The current lack of training for physicians in cell-based therapy combined with the sharply increasing practice of regenerative medicine is concerning for a number of reasons, namely potential harm to patients and avoidable conflicts between governmental regulatory agencies and physicians. This review describes the implementation of a Clinical Research Skills Development Program with the purpose of promoting the further development of academic fellowship programs in cell-based regenerative medicine.
Summary
Cell-based therapy aimed at restoring organ function is one of the most exciting and promising areas of medical research. However, a novel intervention like cell-based therapy requires physician education and training. An increasing number of physicians untrained in regenerative medicine are using cell-based therapy to treat patients for a wide variety of chronic illnesses. The current lack of training for physicians in this area combined with the sharply increasing practice of regenerative medicine is concerning for a number of reasons, namely potential harm to patients and avoidable conflicts between governmental regulatory agencies and physicians. Academic medical fellowship training programs are needed that specifically prepare physicians for treating patients with cell-based therapies for various organ systems and chronic diseases. The National Heart, Lung, and Blood Institute established the Cardiovascular Cell Therapy Network to design and conduct clinical trials that advance the field of cell-based therapy for patients with cardiovascular disease. As part of the network, a two-year Clinical Research Skills Development Program was supported at two centers with the goal of training early career investigators in cell-based clinical and translational research. In this review, we describe the implementation of this training program at our institution with the purpose of promoting the further development of academic fellowship programs in cell-based regenerative medicine.
Introduction
The promising cell-based therapy field aimed at restoring organ function has exploded in the past decade. Currently, stem cells from various sources are being evaluated in phase I/II trials for a myriad of chronic, disabling disorders that have no effective therapies available. Although preclinical studies have provided mechanistic insights and phase I/II studies have provided evidence of safety in the short term, questions regarding the most effective cell type, dose, timing and route of administration, interaction with other concurrent therapies, sustainability of effect, and adverse effects, such as opportunistic infections and tumor development or progression, remain to be resolved. These questions need to be answered through rigorously conducted, multicenter clinical trials with well-defined clinical endpoints, a longer duration of follow-up, and a greater number of patients [1]. Moreover, a novel intervention like stem cell therapy requires innovative evaluation and assessment tools that place an emphasis on elucidating mechanisms of action and defining clinically relevant outcomes. From dosing and delivery to evaluating safety and efficacy, stem cell therapy provides not only opportunities but also challenges in our mission to develop effective and sustainable therapeutic interventions for chronic diseases.
Although there are many clinical trials throughout the world being conducted and reported through ClinicalTrials.gov and governmental regulatory bodies provide some oversight [2–4], an increasing number of physicians untrained in stem cell-based therapy are administering stem cells into innumerable patients for a broad diversity of conditions, with little or no evidence of safety or efficacy [5, 6]. Furthermore, as stem cell technologies advance, a growing number of academic physicians and institutions are interested in practicing regenerative medicine [5, 6]. To this end, academic medical fellowship training programs are needed that specifically prepare physicians for treating patients with stem cell-based therapies for various organ systems and chronic diseases.
The National Heart, Lung, and Blood Institute established the Cardiovascular Cell Therapy Network (CCTRN) to design and conduct clinical trials that advance the field of cell-based therapy for patients with cardiovascular disease [7, 8]. As part of the network, a 2-year Clinical Research Skills Development Program was supported at two centers with the goal of training early career (fellows and junior faculty) investigators in cell-based clinical and translational research. