Abstract
Oncolytic viruses (OVs) selectively replicate in and kill cancer cells, and spread within the tumor, while not harming normal tissue. In addition to this direct oncolytic activity, OVs are also very effective at inducing immune responses to themselves and to the infected tumor cells. OVs encompass a broad diversity of DNA and RNA viruses that are naturally cancer-selective or can be genetically-engineered. OVs provide a diverse platform for immunotherapy; they act as in situ vaccines, and can be armed with immune modulatory transgenes or combined with other immunotherapies. However, the interactions of OVs with the immune system may affect therapeutic outcomes in opposing fashions: negatively by limiting virus replication and/or spread, or positively by inducing antitumor immune responses. Many aspects of the OV-tumor/host interaction are important in delineating the effectiveness of therapy; they include: (i) innate immune responses and the degree of inflammation induced, (ii) types of virus-induced cell death, (iii) inherent tumor physiology, such as infiltrating and resident immune cells, vascularity/hypoxia, lymphatics, and stromal architecture, and (iv) tumor cell phenotype, including alterations in IFN signaling, oncogenic pathways, cell surface immune markers (MHC, co-stimulatory, NK receptors), and the expression of immunosuppressive factors. Recent clinical trials with a variety of OVs, especially those expressing GM-CSF, have demonstrated efficacy and induction of antitumor immune responses in the absence of significant toxicity. Manipulating the balance between anti-virus and antitumor responses, often involving overlapping immune pathways, will be critical to the clinical success of OVs.
Oncolytic Viruses
Oncolytic virus (OV) therapy is based on selective replication of viruses in cancer cells and their subsequent spread within a tumor without causing damage to normal tissue (1, 2). It represents a unique class of cancer therapeutics with distinct mechanisms of action. The activity of OVs is very much a reflection of the underlying biology of the viruses from which they are derived and the host-virus interactions that have evolved in the battle between pathogenesis and immunity. This provides a diverse set of activities that can be harnessed and manipulated. Typically, OVs fall into 2 classes: (i) viruses that naturally replicate preferentially in cancer cells and are non-pathogenic in humans often due to elevated sensitivity to innate antiviral signaling or dependence on oncogenic signaling pathways. These include autonomous parvoviruses, myxoma virus (MYXV; poxvirus), Newcastle disease virus (NDV; paramyxovirus), reovirus, and Seneca valley virus (SVV; picornavirus); and (ii) viruses that are genetically-manipulated for use as vaccine vectors including measles virus (MV; paramyxovirus), poliovirus (PV; picornavirus), and vaccinia virus (VV; poxvirus), and/or those genetically-engineered with mutations/deletions in genes required for replication in normal, but not cancer cells including adenovirus (Ad), herpes simplex virus (HSV), VV, and vesicular stomatitis virus (VSV; rhabdovirus) (1, 3). Genetic engineering has facilitated the rapid expansion of oncolytic viruses in the last 2 decades, enabling a broad range of potentially pathogenic viruses to be manipulated for safety and targeting (3). Many of the ‘Hallmarks of Cancer’ described by Hanahan and Weinberg (4) provide a permissive environment for oncolytic viruses; they include sustained proliferation, resisting cell death, evading growth suppressors, genome instability, DNA damage stress, and avoiding immune destruction. In addition, insertion of foreign sequences can endow further selectivity for cancer cells and safety, as well as alter virus tropism through targeting of translation with IRES’s or miRNAs (PV, VSV), transcription with cell-specific promoter/enhancers (Ad, HSV), or transduction with altered virus receptors (HSV, Ad, MV, VSV) (1, 3). These strategies are also being used to target replication-deficient viral vectors for gene therapy applications in cancer immunotherapy.
OVs have many features that make them advantageous and distinct from current therapeutic modalities: (i) there is a low probability for the generation of resistance (not seen so far), as OVs often target multiple oncogenic pathways and employ multiple means for cytotoxicity; (ii) they replicate in a tumor-selective fashion and are non-pathogenic, only minimal systemic toxicity has been detected; (iii) virus dose in the tumor increases with time due to in situ virus amplification, as opposed to classical drug pharmacokinetics that decreases with time; and (iv) safety features can be built in, such as drug and immune sensitivity. These features should result in a very high therapeutic index. An important issue for OV therapy is delivery. While systemic intravenous administration is simpler than intratumoral injection and can target multiple tumors, it has drawbacks including non-immune human serum containing anti-OV antibodies that pre-exist for human viruses or induce by multiple administrations; lack of extravasation into tumors; and sequestration in the liver (1). Cell carriers (i.e., mesenchymal stromal cells, myeloid-derived suppresser cells (MDSCs), neural stem cells, T cells, cytokine-induced killer cells, or irradiated tumor cells) can shield virus from neutralization and facilitate virus delivery to the tumor (5). The effectiveness will vary depending upon the cell phenotype, permissiveness to virus infection, tumor homing ability, and transfer of infectious virus to tumor cells. To block virus neutralization and extend vascular circulation, viruses can also be coated in nanoparticles (i.e., PEGylation) (1).
