1 (4 %) | |
| Vision changes | 10 (39 %) | 2 (8 %) | |
| Neuropathy | 1 (4 %) | ||
| Other | |||
| Proteinuria | 2 (8 %) | 1 (4 %) | |
| Epistaxis | 22 (85 %) | ||
| Rectal fistula | 1 (4 %) | ||
| Wound complication | 2 (8 %) | ||
| Mucositis | 18 (69 %) | 1 (4 %) | |
| increased lacrimation | 10 (39 %) | 2 (8 %) |
This patient died of septic shock, GI, gastrointestinal
Efficacy
At median follow-up time was 7.5 months (range 0.3–53 months), the median PFS was 14.3 months 95 % confidence interval (95% CI): 9.3–35 months; (Table 3 and Fig. 1). Twelve patients (46 %) had best response as partial response (PR), ten had stable disease (SD, six of them had duration of SD>24 weeks), four patients were not evaluable for best response as they came off therapy for toxicity prior to response assessment. The objective response rate (ORR) including the best response of CR or PR was (12/26) 46 %. The clinical benefit rate (CBR) including CR or PR or SD> 24 weeks was (18/26) 69 % (95 % CI: 48–86 %). The CBR among the ER positive (n=14) and the ER negative (n=12) patients in the study was comparable at 71 % and 67 % (p=0.79). The median duration of response was 7 months (range 2–31 months). The median duration of stable disease in 10 patients was 7 months (range 2–51 months).
Table 3.
Efficacy endpoints and responses by RECIST (intention –to-treat population)
| Response | No. of patients (N=26) |
|---|---|
| Complete response | 0 |
| Partial response | 12 |
| Stable disease | 10 |
| Clinical benefit response* | 18 |
| Progressive disease | 12 |
| Nonevaluable | 4 |
| Response duration (95 % CI) | 7.2 mo (1.9–31 mo) |
| PFS (95 % CI) | 14.3 mo (9.3–35 mo) |
Patients were evaluated for efficacy by Response Evaluation Criteria in Solid Tumors (RECIST) version 1.0
Clinical benefit response is defined as the best response of partial response (PR) or complete response (CR), or stable disease (SD) for at least 24 weeks
Fig. 1.
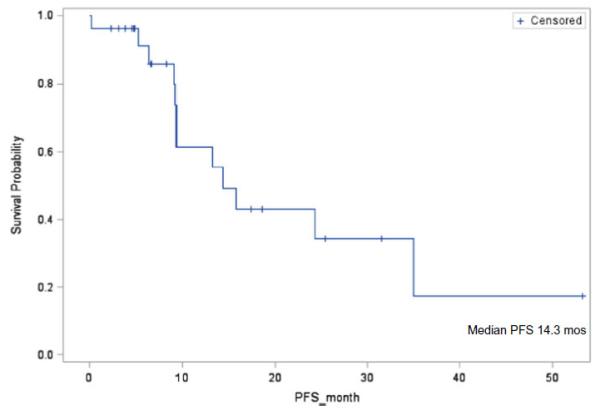
Kaplan-Meier curve of progression-free survival
CTCs
Plasma samples were available for isolating CTCs in 13 (50 %) patients pretreatment and after cycle 1. CTCs were detected in 7 patients at baseline, 4 of them with CTCs≥5 (per 7.5 mL sample) and all of these 4 patients converted to<5/7.5 ml CTCs after one cycle. One of these four patients had PR and three had SD with PFS of 20, 9, 7 and 5 months. CTCs became undetectable in three patients who had 1/7.5 ml or 2/7.5 ml CTCs at baseline after one cycle (Fig. 2). Five patients had undetectable CTCs at baseline and remained undetectable after one cycle of treatment. Only one patient had zero CTCs at baseline and 5/7.5 ml CTCs after one cycle of treatment. This patient achieved PR after being on trial for two months. She was later taken off trial due to requirement of hemorrhoidectomy.
Fig. 2.
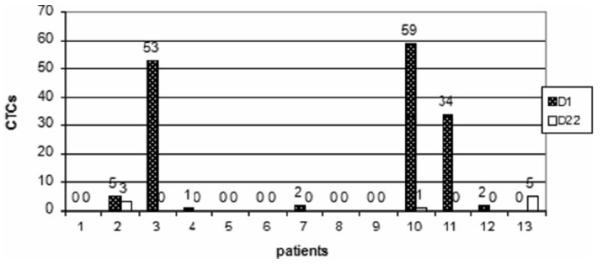
CTCs (Circulating tumor cells): pretreatment (D1) and after cycle 1 (D22)
Several publications demonstrated the poor prognosis for MBC patients that presented basal CTC count≥5 in 7.5 mL of blood. Moreover, a substantial decrease in CTC count is an early marker of individual response to treatment [20, 21]. We did not detect statistically significant difference in terms of best treatment response or PFS between patients with≥5/7.5 ml CTCS and<5/7.5 ml CTCs at baseline. Because of the small sample size, we could not perform any statistical analysis to correlate the baseline CTCs, first follow-up CTCs, and the CTC response rate with PFS.
