Abstract
Cyclic nucleotides [e.g., cyclic adenosine monophosphate (cAMP) and cyclic guanosine monophosphate (cGMP)] are ubiquitous second messengers that affect multiple cell functions from maturation of the egg to cell division, growth, differentiation, and death. The concentration of cAMP can be regulated by processes within membrane domains (local regulation) as well as throughout a cell (global regulation). The phosphodiesterases (PDEs) that degrade cAMP have well-known roles in both these processes. It has recently been discovered that ATP-binding cassette (ABC) transporters contribute to both local and global regulation of cAMP. This regulation may require the formation of macromolecular complexes. Some of these transporters are ubiquitously expressed, whereas others are more tissue restricted. Because some PDE inhibitors are also ABC transporter inhibitors, it is conceivable that the therapeutic benefits of their use result from the combined inhibition of both PDEs and ABC transporters. Deciphering the individual contributions of PDEs and ABC transporters to such drug effects may lead to improved therapeutic benefits.
Keywords: CFTR, cAMP, efflux, export, MRP4
EARLY EVIENCE FOR CYCLIC NUCLEOTIDE COMPARTMENTALIZATION
The first evidence for a nonuniform cellular effect of increases in cAMP was provided by the work of Buxton & Brunton (1), who compared cAMP activation of protein kinase A (PKA) in response to treatment of perfused rabbit heart with agonists of different G protein–coupled receptors (GPCRs): prostaglandin E1 (PGE1, a prostanoid receptor agonist) or isoproterenol (a β-adrenergic receptor agonist). After treatment, the heart was homogenized and separated into soluble and particulate fractions, and the amount of PKA in each fraction was determined. This analysis revealed that the ratio of activated PKA in soluble fractions to activated PKA in particulate fractions was ligand dependent. Notably, PGE1 increased the activity primarily of the soluble PKA, whereas isoproterenol increased particulate PKA; moreover, the increase in particulate, but not soluble, PKA activity was associated with positive cardiac inotropy. These studies (1) suggested that after its formation, the “cAMP message” was compartmentalized and might produce different biological effects if cAMP signals were spatially segregated within a cell.
An elegant series of experiments by Jurevicius & Fischmeister (2) provided evidence for such spatial regulation. The authors used patch clamps to record cAMP-activated calcium currents (L-type Ca2C) from two physically separated sites on a single isolated cardiac myocyte after β-adrenergic receptor stimulation. These investigators hypothesized that if cAMP activation occurs throughout the cell, then locally applied adrenergic stimulation would activate calcium currents at both sites. They found that local application of isoproterenol produced a restricted activation of calcium currents (i.e., compartmentalized effect), whereas local application of forskolin (a general adenylyl cyclase activator) activated the calcium current throughout the cell (i.e., produced global effects). Many factors contribute to cAMP compartmentalization, but here we primarily describe recent evidence for the role of ATP-binding cassette (ABC) transporters in the process. We relate this role to that of phosphodiesterases (PDEs) because they are important therapeutic targets, and some PDE inhibitors might exert part of their effect owing to inhibition of ABC transporters.
PHOSPHODIESTERASES MODULATE LOCAL cAMP CONCENTRATION
Activation of PKA requires the binding of cyclic adenosine monophosphate (cAMP) to the PKA holoenzyme, which produces dissociation of its regulatory and catalytic subunits. However, to elicit PKA activation, cellular cAMP concentrations need to increase in excess of the Km of PKA [~3 μM (3)]. Because cAMP has a high diffusion rate (500–700 μm s−1), PKA activation could theoretically occur throughout a cell by any stimulus that elevated intracellular cAMP. This leads to the question: How is PKA specifically activated in response to receptor-mediated elevation in cAMP? In other words, what prevents cAMP, once formed, from always producing global activation of both the soluble and particulate forms of PKA?
The limitation of PKA activation by freely diffusible cAMP may occur via physical barriers (e.g., endoplasmic reticulum below the plasma membrane or membrane invaginations) or enzymatic barriers that restrict intracellular diffusion of cAMP (4, 5, 6, 7). An enzymatic barrier appears to be provided by PDEs. Cyclic nucleotide PDEs constitute a group of enzymes responsible for the degradation of the phosphodiester bond in cAMP and cyclic guanosine monophosphate (cGMP); this degradation results in the hydrolysis of cAMP and cGMP and the formation of adenosine 5′-monophosphate (AMP) and guanine 5′-monophosphate (GMP), respectively. PDEs localize either to the cytosol or to the subcellular compartments, including the plasma membrane, but they can be recruited into multiprotein signaling complexes (8). The amino terminal sequence of a PDE can determine its subcellular localization, and such localization appears to determine whether a PDE is activated.
Eleven mammalian PDE gene families containing 21 genes (9, 10) have been described. The families are clustered according to criteria that include structural similarity, sequence homology, protein domains, and various enzymatic properties such as sensitivity to endogenous regulators and inhibitors, kinetic properties, and substrate specificity. PDEs are grouped into three categories with respect to their substrate specificities: cAMP-specific (PDE4, PDE7, and PDE8), cGMP-specific (PDE5, PDE6, and PDE9), and dual-specific (PDE1, PDE2, PDE3, PDE10, and PDE11) (4). This review focuses on several PDEs that have well-defined roles in compartmental signaling.
Brechler et al. (11) provided evidence for a role of PDEs in compartmentalized cAMP signaling that is initiated by the hormone glucagon in heart cells. Prior studies had shown that glucagon increased cAMP, leading to activation of calcium channels (12). However, glucagon did not activate adenylyl cyclase; instead, by acting through a pertussis toxin–sensitive pathway, it inhibited a membrane-bound PDE that has a high affinity for cAMP. The increase in cAMP was not attributable to inhibition of a soluble form of PDE. Through the judicious use of PDE inhibitors, the authors suggested that an elevation of cAMP in the membrane contributed to the positive inotropic effect of glucagon. Because pharmacologic inhibition of PDE affects cAMP concentrations in specific subcellular compartments, considerable effort has been directed toward understanding how compartmentalized signaling works, with cAMP degradation by PDEs underpinning the mechanism.
Recent studies that utilize live-cell imaging and florescence resonance energy transfer (FRET) have provided strong support for the idea that PDEs have a role in compartmentalized cAMP signaling. Experiments performed by Leroy et al. (13) revealed the contribution of PDEs to the intracellular spatiotemporal dynamics of cAMP concentration in ventricular myocytes. By using pulse treatments of a β-adrenergic receptor agonist, these authors showed that cAMP peaks are observed faster at the membrane than in the cytosol. This finding suggests that cAMP is rapidly synthesized and hydrolyzed at the plasma membrane and that diffusion is restricted in the cytoplasm (13). Interestingly, Nikolaev et al. (14) showed that localized β1-adrenergic receptor stimulation generates a cAMP gradient that propagates throughout the cells, whereas localized β2-adrenergic receptor stimulation does not elicit cAMP diffusion. If the cAMP spatiotemporal dynamics depends on the source of cAMP, then the contributors to compartmentalization may be part of a specific organization. Additional studies using FRET technology showed that two different PDE4 subfamilies, PDE4B and PDE4D, are responsible for cAMP dynamics in defined compartments of the cell (Figure 1). Results from this study suggest that PDE4B regulates a subplasma membrane cAMP compartment, whereas PDE4D contributes mainly to cytosolic cAMP regulation. This finding led to a model in which cytosolic PDE4D acts as a sink to locally drain cAMP (15).
Figure 1.
Role of compartmentalized phosphodiesterases (PDEs). (a) In response to stimulation by an agonist, concerted activity of PDE4B at the subplasma membrane and PDE4D in the cytosol generates an intracellular cyclic adenosine monophosphate (cAMP) gradient. (b) cAMP concentrations in the cytosol are regulated by the compartmentalized PDE4D. Abbreviation: AC, adenylate cyclase.
The concept that PDE4D controls localized cAMP concentration suggests the possibility that multiple cAMP gradients can be simultaneously generated in different cellular locations. Because of the rapid diffusion of cAMP (see above), this gradient would depend not on the distance from adenylyl cyclase but instead on its proximity to the closest PDE4 sink. Localized pools of PDE4 might then restrict inappropriate, excess activation of cAMP effectors such as PKA. This concept of PDE sinks is supported by studies using FRET reporters fused to individual PDEs; such studies reveal the formation of cAMP gradients around PDE molecules (15). To further explore intracellular cAMP gradients, investigators modified the cAMP binding protein, Epac (Exchange protein activated by cAMP), as a FRET reporter for cAMP (CFP-Epac-YFP, with CFP fused to the N terminus and YFP to the C terminus). HEK293 cells were transfected with CFP-Epac-YFP and then treated with a β-adrenergic receptor agonist to increase cAMP production in the presence and absence of PDE4 inhibitors, rolipram, and Ro20-1724. Cells treated with either of these two compounds produced a rapid decrease in the YFP-FRET, indicating an increase in intracellular cAMP levels upon inhibition of PDE4. The authors demonstrated the selective nature of this effect by using PDE3 and PDE5 inhibitors, which produced no change in FRET (15, 16). These results demonstrated that specific PDEs regulate cAMP concentrations and that this regulation depends on the location of the PDEs.
PDEs are located in multiple subcellular compartments, and putative or established targeting domains have been identified for most of the PDE families (17). PDE3s target the endoplasmic reticulum by a transmembrane domain consisting of six transmembrane helices (18), and PDE4D5 interacts with RACK-1, a scaffolding protein that binds certain PKC isoforms after their activation by diacylglycerol (19). PDE4D3 is targeted to the Golgi/centrosomal region through anchoring by myomegalin (20). Some PDE4D and PDE4A variants bind Src homology 3 domains of, e.g., Src kinases (21–23); furthermore, PDE4 isoforms, via their catalytic domain, bind to and are phospho-rylated by Erk (24). PDE4A1 contains a novel lipid binding domain, TAPAS, that has specificity for phosphatidic acid and that serves to target this PDE to specific cellular membranes (25).