OV Immunotherapy
Virus infection and pathogenicity have been major drivers in the evolution of the human immune system, and vaccination against viruses is the quintessential exploitation of adaptive immunity. A major goal of OV-mediated immunotherapy is to activate and redirect functional innate and adaptive immune responses towards the tumor. Interactions between innate and adaptive immune cells and signaling factors (i.e., cytokines, chemokines), often involved in virus infections, play a large role in antitumor immunity or lack thereof, as well as successful immunotherapies (Figure 1). Virus infection induces an inflammatory response leading to adaptive anti-virus immunity. Thus, the immune system was seen initially as a negative factor in OV therapy for limiting virus infection/delivery because of preexisting or therapy-induced immunity, virus replication because of innate antiviral responses, and virus spread because of the infiltration of innate immune cells (6). In addition, most early studies were performed in human xenograft tumor models in immune-deficient mice lacking adaptive immune responses because some viruses were species-selective or replicated better in human cells, and the availability of a broad diversity of human cancer cell lines. With the use of syngeneic tumor models in immunocompetent mice, it became clear that the consequences of the immune system were complex but that the induction of antitumor immunity was feasible and efficacious (6). In particular, many OVs act as in situ vaccines, inducing robust and specific adaptive antitumor responses, often CD8+ T cell-mediated and long lasting (7, 8). Interestingly, adaptive antiviral immunity can enhance antitumor immunity for HSV, but not for VSV (8, 9).
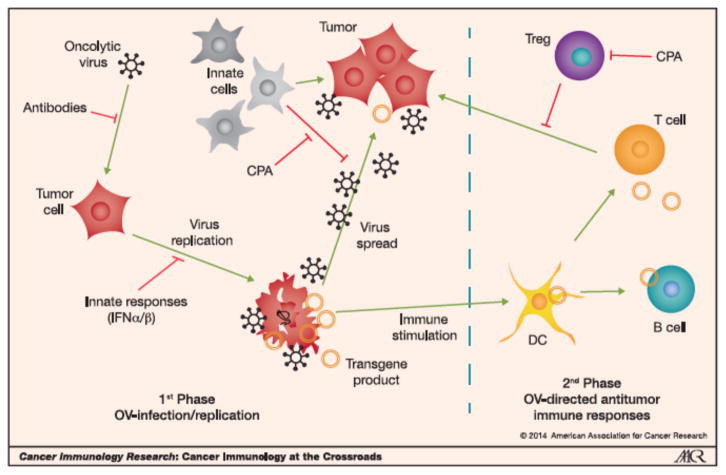
Figure 1. Cartoon of OV-mediated effects in tumor.
1st Phase. OV delivered intratumorally or systemically, infects tumor cells (can be blocked by humoral defense systems; Antibodies). After infection, OV replicates (can be blocked by innate responses; i.e., IFNα/β), kills cells often by immunogenic cell death (ICD), and spreads throughout the tumor (can be blocked by innate immune cells, i.e., NK cells, macrophages), eliciting an inflammatory response. When an armed OV is used, the immunomodulatory transgene is expressed (Transgene product). 2nd Phase. ICD and inflammation recruit dendritic cells (DC) to the tumor where they take-up TAAs and induce an adaptive immune response (T and B cells), which targets the tumor (can be blocked by Tregs and MDSCs). Innate cells such as NK cells also have antitumor activities. Antitumor immune responses can be further enhanced by transgene products.
The inflammatory cascade and immunogenic cell death (ICD) induced by OV infection of tumors makes OVs particularly powerful inducers of antitumor immunity (8, 10). Among the many different types of cell death, some are immunogenic and characterized by the release of danger-associated molecular patterns (DAMPs) such as calreticulin, high-mobility group protein B1 (HMGB1), and ATP, along with tumor-associated antigens (TAAs) (10). Multiple forms of ICD have been observed after OV (Ad, VV, HSV, MV, coxsackievirus) infection of cancer cells, and there is a suggestion that ICD occurs in patients after treatment with oncolytic Ad and TMZ (11). However, much remains to be learned about the mechanisms of OV-mediated cell death and how it can be exploited to enhance immunogenicity. Inflammation, typically chronic, can also promote tumorigenesis and inhibit T cell antitumor activity (12). Restraining anti-viral immune responses and minimizing pathology, while promoting antitumor immune responses is a complex and poorly understood balancing act that will dictate OV therapy outcomes. In some cases, where minimal OV replication occurs in mouse tumors (i.e., HSV) or no replication is required (i.e. reovirus) (13), antitumor efficacy is principally due to OV-induced immune responses. Understanding, harnessing, modulating, and/or enhancing OV-mediated immune responses for effective antitumor immunity is a major focus of current research and one that intersects with other immunotherapeutic strategies.
Many viruses express immune evasion genes that enable them to establish infections and spread within their host (14). Mutations in these genes (i.e., HSV Us11, VV E3L, MYXV M156R, Ad VAI, and reovirus σ2/σ3, inhibitors of PKR; HSV ICP0, VV N2, NDV V, and MV V, inhibitors of IRF3; HSV ICP0, MYXV M13L, MV V, PV 3C and VSV M, inhibitors of NFκB; VV B8R and MYXV MT-7, inhibitors of IFNγ; HSV ICP47 and Ad E3-19K, inhibitors of MHC class I presentation; MV gp, inhibitor of T cells; and MYXV M128L and MV H, inhibitors of CD46) are likely to enhance the induction of immunity and possibly cross-presentation of TAAs. Such mutations should improve the safety of OVs by making them more ‘visible’ to the immune system, as well as increasing antitumor immune responses. Conversely, they may diminish virus replication and spread. An additional problem not as easily addressed is OV infection of immune cells, especially DCs, that interferes with their function (15, 16).
Innate Immunity
Although adaptive immunity appears to provide and, in fact, represent even the major mode of anticancer action for OVs, it is also evident that an initial host response against an administered OV could destroy it along with the infected cells, before the OV has a chance to replicate and induce cytotoxicity of a magnitude that is sufficient to set up an effective vaccination response (17). Location and site of OV administration is an important determinant of the characteristics of these initial host responses against the OV. For instance, intravenous or intra-arterial administration of OVs such as recombinant HSV1 leads to its rapid recognition and elimination by the circulating complement and antibodies of the humoral defense system (18, 19). This also has been shown for VV (20), NDV (21), MV (22), and Ad (23, 24). Intratumoral administration also can lead to complement- and antibody-mediated destruction of the OV. In addition, intracellular and microenvironmental antiviral defense responses in infected tumor cells also can greatly limit the magnitude of OV replication (25–31). Finally, innate immune cells can rapidly respond to an administered OV further limiting its survival and that of OV-infected tumor cells (32–35). In all these models, circumvention of such responses using pharmacologic agents, such as histone deacetylase (HDAC) inhibitors or immunomodulating drugs, or genes that block antiviral defense mechanisms, have led to improved OV replication and tumor cytotoxicity (reviewed in ref. (36)). When pharmacologic agents are utilized, the interference of antiviral responses can be applied in a transient fashion usually right before or at the time of OV administration. This should lead to an initial burst of OV replication leading to tumor cell lysis. As the pharmacologic effects against host innate immunity wane, a large debris field of OVs and tumor antigens could be more promptly recognized by the antiviral host response leading to a secondary long-term vaccination effect responsible for effective tumor immunity (Figure 1). However, quantification of responses to OV therapy is a sorely needed area of investigation. For instance, the number of OV replicative rounds, the tumor cell-OV burst size, the number of OV replicative tumor foci, and the temporal kinetics of innate response suppression that are needed for an efficient lytic and vaccination effect still are undetermined. In fact, current applications of innate immunity modulation with OV administration remain determined in an empirical manner.
Clinical Outcomes
There have been numerous clinical trials of OVs for cancer. As expected, most have been phase I with a few phase II trials. Currently there is a phase III trial of an oncolytic HSV1 expressing GM-CSF for melanoma (talimogene laherparepvec, T-Vec) sponsored by Amgen, Inc. Subjects were randomized to intratumoral injections of the agent T-Vec or of GM-CSF alone. Preliminary reports show a 16% durable response rate for the T-vec arm compared to only 2% for the GM-CSF alone arm, and a 26% overall response rate (vs. only 6% for the GM-CSF alone arm). There were also encouraging trends towards improved overall survival at an interim analysis (http://www.fiercebiotech.com/story/amgen-trumpets-t-vec-oncolytic-virus-results-phiii-melanoma-study/2013-06-01). Although there is hope that the final analysis will show results that will lead to the first US FDA approval of an OV for tumors, detractors already have pointed out that there has been a recent explosion of several new promising pharmacologic agents against melanoma, which may impede approval or, if approved, the market utilization of T-Vec.