Discussion
We evaluated the efficacy and safety of bevacizumab combined with trastuzumab and docetaxel in patients with HER2-positive MBC. Docetaxel is one of the most active cytotoxic agents for advanced breast cancer treatment and it is associated with dose-dependent toxicity. Higher rates of grade 3 or 4 febrile neutropenia and infection have been reported in patients with MBC receiving the 100 mg/m2 dose of docetaxel compared to patients receiving the lower dose of 75 mg/m2. The AVADO study is the phase III trial provided the clinical rationale for combining full-dose docetaxel (100 mg/m2) with bevacizumab in patients with MBC [10]. The incidences of grade 3 or 4 adverse events (AEs) in both study arms were relatively high, with the treatment arm (bevacizumab - docetaxel) carrying a greater incidence of grade 3/4 neutropenia (20 % [49 of 247] vs. 17 % [40 of 231]), febrile neutropenia (16 % vs. 11 %). In the AVEREL study, patients with HER2- positive MBC were randomly assigned to receive docetaxel (100 mg/m2) plus trastuzumab with or without bevacizumab every 3 weeks. Again the grade 3 or 4 AEs incidence in both study arms were high, e.g. grade 3/4 neutropenia (20.5 % [n = 215] vs. 25.7 % [n = 206]) in bevacizumab and placebo groups, respectively. The treatment arm (bevacizumab-trastuzumab-docetaxel) exhibited a greater incidence of febrile neutropenia (11.6 % [n=215] vs. 8.7 % [n=206]) [19].
Our study was amended to a reduced dose of docetaxel at 75 mg/m2 after two of the first four patients treated with 100 mg/m2 of docetaxel had developed grade 3 fatigue. The rate of febrile neutropenia was 8 % in our study, which compared favorably to the AVERAL trial where the rate of febrile neutropenia was 12 % in the bevacizumab arm [19]. This improved therapeutic ratio for docetaxel at 75 mg/m2 was supported by Schwartzberg et al. in their phase II study of bevacizumab in combination with 75 mg/m2 docetaxel +/− trastuzumab in MBC [22].
The safety profile of this regimen was consistent with prior clinical trials with fatigue (27 %), mylagia and/or arthraligia (20 %) and infection (15 %) being the most frequently reported grade≥3 treatment-related adverse events. Most of the adverse effects attributable to bevacizumab were tolerable and similar to those observed in other clinical trials. Notably only one (4 %) patient developing grade 3 hypertension consistent with our previous published phase II trial with bevacizumab and docetaxel in MBC [8]. Recent phase II studies evaluating bevacizumab and trastuzumab for HER2- positive MBC, one with capecitabine and one in combination with vinorelbine, report similar rates of grade 3 hypertension [23, 24]. In comparison, higher incidence (11 % to 18 %) of grade 3 hypertension was observed in patients in earlier trials of bevacizumab with taxanes in MBC [11, 10, 9], other solid tumors [25, 26], and a more recent trial of adjuvant chemotherapy +/− bevacizumab for women with triple negative breast cancer [27]. Interestingly an association between VEGF genotype and bevacizumab toxicity including grade 3/4 hypertension in MBC has been observed by several groups. For example, VEGF+405G>C polymorphism has been reported to be associated with bevacizumab toxicity in breast cancer, although the directionality of association is controversial [28, 29]. For example, Schneider et al. found the C allele to be protective against developing hypertension, while Etienne-Grimaldi et al. found the G allele to be protective against developing toxicity in general (i.e., hypertension, arterial or venous thromboembolism or hemorrhage) [29, 28].
This trial was closed early because of slow accrual and because the report of a randomized phase III trial (AVEREL) showed the addition of bevacizumab to a regimen of trastuzumab and high-dose docetaxel (100 mg/m2) as first line treatment did not significantly improve outcome for HER2- positive MBC. There was a trend favoring bevacizumab in investigator-assessed PFS (median PFS, 16.5 vs. 13.7 months, HR 0.82, P=0.0775), with higher response rate in the bevacizumab arm (74 % versus 70 %). The median PFS by independent review committee were 16.8 months and 13.9 months for the bevacizumab- versus non-bevacizumab arms, respectively, with similar 3 month increase in median PFS (P=0.0162). No difference in overall survival, a finding consistently established in trials adding bevacizumab to chemotherapy in MBC [19]. Our study demonstrated a clinical benefit rate of 69 % and a median PFS of 14.3 months. At the time of study close, one year PFS was 60 %. We did not reach the prior planed efficacy of 70 % one-year PFS. Despite this limited sample size, our results demonstrated a favorable efficacy than what was reported in the control arm of the CLEOPATRA trial with the same doses of docetaxel (75 mg/m2) and trastuzumab with an ORR of 69 % and PFS of 12.4 months [30].
The degree of increase in PFS with addition of bevacizumb in AVEREL study is less pronounced than that observed for novel HER2-targeted therapies. For example, the addition of a second HER2-targeted therapy, pertuzumab, to docetaxel and trastuzumab was associated with a 6-month increase in median PFS (12.4 to 18.5 months, HR of 0.62, P<0.001) in the CLEOPATRA study [30]. Nevertheless, the benefit with bevacizumab is likely to be independent of that provided by other HER2- directed therapies. Better patient selection will likely lead to a more marked benefit of bevacizumab in specific subgroups of patients. Therefore, the identification of biomarkers to define a subset of patients who derive benefit from antiangiogenic agents such as bevacizumab is necessary. However, this task has proven to be challenging for various reasons. Angiogenesis is a complex and highly adaptive process, as a result, alternative angiogenic signals can compensate when VEGF is inhibited by bevacizumab. Furthermore, it is unclear that which clinical end points accurately predict a benefit from bevacizumab since it is usually used in combination with chemotherapy [31].
