Abstract
Background:
A single subanesthetic infusion of the N-methyl-D-aspartate (NMDA) receptor antagonist ketamine has rapid and potent antidepressant properties in treatment-resistant major depressive disorder (TRD). As a family history of an alcohol use disorder is a positive predictor of ketamine’s antidepressant response and the strength of the association increases over time, we hypothesized that depressed subjects with a family history of an alcohol use disorder would have greater antidepressant durability and that riluzole would augment and/or extend ketamine’s antidepressant efficacy.
Methods:
Fifty-two TRD subjects received an open-label infusion of ketamine (0.5mg/kg over 40 minutes), and, four to six hours post-infusion, were randomized to either flexible-dose (100–200mg/day) riluzole or placebo in the following proportions: Family History Positive (FHP) riluzole (n = 10), FHP placebo (n = 9), Family History Negative (FHN) riluzole (n = 16), and FHN placebo (n = 17).
Results:
FHP subjects randomized to placebo had a greater antidepressant response than FHN subjects; however, contrary to our initial hypothesis, there was no significant difference in antidepressant efficacy with riluzole. Although potentially underpowered, there was no difference in overall time-to-relapse based on randomization status (riluzole responders: n = 15, placebo responders: n = 17). Yet, time-to-relapse was longer in FHP placebo responders (n = 8) compared to FHN placebo responders (n = 9) with, again, no significant difference in time-to-relapse in FHP riluzole responders (n = 6) compared to FHN riluzole responders (n = 9).
Conclusions:
Ketamine’s extended antidepressant durability in FHP TRD should be considered in the design and analysis of ketamine depression trials.
Keywords: alcohol use disorder, family history, ketamine, major depressive disorder, riluzole
Introduction
Major depressive disorder (MDD) has one of the highest morbidities worldwide (Kessler et al., 2003; Ustun et al., 2004; Ormel et al., 2008), and, as demonstrated in large real-world effectiveness trials (Rush et al., 2006, 2011), standard antidepressants are effective in only a proportion of patients. Additionally, there is a substantial time lag in response: 2–4 weeks for initial effect and 6–12 weeks for maximal efficacy. Treatment-resistant MDD (TRD) is associated with substantial psychosocial dysfunction, morbidity, and mortality, due in part to suicide and undertreated medical comorbidities. As a result, there is a critical need for better and more rapid-acting antidepressants to quickly alleviate the burden of depression for patients, their families and friends, and society at large.
The noncompetitive N-methyl-D-aspartate (NMDA) receptor antagonist ketamine, a US Food and Drug Administration–approved dissociative anesthetic, acted as a rapid-acting antidepressant in several randomized, double-blind, placebo-controlled (Berman et al., 2000; Zarate et al., 2006, 2012; Diazgranados et al., 2010; Valentine et al., 2011; Murrough, Iosifescu, et al., 2013) and open-label (Ibrahim et al., 2012; Murrough, Perez, et al., 2013) studies. Unlike monoaminergic antidepressants, which often require weeks to months to achieve maximal efficacy, a single subanesthetic dose of ketamine has rapid (within hours) and potent (at least one week) antidepressant efficacy. As a result, there have been numerous efforts to maintain ketamine’s antidepressant efficacy beyond this one-week window. In several case reports (Liebrenz et al., 2009; Murrough et al., 2011; Blier et al., 2012) and an open-labeled trial (Murrough, Perez, et al., 2013), repeated-dose ketamine prolonged the initial antidepressant response. However, there are as yet no controlled long-term studies demonstrating that repeated-dose ketamine is safe and tolerable. Based on preliminary antidepressant efficacy in MDD (Sanacora et al., 2004, 2007; Zarate et al., 2004), the glutamatergic modulator riluzole has been investigated as an oral means of prolonging ketamine’s antidepressant response. Mathew et al. (2010) administered double-blind flexible dose (100–200mg/day) riluzole to 17 TRD ketamine responders in a randomized, placebo-controlled, 32-day extension trial. In an interim analysis, riluzole did not delay time to relapse and, as a result, the trial was stopped for futility. Next, our group designed and recently reported a four-week, randomized, double-blind, placebo-controlled riluzole extension trial following open-label subanesthetic dose ketamine infusion in 42 TRD patients (Zarate et al., 2012). In that report, there was also no improvement in depression between riluzole and placebo groups.
We have explored demographic and clinical factors to identify subgroups associated with better response in order to maximize ketamine’s antidepressant effects. In both treatment-resistant MDD (Phelps et al., 2009) and bipolar depression (Luckenbaugh et al., 2012), subjects with a family history of alcohol dependence in a first-degree relative had a more robust and sustained antidepressant response to ketamine. Also, in a recent pooled correlative analysis of all our reported ketamine trials at the National Institute of Mental Health, at one week post-infusion, family history of an alcohol use disorder in a first-degree relative was the strongest studied demographic and clinical predictor of ketamine response, alone accounting for up to 22% of the variance in percent change in the 17-item Hamilton Depression Rating Scale (HDRS17) scores (Niciu, Luckenbaugh, et al., 2014). Finally, in an independent sample of 42 patients with treatment-resistant bipolar depression, a family history of alcoholism also correlated with improved antidepressant response to ketamine: 17 of 22 responders vs. 4 of 20 non-responders had a positive family history (Permoda-Osip et al., 2014). And, in two bipolar depression samples (Luckenbaugh et al., 2012; Permoda-Osip et al., 2014) but not in our unipolar depressed sample (Phelps et al., 2009), a lifetime personal history of an alcohol use disorder also predicted improved antidepressant efficacy.
After our initial ketamine riluzole extension trial report (Ibrahim et al., 2012), enrollment continued with the aim of identifying biomarkers and clinical predictors of treatment response. Here, we report the effect of family history of an alcohol use disorder in a first-degree relative over the full 28-day trial, and include 10 additional patients. We hypothesized that improvement in depressive symptoms resulting from a single ketamine infusion would be prolonged beyond one week in subjects with a family history of an alcohol use disorder in a first-degree relative (Family History Positive [FHP]) compared to those without an alcohol use disorder in an immediate relative (Family History Negative [FHN]). We also hypothesized that riluzole would augment and/or extend ketamine’s antidepressant efficacy in FHP but not FHN subjects. Finally, as in our initial report (Phelps et al., 2009), we hypothesized that a lifetime personal history of an alcohol use disorder would not predict ketamine’s antidepressant efficacy in this larger TRD sample.
Methods
Patient Selection
All subjects (18–65 years old) had a diagnosis of MDD without psychotic features as assessed by face-to-face evaluation with a licensed independent psychiatric practitioner and Structured Clinical Interview for Axis I DSM-IV Disorders-Patient Version. They were admitted to the Clinical Research Center of the National Institute of Mental Health in Bethesda, Maryland, between January 2006 and September 2013 and had a Montgomery Asberg Depression Rating Scale (MADRS) severity score of ≥22 both at screening and on the day of ketamine infusion (with no greater than a 25% decrease between screening and infusion). Additionally, participants were in a major depressive episode of at least four weeks duration at the time of screening. Treatment resistance was confirmed by prior failure of at least 2 adequate antidepressant trials using a modified version of the antidepressant treatment history form (Sackeim, 2001).
Stable physical health was assessed by medical history, physical examination, standard laboratory measures, electrocardiogram, and urine toxicology. All subjects could not meet criteria for an active substance use disorder (excluding nicotine or caffeine) for at least 3 months prior to enrollment. Comorbid axis I anxiety disorders were permitted if they were not the primary focus of treatment within 12 months prior to screening. Exclusion criteria included serious unstable medical conditions (e.g. uncontrolled asthma or hypertension), previous use of ketamine, riluzole, or phencyclidine, and concomitant treatment with psychotropic medications or electroconvulsive therapy in the 2 weeks prior to infusion (at least 5 weeks for fluoxetine). Female subjects could not be pregnant or nursing and agreed to use approved methods of birth control or complete abstinence during the entirety of the protocol.
The written protocol was approved by the Combined Neuroscience Institutional Review Board of the National Institutes of Health (NIH). All subjects provided written informed consent for screening and this specific protocol, and were assigned an independent clinical research advocate to impartially monitor the consent process and ensure ethical research participation.
Study Design and Medications
As previously described (Ibrahim et al., 2012), this was a double-blind, randomized, parallel-group, placebo-controlled, flexible-dose, inpatient study conducted to determine the antidepressant efficacy of an intravenous ketamine infusion followed by oral riluzole. Following a 2-week drug-free period (5 weeks for fluoxetine), 52 subjects received a single open-label infusion of 0.5mg/kg ketamine hydrochloride over 40 minutes. Four to six hours post-infusion, subjects were randomized to either flexible-dose (100–200mg/day) riluzole or placebo twice daily for 4 weeks. Dose escalations occurred on a weekly basis until the appearance of treatment-limiting side effects or study completion. Dose reductions were permitted by one capsule (50mg/week to a minimum of 100mg/day) in the case of intolerable side effects. Nursing staff monitored medication adherence. No concomitant medications with central nervous system effects were permitted throughout the 4-week trial.
Outcome Measures and Statistical Analyses
As in our prior studies (Phelps et al., 2009; Luckenbaugh et al., 2012; Niciu, Luckenbaugh, et al., 2014), family history was assessed pre-infusion with the Family Interview for Genetic Studies. FHP was defined as having at least one first-degree relative with an alcohol use disorder, and, as corollary, FHN was defined as an absence of a first-degree relative with an alcohol use disorder. Subjects were rated with a battery of neuropsychiatric measures at 60 minutes prior to infusion, at several post-infusion time points on infusion day (+40, +80, +120, and +230 minutes) and throughout the following 28 days. The following measures were rated daily: the MADRS (Montgomery and Asberg, 1979), HDRS17 (Hamilton, 1960), the Beck Depression Inventory (Beck and Beamesderfer, 1974), the Scale of Suicide Ideation (Beck et al., 1979), and the Young Mania Rating Scale (YMRS; Young et al., 1978). The Hamilton Anxiety Rating Scale (Hamilton, 1959) was obtained at days 1, 3, 7, 14, 21, and 28. Change in MADRS was primary and the remaining measures were secondary outcomes. All assessments were administered by research nurses, licensed independent practitioners (including psychiatrists), and psychologists who often evaluated the same patients concurrently to maximize reliability: for the MADRS, the inter-class correlation coefficient (ICC) = 0.94; HDRS17: ICC = 0.92; and YMRS: ICC = 0.92. Whenever possible, the same blinded rater conducted the clinician-administered ratings for an individual patient. As all subjects were inpatients, daily ratings were obtained for the entirety of the 28-day trial, even if the subjects relapsed post-ketamine infusion (see Results for additional information on treatment retention).
Factorial linear mixed models with restricted maximum likelihood estimation and an autoregressive moving average covariance structure were used to examine the change in clinical ratings over the treatment course with study day as the within-subjects factor and family history of an alcohol use disorder and medication (riluzole vs. placebo) extension as between-subjects factors. Baseline rating scale scores were included as covariates. All potential interactions between time, family history of an alcohol use disorder, and drug were included in the model. The fixed intercept was included, but the random intercept and random subject effect were not included because they did not contribute significantly to the model. The primary analysis used the intent-to-treat sample, where all participants had at least one post-baseline measure.
Kaplan-Meier survival analyses were performed using patients who responded (≥50% MADRS improvement from baseline) to ketamine at ≤230 minutes post-infusion. Relapse was defined by 2 consecutive days where the patient had ≤25% improvement from baseline MADRS. A log-rank test was used to compare drug effects, and family history of an alcohol use disorder was examined within each drug group.
Additional models examined a lifetime personal history of an alcohol use disorder in the same fashion as described above.
All analyses used two-tailed significance criteria of p < .05 and were performed with SPSS 21 (IBM).
Results
A total of 142 subjects were assessed under our screening protocol, 59 subjects were enrolled, and 52 subjects received ketamine followed by randomization to riluzole or placebo (Supplemental Figure S1). The demographics and clinical characteristics of the sample are reported in Supplemental Table S1. Family history and drug groups resulting from randomization were in the following proportions: FHN placebo (n = 17), FHP placebo (n = 9), FHN riluzole (n = 16), and FHP riluzole (n = 10).
On controlling for baseline MADRS, we first observed a main effect of group [MADRS: FHN (n = 33) < FHP (n = 19); F(1,49) = 5.25, p = .03] over the course of 4 weeks but no main effect of drug [F(1,50) = 0.07, p = .79]. Yet, there was a significant group-by-drug interaction [F(1,49) = 5.18, p = .03], such that FHP subjects had less depression than FHN subjects [F(1,50) = 9.69, p = .003] when randomized to placebo (Figure 1A). An analogous effect was not evident with riluzole [F(1,48) = 0.003, p = .95; Figure 1B]. Although potentially underpowered, the three-way interaction (group-by-drug-by-time) did not reach statistical significance [F(27,403) = 1.50, p = .053].
Figure 1.
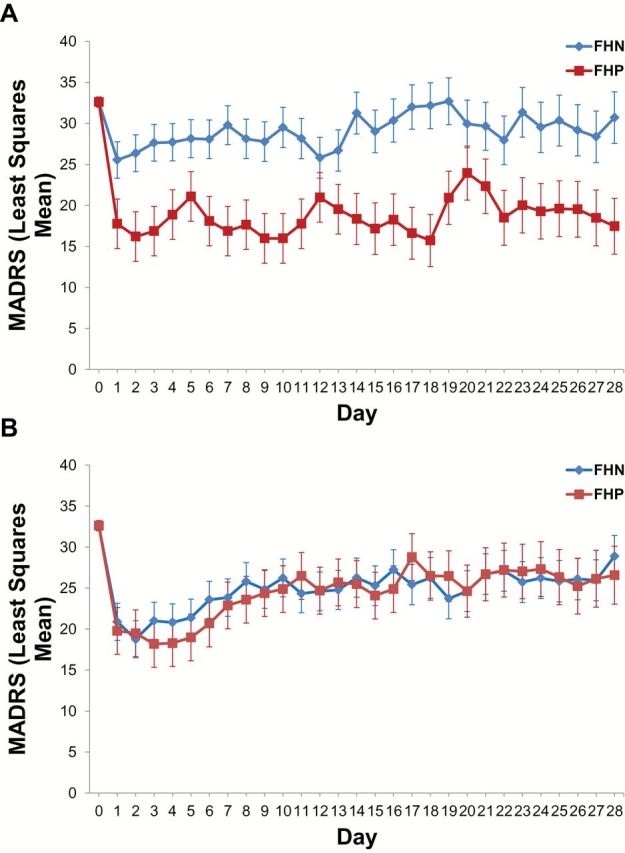
Ketamine’s antidepressant efficacy is improved for at least four weeks in treatment-resistant unipolar depressed subjects with a family history of an alcohol use disorder. (A) When randomized to placebo 4–6 hours after a single subanesthetic intravenous ketamine infusion, treatment-resistant unipolar depressed subjects with a family history of an alcohol use disorder displayed a greater antidepressant response over the next four weeks [group x time interaction: F(1,50) = 9.69, p = .003]. (B) When randomized to flexible-dose riluzole (100–200mg/day) 4–6 hours after a single subanesthetic intravenous infusion of ketamine, there was no statistically significant difference in antidepressant response based on family history status [group x time interaction: F(1,68) = .003, p = .95]. Abbreviations: FHP: family history positive; FHN: family history negative.
A similar group-by-drug interaction was observed for the HDRS17 [F(1,48) = 9.82, p = .003]. There were no significant group-by-drug effects, however, observed for the Beck Depression Inventory [F(1,49) = 1.95, p = .17], Hamilton Anxiety Rating Scale [F(1,44) = 3.41, p = .09], Scale of Suicide Ideation [F(1,30) = 0.09, p = .77] or YMRS [F(1,48) = 1.83, p = .18]. There were no statistically-significant three-way interactions on any of these secondary measures.
We next performed time-to-relapse survival analyses in ketamine responders (placebo: n = 17; riluzole: n = 15; Figure 2). Riluzole did not significantly delay time-to-relapse (χ2 = 3.73, p = .053; again, the analysis may be underpowered to detect this effect as the effect size was large [Cohen’s d = 0.78]; Figure 2A). The group-by-drug responder breakdown was as follows: FHN placebo responders: n = 9; FHP placebo responders, n = 8; FHN riluzole responders: n = 9; and FHP riluzole responders, n = 6. The FHN group relapsed more quickly than the FHP group on randomization to placebo (χ2 = 7.38, p = .007; FHN placebo responders [3.6 days, SE = 1.0] vs. FHP placebo responders [17.0 days, SE = 3.9]; Figure 2B). Five of the nine FHN placebo responders dropped out, on average, 14±3.9 days into the 28-day trial due to worsening mood and anxiety. Only 1 of the 8 FHP placebo responders dropped out before study completion: at day 18, again due to worsening depression. There was no significant difference between FHP and FHN subjects randomized to riluzole post-ketamine infusion (χ2 = 0.16, p = .69; FHN riluzole responders [19.6 days, SE = 3.3] vs. FHP riluzole responders [15.8 days, SE = 3.6]; data not shown).
Figure 2.
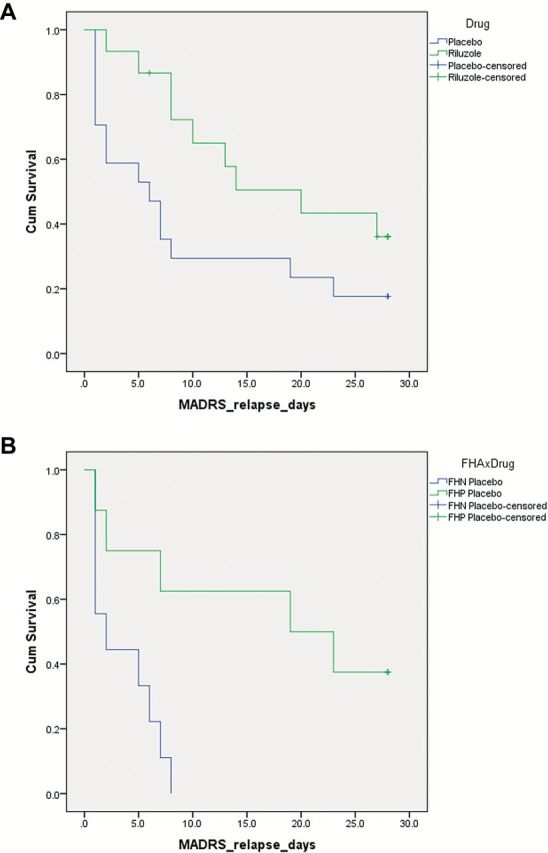
Ketamine’s antidepressant efficacy is maintained in treatment-resistant unipolar depressed subjects with a family history of an alcohol use disorder. (A) Prior to stratification by family history status, in a Kaplan-Meier survival analysis, riluzole did not delay time-to-relapse in treatment-resistant MDD antidepressant responders (χ2 = 3.73, p = .053). Response was defined as ≥50% MADRS improvement from baseline at any time point before 230 minute post-infusion, and relapse was defined as two consecutive days where patients had <25% improvement from baseline MADRS. (B) In the subgroup analysis, ketamine’s antidepressant response was extended in FHP patients randomized to placebo post–ketamine infusion. Abbreviations: FHP: family history positive; FHN: family history negative.
Similar predictor analyses were performed with a lifetime personal history of an alcohol use disorder (see Supplemental Materials).
Discussion
Ketamine has rapid-acting antidepressant effects in both treatment-resistant unipolar (Berman et al., 2000; Zarate et al., 2006; Valentine et al., 2011; Murrough, Iosifescu, et al., 2013) and bipolar (Diazgranados et al., 2010; Zarate et al., 2012) depression. Although the effect size is large to very large, even in refractory populations (Aan Het Rot et al., 2012), not all patients have an antidepressant (or even a positive) response (Niciu, Grunschel, et al., 2013; Szymkowicz et al., 2014). In order to better predict response, our group has extensively investigated treatment response biomarkers (Zarate et al., 2013; Niciu, Mathews, et al., 2014), and one of the strongest positive predictors is a family history of an alcohol use disorder in a first-degree relative (Phelps et al., 2009; Luckenbaugh et al., 2012; Niciu, Luckenbaugh, et al., 2014). In our combined dataset, the strength of this association increased over time, such that it was the strongest identified predictor at one week, alone explaining up to 22% of the antidepressant variance (Niciu, Luckenbaugh, et al., 2014). Yet, this mediating effect in both unipolar and bipolar depression has only been studied up to one week after ketamine infusion. In the present report, ketamine’s antidepressant efficacy was extended for four weeks (and potentially even longer, as the study completed at this time) in FHP subjects. Additionally, FHP extended the duration of antidepressant response by, on average, 13.4 days. These differences, however, were not due to poorer tolerability of ketamine in the FHN group, as the 5 FHN placebo patients who dropped out discontinued their participation, on average, two weeks into the trial due to worsening mood and anxiety.
Next, the lack of antidepressant efficacy in the FHP riluzole group was contrary to our initial hypothesis. As a potential explanation, we hypothesize that the acute “glutamate surge” is greater in the FHP group, which increases AMPA-to-NMDA receptor throughput and intracellular second messenger/signal transduction cascades critical for ketamine’s antidepressant response (Niciu, Ionescu, et al., 2013). The acute post-infusion administration of riluzole may decrease this synaptic glutamate release by antagonizing presynaptic ionotropic sodium channels, thereby preferentially attenuating ketamine’s antidepressant efficacy. Although too rapid to explain the acute effects, increased riluzole-induced astrocytic GLT-1/EAAT-2 expression also may abrogate the extended antidepressant efficacy of ketamine in the FHP group.
We view a family history of an alcohol use disorder as a proxy for genetic or epigenetic risk. As alcohol use disorders are estimated to be at least 50% heritable (Enoch, 2013), we hypothesize that at least a portion of the increased antidepressant efficacy in FHP TRD is attributable to common genetic variation: e.g., single nucleotide polymorphisms and variable number of tandem repeats. Differential glutamate receptor sensitivity may be based on such variation in NMDA receptor subunits (Schumann et al., 2008) and other downstream effectors proteins (Niciu, Ionescu, et al., 2013). However, a family history of an alcohol use disorder also predisposes to other factors, (e.g., an increased risk of physical abuse which, of note, was the only investigated demographic factor significantly increased in the FHP group) that may have long-lasting epigenetic effects (e.g., differential methylation, acetylation and microRNA expression), contributing to this enhanced antidepressant efficacy. Differential methylation (Ressler et al., 2011) and microRNA expression (Zhou et al., 2014) have been observed in post-traumatic stress disorder. Future research should be aimed at identifying the genetic and neural substrates of this differential sensitivity, which may ultimately allow patient stratification based on more objective, continuous measures than the subjective, categorical domain of family history.
Contrary to family history, a lifetime personal history of an alcohol use disorder did not predict ketamine’s antidepressant efficacy in this (potentially underpowered) sample. In post hoc analyses from our ketamine bipolar depression studies (Diazgranados et al., 2010; Zarate et al., 2012), however, a lifetime personal history of an alcohol use disorder moderated improved antidepressant response (Luckenbaugh et al., 2012), a finding which has been replicated in an independent Polish bipolar depression ketamine cohort (Permoda-Osip et al., 2014). In addition to its γ-aminobutyric acid effects, alcohol is also a weak NMDA receptor antagonist (Lovinger, 1995; Fink and Gothert, 1996; Kash et al., 2008). We hypothesize that, due to lingering NMDA receptor blockade, chronic alcohol exposure produces long-term glutamatergic dysfunction—i.e., differential expression of ionotropic (postsynaptic) and/or metabotropic (both pre- and postsynaptic) receptors—that persists even after prolonged abstinence. In support of this hypothesis, central glutamate perturbations have been reported in alcohol use disorders alone and in combination with bipolar disorder, even after ≥1 year abstinence (Thoma et al., 2011). Decreased dorsolateral prefrontal cortical “Glx” (magnetic resonance-detectable glutamate + glutamine) has also been observed in (primarily male) alcohol-dependent bipolar patients compared to non-alcohol dependent bipolar and healthy control subjects (Nery et al., 2010). Taken together, ketamine’s differential effects in PHP treatment-resistant unipolar vs. bipolar depression may represent a critical avenue for future neurobiological and pharmacological investigations.
In conclusion, we again present compelling evidence that FHP treatment-resistant unipolar depressed subjects have a more robust antidepressant response to ketamine. Due to the length of this study, we report for the first time that the antidepressant effect of a single infusion is sustained over an entire for at least four weeks. FHP also delayed time-to-relapse in ketamine responders. Finally, although potentially underpowered, total MADRS change was not predicted by personal history status. Due to the strength and longevity of ketamine’s antidepressant efficacy in FHP patients, we encourage all future ketamine depression studies to assess, report, and potentially co-vary based on this variable.
Supplementary Material
For supplementary material accompanying this paper, visit http://www.ijnp.oxfordjournals.org/
Statement of Interest
Drs Niciu, Ionescu, Richards, Vande Voort, and Ballard, Ms. Brutsche, and Mr. Luckenbaugh have no potential financial conflicts of interest to disclose. Dr Furey is listed as a co-inventor on a patent application for the use of scopolamine in major depression, and Dr Zarate is listed as a co-inventor on a patent application for the use of ketamine and its metabolites in major depression. Drs Furey and Zarate have assigned their rights in the patent to the US Government but will share a percentage of any royalties that may be received by the Government.
Supplementary Material
Acknowledgments
The authors gratefully acknowledge the support of the Intramural Research Program of the National Institute of Mental Health and National Institutes of Health (IRP-NIMH-NIH), and thank the 7SE Inpatient Mood and Anxiety Disorders Research Unit of the NIMH-NIH for their support.
The authors gratefully acknowledge the support of the IRP-NIMH-NIH and the National Alliance for Research in Schizophrenia and Affective Disorders Independent Investigator Award and Brain and Behavior Foundation Bipolar Research Award (Dr Zarate). Salary support was also provided by the IRP-NIMH-NIH (Drs Niciu, Ionescu, Richards, Vande Voort, Ballard, Furey, and Zarate, Ms. Brutsche, and Mr. Luckenbaugh). Other than the aforementioned, the IRP-NIMH-NIH played no other role.
References
- Aan Het Rot M, Zarate CA, Jr., Charney DS, Mathew SJ. (2012). Ketamine for depression: where do we go from here? Biol Psychiatry 72:537–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Beamesderfer A. (1974). Assessment of depression: the depression inventory. Mod Probl Pharmacopsychiatr 7:151–169. [DOI] [PubMed] [Google Scholar]
- Beck AT, Kovacs M, Weissman A. (1979). Assessment of suicidal intention: the scale for suicide ideation. J Consult Clin Psychol 47:343–352. [DOI] [PubMed] [Google Scholar]
- Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, Krystal JH. (2000). Antidepressant effects of ketamine in depressed patients. Biol Psychiatry 47:351–354. [DOI] [PubMed] [Google Scholar]
- Blier P, Zigman D, Blier J. (2012). On the safety and benefits of repeated intravenous injections of ketamine for depression. Biol Psychiatry 72:e11–12. [DOI] [PubMed] [Google Scholar]
- Diazgranados N, Ibrahim L, Brutsche NE, Newberg A, Kronstein P, Khalife S, Kammerer WA, Quezado Z, Luckenbaugh DA, Salvadore G, Machado-Vieira R, Manji HK, Zarate CA., Jr (2010). A randomized add-on trial of an N-methyl-D-aspartate antagonist in treatment-resistant bipolar depression. Arch Gen Psychiatry 67:793–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enoch MA. (2013). Genetic influences on the development of alcoholism. Curr Psychiatry Rep 15:412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink K, Gothert M. (1996). Both ethanol and ifenprodil inhibit NMDA-evoked release of various neurotransmitters at different, yet proportional potency: potential relation to NMDA receptor subunit composition. Naunyn Schmiedebergs Arch Pharmacol 354:312–319. [DOI] [PubMed] [Google Scholar]
- Hamilton M. (1959). The assessment of anxiety states by rating. Br J Med Psychol 32:50–55. [DOI] [PubMed] [Google Scholar]
- Hamilton M. (1960). A rating scale for depression. J Neurol Neurosurg Psychiatry 23:56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim L, Diazgranados N, Franco-Chaves J, Brutsche N, Henter ID, Kronstein P, Moaddel R, Wainer I, Luckenbaugh DA, Manji HK, Zarate CA., Jr. (2012). Course of improvement in depressive symptoms to a single intravenous infusion of ketamine vs add-on riluzole: results from a 4-week, double-blind, placebo-controlled study. Neuropsychopharmacology 37:1526–1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kash TL, Matthews RT, Winder DG. (2008). Alcohol inhibits NR2B-containing NMDA receptors in the ventral bed nucleus of the stria terminalis. Neuropsychopharmacology 33:1379–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, Rush AJ, Walters EE, Wang PS. (2003). The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R). JAMA 289:3095–3105. [DOI] [PubMed] [Google Scholar]
- Liebrenz M, Stohler R, Borgeat A. (2009). Repeated intravenous ketamine therapy in a patient with treatment-resistant major depression. World J Biol Psychiatry 10:640–643. [DOI] [PubMed] [Google Scholar]
- Lovinger DM. (1995). Developmental decrease in ethanol inhibition of N-methyl-D-aspartate receptors in rat neocortical neurons: relation to the actions of ifenprodil. J Pharm Exp Ther 274:164–172. [PubMed] [Google Scholar]
- Luckenbaugh DA, Ibrahim L, Brutsche N, Franco-Chaves J, Mathews D, Marquardt CA, Cassarly C, Zarate CA., Jr. (2012). Family history of alcohol dependence and antidepressant response to an N-methyl-D-aspartate antagonist in bipolar depression. Bipolar Disord 14:880–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathew SJ, Murrough JW, aan het Rot M, Collins KA, Reich DL, Charney DS. (2010). Riluzole for relapse prevention following intravenous ketamine in treatment-resistant depression: a pilot randomized, placebo-controlled continuation trial. Int J Neuropsychop 13:71–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery SA, Asberg M. (1979). A new depression scale designed to be sensitive to change. Br J Psychiatry 134:382–389. [DOI] [PubMed] [Google Scholar]
- Murrough JW, Perez AM, Mathew SJ, Charney DS. (2011). A case of sustained remission following an acute course of ketamine in treatment-resistant depression. J Clin Psychiatry 72:414–415. [DOI] [PubMed] [Google Scholar]
- Murrough JW, Iosifescu DV, Chang LC, Al Jurdi RK, Green CE, Perez AM, Iqbal S, Pillemer S, Foulkes A, Shah A, Charney DS, Mathew SJ. (2013). Antidepressant efficacy of ketamine in treatment-resistant major depression: A two-site randomized controlled trial. Am J Psych 170:1134–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murrough JW, Perez AM, Pillemer S, Stern J, Parides MK, aan het Rot M, Collins KA, Mathew SJ, Charney DS, Iosifescu DV. (2013). Rapid and longer-term antidepressant effects of repeated ketamine infusions in treatment-resistant major depression. Biol Psychiatry 74:250–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nery FG, Stanley JA, Chen HH, Hatch JP, Nicoletti MA, Monkul ES, Lafer B, Soares JC. (2010). Bipolar disorder comorbid with alcoholism: a 1H magnetic resonance spectroscopy study. J Psychiatr Res 44:278–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niciu MJ, Grunschel BD, Corlett PR, Pittenger C, Bloch MH. (2013). Two cases of delayed-onset suicidal ideation, dysphoria and anxiety after ketamine infusion in patients with obsessive-compulsive disorder and a history of major depressive disorder. J Psychopharmacol 27:651–654. [DOI] [PubMed] [Google Scholar]
- Niciu MJ, Ionescu DF, Mathews DC, Richards EM, Zarate CA. (2013). Second messenger/signal transduction pathways in major mood disorders: moving from membrane to mechanism of action, part I: major depressive disorder. CNS Spectr: 18:231–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niciu MJ, Luckenbaugh DA, Ionescu DF, Guevara S, Machado-Vieira R, Richards EM, Brutsche NE, Nolan NM, Zarate CA., Jr (2014). Clinical predictors of ketamine response in treatment-resistant major depression. J Clin Psychiatry 75:e417–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niciu MJ, Mathews DC, Nugent AC, Ionescu DF, Furey ML, Richards EM, Machado-Vieira R, Zarate CA., Jr (2014). Developing biomarkers in mood disorders research through the use of rapid-acting antidepressants. Depress Anxiety 31:297–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ormel J, et al. (2008). Disability and treatment of specific mental and physical disorders across the world. Br J Psychiatry 192:368–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Permoda-Osip A, Skibinska M, Bartkowska-Sniatkowska A, Kliwicki S, Chlopocka-Wozniak M, Rybakowski JK. (2014). Factors connected with efficacy of single ketamine infusion in bipolar depression. Psychiatr Pol 48:35–47. [PubMed] [Google Scholar]
- Phelps LE, Brutsche N, Moral JR, Luckenbaugh DA, Manji HK, Zarate CA., Jr. (2009). Family history of alcohol dependence and initial antidepressant response to an N-methyl-D-aspartate antagonist. Biol Psychiatry 65:181–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ressler KJ, Mercer KB, Bradley B, Jovanovic T, Mahan A, Kerley K, Norrholm SD, Kilaru V, Smith AK, Myers AJ, Ramirez M, Engel A, Hammack SE, Toufexis D, Braas KM, Binder EB, May V. (2011). Post-traumatic stress disorder is associated with PACAP and the PAC1 receptor. Nature 470:492–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rush AJ, Trivedi MH, Stewart JW, Nierenberg AA, Fava M, Kurian BT, Warden D, Morris DW, Luther JF, Husain MM, Cook IA, Shelton RC, Lesser IM, Kornstein SG, Wisniewski SR. (2011). Combining medications to enhance depression outcomes (CO-MED): acute and long-term outcomes of a single-blind randomized study. Am J Psych 168:689–701. [DOI] [PubMed] [Google Scholar]
- Rush AJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart JW, Warden D, Niederehe G, Thase ME, Lavori PW, Lebowitz BD, McGrath PJ, Rosenbaum JF, Sackeim HA, Kupfer DJ, Luther J, Fava M. (2006). Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psych 163:1905–1917. [DOI] [PubMed] [Google Scholar]
- Sackeim HA. (2001). The definition and meaning of treatment-resistant depression. J Clin Psychiatry 62(Suppl 16):10–17. [PubMed] [Google Scholar]
- Sanacora G, Kendell SF, Fenton L, Coric V, Krystal JH. (2004). Riluzole augmentation for treatment-resistant depression. Am J Psych 161:2132. [DOI] [PubMed] [Google Scholar]
- Sanacora G, Kendell SF, Levin Y, Simen AA, Fenton LR, Coric V, Krystal JH. (2007). Preliminary evidence of riluzole efficacy in antidepressant-treated patients with residual depressive symptoms. Biol Psychiatry 61:822–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumann G, et al. (2008). Systematic analysis of glutamatergic neurotransmission genes in alcohol dependence and adolescent risky drinking behavior. Arch Gen Psychiatry 65:826–838. [DOI] [PubMed] [Google Scholar]
- Szymkowicz SM, Finnegan N, Dale RM. (2014). Failed response to repeat intravenous ketamine infusions in geriatric patients with major depressive disorder. J Clin Psychopharmacol 34:285–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoma R, Mullins P, Ruhl D, Monnig M, Yeo RA, Caprihan A, Bogenschutz M, Lysne P, Tonigan S, Kalyanam R, Gasparovic C. (2011). Perturbation of the glutamate-glutamine system in alcohol dependence and remission. Neuropsychopharmacology 36:1359–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ustun TB, Ayuso-Mateos JL, Chatterji S, Mathers C, Murray CJ. (2004). Global burden of depressive disorders in the year 2000. Br J Psychiatry 184:386–392. [DOI] [PubMed] [Google Scholar]
- Valentine GW, Mason GF, Gomez R, Fasula M, Watzl J, Pittman B, Krystal JH, Sanacora G. (2011). The antidepressant effect of ketamine is not associated with changes in occipital amino acid neurotransmitter content as measured by [(1)H]-MRS. Psychiatry Res 191:122–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young RC, Biggs JT, Ziegler VE, Meyer DA. (1978). A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry 133:429–435. [DOI] [PubMed] [Google Scholar]
- Zarate CA, Jr, Payne JL, Quiroz J, Sporn J, Denicoff KK, Luckenbaugh D, Charney DS, Manji HK. (2004). An open-label trial of riluzole in patients with treatment-resistant major depression. Am J Psych 161:171–174. [DOI] [PubMed] [Google Scholar]
- Zarate CA, Jr, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, Charney DS, Manji HK. (2006). A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry 63:856–864. [DOI] [PubMed] [Google Scholar]
- Zarate CA, Jr, Brutsche NE, Ibrahim L, Franco-Chaves J, Diazgranados N, Cravchik A, Selter J, Marquardt CA, Liberty V, Luckenbaugh DA. (2012). Replication of ketamine’s antidepressant efficacy in bipolar depression: a randomized controlled add-on trial. Biol Psychiatry 71:939–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarate CA, Jr, Mathews DC, Furey ML. (2013). Human biomarkers of rapid antidepressant effects. Biol Psychiatry 73:1142–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Nagarkatti P, Zhong Y, Ginsberg JP, Singh NP, Zhang J, Nagarkatti M. (2014). Dysregulation in microRNA expression Is associated with alterations in immune functions in combat veterans with post-traumatic stress disorder. PLOS ONE 9:e94075. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