Two OV therapy phase II trials have been completed recently. An oncolytic VV that expresses GM-CSF (Pexa-Vec, JX-594) has completed a randomized phase II trial in subjects with hepatocellular carcinoma (HCC), sponsored by Jennerex Inc. (37). Low- or high-dose JX-594 were administered intravascularly into liver tumors. Surprisingly, the authors discovered that response rates and intrahepatic disease control were equivalent in both injected and distant non-injected tumors at either dose, suggesting that an immune response might be responsible for the observed anticancer effects. They also reported that JX-594 anticancer immunity occurred after JX-594 replication and GM-CSF expression. While such radiologic tumor responses were independent of dosage, subject survival duration was related to dosage (median survival of 14.1 months compared to 6.7 months on the high and low dose, respectively; hazard ratio 0.39; P=0.020). While these results appear highly encouraging, some of the data related to immunity was equivocal. For instance, although the authors directly injected only some tumors, viral genomes were detected in the peripheral blood within 15 minutes. This implied that even the non-injected tumors likely were exposed to JX-594, rendering more equivocal the conclusion of an immunologic effect was responsible for the observed response in the non-injected tumors (38). In addition, the authors observed humoral immunity in subject sera raised against HCC cell lines rather than the actual subject tumors. Although they posited that such humoral immunity, based on antibody-mediated complement activation against tumor antigens, is a critical determinant of the JX-594 effects (39), assays of direct T cell-mediated cytotoxicity against autologous HCC cells would enhance the evidence for an immunologic mechanism as a critical determinant for the observed antitumor response.
A phase II trial has completed for an oncolytic reovirus (Reolysin, sponsored by Oncolytic Biotech) intravenous administration in subjects with metastatic melanoma (40). Although the authors report no objective responses in the 21 treated patients, they were able to obtain post-treatment biopsies that were evaluable for 13 patients. In two patients there was immunohistochemical suggestion of productive reoviral replication in the tumors. The authors also noted that neutralizing antibody titers increased in these subjects. They concluded that the lack of a response precluded further progression of the trial to subsequent phases and reasoned that this failure was due to the rising neutralizing antibody titers against reovirus. In fact, they discussed that a trial of Reolysin with cyclophosphamide to reduce serum neutralization of reovirus was needed. In summary, these advanced clinical trials are providing tantalizing pieces of evidence that seem to invoke the critical role of an immunologic mechanism of anticancer action for effective virotherapy. However, there still is a lack of unequivocal demonstration of OV-induced immunologic activity, and the alternative explanation related to direct viral cytotoxicity as a predominant mechanism is still possible. This would be important to establish in the context of clinical trials rather than in preclinical studies. This knowledge would point clinicians towards avenues of even more stimulation of immune responses vs. blocking antiviral immunity, as suggested by the Reolysin trial discussed above.
Enhancing OV Immunotherapy
Many OVs can accommodate gene insertions and thus can be ‘armed’ with therapeutic transgenes, combining local gene delivery with oncolytic activity (41). Local expression in the tumor obviates toxicity arising from systemic administration of potent immune modulators. GM-CSF, based on its effects in cytokine-transduced cancer cell vaccines (i.e, clinically approved Sipuleucel-T), has been incorporated into a number of OVs (HSV T-Vec, VV JX-594, Ad Ad5/3-D24-GMCSF (42), and CG0070 (43)) that have entered clinical trials (8). GM-CSF-expressing OVs demonstrated only moderate activity in preclinical studies (44, 45), while JX-594 was not compared to a VV lacking GM-CSF (46). Other therapeutic transgenes include: IL-2 (NDV, HSV, parvovirus), IL-12 (Ad, HSV), IL-15 (VSV), IL-18 (HSV), IFNα/β (Ad, VSV, VV), soluble CD80 (Ad, HSV), 4-1BB (VV), CD40L (Ad, and no effect with VSV), Flt3L (Ad, HSV), CCL3 (Ad), CCL5 (Ad, VV), and combinations thereof (2). In addition to transgenes that enhance adaptive immune responses, cytokines/chemokines directed at the tumor microenvironment can alter the immune cell balance towards productive therapeutic immunity (Figure 1). IL-12, a potent antitumor cytokine with anti-angiogenic activities, when expressed from oncolytic HSV reduced neovasculature and tumor Tregs, and induced T cell-mediated immunity in an immunocompetent cancer stem cell model (47). Expression of a CXCR4 antagonist from oncolytic VV reduced tumor vasculature and accumulation of bone marrow-derived epithelial and myeloid cells, and induced antitumor humoral responses (48).