Among the multiple genomic, serological and dynamic biomarkers studied, circulating VEGF-A isoforms and expression of VEGFRs are considered as strong biomarker candidates. Patients with high baseline plasma VEGF-A concentrations derived more pronounced PFS improvement from the combination of bevacizumab with docetaxel than those with low plasma VEGF-A concentrations in the AVADO study [32, 10]. Likewise, in the AVEREL study, patients with high plasma VEGF-A concentrations had more substantial benefit from bevacizumab than those with low plasma VEGF-A concentrations [19]. Higher levels of plasma VEGFR-2 were found to be associated with predicting benefits of bevacizumab in the large adjuvant BEATRICE trial [27]. Moreover, an association between VEGF genetic polymorphisms and median OS when using bevacizumab has been observed in E2100, a phase III study comparing paclitaxel versus paclitaxel plus bevacizumab as initial chemotherapy for MBC [28]. In the first prospective biomarker study (MERiDiAN), patients with HER2-negative MBC receive paclitaxel with or without bevacizumab as stratified for VEGF-A high and VEGF-A low cohorts. Clinical studies with prospective use of biomarkers for patient selection need to be done, to allow us to target the appropriate patients who likely to benefit from bevacizumab.
The presence and number of CTCs are associated with inferior prognosis in patients with MBC [13, 18, 33]. The management of breast cancer has evolved based on our better understanding of the differences among breast cancer subtypes in terms of biology, prognosis, and response to therapy including molecularly targeted therapies. These important advances emphasized the need to further explore the prognostic value of CTCs in relation to disease subtype and particular type of therapy [34]. This trial was designed to estimate the frequency of CTCs in the population of women with HER-2 positive MBC initiating taxane base-chemotherapy with two targeted therapies (trastuzumab and bevacizumab), and the frequency with which patients convert from≥5 CTCs to<5 CTCs per 7.5 mL blood after one cycle of therapy. Because of early termination prior to the accrual goal of all 39 subjects and CTCs were available in only 50 % of patients, we could not perform statistical analysis to correlate the baseline CTCs, first follow-up CTCs, or the CTC response rate with PFS based on the small sample size. Our study therefore cannot evaluate whether CTC number at baseline could predict the benefit from addition of bevaciumab to standard therapy for HER-2 positive MBC.
Nevertheless, the past year has seen conflicting results about the implication of CTCs in the HER2- positive MBC patients in the era of targeted therapies. Giordano et al. reported that CTCs were strongly predictive of survival in all MBC subtypes except HER2- positive patients who had been treated with targeted therapy in their retrospective study with 517 MBC patients. The authors concluded that this highly effective anti-HER2 therapy eliminates CTCs with HER2 amplification, thus impairing the prognostic value of CTCs in HER2- positive patients [35]. Accordingly, Bidard et al. found that changes in CTC count during treatment was not a surrogate for time to progression of first-line chemotherapy combined with bevacizumab in MBC patients [36]. Similarly, CTCs levels appeared to decrease more markedly in patients who received first –line chemotherapy plus targeted therapy (trastuzumab and bevacizumab) in Pierga et al.’s retrospective study [21]. It is suggested that bevacizumab has potential to eradicate CTCs. The inclusion of bevacizumab in treatment regimens may modify the predictive value of CTC during treatment possibly due to impaired tumor cells intravasation through vessels endothelium.
Additionally, Giuliano et al. reported that chemotherapy bevacizumab was superior to chemotherapy alone with regards to PFS, but only in patients with a high baseline CTC count (≥5/7.5 ml) (HR=0.28, 95 % CI=0.12 to 0.64, P=0.04), not in patients with CTCs<5/7.5 ml. In contrast, combination chemotherapy was superior to single-agent chemotherapy in both CTC high or low groups. The authors also suggested MBC patients with baseline CTCs≥5 received a survival benefit from HER2 targeted therapy [20]. Most recently, the independent prognostic value of CTC enumeration in MBC patients was confirmed in a large retrospective study included 486 patients, and no association was detected between primary tumor molecular subtypes and the impact of CTC status on OS [37]. The clinical value of CTC enumeration, its relationship to phenotypic subtypes and particular type of therapy are now being assessed in two ongoing randomized studies: the USA-based SWOG S0500 study focusing on first-line treatment and the French CirCe01 study focusing on the third and subsequent lines of treatment [34].
Since our trial was initiated, results have been reported from several studies investigating bevacizumab and trastuzumab for HER2 positive MBC (Table 4). A consistent pattern: improvements in response rate and PFS, but no impact on OS have been observed. In the phase II study, Martin et al. reported bevacizumab and trastuzumab in combination with capecitibine as first-line treatment demonstrated a 73 % ORR and median PFS of 14.4 months [23]. Lin et al. reported the combination of bevacizumab, trastuzumab and vinorelbine is active with ORR of 16/22 (73 %) and 5/7 (71 %) respectively in the first- and second-line settings, however, associated with higher-than-expected rates of grade 3/4 toxicity compared with doublet combinations [24]. Recently, in a phase II trial with 21 patients with HER2 positive MBC, bevacizumab and trastuzumab in combination with docetaxel at 75 mg/m2 was reported with PFS of 13.3 months and CBR of 81 % [22]. The randomized phase III study (ECOG 1105), designed to evaluate the value of adding bevacizumab to a trastuzumab/paclitaxel (±carboplatin) backbone in the first-line setting, was terminated due to poor accrual after a total of 96/489 planned patients were enrolled [38]. The combination of bevacizumab and trastuzumab are being investigated in both neoadjuvant and adjuvant setting for HER2- positive breast cancer as well. No additional benefit was achieved from adding bevacizumab to adjuvant therapy for HER2-positive breast cancer as suggested by results of the BETH trial, which was recently presented at 2013 San Antonio Breast Cancer Symposium [39]. The results of two recent neoadjuvant trials indicate the addition of bevacizumab to neoadjuvant chemotherapy significantly increased the rate of pathological complete response in patients with breast cancer, but also had an increase in adverse effects [40, 41]. In contrast, the recently reported BEATRICE trial in which over 2,500 women with triple negative breast were randomized to adjuvant chemotherapy +/− bevacizumab showed no differences in invasive disease free survival [27].
Table 4.
Summary of studies investigating bevacizumab and trastuzumab for HER2 positive MBC
| First author | Ref. | year | phase | N | Investigational therapy | mPFS (months) | ORR (%) |
|---|---|---|---|---|---|---|---|
| Gianni | [19] | 2013 | III | 424 | A: Docetaxel+Transtuzumab+Bevacizumab B: Docetaxel+Transtuzumab |
A:16.5 B:13.7 |
A:74.3 B:69.9 |
| Martin | [23] | 2012 | II | 88 | Capecitabine+Transtuzumab+Bevacizumab | 14.4 | 73 |
| Arteaga | [38] | 2012 | III | 96 | A:paclitaxel±carboplatin+trastuzumab+bevacizumab B: Paclitaxel±carboplatin+trastuzumab |
A:12.2 B:11.1 |
A:14 B:12 |
| Lin | [24] | 2013 | II | 29 | Vinorelbine+Transtuzumab+Bevacizumab | 9.9 (1st line) 7.8 (2nd line) |
73 % (1st line) (71 %(2nd line) |
| Schwartzberg | [22] | 2013 | II | 21 | Docetaxel+Transtuzumab+Bevacizumab | 13.3 | 81 % |
Although bevacizumab in combination with trastuzumab and docetaxel has anti-tumor activity, the side effects of this regimen were high. Further, data from other randomized trials showed that bevizumab when added to taxanes and trastuzumab does not improve therapeutic efficacy but increases toxicities. However, based on the results of this study and other reports, some patients do have prolonged benefit from bevacizumab containing treatment regimen for HER-2 over-expressing breast cancer. In our current trial, 13 (50 %) patients stayed on the biological therapy alone (trastuzumab and/or bevacizumab) for a median of 7.5 cycles (range 1–70). Because only a subset of patients responds, the overall clinical benefit is limited. Future research should focus on identification of biomarkers that could accurately identify patients who will derive significant clinical benefit from the addition of bevacizumab in breast cancer.
In summary, the combination of trastuzumab, docetaxel, and bevacizumab has clinical activity in HER2-positive MBC with some patients receiving prolonged benefit on the biological therapy alone with minimal toxicity. Identification of predictive biomarkers and careful patient selection should be incorporated in further investigation of anti-VEGF in combination of HER2 targeted therapy in HER2 positive breast cancer.
Acknowledgments
Funding This work was supported by Genentech.
Footnotes
Conflict of interest disclosure Dr. Bhuvaneswari Ramaswamy had disclosed that she had served on the advisory board for Genentech.
Ethical approval All patients signed an informed consent form prior to enrollment in the study. Local ethical approval was received and the study was conducted in accordance with the Ohio State University and University of Pittsburgh Institutional Review Boards.
Presented in part at 2010 ASCO Breast Cancer Symposium
Contributor Information
Meng Zhao, Division of Medical Oncology, The Ohio State University Comprehensive Cancer Center, The Ohio State University’s Wexner Medical Center, Starling-Loving Hall, 320 W 10th Ave, Columbus, OH 43210, USA.
Xueliang Pan, Center for Biostatistics, The Ohio State University, 2012 Kenny Road, Columbus, OH 43221, USA.
Rachel Layman, Division of Medical Oncology, The Ohio State University Comprehensive Cancer Center, The Ohio State University’s Wexner Medical Center, Starling-Loving Hall, 320 W 10th Ave, Columbus, OH 43210, USA.
Maryam B. Lustberg, Division of Medical Oncology, The Ohio State University Comprehensive Cancer Center, The Ohio State University’s Wexner Medical Center, Starling-Loving Hall, 320 W 10th Ave, Columbus, OH 43210, USA
Ewa Mrozek, Division of Medical Oncology, The Ohio State University Comprehensive Cancer Center, The Ohio State University’s Wexner Medical Center, Starling-Loving Hall, 320 W 10th Ave, Columbus, OH 43210, USA.
Erin R. Macrae, Division of Medical Oncology, The Ohio State University Comprehensive Cancer Center, The Ohio State University’s Wexner Medical Center, Starling-Loving Hall, 320 W 10th Ave, Columbus, OH 43210, USA
Robert Wesolowski, Division of Medical Oncology, The Ohio State University Comprehensive Cancer Center, The Ohio State University’s Wexner Medical Center, Starling-Loving Hall, 320 W 10th Ave, Columbus, OH 43210, USA.
Sarah Carothers, Division of Medical Oncology, The Ohio State University Comprehensive Cancer Center, The Ohio State University’s Wexner Medical Center, Starling-Loving Hall, 320 W 10th Ave, Columbus, OH 43210, USA.
Shannon Puhalla, University of Pittsburgh Medical Center, 300 Halket Str. Suite 4628, Pittsburgh, PA 15123, USA.
Charles L. Shapiro, Division of Medical Oncology, The Ohio State University Comprehensive Cancer Center, The Ohio State University’s Wexner Medical Center, Starling-Loving Hall, 320 W 10th Ave, Columbus, OH 43210, USA
Bhuvaneswari Ramaswamy, Division of Medical Oncology, The Ohio State University Comprehensive Cancer Center, The Ohio State University’s Wexner Medical Center, Starling-Loving Hall, 320 W 10th Ave, Columbus, OH 43210, USA.
References
- 1.Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M, Baselga J, Norton L. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344(11):783–792. doi: 10.1056/NEJM200103153441101. doi:10.1056/nejm200103153441101. [DOI] [PubMed] [Google Scholar]
- 2.Marty M, Cognetti F, Maraninchi D, Snyder R, Mauriac L, Tubiana-Hulin M, Chan S, Grimes D, Anton A, Lluch A, Kennedy J, O’Byrne K, Conte P, Green M, Ward C, Mayne K, Extra JM. Randomized phase II trial of the efficacy and safety of trastuzumab combined with docetaxel in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer administered as first-line treatment: the M77001 study group. J Clin Oncol Off J Am Soc Clin Oncol. 2005;23(19):4265–4274. doi: 10.1200/JCO.2005.04.173. doi:10.1200/jco.2005.04.173. [DOI] [PubMed] [Google Scholar]
- 3.Romond EH, Perez EA, Bryant J, Suman VJ, Geyer CE, Jr, Davidson NE, Tan-Chiu E, Martino S, Paik S, Kaufman PA, Swain SM, Pisansky TM, Fehrenbacher L, Kutteh LA, Vogel VG, Visscher DW, Yothers G, Jenkins RB, Brown AM, Dakhil SR, Mamounas EP, Lingle WL, Klein PM, Ingle JN, Wolmark N. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353(16):1673–1684. doi: 10.1056/NEJMoa052122. doi:10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- 4.Dang C, Fornier M, Sugarman S, Troso-Sandoval T, Lake D, D’Andrea G, Seidman A, Sklarin N, Dickler M, Currie V, Gilewski T, Moynahan ME, Drullinsky P, Robson M, Wasserheit-Leiblich C, Mills N, Steingart R, Panageas K, Norton L, Hudis C. The safety of dose-dense doxorubicin and cyclophosphamide followed by paclitaxel with trastuzumab in HER-2/neu overexpressed/amplified breast cancer. J Clin Oncol Off J Am Soc Clin Oncol. 2008;26(8):1216–1222. doi: 10.1200/JCO.2007.12.0733. doi:10.1200/jco.2007.12.0733. [DOI] [PubMed] [Google Scholar]
- 5.Yen L, You XL, Al Moustafa AE, Batist G, Hynes NE, Mader S, Meloche S, Alaoui-Jamali MA. Heregulin selectively upregulates vascular endothelial growth factor secretion in cancer cells and stimulates angiogenesis. Oncogene. 2000;19(31):3460–3469. doi: 10.1038/sj.onc.1203685. doi:10.1038/sj.onc.1203685. [DOI] [PubMed] [Google Scholar]
- 6.Yang W, Klos K, Yang Y, Smith TL, Shi D, Yu D. ErbB2 overexpression correlates with increased expression of vascular endothelial growth factors A, C, and D in human breast carcinoma. Cancer. 2002;94(11):2855–2861. doi: 10.1002/cncr.10553. doi:10.1002/cncr.10553. [DOI] [PubMed] [Google Scholar]
- 7.Konecny GE, Meng YG, Untch M, Wang HJ, Bauerfeind I, Epstein M, Stieber P, Vernes JM, Gutierrez J, Hong K, Beryt M, Hepp H, Slamon DJ, Pegram MD. Association between HER-2/neu and vascular endothelial growth factor expression predicts clinical outcome in primary breast cancer patients. Clin Cancer Res: Off J Am Assoc Cancer Res. 2004;10(5):1706–1716. doi: 10.1158/1078-0432.ccr-0951-3. [DOI] [PubMed] [Google Scholar]
- 8.Ramaswamy B, Elias AD, Kelbick NT, Dodley A, Morrow M, Hauger M, Allen J, Rhoades C, Kendra K, Chen HX, Eckhardt SG, Shapiro CL. Phase II trial of bevacizumab in combination with weekly docetaxel in metastatic breast cancer patients. Clin Cancer Res: Off J Am Assoc Cancer Res. 2006;12(10):3124–3129. doi: 10.1158/1078-0432.CCR-05-2603. doi:10.1158/1078-0432.ccr-05-2603. [DOI] [PubMed] [Google Scholar]
- 9.Miller K, Wang M, Gralow J, Dickler M, Cobleigh M, Perez EA, Shenkier T, Cella D, Davidson NE. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med. 2007;357(26):2666–2676. doi: 10.1056/NEJMoa072113. doi:10.1056/NEJMoa072113. [DOI] [PubMed] [Google Scholar]
- 10.Miles DW, Chan A, Dirix LY, Cortes J, Pivot X, Tomczak P, Delozier T, Sohn JH, Provencher L, Puglisi F, Harbeck N, Steger GG, Schneeweiss A, Wardley AM, Chlistalla A, Romieu G. Phase III study of bevacizumab plus docetaxel compared with placebo plus docetaxel for the first-line treatment of human epidermal growth factor receptor 2-negative metastatic breast cancer. J Clin Oncol Off J Am Soc Clin Oncol. 2010;28(20):3239–3247. doi: 10.1200/JCO.2008.21.6457. doi:10.1200/jco.2008.21.6457. [DOI] [PubMed] [Google Scholar]