PHOSPHODIESTERASES CLUSTER IN CAVEOLAE
Whereas PDEs reside in various subcellular compartments, such as the plasma membrane, cytoplasm, and subnuclear areas, they may also cluster in domains that modulate multiple incoming cAMP signals. Plasma membrane caveolae are one type of structure in which PDEs are found. Caveolae (“little caves”), flask-like invaginations of the plasma membrane, are proposed to be organizers of signal transduction in numerous cell types, in particular pulmonary vascular endothelial cells (26, 27). Caveolae are morphologically distinct entities that constitute a subset of lipid rafts that organize membrane lipid and protein components. In addition, they are enriched with particular lipids (e.g., cholesterol, glycosphingolipids) and scaffolding proteins (e.g., caveolins) that interact with multiple proteins (28).
One might expect that some PDEs would localize to caveolae as a means to modulate membrane changes in cAMP concentration. Indeed, PDE3B localizes to caveolae in primary adipocytes, as demonstrated by coimmunoprecipitation with caveolin-1 (29). This physical interaction appears to play a regulatory role because disruption of caveolae with methyl-β-cyclodextrin (by removing cholesterol from the membrane) reduces PDE3B expression. PDE3B expression is also reduced in caveolin−/− mice, as shown by Nilsson et al. (29). The authors proposed that caveolin-1 is required to stabilize PDE3B. However, not all PDEs are stabilized by caveolin-1. For instance, the relationship between PDE5 and caveolin-1 in pulmonary artery smooth muscle cells shows that when caveolin-1 is overexpressed, PDE5 expression is decreased; Murray et al. (30) speculated that this inverse relationship arose not from a direct interaction but from an undefined mechanism. Further knowledge of the interactions of PDEs and caveolin is necessary to fully define the contribution of caveolae to cAMP compartmentalization.
PHOSPHODIESTERASE INHIBITORS
Multiple PDE inhibitors have been developed as therapies for pathological conditions that might be modulated by altering cyclic nucleotide levels. PDEs are good therapeutic targets because of their high affinity for substrates and unique substrate binding requirements that make them amenable to small-molecule inhibitors (31). However, because overall concentrations of cAMP and cGMP in most cell types range from <1 μM to ~10 μM, developing competitive inhibitors that block PDE activity is a challenge. Nonetheless, pharmacologic inhibition of PDEs has been achieved and used to support the idea of cAMP compartmentalization.
The location of a PDE within a cell might impact the efficacy of a PDE inhibitor (Figure 1). For example, within the PDE3 subfamily, PDE3A is either membrane associated or cytosolic, whereas PDE3B is predominantly membrane associated. Several isoform-selective PDE3 inhibitors such as amrinone, milrinone, cilostamide, cilostazol, and trequinsin are available, but none of these compounds distinguish between PDE3A and PDE3B. One compound, OPC-33450, shows selectivity between the isoforms (32). Both PDE3A and PDE3B are expressed in vascular smooth muscle cells and regulate vascular contractility (33). Recent studies found that PDE3A is expressed at the apical plasma membrane of epithelial cells, where it interacts directly with the cystic fibrosis transmembrane conductance regulator (CFTR) channel. Notably, PDE3 inhibition by cilostazol generated compartmentalized cAMP, which potentiated CFTR channel function. These interactions required an intact actin cytoskeleton because the actin polymerization inhibitor latrunculin B disrupted both the PDE3A-CFTR interaction and the compartmentalization of cAMP (34).
PHOSPHODIESTERASE RECRUITMENT
PDEs are vital to maintaining proper cAMP concentration within a cell. Knockout models of PDE4B (31) have illustrated how signaling processes that regulate biological processes are altered. If a cell lacks PDEs, it might become overrun by cAMP and produce promiscuous activation of multiple cAMP-dependent pathways. The close proximity of PDEs to the site of cAMP formation constrains cAMP dissemination throughout a cell. Thus, compartmentalized PDEs produce low local concentrations of cAMP (Figure 1), and PDEs can be recruited to protein complexes to attenuate cAMP signaling.
A well-characterized example of PDE recruitment is the relationship among β2-adrenergic receptor (β2AR), β-arrestin, and PDE4. In a study performed on HEK293 cells, agonist stimulation of β2AR increased cAMP concentration and activated PKA. Activated PKA, in turn, phosphory-lated β2AR, which recruited β-arrestin to PDE4 in close proximity to the β2AR. This attenuated the local cAMP concentration and, concurrently, abrogated further phosphorylation of the β2AR by PKA (35, 36). This interaction provides a mechanism whereby cAMP-degrading enzymes localize close to the origin of cAMP synthesis in an agonist-dependent fashion.
MEMBRANE EFFLUX TRANSPORTERS IN INTRACELLULAR CYCLIC NUCLEOTIDE HOMEOSTASIS
Although regulation of cyclic nucleotides occurs by PDEs, other processes can also alter the intracellular cyclic nucleotide concentration. Export of monophosphorylated nucleotides was first noted by Davoren and Sutherland (37, 38), who discovered the biosynthesis of cAMP in response to hormonal stimulation and adenylyl cyclase activation. The authors observed not only an increase in intracellular cAMP but also a concurrent rise in extracellular cAMP. This finding was reported in the 1960s (37). Subsequent studies conducted in the 1970s (39, 40) showed that nucleoside monophosphate analogs exhibit similar export properties, suggesting a common mediator. Many different types of cells export cAMP, including erythrocytes, hepatocytes, endothelial and epithelial cells, neuronal cells, and fibroblasts. However, the ability to export cAMP varies among cell types. Some cells have first-order elimination-rate constants for cAMP that range from 0.14 min−1 to 0.014 min−1, corresponding to a half-life of intracellular cAMP from a few minutes to almost one hour. This range could be due to differences in the amount of transporter or perhaps the presence of multiple cAMP export transporters (see below). Furthermore, this nucleotide extrusion process has several general properties, such as being unidirectional, inhibited by depletion of cellular energy [i.e., adenosine triphosphate (ATP)], and chemically inhibited by compounds such as probenecid (an organic anion transport inhibitor) and prostaglandins, as well as having saturable kinetics (41–44).
Akin to cAMP, cGMP is another natural nucleotide that undergoes active efflux from cells. For example, isolated rat hepatocytes stimulated with cytokines and lipopolysaccharide produce nitric oxide (which activates guanylyl cyclase to produce cGMP), but only a small elevation occurs in intracellular cGMP concentration compared with a large increase in extracellular cGMP levels. As observed for cAMP extrusion, cGMP export is inhibited by probenecid (45). Further studies have demonstrated that cGMP efflux is not unique to the liver or kidney; it also occurs in other cells such as vascular smooth muscle cells, endothelial cells, and fibroblasts (46).
In 1999, investigators identified a transporter termed multidrug resistance protein 4 (MRP4; also known as ABCC4), a member of the ABC transporter family (see Mechanism of ATP-Binding Cassette Transporters and Substrate Recognition, below). MRP4 can export nucleotide analogs such as a purine nucleotide monophosphate analog that has antiviral properties (47). The resemblance of this substrate to cAMP prompted subsequent studies to evaluate if MRP4 was capable of ATP-dependent transport of cAMP and cGMP (Figure 2). Two approaches were used: membrane vesicle transport systems and mammalian cells engineered to stably overexpress MRP4. MRP4-dependent transport activity was assayed through the employment of purified, everted membrane vesicles prepared from insect cells programmed to express MRP4. The everted MRP4-containing vesicles allowed determination of the kinetic properties and ATP dependence of transport. These studies showed that vesicular uptake of cAMP and cGMP requires ATP. Moreover, collapse of the osmotic gradient disrupted transport, confirming that the cyclic nucleotide was adsorbing to the surface of the vesicle not in a nonspecific fashion but instead, in an ATP-dependent fashion (48, 49). These initial studies indicated that the affinity constants of MRP4 for cAMP and cGMP are 45 μM and 10 μM, respectively; however, subsequent studies using other systems have suggested that the Km for cyclic nucleotide transport is in the 0.5-mM range (50). The reason for the discrepancy is unclear. Nonetheless, these findings provided a mechanism to account for the energy-dependent export of cyclic nucleotides from cells originally observed by Davoren & Sutherland (37).
Figure 2.

ABCC4 at the plasma membrane regulates the concentrations of intracellular cyclic nucleotides and NMP analogs. Abbreviations: AC, adenylate cyclase; cAMP, cyclic adenosine monophosphate; NMP, nucleotide monophosphate.
MECHANISM OF ATP-BINDING CASSETTE TRANSPORTERS AND SUBSTRATE RECOGNITION
ABC transporters mediate the ATP-dependent extrusion of a diverse array of endogenous and exogenous substances (52). These transporters are phylogenetically conserved and expressed in microbial pathogens, plants, and higher organisms. There are 48 transporters belonging to the ABC superfamily in the human genome. Defects in certain genes are the basis of inherited diseases that include a neonatal surfactant deficiency (53), a bleeding disorder and macular degeneration (54), and liver diseases (55), all of which are caused by the failure to export a specific ligand across a lipid bilayer (52). Some human ABC transporters also appear to function in a protective capacity by exporting cytotoxic compounds (e.g., dietary cytotoxics and therapeutic drugs) out of cells. These transporters [e.g., P-glycoprotein (ABCB1), BCRP (ABCG2), and MRP1 (ABCC1)] are highly expressed in the gut, liver, and kidneys, where they also restrict the bioavailability of administered drugs (56–58).
In the ABC transporter superfamily, ABC exporters can be distinguished from ABC importers by the directionality of transport and the distinct structural arrangements of the membrane-spanning domains (MSDs) (Figure 3). All ABC transporters contain two nucleotide-binding domains (NBDs), each of which carries a canonical ABC motif and two MSDs; each of these MSDs usually contains six transmembrane helices in exporters. The MSDs are not highly conserved and form the ligand binding sites to provide substrate specificity. The NBDs are highly homologous throughout the ABC superfamily and have several characteristic motifs, including the Walker A and B motifs common to many nucleotide-binding proteins (59). However, other motifs, such as the ABC signature and the D, H, and Q loops, are unique to the family (60). Interestingly, bacterial and archaeal ABC transporters are typically expressed as half-transporters, with one NBD and one MSD on a single polypeptide chain. The two polypeptides then assemble into a functional homo-or heterodimer. In eukaryotes, however, ABC exporters are often expressed as a single polypeptide chain containing all four domains (two NBDs and two MSDs) (61).
Figure 3.
(a) Membrane topology of full and half ATP-binding cassette (ABC) transporters. Blue bars represent predicted membrane-spanning domains (MSDs); green circles represent nucleotide-binding domains (NBDs). (b) The core domain structure of ABC transporters with the conserved key motifs in a NBD. Abbreviation: Pi, inorganic phosphate.
The concept of export of drugs was first proposed by Dano (62) as a mechanism of cancer chemotherapeutic drug resistance. The multidrug resistance P-glycoprotein (ABCB1) was first characterized by Juliano & Ling (63) in the 1970s and was cloned in the 1980s. In 1992, Cole, Deeley, and colleagues (64) cloned the multidrug resistance-associated protein gene, now known as ABCC1 or MRP1. The expansion of the human ABC family accelerated in the 1990s by the expressed sequence tag (est) database, a collection of DNA sequence information from cDNA libraries, and subsequent efforts using computer-aided data mining to identify homologous genes. These tools allowed investigators to expand the members of the ABC superfamily.
In general, the NBDs of ABC transporters bind ATP and hydrolyze ATP (there is no evidence for autophosphorylation of the transporter). Current models indicate that vectorial transport of substrates is provided by ATP binding and hydrolysis (52, 65). Substrate transport occurs against a transmembrane concentration gradient for hydrophilic substrates and against the lipid-water partition coefficient for hydrophobic substrates. In general, the affinity of the MSDs for the ligand is coupled to ATP binding to the NBDs: In the absence of ATP, the MSDs have high affinity for ligand, whereas in the presence of ATP, the affinity is reduced. More detailed models have been proposed to explain this catalytic cycle of energetic coupling between the NBDs and the transport by the MSDs (52).
ABCC4/MRP4 AS A REGULATOR OF cAMP
The domain organization of MRP4 is typical of ABC transporters: The core structure is composed of two MSDs, each of which consists of six transmembrane helices and two cytosolic ATP-binding domains, which bind and hydrolyze ATP to power substrate transport. MRP4 is ubiquitously expressed in many tissues, including the prostate, liver, testis, ovary, and kidney (66); the blood-brain barrier (67); the cardiovascular cells (see Transporters as Regulators in Disease, below); the adrenal gland (48); and the Leydig cells, where it has a role in regulating cAMP-activated de novo testosterone biosynthesis (68). Among the ABCC subfamily, MRP4 is unique, because in polarized cells it can localize either to the apical or basolateral membrane (67). Recent studies have proposed that interactions through MRP4’s C-terminal PDZ interaction motif contribute to its polarized expression in certain cells (69, 70).
A biological role for MRP4 in regulating cAMP export was described by studies on a gut epithelial cell line, HT29-CL19A, which expresses both MRP4 and CFTR on the apical plasma membrane (71). These polarized cells vectorially transport cAMP across the apical membrane. This transport is inhibited by the MRP4 inhibitor MK571 or the MRP4 substrate PMEA [9-(2-phosphonyl methoxyethyl)adenine], demonstrating that MRP4 functions as a cAMP exporter. Because the CFTR channel is activated by cAMP, inhibition of MRP4 transport activity potentiates the cAMP-activated CFTR channel in these cells. Whereas MRP4 can export cAMP, it appears that MRP4 also regulates the CFTR channel by modulating cAMP in a compartmentalized fashion. The potentiation of the CFTR channel by either inhibition or silencing of MRP4 was most prominent in response to treatment with low concentrations (<20 μM) of the cAMP-elevating agent adenosine but not with higher concentrations (>20 μM). Real-time monitoring of cAMP dynamics (using the aforementioned CFP-Epac-YFP) in response to adenosine and another cAMP-elevating agent, forskolin, demonstrated that MRP4 inhibition induces localized cAMP accumulation near the plasma membrane. In addition, low concentrations (2 μM) of adenosine produce a further increase in cAMP concentration near the plasma membrane. This increase revealed how MRP4 regulates cAMP levels in a compartmentalized fashion. However, MRP4 inhibition failed to show local cAMP effects at adenosine concentrations that strongly elevate intracellular cAMP concentrations, suggesting that compartmentalization is overridden at high intracellular cAMP concentrations. Nonetheless, MRP4 can regulate CFTR at low adenosine concentrations by the physical association of MRP4 and CFTR through the PDZ-binding protein PDZK1. [MRP4 has a conserved C-terminal PDZ motif (ETAL).] How the formation of this macromolecular complex is regulated is unknown, but its components may exist within larger domains. In this respect, it is notable that a recent study found that MRP4 localizes in caveolin-enriched fractions (72). Because caveolins are integral membrane proteins that act as a scaffold for membrane proteins in microdomains, it is conceivable that caveolins physically anchor MRP4 in microdomains.
Recently, Sellers et al. (73) determined that MRP4 is expressed in mouse ventricular my-ocytes. Using a cAMP FRET reporter, they found that MRP4 has a role in β-adrenergic-receptor-stimulated contraction because MRP4 inhibition potentiates submaximal (but not maximal) isoproterenol-stimulated cAMP accumulation and contraction rate. One notable finding was the demonstration that MRP4-dependent regulation of myocyte contraction rate was CFTR dependent, whereas PDE4-dependent potentiation of contraction rate was CFTR independent. The study suggests that PDE4 and MRP4 use independent processes to regulate localized cAMP levels. Moreover, these studies suggest that MRP4 function might be modulated through the formation of a macromolecular complex with CFTR.
Akin to what occurs with certain PDEs, MRP4 promotes the modulation of local membrane cAMP concentrations that are coupled to some GPCR-mediated events. To accomplish local regulation of cAMP, MRP4 forms macromolecular complexes in specialized subcellular domains (see Transporters as Regulators in Disease, below).
OTHER ABC TRANSPORTERS CAPABLE OF EXPORTING CYCLIC NUCLEOTIDES
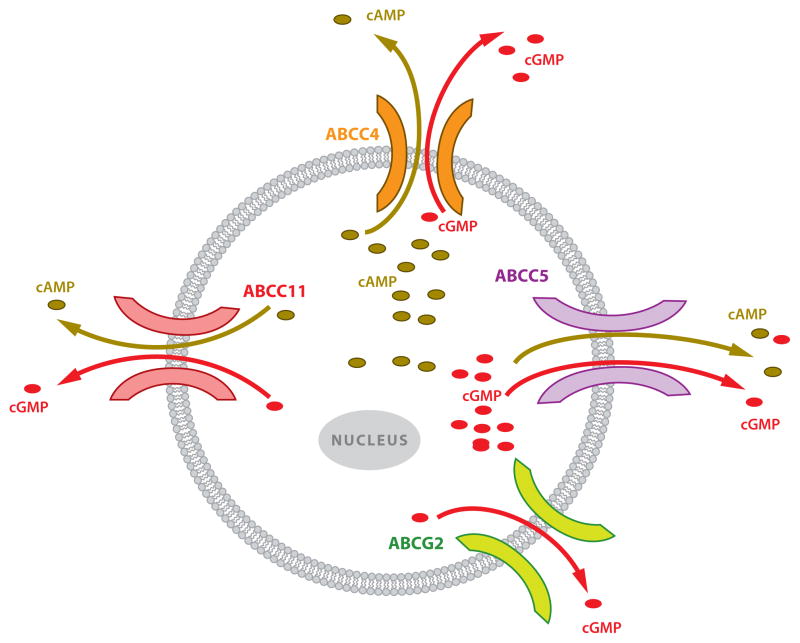
Additional ABC transporters have been shown to export cyclic nucleotides (Figure 4 and Table 1). For instance, Jedlitschky et al. (74) used membrane vesicles to show that a close relative of MRP4, MRP5 (ABCC5), transports cGMP with micromolar affinity (and cAMP with much lower affinity). Studies using intact cells showed that MRP5 was capable of exporting cAMP from cells engineered to overexpress MRP5 at rates greater than the export rates from unmodified cells and in an ATP-dependent fashion (50).
Figure 4.
Multidrug transporters export intracellular cyclic nucleotides with different specificity: ABCC4, ABCC5, and ABCC11 can transport both cAMP and cGMP, whereas ABCG2 transports only cGMP. Abbreviations: cAMP, cyclic adenosine monophosphate; cGMP, cyclic guanosine monophosphate.
Table 1.
ABC transporters that transport cyclic nucleotides
| Transporter | Species | System | Nucleotide | Km | Reference |
|---|---|---|---|---|---|
| ABCG2 | Human | Erythrocyte vesicles | cGMP | 132 μM | 80 |
| Abcg2 | Mouse | Erythrocyte vesicles | cGMP | 9 mM | |
| Abcc4 | Mouse | Erythrocyte vesicles | cGMP | 2.9 mM | 80 |
| ABCC4 | Human | SF9 insect cell vesicles | cAMP | 44.5 μM | 48 |
| cGMP | 9.69 μM | ||||
| ABCC4 | Human | SF9 insect cell vesicles | cAMP | Not reported | 49 |
| cGMP | |||||
| ABCC4 | Human | HEK293 cells | cAMP | Not reported | 50 |
| cGMP | |||||
| ABCC5 | Human | SF9 insect cell vesicles | cAMP | Not reported | 49 |
| cGMP | |||||
| ABCC5 | Human | HEK293 cells | cAMP | Not reported | 50 |
| cGMP | |||||
| ABCC5 | Human | V79 cells | cAMP | 379 μM | 74 |
| cGMP | 2.1 μM | ||||
| ABCC11 | Human | LLC-PK1 vesicles | cAMP | Not reported | 106 |
| cGMP |
Abbreviations: cAMP, cyclic adenosine monophosphate; cGMP, cyclic guanosine monophosphate; LLC-PK1, pig kidney epithelial cells.
These studies on intact cells suggest that MRP4 and MRP5 are low-affinity cyclic nucleotide exporters that may function as overflow pumps when phosphodiesterases are limiting (50). In the absence of adenylyl cyclase activation, inhibition of MRP4 transport does not lead to substantial increases in intracellular cyclic nucleotide concentrations, and overexpression of MRP4 does not lead to a substantial decrease in intracellular cAMP levels, even when the total cAMP concentrations are high (50). These findings fostered the idea that MRP4 is a cAMP overflow pump. However, this idea arose prior to the demonstration that MRP4 modulated local membrane concentrations of cAMP (71). Regulation by MRP4 of local and global cAMP concentrations may depend on the amount of transporter at the plasma membrane, and MRP4 may remain in a nonfunctional state prior to changes in cAMP concentration that occur after adenylyl cyclase activation. Recent studies with sea urchins revealed that although the total amount of an ABC transporter is constant, the functional amount in the plasma membrane is regulated by engagement with the cytoskeleton (75).