Like many cancer vaccine strategies, OVs expressing TAAs can be used to induce tumor-selective adaptive immune responses. The combination of TAA expression in the tumor and OV-mediated cell killing induces enhanced T cell migration and activation compared to OV-infected tumor cells expressing the TAA (49). This can be coupled to a prime (replication-deficient Ad or oncolytic Semliki Forest virus expressing a TAA) - boost (oncolytic VSV or VV expressing the same TAA) vaccine strategy, where the boosted secondary response to the tumor dominates the primary anti-OV response (6, 8). To expand the antigenic repertoire, cDNA libraries from normal tissue (e.g., prostate for prostate tumors) or recurrent tumors have been inserted into VSV, and induced therapeutic immunity (50). Further enhancement was obtained by expressing xenogeneic TAAs (50, 51). The ability of oncolytic VSV expressing TAAs to induce IL-17 in the context of tumor immunity has been exploited to screen tumor cDNA libraries for individual TAAs and optimal TAA combinations, limiting potentially inappropriate responses of whole cell or cDNA vaccines (52). Developing a similar strategy in a human setting would be a major advance.
There are a number of immunomodulatory agents that have been examined to restrain antiviral immune responses and promote OV replication and spread. Cyclophosphamide (CPA) can increase OV replication and inhibit tumor growth by suppressing innate immune cell (34) and antibody responses (53), depleting Tregs, and enhancing the antitumor activity of CTL (8) (Figure 1). A challenge is to identify immune-suppressive strategies that can blunt acute innate cells from blocking virus replication and spread, while permitting sufficient inflammation and cross-priming for robust antitumor immunity. Conversely, it will be of interest to combine OV with chemotherapies that induce immunogenic cell death (e.g. CPA, oxaloplatin, or anthracyclines such as doxorubicin and mitoxantrone), increase tumor cell antigenicity (e.g. gemcitabine, cisplatin, or etoposide), or susceptibility to immune cells (e.g. HDAC inhibitors, paclitaxel, or doxorubicin), or suppress MDSCs (e.g. gemcitabine, paclitaxel) and Tregs (e.g. CPA or sunitinib) (54) in immune competent preclinical models.
References
- 1.Russell SJ, Peng KW, Bell JC. Oncolytic virotherapy. Nat Biotechnol. 2012;30:658–70. doi: 10.1038/nbt.2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Atherton MJ, Lichty BD. Evolution of oncolytic viruses: novel strategies for cancer treatment. Immunotherapy. 2013;5:1191–206. doi: 10.2217/imt.13.123. [DOI] [PubMed] [Google Scholar]
- 3.Cattaneo R, Miest T, Shashkova EV, Barry MA. Reprogrammed viruses as cancer therapeutics: targeted, armed and shielded. Nat Rev Microbiol. 2008;6:529–40. doi: 10.1038/nrmicro1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 5.Nakashima H, Kaur B, Chiocca EA. Directing systemic oncolytic viral delivery to tumors via carrier cells. Cytokine Growth Factor Rev. 2010;21:119–26. doi: 10.1016/j.cytogfr.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Melcher A, Parato K, Rooney CM, Bell JC. Thunder and lightning: immunotherapy and oncolytic viruses collide. Mol Ther. 2011;19:1008–16. doi: 10.1038/mt.2011.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Toda M, Rabkin SD, Kojima H, Martuza RL. Herpes simplex virus as an in situ cancer vaccine for the induction of specific anti-tumor immunity. Hum Gene Ther. 1999;10:385–93. doi: 10.1089/10430349950018832. [DOI] [PubMed] [Google Scholar]
- 8.Bartlett DL, Liu Z, Sathaiah M, Ravindranathan R, Guo Z, He Y, et al. Oncolytic viruses as therapeutic cancer vaccines. Mol Cancer. 2013;12:103. doi: 10.1186/1476-4598-12-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miller CG, Fraser NW. Role of the immune response during neuro-attenuated herpes simplex virus-mediated tumor destruction in a murine intracranial melanoma model. Cancer Res. 2000;60:5714–22. [PubMed] [Google Scholar]