- 11.Gray R, Bhattacharya S, Bowden C, Miller K, Comis RL. Independent review of E2100: a phase III trial of bevacizumab plus paclitaxel versus paclitaxel in women with metastatic breast cancer. J Clin Oncol Off J Am Soc Clin Oncol. 2009;27(30):4966–4972. doi: 10.1200/JCO.2008.21.6630. doi:10.1200/jco.2008.21.6630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Robert NJ, Dieras V, Glaspy J, Brufsky AM, Bondarenko I, Lipatov ON, Perez EA, Yardley DA, Chan SY, Zhou X, Phan SC, O’Shaughnessy J. RIBBON-1: randomized, double-blind, placebo-controlled, phase III trial of chemotherapy with or without bevacizumab for first-line treatment of human epidermal growth factor receptor 2-negative, locally recurrent or metastatic breast cancer. J Clin Oncol Off J Am Soc Clin Oncol. 2011;29(10):1252–1260. doi: 10.1200/JCO.2010.28.0982. doi:10.1200/jco.2010.28.0982. [DOI] [PubMed] [Google Scholar]
- 13.Cristofanilli M, Budd GT, Ellis MJ, Stopeck A, Matera J, Miller MC, Reuben JM, Doyle GV, Allard WJ, Terstappen LW, Hayes DF. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med. 2004;351(8):781–791. doi: 10.1056/NEJMoa040766. doi:10.1056/NEJMoa040766. [DOI] [PubMed] [Google Scholar]
- 14.Nole F, Munzone E, Zorzino L, Minchella I, Salvatici M, Botteri E, Medici M, Verri E, Adamoli L, Rotmensz N, Goldhirsch A, Sandri MT. Variation of circulating tumor cell levels during treatment of metastatic breast cancer: prognostic and therapeutic implications. Ann Oncol: Off J Eur Soc Med Oncol/ESMO. 2008;19(5):891–897. doi: 10.1093/annonc/mdm558. doi:10.1093/annonc/mdm558. [DOI] [PubMed] [Google Scholar]
- 15.Nakamura S, Yagata H, Ohno S, Yamaguchi H, Iwata H, Tsunoda N, Ito Y, Tokudome N, Toi M, Kuroi K, Suzuki E. Multi-center study evaluating circulating tumor cells as a surrogate for response to treatment and overall survival in metastatic breast cancer. Breast cancer (Tokyo, Japan) 2010;17(3):199–204. doi: 10.1007/s12282-009-0139-3. doi:10.1007/s12282-009-0139-3. [DOI] [PubMed] [Google Scholar]
- 16.Allard WJ, Matera J, Miller MC, Repollet M, Connelly MC, Rao C, Tibbe AG, Uhr JW, Terstappen LW. Tumor cells circulate in the peripheral blood of all major carcinomas but not in healthy subjects or patients with nonmalignant diseases. Clin Cancer Res: Off J Am Assoc Cancer Res. 2004;10(20):6897–6904. doi: 10.1158/1078-0432.CCR-04-0378. doi:10.1158/1078-0432.ccr-04-0378. [DOI] [PubMed] [Google Scholar]
- 17.Maestro LM, Sastre J, Rafael SB, Veganzones SB, Vidaurreta M, Martin M, Olivier C, DELO VB, Garcia-Saenz JA, Alfonso R, Arroyo M, Diaz-Rubio E. Circulating tumor cells in solid tumor in metastatic and localized stages. Anticancer Res. 2009;29(11):4839–4843. [PubMed] [Google Scholar]
- 18.Liu MC, Shields PG, Warren RD, Cohen P, Wilkinson M, Ottaviano YL, Rao SB, Eng-Wong J, Seillier-Moiseiwitsch F, Noone AM, Isaacs C. Circulating tumor cells: a useful predictor of treatment efficacy in metastatic breast cancer. J Clin Oncol Off J Am Soc Clin Oncol. 2009;27(31):5153–5159. doi: 10.1200/JCO.2008.20.6664. doi:10.1200/jco.2008.20.6664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gianni L, Romieu GH, Lichinitser M, Serrano SV, Mansutti M, Pivot X, Mariani P, Andre F, Chan A, Lipatov O, Chan S, Wardley A, Greil R, Moore N, Prot S, Pallaud C, Semiglazov V. AVEREL: a randomized phase III Trial evaluating bevacizumab in combination with docetaxel and trastuzumab as first-line therapy for HER2-positive locally recurrent/metastatic breast cancer. J Clin Oncol Off J Am Soc Clin Oncol. 2013;31(14):1719–1725. doi: 10.1200/JCO.2012.44.7912. doi:10.1200/jco.2012.44.7912. [DOI] [PubMed] [Google Scholar]
- 20.Giuliano M, Giordano A, Jackson S, Hess KR, De Giorgi U, Mego M, Handy BC, Ueno NT, Alvarez RH, De Laurentiis M, De Placido S, Valero V, Hortobagyi GN, Reuben JM, Cristofanilli M. Circulating tumor cells as prognostic and predictive markers in metastatic breast cancer patients receiving first-line systemic treatment. Breast cancer research : BCR. 2011;13(3):R67. doi: 10.1186/bcr2907. doi:10.1186/bcr2907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pierga JY, Hajage D, Bachelot T, Delaloge S, Brain E, Campone M, Dieras V, Rolland E, Mignot L, Mathiot C, Bidard FC. High independent prognostic and predictive value of circulating tumor cells compared with serum tumor markers in a large prospective trial in first-line chemotherapy for metastatic breast cancer patients. Ann Oncol: Off J Eur Soc Med Oncol/ESMO. 2012;23(3):618–624. doi: 10.1093/annonc/mdr263. doi:10.1093/annonc/mdr263. [DOI] [PubMed] [Google Scholar]