At least two other ABC transporters have been shown to export cyclic nucleotides: ABCG2 and ABCC11 (MRP8). MRP8, like MRP4 and MRP5, lacks the third N-terminal MSD found in MRP1, MRP2, MRP3, MRP6, and MRP7. Overexpression of MRP8 in an LLC-PK1 pig kidney cell line led to the following result: Upon adenylyl cyclase activation, intracellular cAMP was reduced in MRP8-overexpressing cells, and cAMP export was enhanced. Notably, an MRP8 loss-of-function allele determines the dry earwax phenotype that is frequently found in East Asians. However, because earwax contains many aliphatic compounds that are potential substrates, it is unlikely that a cyclic nucleotide is its endogenous substrate.
In addition to its role in transporting chemotherapy drugs (76), ABCG2 also serves as a progenitor cell marker (77). Although cyclic nucleotides can impact progenitor cell differentiation (78, 79), whether ABCG2 is a cAMP transporter is not known. However, utilizing combinations of knockout mice, Borst and colleagues (80) demonstrated that Abcg2 and Abcc4 (Mrp4) are the predominant cGMP transporters in murine erythrocytes, whereas Mrp5 has no obvious role in erythrocyte cGMP transport. Because Abcg2 exhibits additional functional overlap with Abcc4 [it transports similar chemotherapeutic nucleotide analogs (81)], it is possible that one endogenous role is the transport of cAMP.
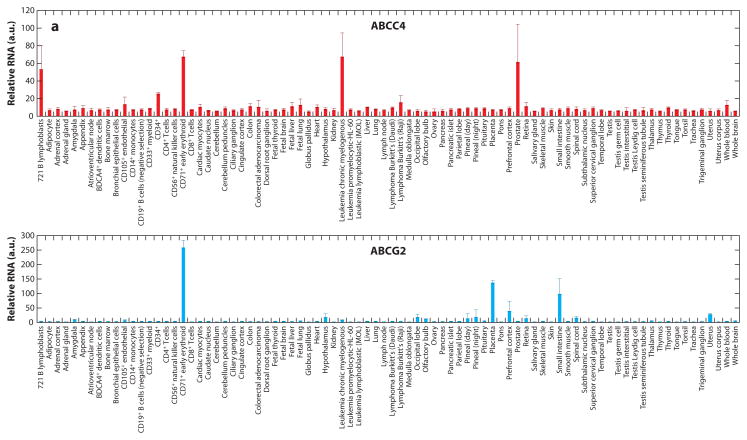
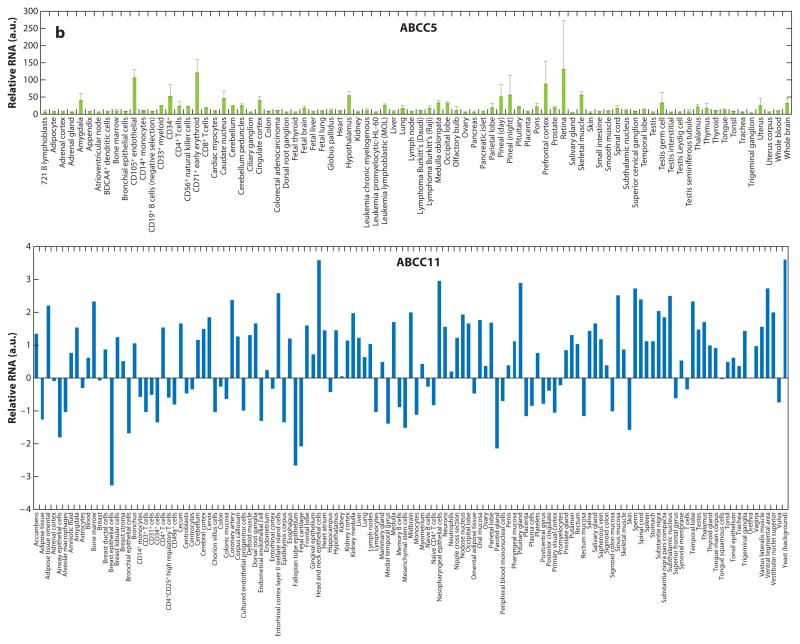
The ABC transporters ABCC4, ABCC5, ABCC11, and ABCG2 are widely expressed in human tissues (Figure 5). ABCC5 is highly expressed in multiple areas in the brain. ABCG2 is highly expressed in the intestine and placenta, unlike ABCC4 and ABCC5, which are expressed at low levels. One could infer from these findings that regulation of cyclic nucleotides by ABC transporters in certain tissues depends on both the amount of transporter and the number of different types of ABC transporters present. Perhaps future studies on tissues from knockout animals will reveal these roles.
Figure 5.
Tissue expression of ATP-binding cassette (ABC) transporters that export cyclic nucleotides. Each cyclic nucleotide–transporting ABC transporter has a distinct tissue expression pattern. The overlap in tissue distribution suggests that some tissues may have redundant backup cyclic nucleotide export routes. In contrast, ABCC11 is broadly expressed and may impact multiple tissues. Data from http://www.biogps.org. Abbreviation: a.u., arbitrary units. Figure continues on next page.
INTERACTIONS BETWEEN PHOSPHODIESTERASES AND MRP4
Prior to the identification of MRP4 as a transporter that can efflux intracellular monophosphory-lated nucleotides, some cell types were noted to export a substantial portion of their intracellular cAMP, whereas others exported much less and appeared to rely on PDEs to modulate intracellular cAMP concentrations (82). In certain cell culture systems, such as fibroblasts (83) and smooth muscle cells (84), inhibiting cAMP efflux by a nonspecific organic anion inhibitor (probenecid) did not elevate intracellular cAMP levels compared with untreated cells. From these studies, it was concluded that PDEs have a major role in regulating intracellular cyclic nucleotide concentrations. In contrast, in avian and mammalian erythrocytes, cyclic nucleotide export was the predominant mechanism regulating intracellular cyclic nucleotide concentrations (40, 85). Furthermore, in mammalian reticulocytes, β-adrenergic receptor activation was associated with cAMP export. Export could be inhibited by ATP-depleting agents (iodoacetate and dinitrophenol) as well as by probenecid and prostaglandin A1 (40). Moreover, this cyclic nucleotide egress occurred even after cessation of adenylyl cyclase stimulation and was dependent only on the availability of intracellular cAMP.
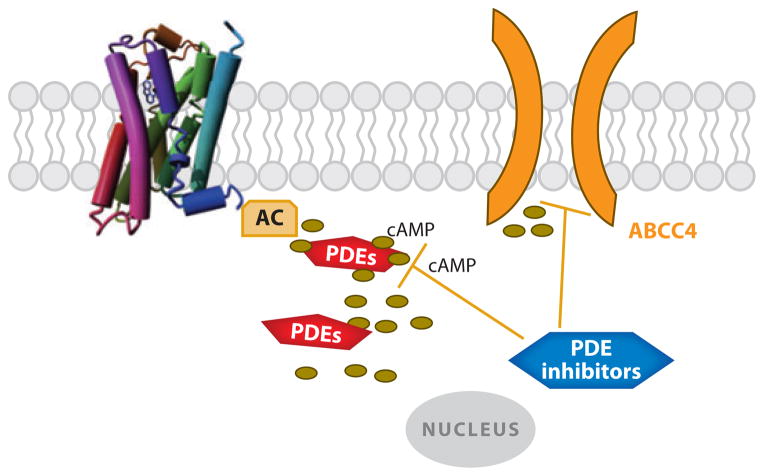
Some PDE inhibitors are effective inhibitors of MRP4 and MRP5 transport (86) (Table 2). This raises the possibility that the therapeutic benefit and/or untoward side effects might be attributable to MRP4 inhibition as well as PDE inhibition. Jedlitschky et al. (74), using membrane vesicles, found that PDE inhibitors potently inhibit MRP5 transport function. A subsequent study found that the PDE inhibitors trequinsin, zaprinast, and sildenafil inhibit transport by MRP4 (87). Among the PDEs that inhibit MRP4, trequinsin was reported to be the most potent inhibitor (87). The concentrations producing 50% inhibition of MRP4 by these PDE inhibitors are in the low micromolar range (Table 2), which, for some of these compounds, is comparable with the concentration required for PDE inhibition. Interestingly, an estimate of the typical therapeutic dose for the PDE inhibitor sildenafil (~3 μM) suggests a disconnect between the dose required to inhibit its target PDE, PDE5, in vitro (3–350 nM) and therapeutic efficacy. Thus, it is possible that MRP4 inhibition in vivo accounts for some of its therapeutic effect. Furthermore, our recent studies show that low concentrations of cilostazol increase cAMP concentrations close to the plasma membrane (34). This is consistent with our findings demonstrating that this compound is a potent Mrp4 inhibitor (S. Cheepala & J.D. Schuetz, unpublished data). Reciprocally, a recent study reported that treatment with sildenafil led to a progressive increase in MRP4 expression in cultured smooth muscle cells (88). This suggests that MRP4 overexpression could compensate for PDE inhibition. In cardiomyocytes, MRP4 deficiency is associated with changes in the expression profile of PDEs. PDE3A and PDE4A expression and activities were increased, perhaps as a way to compensate for the loss of cAMP extrusion (89). Counteracting PDEs may thus blunt the impact of MRP4 deficiency, and the reverse is likely true even if it has not been formerly established. With aging, the overactivity of PDEs progressively decreases, and MRP4-deficient mice display enhanced cardiac myocyte cAMP formation and an enhanced effect on cardiac contractility and remodeling (89). Clearly, more thorough investigations of PDE inhibitors, their potential to inhibit MRP4, and MRP4’s potential to blunt their effect are needed to assess whether MRP4 inhibition accounts for some of the therapeutic effect of PDE inhibitors (Figure 6).