- 10.Inoue H, Tani K. Multimodal immunogenic cancer cell death as a consequence of anticancer cytotoxic treatments. Cell Death Differ. 2013 doi: 10.1038/cdd.2013.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liikanen I, Ahtiainen L, Hirvinen ML, Bramante S, Cerullo V, Nokisalmi P, et al. Oncolytic adenovirus with temozolomide induces autophagy and antitumor immune responses in cancer patients. Mol Ther. 2013;21:1212–23. doi: 10.1038/mt.2013.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elinav E, Nowarski R, Thaiss CA, Hu B, Jin C, Flavell RA. Inflammation-induced cancer: crosstalk between tumours, immune cells and microorganisms. Nat Rev Cancer. 2013;13:759–71. doi: 10.1038/nrc3611. [DOI] [PubMed] [Google Scholar]
- 13.Prestwich RJ, Ilett EJ, Errington F, Diaz RM, Steele LP, Kottke T, et al. Immune-mediated antitumor activity of reovirus is required for therapy and is independent of direct viral oncolysis and replication. Clin Cancer Res. 2009;15:4374–81. doi: 10.1158/1078-0432.CCR-09-0334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Versteeg GA, Garcia-Sastre A. Viral tricks to grid-lock the type I interferon system. Curr Opin Microbiol. 2010;13:508–16. doi: 10.1016/j.mib.2010.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cunningham AL, Donaghy H, Harman AN, Kim M, Turville SG. Manipulation of dendritic cell function by viruses. Curr Opin Microbiol. 2010;13:524–9. doi: 10.1016/j.mib.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 16.Leveille S, Goulet ML, Lichty BD, Hiscott J. Vesicular stomatitis virus oncolytic treatment interferes with tumor-associated dendritic cell functions and abrogates tumor antigen presentation. J Virol. 2011;85:12160–9. doi: 10.1128/JVI.05703-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chiocca EA. The host response to cancer virotherapy. Curr Opin Mol Ther. 2008;10:38–45. [PubMed] [Google Scholar]
- 18.Wakimoto H, Fulci G, Tyminski E, Chiocca EA. Altered expression of antiviral cytokine mRNAs associated with cyclophosphamide’s enhancement of viral oncolysis. Gene Ther. 2004;11:214–23. doi: 10.1038/sj.gt.3302143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kueberuwa G, Cawood R, Seymour LW. Blood compatibility of enveloped viruses. Curr Opin Mol Ther. 2010;12:412–20. [PubMed] [Google Scholar]
- 20.Magge D, Guo ZS, O’Malley ME, Francis L, Ravindranathan R, Bartlett DL. Inhibitors of C5 complement enhance vaccinia virus oncolysis. Cancer Gene Ther. 2013;20:342–50. doi: 10.1038/cgt.2013.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Biswas M, Johnson JB, Kumar SR, Parks GD, Elankumarana S. Incorporation of host complement regulatory proteins into Newcastle disease virus enhances complement evasion. J Virol. 2012;86:12708–16. doi: 10.1128/JVI.00886-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Russell SJ, Peng KW. Measles virus for cancer therapy. Curr Top Microbiol Immunol. 2009;330:213–41. doi: 10.1007/978-3-540-70617-5_11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tomita K, Sakurai F, Tachibana M, Mizuguchi H. Correlation between adenovirus-neutralizing antibody titer and adenovirus vector-mediated transduction efficiency following intratumoral injection. Anticancer Res. 2012;32:1145–52. [PubMed] [Google Scholar]
- 24.Raki M, Sarkioja M, Escutenaire S, Kangasniemi L, Haavisto E, Kanerva A, et al. Switching the fiber knob of oncolytic adenoviruses to avoid neutralizing antibodies in human cancer patients. J Gene Med. 2011;13:253–61. doi: 10.1002/jgm.1565. [DOI] [PubMed] [Google Scholar]