- 22.Schwartzberg LS, Badarinath S, Keaton MR, Childs BH. Phase II multicenter study of docetaxel and Bevacizumab with or without Trastuzumab as first-line treatment for patients with metastatic breast cancer. Clinical breast cancer. 2013 doi: 10.1016/j.clbc.2013.12.003. doi:10.1016/j.clbc.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 23.Martin M, Makhson A, Gligorov J, Lichinitser M, Lluch A, Semiglazov V, Scotto N, Mitchell L, Tjulandin S. Phase II study of bevacizumab in combination with trastuzumab and capecitabine as first-line treatment for HER-2-positive locally recurrent or metastatic breast cancer. Oncologist. 2012;17(4):469–475. doi: 10.1634/theoncologist.2011-0344. doi:10.1634/theoncologist.2011-0344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin NU, Seah DS, Gelman R, Desantis S, Mayer EL, Isakoff S, Dipiro P, Krop IE, Come SE, Weckstein D, Winer EP, Burstein HJ. A phase II study of bevacizumab in combination with vinorelbine and trastuzumab in HER2-positive metastatic breast cancer. Breast Cancer Res Treat. 2013;139(2):403–410. doi: 10.1007/s10549-013-2551-9. doi:10.1007/s10549-013-2551-9. [DOI] [PubMed] [Google Scholar]
- 25.Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S, Holmgren E, Ferrara N, Fyfe G, Rogers B, Ross R, Kabbinavar F. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350(23):2335–2342. doi: 10.1056/NEJMoa032691. doi:10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 26.Hurwitz HI, Fehrenbacher L, Hainsworth JD, Heim W, Berlin J, Holmgren E, Hambleton J, Novotny WF, Kabbinavar F. Bevacizumab in combination with fluorouracil and leucovorin: an active regimen for first-line metastatic colorectal cancer. J Clin Oncol Off J Am Soc Clin Oncol. 2005;23(15):3502–3508. doi: 10.1200/JCO.2005.10.017. doi:10.1200/jco.2005.10.017. [DOI] [PubMed] [Google Scholar]
- 27.Cameron D, Brown J, Dent R, Jackisch C, Mackey J, Pivot X, Steger GG, Suter TM, Toi M, Parmar M, Laeufle R, Im YH, Romieu G, Harvey V, Lipatov O, Pienkowski T, Cottu P, Chan A, Im SA, Hall PS, Bubuteishvili-Pacaud L, Henschel V, Deurloo RJ, Pallaud C, Bell R. Adjuvant bevacizumab-containing therapy in triple-negative breast cancer (BEATRICE): primary results of a randomised, phase 3 trial. Lancet Oncol. 2013;14(10):933–942. doi: 10.1016/S1470-2045(13)70335-8. doi:10.1016/s1470-2045(13)70335-8. [DOI] [PubMed] [Google Scholar]
- 28.Schneider BP, Wang M, Radovich M, Sledge GW, Badve S, Thor A, Flockhart DA, Hancock B, Davidson N, Gralow J, Dickler M, Perez EA, Cobleigh M, Shenkier T, Edgerton S, Miller KD. Association of vascular endothelial growth factor and vascular endothelial growth factor receptor-2 genetic polymorphisms with outcome in a trial of paclitaxel compared with paclitaxel plus bevacizumab in advanced breast cancer: ECOG 2100. J Clin Oncol Off J Am Soc Clin Oncol. 2008;26(28):4672–4678. doi: 10.1200/JCO.2008.16.1612. doi:10.1200/jco.2008.16.1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Etienne-Grimaldi MC, Formento P, Degeorges A, Pierga JY, Delva R, Pivot X, Dalenc F, Espie M, Veyret C, Formento JL, Francoual M, Piutti M, de Cremoux P, Milano G. Prospective analysis of the impact of VEGF-A gene polymorphisms on the pharmacodynamics of bevacizumab-based therapy in metastatic breast cancer patients. Br J Clin Pharmacol. 2011;71(6):921–928. doi: 10.1111/j.1365-2125.2010.03896.x. doi:10.1111/j.1365-2125.2010.03896.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baselga J, Cortes J, Kim SB, Im SA, Hegg R, Im YH, Roman L, Pedrini JL, Pienkowski T, Knott A, Clark E, Benyunes MC, Ross G, Swain SM. Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med. 2012;366(2):109–119. doi: 10.1056/NEJMoa1113216. doi:10.1056/NEJMoa1113216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lambrechts D, Lenz HJ, de Haas S, Carmeliet P, Scherer SJ. Markers of response for the antiangiogenic agent bevacizumab. J Clin Oncol Off J Am Soc Clin Oncol. 2013;31(9):1219–1230. doi: 10.1200/JCO.2012.46.2762. doi:10.1200/jco.2012.46.2762. [DOI] [PubMed] [Google Scholar]