Table 2.
Phosphodiesterase inhibitors that inhibit MRP4/ABCC4
| Inhibitor | PDE | IC50 | Reference | MRP4 IC50 | Estimated human concentrationb |
|---|---|---|---|---|---|
| Cilostazol | 13.5 μM | ||||
| PDE2 | 0.2 μM | 32 | |||
| PDE3 | 45.2 μM | ||||
| Dipyridamolea | 105 | 2 μM | 2.9 μM | ||
| PDE5 | 0.9 μM | 94 | |||
| PDE6 | 0.38 μM | ||||
| PDE7 | 9 μM | ||||
| PDE8 | 4.5 μM | ||||
| PDE10 | 1 μM | 95 | |||
| PDE11 | 0.37 μM | ||||
| Sildenafila | 105 | 20 μM | 2.6 μM | ||
| PDE1 | 280 nM | 96 | |||
| PDE5 | 3.5 nM | ||||
| PDE6 | 37 nM | ||||
| Tadalafil | |||||
| PDE5 | 6.7 nM | 96 | |||
| PDE11 | 37 nM | ||||
| Trequinsina | 105 | 10 μM | |||
| PDE2 | ~1 μM | 97 | |||
| PDE3 | 0.3 nM | 98 | |||
| PDE4 | 230–790 nM | 99 | |||
| Vardenafil | |||||
| PDE1 | 180 nM | 100 | |||
| PDE2 | >1,000 nM | ||||
| PDE3 | >1,000 nM | ||||
| PDE4 | >1,000 nM | ||||
| PDE5 | 0.7 nM | ||||
| PDE6 | 11 nM | ||||
| Zaprinasta | 105 | 250 μM | |||
| PDE1 | 6 μM | 101 | |||
| PDE5 | 0.76 μM | 102 | |||
| PDE6 | 0.15 μM | ||||
| PDE9 | 35 μM | 103 | |||
| PDE10 | 11–22 μM | 95 | |||
| PDE11 | 12 μM | ||||
| Zardaverine | |||||
| PDE3 | 0.58 μM | 104 | |||
| PDE4 | 0.17 μM |
From Reference 105.
Formula for calculation of human dosage concentration:
Based on assumption for average 75-kg person.
Abbreviations: ABC, ATP-binding cassette; PDE, phosphodiesterase.
Figure 6.
Phosphodiesterase (PDE) inhibitors may dually inhibit ABCC4 and PDEs. Abbreviations: AC, adenylate cyclase; cAMP, cyclic adenosine monophosphate; PDE, phosphodiesterase.
TRANSPORTERS AS REGULATORS IN DISEASE
Cardiovascular System
Cyclic nucleotides are key determinants of numerous cardiovascular functions. In the vascular system, cAMP causes vasorelaxation, inhibits smooth muscle cell proliferation, and regulates en-dothelial permeability. The underlying mechanisms involve activation of cAMP-dependent PKA and phosphorylation of PKA substrate proteins. cAMP can also act in a PKA-independent manner through its direct binding to Epac (see above). In contrast, soluble guanylyl cyclase activation in response to nitric oxide (NO) stimulation increases the cGMP levels and causes vasodilation and inhibition of smooth muscle cell proliferation. Determinants of the NO/cGMP pathway remain incompletely described, but the antiproliferative effect of cGMP may involve inhibition of key targets through PKA and/or protein kinase G activation (90). One potential mechanism accounting for this could be increased cAMP levels, which produce inhibition of cAMP-hydrolyzing PDE3, as shown in proliferating vascular smooth muscle cells.
Reagents that can augment cyclic nucleotide levels represent good candidates to limit vascular remodeling and promote vasodilation. Targeting cyclic nucleotide degradation by inhibition of PDEs has been a therapeutic goal for many years. Members of the PDE family have specificity for cAMP or cGMP, but also differential tissue expression. In the vascular system, PDE5 represents the major metabolic pathway for cGMP, whereas PDE3/4 catalyzes cAMP degradation. PDE5 inhibitors are used in patients affected with vasculoproliferative disorders such as pulmonary hypertension.
Recent evidence has highlighted the role of transporters in regulating the levels of cyclic nucleotides in the vascular system. Two recently published studies reported that MRP4 acts as an endogenous regulator of intracellular cyclic nucleotide levels and as a mediator of related signaling pathways in vascular smooth muscle cells (72, 89). MRP4 knockdown, achieved by RNA interference (RNAi), in human arterial smooth muscle cells significantly increased cAMP and cGMP intracellular levels while concomitantly decreasing extracellular levels of the cyclic nucleotides. However, the maximal increase was observed by inhibition of both PDE and MRP4, suggesting that the intracellular cyclic nucleotide content is determined by two independent mechanisms, efflux by MRP4 and catabolism by PDEs.
Vascular remodeling under pathological conditions is influenced by MRP4 function. MRP4 is highly expressed in proliferating smooth muscle cells, but expression is low in quiescent vascular cells (72, 89, 91); this result suggests that MRP4 may play a minor physiological role in the normal vascular system. However, MRP4 expression and the contribution of MRP4 to function may increase under pathological conditions because MRP4 silencing significantly inhibits proliferation of cultured smooth muscle cells (72). In vivo, MRP4 silencing achieved by RNAi-based gene transfer has the potential to significantly reduce the formation of the pathological proliferative layer (i.e., the neointima) after injury of rat carotids (72). Interestingly, these beneficial effects were linked mainly to the activation of PKA, which amplifies the phosphorylation of CREB (cAMP-responsive element binding protein), a classical repressor of smooth muscle cell proliferation (91).
Recent studies on mice and in patient samples suggest that inhibiting MRP4 could help in the treatment of pulmonary arterial hypertension (PAH), a vasculoproliferative and vasospastic disorder involving abnormalities in the homeostasis of cyclic nucleotides and smooth muscle cell proliferation. In a hypoxia-induced mouse model of PAH, MRP4-deficient mice, unlike wild-type mice, did not develop disease, and a small-molecule MRP4 inhibitor reversed development of PAH (88).
MRP4 in Secretory Diarrhea
CFTR is expressed at the apical surfaces of secretory epithelial cells that line the lumen of the gut, where it forms a macromolecular complex with MRP4 that is mediated by PDZK1 (see ABCC4/MRP4 as a Regulator of cAMP, above). Exposure of the gut lumen to toxins secreted by colonizing pathogenic microorganisms (e.g., Escherichia coli, Vibrio cholerae) elicits excessive production of cAMP and/or cGMP. This production leads to the hyperactivation of the CFTR channel with concurrent inhibition of fluid absorption, a process mediated by Na+/H+ exchangers (e.g., NHE3) and the epithelial sodium channel. This dysregulation in ionic balance causes secretory diarrhea. As described above, MRP4 functions as a cAMP and/or cGMP efflux transporter to ensure compartmentalized regulation of cAMP and/or cGMP levels. This function can be accomplished by MRP4 localization in plasma membrane microdomains, suggesting that MRP4 plays a regulatory role in the pathogenic process of enterotoxin-induced secretory diarrhea. Indeed, in vivo studies have shown that inhibition of MRP4 potentiates cholera toxin–induced and CFTR-dependent fluid secretion; MRP4-deficient mice are more prone to CFTR-mediated secretory diarrhea (71). The findings have therapeutic implications for the development of pharmacologic approaches to treat disorders such as secretory diarrhea, irritable bowel syndrome, and perhaps inflammatory bowel disease.
CONCLUSIONS AND IMPLICATIONS
The general view has been that PDEs provide the sole means of modulating cAMP in cells (92, 93). However, as we discuss here, ABC transporters provide an additional means of regulating cAMP levels. These transporters can form macromolecular complexes, but whether the formation of the complex is dictated by the stimulus is unknown. Also unknown is the extent to which the overlap in activity between inhibitors of PDEs and ABC transporters provides therapeutic benefit or untoward side effects. Such issues, in addition to efforts to sort out the roles of certain less characterized ABC transporters, are challenges for the future.
SUMMARY POINTS.
PDEs can regulate cyclic nucleotide concentrations at the plasma membrane as well as in the cytosol.
Membrane cyclic nucleotide concentrations can be regulated by PDEs and ABC transporters.
Some PDE inhibitors also inhibit ABC transporters.
Multiple ABC transporters can transport cyclic nucleotides.
ABC transporters form multiprotein regulatory complexes.
ABC transporters modulate PKA signaling.
FUTURE ISSUES.
Do drugs that inhibit ABC transporters contribute to their thereapuetic efficacy (e.g., antiplatelet drugs)?
Does the composition of the multiprotein complex have an impact on ABC-transporter activity?
What is the interplay between ABC transporters in regulating cyclic nucleotides?
How do PDEs and ABC transporters coordinately regulate membrane and cytosolic cyclic nucleotide concentrations?
Acknowledgments
This work was supported by NIH grants 2R01 GM60904, P30 CA21745, and CA21865 and by the American Lebanese Syrian Associated Charities (ALSAC).