- 25.Ben Yebdri F, Van Grevenynghe J, Tang VA, Goulet ML, Wu JH, Stojdl DF, et al. Triptolide-mediated inhibition of interferon signaling enhances vesicular stomatitis virus-based oncolysis. Mol Ther. 2013;21:2043–53. doi: 10.1038/mt.2013.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alvarez-Breckenridge CA, Yu J, Price R, Wei M, Wang Y, Nowicki MO, et al. The histone deacetylase inhibitor valproic acid lessens NK cell action against oncolytic virus-infected glioblastoma cells by inhibition of STAT5/T-BET signaling and generation of gamma interferon. J Virol. 2012;86:4566–77. doi: 10.1128/JVI.05545-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Otsuki A, Patel A, Kasai K, Suzuki M, Kurozumi K, Chiocca EA, et al. Histone deacetylase inhibitors augment antitumor efficacy of herpes-based oncolytic viruses. Mol Ther. 2008;16:1546–55. doi: 10.1038/mt.2008.155. [DOI] [PubMed] [Google Scholar]
- 28.Liu YP, Suksanpaisan L, Steele MB, Russell SJ, Peng KW. Induction of antiviral genes by the tumor microenvironment confers resistance to virotherapy. Sci Rep. 2013;3:2375. doi: 10.1038/srep02375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Okemoto K, Kasai K, Wagner B, Haseley A, Meisen H, Bolyard C, et al. DNA Demethylating Agents Synergize with Oncolytic HSV1 against Malignant Gliomas. Clin Cancer Res. 2013;19:5952–9. doi: 10.1158/1078-0432.CCR-12-3588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Okemoto K, Wagner B, Meisen H, Haseley A, Kaur B, Chiocca EA. STAT3 activation promotes oncolytic HSV1 replication in glioma cells. PLoS One. 2013;8:e71932. doi: 10.1371/journal.pone.0071932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Berchtold S, Lampe J, Weiland T, Smirnow I, Schleicher S, Handgretinger R, et al. Innate immune defense defines susceptibility of sarcoma cells to measles vaccine virus-based oncolysis. J Virol. 2013;87:3484–501. doi: 10.1128/JVI.02106-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fulci G, Dmitrieva N, Gianni D, Fontana EJ, Pan X, Lu Y, et al. Depletion of peripheral macrophages and brain microglia increases brain tumor titers of oncolytic viruses. Cancer Res. 2007;67:9398–406. doi: 10.1158/0008-5472.CAN-07-1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lamfers ML, Fulci G, Gianni D, Tang Y, Kurozumi K, Kaur B, et al. Cyclophosphamide increases transgene expression mediated by an oncolytic adenovirus in glioma-bearing mice monitored by bioluminescence imaging. Mol Ther. 2006;14:779–88. doi: 10.1016/j.ymthe.2006.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fulci G, Breymann L, Gianni D, Kurozomi K, Rhee SS, Yu J, et al. Cyclophosphamide enhances glioma virotherapy by inhibiting innate immune responses. Proc Natl Acad Sci U S A. 2006;103:12873–8. doi: 10.1073/pnas.0605496103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alvarez-Breckenridge CA, Yu J, Caligiuri MA, Chiocca EA. Uncovering a novel mechanism whereby NK cells interfere with glioblastoma virotherapy. Oncoimmunology. 2013;2:e23658. doi: 10.4161/onci.23658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alvarez-Breckenridge C, Kaur B, Chiocca EA. Pharmacologic and chemical adjuvants in tumor virotherapy. Chem Rev. 2009;109:3125–40. doi: 10.1021/cr900048k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Heo J, Reid T, Ruo L, Breitbach CJ, Rose S, Bloomston M, et al. Randomized dose-finding clinical trial of oncolytic immunotherapeutic vaccinia JX-594 in liver cancer. Nat Med. 2013;19:329–36. doi: 10.1038/nm.3089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tanabe KK, Chiocca EA. “Infectious” optimism for treatment of hepatocellular carcinoma. Mol Ther. 2013;21:722–4. doi: 10.1038/mt.2013.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim MK, Breitbach CJ, Moon A, Heo J, Lee YK, Cho M, et al. Oncolytic and immunotherapeutic vaccinia induces antibody-mediated complement-dependent cancer cell lysis in humans. Sci Transl Med. 2013;5:185ra63. doi: 10.1126/scitranslmed.3005361. [DOI] [PubMed] [Google Scholar]