- 32.Miles DW, de Haas SL, Dirix LY, Romieu G, Chan A, Pivot X, Tomczak P, Provencher L, Cortes J, Delmar PR, Scherer SJ. Biomarker results from the AVADO phase 3 trial of first-line bevacizumab plus docetaxel for HER2-negative metastatic breast cancer. Br J Cancer. 2013;108(5):1052–1060. doi: 10.1038/bjc.2013.69. doi:10.1038/bjc.2013.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Munzone E, Botteri E, Sandri MT, Esposito A, Adamoli L, Zorzino L, Sciandivasci A, Cassatella MC, Rotmensz N, Aurilio G, Curigliano G, Goldhirsch A, Nole F. Prognostic value of circulating tumor cells according to immunohistochemically defined molecular subtypes in advanced breast cancer. Clinical breast cancer. 2012;12(5):340–346. doi: 10.1016/j.clbc.2012.07.001. doi:10.1016/j.clbc.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 34.Bidard FC, Fehm T, Ignatiadis M, Smerage JB, Alix-Panabieres C, Janni W, Messina C, Paoletti C, Muller V, Hayes DF, Piccart M, Pierga JY. Clinical application of circulating tumor cells in breast cancer: overview of the current interventional trials. Cancer Metastasis Rev. 2013;32(1–2):179–188. doi: 10.1007/s10555-012-9398-0. doi:10.1007/s10555-012-9398-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Giordano A, Giuliano M, De Laurentiis M, Arpino G, Jackson S, Handy BC, Ueno NT, Andreopoulou E, Alvarez RH, Valero V, De Placido S, Hortobagyi GN, Reuben JM, Cristofanilli M. Circulating tumor cells in immunohistochemical subtypes of meta-static breast cancer: lack of prediction in HER2-positive disease treated with targeted therapy. Ann Oncol: Off J Eur Soc Med Oncol/ESMO. 2012;23(5):1144–1150. doi: 10.1093/annonc/mdr434. doi:10.1093/annonc/mdr434. [DOI] [PubMed] [Google Scholar]
- 36.Bidard FC, Mathiot C, Degeorges A, Etienne-Grimaldi MC, Delva R, Pivot X, Veyret C, Bergougnoux L, de Cremoux P, Milano G, Pierga JY. Clinical value of circulating endothelial cells and circulating tumor cells in metastatic breast cancer patients treated first line with bevacizumab and chemotherapy. Ann Oncol: Off J Eur Soc Med Oncol/ESMO. 2010;21(9):1765–1771. doi: 10.1093/annonc/mdq052. doi:10.1093/annonc/mdq052. [DOI] [PubMed] [Google Scholar]
- 37.Wallwiener M, Hartkopf AD, Baccelli I, Riethdorf S, Schott S, Pantel K, Marme F, Sohn C, Trumpp A, Rack B, Aktas B, Solomayer EF, Muller V, Janni W, Schneeweiss A, Fehm TN. The prognostic impact of circulating tumor cells in subtypes of metastatic breast cancer. Breast Cancer Res Treat. 2013;137(2):503–510. doi: 10.1007/s10549-012-2382-0. doi:10.1007/s10549-012-2382-0. [DOI] [PubMed] [Google Scholar]
- 38.Arteaga CL, Mayer IA, O’Neill AM, Swaby RF, Perez EA, Lin NU, Sledge GW. A randomized phase III double-blinded placebo-controlled trial of first-line chemotherapy and trastuzumab with or without bevacizumab for patients with HER2/neu-overexpressing metastatic breast cancer (HER2 positive MBC): A trial of the Eastern Cooperative Oncology Group (E1105) 2012;30(suppl) abstr 605. [Google Scholar]
- 39.Slamon DJ, Swain SM, Buyse M, Martin M, Geyer CE, Im Y-H, Pienkowski T, Kim S-B, Robert NJ, Steger G, Crown J, Verma S, Eiermann W, JP C, Im S-A, Mamouna sE, Schwartzberg L, Paterson A, JR M, L P, MF P, M T, Bee-Munteanu V, Henschel V, Crepelle-Flechais A, Wolmark N. Primary results from BETH, a phase 3 controlled study of adjuvant chemotherapy and trastuzumab± bevacizumab in patients with HER2-positive, node-positive or high risk node-negative breast cancer. 36th Annual San Antonio Breast Cancer Symposium (SABCS)/AACR American Association for Cancer Research:S1-03.2013. [Google Scholar]
- 40.Yardley DA, Raefsky E, Castillo R, Lahiry A, Locicero R, Thompson D, Shastry M, Burris HA, 3rd, Hainsworth JD. Phase II study of neoadjuvant weekly nab-paclitaxel and carboplatin, with bevacizumab and trastuzumab, as treatment for women with locally advanced HER2+ breast cancer. Clinical breast cancer. 2011;11(5):297–305. doi: 10.1016/j.clbc.2011.04.002. doi:10.1016/j.clbc.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 41.Pierga JY, Petit T, Delozier T, Ferrero JM, Campone M, Gligorov J, Lerebours F, Roche H, Bachelot T, Charafe-Jauffret E, Pavlyuk M, Kraemer S, Bidard FC, Viens P. Neoadjuvant bevacizumab, trastuzumab, and chemotherapy for primary inflammatory HER2-positive breast cancer (BEVERLY-2): an open-label, single-arm phase 2 study. Lancet Oncol. 2012;13(4):375–384. doi: 10.1016/S1470-2045(12)70049-9. doi:10.1016/s1470-2045(12)70049-9. [DOI] [PubMed] [Google Scholar]