Footnotes
DISCLOSURE STATEMENT
J.-S.H. holds a patent for inhibitors of MRP4 for the treatment or the prevention of vascular disorders (US 2010/0278809 A1) and has received funding from Agence Nationale de le Recherche (09JCJC0112) and Fondation de France (2006005606). The other authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- 1.Buxton IL, Brunton LL. Compartments of cyclic AMP and protein kinase in mammalian car-diomyocytes. J Biol Chem. 1983;258:10233–39. [PubMed] [Google Scholar]
- 2.Jurevicius J, Fischmeister R. cAMP compartmentation is responsible for a local activation of cardiac Ca2+ channels by β-adrenergic agonists. Proc Natl Acad Sci USA. 1996;93:295–99. doi: 10.1073/pnas.93.1.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dao KK, Teigen K, Kopperud R, Hodneland E, Schwede F, et al. Epac1 and cAMP-dependent protein kinase holoenzyme have similar cAMP affinity, but their cAMP domains have distinct structural features and cyclic nucleotide recognition. J Biol Chem. 2006;281:21500–11. doi: 10.1074/jbc.M603116200. [DOI] [PubMed] [Google Scholar]
- 4.Tasken K, Aandahl EM. Localized effects of cAMP mediated by distinct routes of protein kinase A. Physiol Rev. 2004;84:137–67. doi: 10.1152/physrev.00021.2003. [DOI] [PubMed] [Google Scholar]
- 5.Rich TC, Fagan KA, Nakata H, Schaack J, Cooper DM, Karpen JW. Cyclic nucleotide–gated channels colocalize with adenylyl cyclase in regions of restricted cAMP diffusion. J Gen Physiol. 2000;116:147–61. doi: 10.1085/jgp.116.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rich TC, Fagan KA, Tse TE, Schaack J, Cooper DM, Karpen JW. A uniform extracellular stimulus triggers distinct cAMP signals in different compartments of a simple cell. Proc Natl Acad Sci USA. 2001;98:13049–54. doi: 10.1073/pnas.221381398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zaccolo M, Pozzan T. Discrete microdomains with high concentration of cAMP in stimulated rat neonatal cardiac myocytes. Science. 2002;295:1711–15. doi: 10.1126/science.1069982. [DOI] [PubMed] [Google Scholar]
- 8.Perry SJ, Baillie GS, Kohout TA, McPhee I, Magiera MM, et al. Targeting of cyclic AMP degradation to β2-adrenergic receptors by β-arrestins. Science. 2002;298:834–36. doi: 10.1126/science.1074683. [DOI] [PubMed] [Google Scholar]
- 9.Omori K, Kotera J. Overview of PDEs and their regulation. Circ Res. 2007;100:309–27. doi: 10.1161/01.RES.0000256354.95791.f1. [DOI] [PubMed] [Google Scholar]
- 10.Kleppisch T. Phosphodiesterases in the central nervous system. Handb Exp Pharmacol. 2009;191:71–92. doi: 10.1007/978-3-540-68964-5_5. [DOI] [PubMed] [Google Scholar]
- 11.Brechler V, Pavoine C, Hanf R, Garbarz E, Fischmeister R, Pecker F. Inhibition by glucagon of the cGMP-inhibited low-Km cAMP phosphodiesterase in heart is mediated by a pertussis toxin-sensitive G-protein. J Biol Chem. 1992;267:15496–501. [PubMed] [Google Scholar]
- 12.Mery PF, Brechler V, Pavoine C, Pecker F, Fischmeister R. Glucagon stimulates the cardiac Ca2+ current by activation of adenylyl cyclase and inhibition of phosphodiesterase. Nature. 1990;345:158–61. doi: 10.1038/345158a0. [DOI] [PubMed] [Google Scholar]
- 13.Leroy J, Abi-Gerges A, Nikolaev VO, Richter W, Lechene P, et al. Spatiotemporal dynamics of β-adrenergic cAMP signals and L-type Ca2+ channel regulation in adult rat ventricular myocytes: role of phosphodiesterases. Circ Res. 2008;102:1091–100. doi: 10.1161/CIRCRESAHA.107.167817. [DOI] [PubMed] [Google Scholar]
- 14.Nikolaev VO, Bunemann M, Schmitteckert E, Lohse MJ, Engelhardt S. Cyclic AMP imaging in adult cardiac myocytes reveals far-reaching β1-adrenergic but locally confined β2-adrenergic receptor-mediated signaling. Circ Res. 2006;99:1084–91. doi: 10.1161/01.RES.0000250046.69918.d5. [DOI] [PubMed] [Google Scholar]
- 15.Terrin A, Di Benedetto G, Pertegato V, Cheung YF, Baillie G, et al. PGE1 stimulation of HEK293 cells generates multiple contiguous domains with different [cAMP]: role of compartmentalized phosphodiesterases. J Cell Biol. 2006;175:441–51. doi: 10.1083/jcb.200605050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Herget S, Lohse MJ, Nikolaev VO. Real-time monitoring of phosphodiesterase inhibition in intact cells. Cell Signal. 2008;20:1423–31. doi: 10.1016/j.cellsig.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 17.Francis SH, Houslay MD, Conti M. Phosphodiesterase inhibitors: factors that influence potency, selectivity, and action. Handb Exp Pharmacol. 2011;204:47–84. doi: 10.1007/978-3-642-17969-3_2. [DOI] [PubMed] [Google Scholar]
- 18.Shakur Y, Takeda K, Kenan Y, Yu ZX, Rena G, et al. Membrane localization of cyclic nucleotide phosphodiesterase 3 (PDE3): Two N-terminal domains are required for the efficient targeting to, and association of, PDE3 with endoplasmic reticulum. J Biol Chem. 2000;275:38749–61. doi: 10.1074/jbc.M001734200. [DOI] [PubMed] [Google Scholar]
- 19.Bolger GB, McCahill A, Yarwood SJ, Steele MR, Warwicker J, Houslay MD. Delineation of RAID1, the RACK1 interaction domain located within the unique N-terminal region of the cAMP-specific phosphodiesterase, PDE4D5. BMC Biochem. 2002;3:24. doi: 10.1186/1471-2091-3-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Verde I, Pahlke G, Salanova M, Zhang G, Wang S, et al. Myomegalin is a novel protein of the Golgi/centrosome that interacts with a cyclic nucleotide phosphodiesterase. J Biol Chem. 2001;276:11189–98. doi: 10.1074/jbc.M006546200. [DOI] [PubMed] [Google Scholar]
- 21.Osadchii OE. Myocardial phosphodiesterases and regulation of cardiac contractility in health and cardiac disease. Cardiovasc Drugs Ther. 2007;21:171–94. doi: 10.1007/s10557-007-6014-6. [DOI] [PubMed] [Google Scholar]
- 22.McPhee I, Yarwood SJ, Scotland G, Huston E, Beard MB, et al. Association with the SRC family tyrosyl kinase LYN triggers a conformational change in the catalytic region of human cAMP-specific phosphodiesterase HSPDE4A4B: consequences for rolipram inhibition. J Biol Chem. 1999;274:11796–810. doi: 10.1074/jbc.274.17.11796. [DOI] [PubMed] [Google Scholar]
- 23.Huston E, Beard M, McCallum F, Pyne NJ, Vandenabeele P, et al. The cAMP-specific phospho-diesterase PDE4A5 is cleaved downstream of its SH3 interaction domain by caspase-3: consequences for altered intracellular distribution. J Biol Chem. 2000;275:28063–74. doi: 10.1074/jbc.M906144199. [DOI] [PubMed] [Google Scholar]
- 24.Baillie GS, MacKenzie SJ, McPhee I, Houslay MD. Sub-family selective actions in the ability of Erk2 MAP kinase to phosphorylate and regulate the activity of PDE4 cyclic AMP–specific phosphodi-esterases. Br J Pharmacol. 2000;131:811–19. doi: 10.1038/sj.bjp.0703636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baillie GS, Huston E, Scotland G, Hodgkin M, Gall I, et al. TAPAS-1, a novel microdomain within the unique N-terminal region of the PDE4A1 cAMP-specific phosphodiesterase that allows rapid, Ca2+-triggered membrane association with selectivity for interaction with phosphatidic acid. J Biol Chem. 2002;277:28298–309. doi: 10.1074/jbc.M108353200. [DOI] [PubMed] [Google Scholar]
- 26.Lisanti MP, Scherer PE, Vidugiriene J, Tang Z, Hermanowski-Vosatka A, et al. Characterization of caveolin-rich membrane domains isolated from an endothelial-rich source: implications for human disease. J Cell Biol. 1994;126:111–26. doi: 10.1083/jcb.126.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Patel HH, Murray F, Insel PA. Caveolae as organizers of pharmacologically relevant signal trans-duction molecules. Annu Rev Pharmacol Toxicol. 2008;48:359–91. doi: 10.1146/annurev.pharmtox.48.121506.124841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jacobson K, Mouritsen OG, Anderson RG. Lipid rafts: at a crossroad between cell biology and physics. Nat Cell Biol. 2007;9:7–14. doi: 10.1038/ncb0107-7. [DOI] [PubMed] [Google Scholar]
- 29.Nilsson R, Ahmad F, Sward K, Andersson U, Weston M, et al. Plasma membrane cyclic nucleotide phosphodiesterase 3B (PDE3B) is associated with caveolae in primary adipocytes. Cell Signal. 2006;18:1713–21. doi: 10.1016/j.cellsig.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 30.Murray F, Patel HH, Suda RY, Zhang S, Thistlethwaite PA, et al. Expression and activity of cAMP phosphodiesterase isoforms in pulmonary artery smooth muscle cells from patients with pulmonary hypertension: role for PDE1. Am J Physiol Lung Cell Mol Physiol. 2007;292:L294–303. doi: 10.1152/ajplung.00190.2006. [DOI] [PubMed] [Google Scholar]
- 31.Bender AT, Beavo JA. Cyclic nucleotide phosphodiesterases: molecular regulation to clinical use. Pharmacol Rev. 2006;58:488–520. doi: 10.1124/pr.58.3.5. [DOI] [PubMed] [Google Scholar]
- 32.Sudo T, Tachibana K, Toga K, Tochizawa S, Inoue Y, et al. Potent effects of novel anti-platelet aggregatory cilostamide analogues on recombinant cyclic nucleotide phosphodiesterase isozyme activity. Biochem Pharmacol. 2000;59:347–56. doi: 10.1016/s0006-2952(99)00346-9. [DOI] [PubMed] [Google Scholar]