- 40.Galanis E, Markovic SN, Suman VJ, Nuovo GJ, Vile RG, Kottke TJ, et al. Phase II trial of intravenous administration of Reolysin((R)) (Reovirus Serotype-3-dearing Strain) in patients with metastatic melanoma. Mol Ther. 2012;20:1998–2003. doi: 10.1038/mt.2012.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kaur B, Cripe TP, Chiocca EA. “Buy one get one free”: armed viruses for the treatment of cancer cells and their microenvironment. Curr Gene Ther. 2009;9:341–55. doi: 10.2174/156652309789753329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kanerva A, Nokisalmi P, Diaconu I, Koski A, Cerullo V, Liikanen I, et al. Antiviral and antitumor T-cell immunity in patients treated with GM-CSF-coding oncolytic adenovirus. Clin Cancer Res. 2013;19:2734–44. doi: 10.1158/1078-0432.CCR-12-2546. [DOI] [PubMed] [Google Scholar]
- 43.Burke JM, Lamm DL, Meng MV, Nemunaitis JJ, Stephenson JJ, Arseneau JC, et al. A first in human phase 1 study of CG0070, a GM-CSF expressing oncolytic adenovirus, for the treatment of nonmuscle invasive bladder cancer. J Urol. 2012;188:2391–7. doi: 10.1016/j.juro.2012.07.097. [DOI] [PubMed] [Google Scholar]
- 44.Liu BL, Robinson M, Han ZQ, Branston RH, English C, Reay P, et al. ICP34.5 deleted herpes simplex virus with enhanced oncolytic, immune stimulating, and anti-tumour properties. Gene Ther. 2003;10:292–303. doi: 10.1038/sj.gt.3301885. [DOI] [PubMed] [Google Scholar]
- 45.Varghese S, Rabkin SD, Liu R, Nielsen PG, Ipe T, Martuza RL. Enhanced therapeutic efficacy of IL-12, but not GM-CSF, expressing oncolytic herpes simplex virus for transgenic mouse derived prostate cancers. Cancer Gene Ther. 2006;13:253–65. doi: 10.1038/sj.cgt.7700900. [DOI] [PubMed] [Google Scholar]
- 46.Kim JH, Oh JY, Park BH, Lee DE, Kim JS, Park HE, et al. Systemic armed oncolytic and immunologic therapy for cancer with JX-594, a targeted poxvirus expressing GM-CSF. Mol Ther. 2006;14:361–70. doi: 10.1016/j.ymthe.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 47.Cheema TA, Wakimoto H, Fecci PE, Ning J, Kuroda T, Jeyaretna DS, et al. Multifaceted oncolytic virus therapy for glioblastoma in an immunocompetent cancer stem cell model. Proc Natl Acad Sci U S A. 2013;110:12006–11. doi: 10.1073/pnas.1307935110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gil M, Seshadri M, Komorowski MP, Abrams SI, Kozbor D. Targeting CXCL12/CXCR4 signaling with oncolytic virotherapy disrupts tumor vasculature and inhibits breast cancer metastases. Proc Natl Acad Sci U S A. 2013;110:E1291–300. doi: 10.1073/pnas.1220580110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Diaz RM, Galivo F, Kottke T, Wongthida P, Qiao J, Thompson J, et al. Oncolytic immunovirotherapy for melanoma using vesicular stomatitis virus. Cancer Res. 2007;67:2840–8. doi: 10.1158/0008-5472.CAN-06-3974. [DOI] [PubMed] [Google Scholar]
- 50.Kottke T, Errington F, Pulido J, Galivo F, Thompson J, Wongthida P, et al. Broad antigenic coverage induced by vaccination with virus-based cDNA libraries cures established tumors. Nat Med. 2011;17:854–9. doi: 10.1038/nm.2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Castelo-Branco P, Passer BJ, Buhrman JS, Antoszczyk S, Marinelli M, Zaupa C, et al. Oncolytic herpes simplex virus armed with xenogeneic homologue of prostatic acid phosphatase enhances antitumor efficacy in prostate cancer. Gene Ther. 2010;17:805–10. doi: 10.1038/gt.2010.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pulido J, Kottke T, Thompson J, Galivo F, Wongthida P, Diaz RM, et al. Using virally expressed melanoma cDNA libraries to identify tumor-associated antigens that cure melanoma. Nat Biotechnol. 2012;30:337–43. doi: 10.1038/nbt.2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Peng KW, Myers R, Greenslade A, Mader E, Greiner S, Federspiel MJ, et al. Using clinically approved cyclophosphamide regimens to control the humoral immune response to oncolytic viruses. Gene Ther. 2013;20:255–61. doi: 10.1038/gt.2012.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zitvogel L, Galluzzi L, Smyth MJ, Kroemer G. Mechanism of action of conventional and targeted anticancer therapies: reinstating immunosurveillance. Immunity. 2013;39:74–88. doi: 10.1016/j.immuni.2013.06.014. [DOI] [PubMed] [Google Scholar]