- 33.Maurice DH, Palmer D, Tilley DG, Dunkerley HA, Netherton SJ, et al. Cyclic nucleotide phos-phodiesterase activity, expression, and targeting in cells of the cardiovascular system. Mol Pharmacol. 2003;64:533–46. doi: 10.1124/mol.64.3.533. [DOI] [PubMed] [Google Scholar]
- 34.Penmatsa H, Zhang W, Yarlagadda S, Li C, Conoley VG, et al. Compartmentalized cyclic adenosine 3′,5′-monophosphate at the plasma membrane clusters PDE3A and cystic fibrosis transmembrane conductance regulator into microdomains. Mol Biol Cell. 2010;21:1097–110. doi: 10.1091/mbc.E09-08-0655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baillie GS, Sood A, McPhee I, Gall I, Perry SJ, et al. β-Arrestin-mediated PDE4 cAMP phos-phodiesterase recruitment regulates β-adrenoceptor switching from Gs to Gi. Proc Natl Acad Sci USA. 2003;100:940–45. doi: 10.1073/pnas.262787199. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 36.Baillie GS, Houslay MD. Arrestin times for compartmentalised cAMP signalling and phosphodiesterase-4 enzymes. Curr Opin Cell Biol. 2005;17:129–34. doi: 10.1016/j.ceb.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 37.Davoren PR, Sutherland EW. The effect of L-epinephrine and other agents on the synthesis and release of adenosine 3′,5′-phosphate by whole pigeon erythrocytes. J Biol Chem. 1963;238:3009–15. [PubMed] [Google Scholar]
- 38.Sutherland EW, Rall TW. Fractionation and characterization of a cyclic adenine ribonucleotide formed by tissue particles. J Biol Chem. 1958;232:1077–91. [PubMed] [Google Scholar]
- 39.Barber R, Ray KP, Butcher RW. Turnover of adenosine 3′,5′-monophosphate in WI-38 cultured fibroblasts. Biochemistry. 1980;19:2560–67. doi: 10.1021/bi00553a004. [DOI] [PubMed] [Google Scholar]
- 40.Brunton LL, Buss JE. Export of cyclic AMP by mammalian reticulocytes. J Cycl Nucleotide Res. 1980;6:369–77. [PubMed] [Google Scholar]
- 41.Campbell IL, Taylor KW. The effect of metabolites, papaverine, and probenecid on cyclic AMP efflux from isolated rat Islets of Langerhans. Biochim Biophys Acta. 1981;676:357–64. doi: 10.1016/0304-4165(81)90171-9. [DOI] [PubMed] [Google Scholar]
- 42.Doore BJ, Bashor MM, Spitzer N, Mawe RC, Saier MH., Jr Regulation of adenosine 3′:5′-monophosphate efflux from rat glioma cells in culture. J Biol Chem. 1975;250:4371–72. [PubMed] [Google Scholar]
- 43.Finnegan RB, Carey GB. Characterization of cyclic AMP efflux from swine adipocytes in vitro. Obes Res. 1998;6:292–98. doi: 10.1002/j.1550-8528.1998.tb00352.x. [DOI] [PubMed] [Google Scholar]
- 44.Rindler MJ, Bashor MM, Spitzer N, Saier MH., Jr Regulation of adenosine 3′:5′-monophosphate efflux from animal cells. J Biol Chem. 1978;253:5431–36. [PubMed] [Google Scholar]
- 45.Billiar TR, Curran RD, Harbrecht BG, Stadler J, Williams DL, et al. Association between synthesis and release of cGMP and nitric oxide biosynthesis by hepatocytes. Am J Physiol Cell Physiol. 1992;262:C1077–82. doi: 10.1152/ajpcell.1992.262.4.C1077. [DOI] [PubMed] [Google Scholar]
- 46.Sager G. Cyclic GMP transporters. Neurochem Int. 2004;45:865–73. doi: 10.1016/j.neuint.2004.03.017. [DOI] [PubMed] [Google Scholar]
- 47.Schuetz JD, Connelly MC, Sun D, Paibir SG, Flynn PM, et al. MRP4: a previously unidentified factor in resistance to nucleoside-based antiviral drugs. Nat Med. 1999;5:1048–51. doi: 10.1038/12487. [DOI] [PubMed] [Google Scholar]
- 48.Chen ZS, Lee K, Kruh GD. Transport of cyclic nucleotides and estradiol 17-β-D-glucuronide by multidrug resistance protein 4: resistance to 6-mercaptopurine and 6-thioguanine. J Biol Chem. 2001;276:33747–54. doi: 10.1074/jbc.M104833200. [DOI] [PubMed] [Google Scholar]
- 49.van Aubel RAMH, Smeets PHE, Peters JGP, Bindels RJM, Russel FGM. The MRP4/ABCC4 gene encodes a novel apical organic anion transporter in human kidney proximal tubules: putative efflux pump for urinary cAMP and cGMP. J Am Soc Nephrol. 2002;13:595–603. doi: 10.1681/ASN.V133595. [DOI] [PubMed] [Google Scholar]
- 50.Wielinga PR, van der Heijden I, Reid G, Beijnen JH, Wijnholds J, Borst P. Characterization of the MRP4- and MRP5-mediated transport of cyclic nucleotides from intact cells. J Biol Chem. 2003;278:17664–71. doi: 10.1074/jbc.M212723200. [DOI] [PubMed] [Google Scholar]
- 51.Rall TW, Sutherland EW. Formation of a cyclic adenine ribonucleotide by tissue particles. J Biol Chem. 1958;232:1065–76. [PubMed] [Google Scholar]
- 52.Linton KJ, Holland IB, editors. The ABC Transporters of Human Physiology and Disease: Genetics and Biochemistry of ATP Binding Cassette Transporters. Hackensack, NJ: World Sci; 2011. [Google Scholar]
- 53.Besnard V, Matsuzaki Y, Clark J, Xu Y, Wert SE, et al. Conditional deletion of Abca3 in alveolar type II cells alters surfactant homeostasis in newborn and adult mice. Am J Physiol Lung Cell Mol Physiol. 2010;298:L646–59. doi: 10.1152/ajplung.00409.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Molday RS, Zhong M, Quazi F. The role of the photoreceptor ABC transporter ABCA4 in lipid transport and Stargardt macular degeneration. Biochim Biophys Acta. 2009;1791:573–83. doi: 10.1016/j.bbalip.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kosters A, Karpen SJ. Bile acid transporters in health and disease. Xenobiotica. 2008;38:1043–71. doi: 10.1080/00498250802040584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Borst P, Elferink RO. Mammalian ABC transporters in health and disease. Annu Rev Biochem. 2002;71:537–92. doi: 10.1146/annurev.biochem.71.102301.093055. [DOI] [PubMed] [Google Scholar]
- 57.Gottesman MM, Ambudkar SV. Overview: ABC transporters and human disease. J Bioenerg Biomembr. 2001;33:453–58. doi: 10.1023/a:1012866803188. [DOI] [PubMed] [Google Scholar]
- 58.Schinkel AH, Jonker JW. Mammalian drug efflux transporters of the ATP binding cassette (ABC) family: an overview. Adv Drug Deliv Rev. 2003;55:3–29. doi: 10.1016/s0169-409x(02)00169-2. [DOI] [PubMed] [Google Scholar]
- 59.Oldham ML, Davidson AL, Chen J. Structural insights into ABC transporter mechanism. Curr Opin Struct Biol. 2008;18:726–33. doi: 10.1016/j.sbi.2008.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hohl M, Briand C, Grutter MG, Seeger MA. Crystal structure of a heterodimeric ABC transporter in its inward-facing conformation. Nat Struct Mol Biol. 2012;19:395–402. doi: 10.1038/nsmb.2267. [DOI] [PubMed] [Google Scholar]
- 61.Annilo T, Chen ZQ, Shulenin S, Costantino J, Thomas L, et al. Evolution of the vertebrate ABC gene family: analysis of gene birth and death. Genomics. 2006;88:1–11. doi: 10.1016/j.ygeno.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 62.Dano K. Cross resistance between vinca alkaloids and anthracyclines in Ehrlich ascites tumor in vivo. Cancer Chemother Rep. 1972;56:701–8. [PubMed] [Google Scholar]
- 63.Juliano RL, Ling V. A surface glycoprotein modulating drug permeability in Chinese hamster ovary cell mutants. Biochim Biophys Acta. 1976;455:152–62. doi: 10.1016/0005-2736(76)90160-7. [DOI] [PubMed] [Google Scholar]
- 64.Cole SPC, Bhardwaj G, Gerlach JH, Mackie JE, Grant CE, et al. Overexpression of a transporter gene in a multidrug-resistant human lung cancer cell line. Science. 1992;258:1650–54. doi: 10.1126/science.1360704. [DOI] [PubMed] [Google Scholar]
- 65.Ernst R, Kueppers P, Stindt J, Kuchler K, Schmitt L. Multidrug efflux pumps: substrate selection in ATP-binding cassette multidrug efflux pumps—first come, first served? FEBS J. 2010;277:540–49. doi: 10.1111/j.1742-4658.2009.07485.x. [DOI] [PubMed] [Google Scholar]
- 66.Lee K, Klein-Szanto AJ, Kruh GD. Analysis of the MRP4 drug resistance profile in transfected NIH3T3 cells. J Natl Cancer Inst. 2000;92:1934–40. doi: 10.1093/jnci/92.23.1934. [DOI] [PubMed] [Google Scholar]
- 67.Leggas M, Adachi M, Scheffer GL, Sun D, Wielinga P, et al. Mrp4 confers resistance to topotecan and protects the brain from chemotherapy. Mol Cell Biol. 2004;24:7612–21. doi: 10.1128/MCB.24.17.7612-7621.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Morgan JA, Cheepala SB, Wang Y, Neale G, Adachi M, et al. Deregulated hepatic metabolism exacerbates impaired testosterone production in Mrp4-deficient mice. J Biol Chem. 2012;287:14456–66. doi: 10.1074/jbc.M111.319681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hoque MT, Cole SPC. Down-regulation of Na+/H+ exchanger regulatory factor 1 increases expression and function of multidrug resistance protein 4. Cancer Res. 2008;68:4802–9. doi: 10.1158/0008-5472.CAN-07-6778. [DOI] [PubMed] [Google Scholar]
- 70.Hoque MT, Conseil G, Cole SPC. Involvement of NHERF1 in apical membrane localization of MRP4 in polarized kidney cells. Biochem Biophys Res Commun. 2009;379:60–64. doi: 10.1016/j.bbrc.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 71.Li C, Krishnamurthy PC, Penmatsa H, Marrs KL, Wang XQ, et al. Spatiotemporal coupling of cAMP transporter to CFTR chloride channel function in the gut epithelia. Cell. 2007;131:940–51. doi: 10.1016/j.cell.2007.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sassi Y, Lipskaia L, Vandecasteele G, Nikolaev VO, Hatem SN, et al. Multidrug resistance-associated protein 4 regulates cAMP-dependent signaling pathways and controls human and rat SMC proliferation. J Clin Investig. 2008;118:2747–57. doi: 10.1172/JCI35067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sellers ZM, Naren AP, Xiang Y, Best PM. MRP4 and CFTR in the regulation of cAMP and β-adrenergic contraction in cardiac myocytes. Eur J Pharmacol. 2012;681:80–87. doi: 10.1016/j.ejphar.2012.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jedlitschky G, Burchell B, Keppler D. The multidrug resistance protein 5 functions as an ATP-dependent export pump for cyclic nucleotides. J Biol Chem. 2000;275:30069–74. doi: 10.1074/jbc.M005463200. [DOI] [PubMed] [Google Scholar]
- 75.Shipp LE, Hamdoun A. ATP-binding cassette (ABC) transporter expression and localization in sea urchin development. Dev Dyn. 2012;241:1111–24. doi: 10.1002/dvdy.23786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Krishnamurthy P, Schuetz JD. Role of ABCG2/BCRP in biology and medicine. Annu Rev Pharmacol Toxicol. 2006;46:381–410. doi: 10.1146/annurev.pharmtox.46.120604.141238. [DOI] [PubMed] [Google Scholar]
- 77.Zhou S, Schuetz JD, Bunting KD, Colapietro AM, Sampath J, et al. The ABC transporter Bcrp1/ABCG2 is expressed in a wide variety of stem cells and is a molecular determinant of the side-population phenotype. Nat Med. 2001;7:1028–34. doi: 10.1038/nm0901-1028. [DOI] [PubMed] [Google Scholar]
- 78.Zahir T, Chen YF, MacDonald JF, Leipzig N, Tator CH, Shoichet MS. Neural stem/progenitor cells differentiate in vitro to neurons by the combined action of dibutyryl cAMP and interferon-γ. Stem Cells Dev. 2009;18:1423–32. doi: 10.1089/scd.2008.0412. [DOI] [PubMed] [Google Scholar]
- 79.Asai J, Takenaka H, Katoh N, Kishimoto S. Dibutyryl cAMP influences endothelial progenitor cell recruitment during wound neovascularization. J Investig Dermatol. 2006;126:1159–67. doi: 10.1038/sj.jid.5700188. [DOI] [PubMed] [Google Scholar]
- 80.de Wolf CJ, Yamaguchi H, van der Heijden I, Wielinga PR, Hundscheid SL, et al. cGMP transport by vesicles from human and mouse erythrocytes. FEBS J. 2007;274:439–50. doi: 10.1111/j.1742-4658.2006.05591.x. [DOI] [PubMed] [Google Scholar]
- 81.Takenaka K, Morgan JA, Scheffer GL, Adachi M, Stewart CF, et al. Substrate overlap between Mrp4 and Abcg2/Bcrp affects purine analogue drug cytotoxicity and tissue distribution. Cancer Res. 2007;67:6965–72. doi: 10.1158/0008-5472.CAN-06-4720. [DOI] [PubMed] [Google Scholar]
- 82.Ahlstrom M, Lamberg-Allardt C. Regulation of adenosine 3′,5′-cyclic monophosphate (cAMP) accumulation in UMR-106 osteoblast-like cells: role of cAMP-phosphodiesterase and cAMP efflux. Biochem Pharmacol. 1999;58:1335–40. doi: 10.1016/s0006-2952(99)00199-9. [DOI] [PubMed] [Google Scholar]
- 83.Barber R, Butcher RW. The quantitative relationship between intracellular concentration and egress of cyclic AMP from cultured cells. Mol Pharmacol. 1981;19:38–43. [PubMed] [Google Scholar]
- 84.Fehr TF, Dickinson ES, Goldman SJ, Slakey LL. Cyclic AMP efflux is regulated by occupancy of the adenosine receptor in pig aortic smooth muscle cells. J Biol Chem. 1990;265:10974–80. [PubMed] [Google Scholar]
- 85.Brunton LL, Mayer SE. Extrusion of cyclic AMP from pigeon erythrocytes. J Biol Chem. 1979;254:9714–20. [PubMed] [Google Scholar]
- 86.Russel FGM, Koenderink JB, Masereeuw R. Multidrug resistance protein 4 (MRP4/ABCC4): a versatile efflux transporter for drugs and signalling molecules. Trends Pharmacol Sci. 2008;29:200–7. doi: 10.1016/j.tips.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 87.Chen ZS, Lee K, Walther S, Raftogianis RB, Kuwano M, et al. Analysis of methotrexate and folate transport by multidrug resistance protein 4 (ABCC4): MRP4 is a component of the methotrexate efflux system. Cancer Res. 2002;62:3144–50. [PubMed] [Google Scholar]
- 88.Hara Y, Sassi Y, Guibert C, Gambaryan N, Dorfmüller P, et al. Inhibition of MRP4 prevents and reverses pulmonary hypertension in mice. J Clin Investig. 2011;121:2888–97. doi: 10.1172/JCI45023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sassi Y, Abi-Gerges A, Fauconnier J, Mougenot N, Reiken S, et al. Regulation of cAMP homeostasis by the efflux protein MRP4 in cardiac myocytes. FASEB J. 2012;26:1009–17. doi: 10.1096/fj.11-194027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cornwell TL, Arnold E, Boerth NJ, Lincoln TM. Inhibition of smooth muscle cell growth by nitric oxide and activation of cAMP-dependent protein kinase by cGMP. Am J Physiol Cell Physiol. 1994;267:C1405–13. doi: 10.1152/ajpcell.1994.267.5.C1405. [DOI] [PubMed] [Google Scholar]
- 91.Sassi Y, Hara Y, Lompre AM, Hulot JS. Multi-drug resistance protein 4 (MRP4/ABCC4) and cyclic nucleotides signaling pathways. Cell Cycle. 2009;8:962–63. [PubMed] [Google Scholar]
- 92.Cooper DM. Compartmentalization of adenylate cyclase and cAMP signalling. Biochem Soc Trans. 2005;33:1319–22. doi: 10.1042/BST0331319. [DOI] [PubMed] [Google Scholar]
- 93.Jackson EK, Raghvendra DK. The extracellular cyclic AMP-adenosine pathway in renal physiology. Annu Rev Physiol. 2004;66:571–99. doi: 10.1146/annurev.physiol.66.032102.111604. [DOI] [PubMed] [Google Scholar]
- 94.Hetman JM, Soderling SH, Glavas NA, Beavo JA. Cloning and characterization of PDE7B, a cAMP-specific phosphodiesterase. Proc Natl Acad Sci USA. 2000;97:472–76. doi: 10.1073/pnas.97.1.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lugnier C. Cyclic nucleotide phosphodiesterase (PDE) superfamily: a new target for the development of specific therapeutic agents. Pharmacol Ther. 2006;109:366–98. doi: 10.1016/j.pharmthera.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 96.Gresser U, Gleiter CH. Erectile dysfunction: comparison of efficacy and side effects of the PDE-5 inhibitors sildenafil, vardenafil and tadalafil—review of the literature. Eur J Med Res. 2002;7:435–46. [PubMed] [Google Scholar]
- 97.Ashikaga T, Strada SJ, Thompson WJ. Altered expression of cyclic nucleotide phosphodiesterase isozymes during culture of aortic endothelial cells. Biochem Pharmacol. 1997;54:1071–79. doi: 10.1016/s0006-2952(97)00287-6. [DOI] [PubMed] [Google Scholar]
- 98.Qiu Y, Kraft P, Lombardi E, Clancy J. Rabbit corpus cavernosum smooth muscle shows a different phosphodiesterase profile than human corpus cavernosum. J Urol. 2000;164:882–86. doi: 10.1097/00005392-200009010-00066. [DOI] [PubMed] [Google Scholar]
- 99.Liu S, Veilleux A, Zhang L, Young A, Kwok E, et al. Dynamic activation of cystic fibrosis trans-membrane conductance regulator by type 3 and type 4D phosphodiesterase inhibitors. J Pharmacol Exp Ther. 2005;314:846–54. doi: 10.1124/jpet.105.083519. [DOI] [PubMed] [Google Scholar]
- 100.Saenz de Tejada I, Angulo J, Cuevas P, Fernandez A, Moncada I, et al. The phosphodiesterase inhibitory selectivity and the in vitro and in vivo potency of the new PDE5 inhibitor vardenafil. Int J Impot Res. 2001;13:282–90. doi: 10.1038/sj.ijir.3900726. [DOI] [PubMed] [Google Scholar]
- 101.Francis SH, Sekhar KR, Ke H, Corbin JD. Inhibition of cyclic nucleotide phosphodiesterases by methylxanthines and related compounds. Handb Exp Pharmacol. 2011;200:93–133. doi: 10.1007/978-3-642-13443-2_4. [DOI] [PubMed] [Google Scholar]
- 102.Gibson A. Phosphodiesterase 5 inhibitors and nitrergic transmission—from zaprinast to sildenafil. Eur J Pharmacol. 2001;411:1–10. doi: 10.1016/s0014-2999(00)00824-4. [DOI] [PubMed] [Google Scholar]
- 103.Fisher DA, Smith JF, Pillar JS, St Denis SH, Cheng JB. Isolation and characterization of PDE9A, a novel human cGMP-specific phosphodiesterase. J Biol Chem. 1998;273:15559–64. doi: 10.1074/jbc.273.25.15559. [DOI] [PubMed] [Google Scholar]
- 104.Schudt C, Winder S, Eltze M, Kilian U, Beume R. Zardaverine: a cyclic AMP specific PDE III/IV inhibitor. Agents Actions Suppl. 1991;34:379–402. [PubMed] [Google Scholar]
- 105.Reid G, Wielinga P, Zelcer N, De Haas M, Van Deemter L, et al. Characterization of the transport of nucleoside analog drugs by the human multidrug resistance proteins MRP4 and MRP5. Mol Pharmacol. 2003;63:1094–103. doi: 10.1124/mol.63.5.1094. [DOI] [PubMed] [Google Scholar]
- 106.Chen Z-S, Guo Y, Belinsky M, Kotova E, Kruh G. Transport of bile acids, sulfated steroids, estradiol 17-β-D-glucuronide and leukotriene C4 by human multidrug resistnace protein 8 (ABCC11) Mol Pharmacol. 2005;67:545–57. doi: 10.1124/mol.104.007138. [DOI] [PubMed] [Google Scholar]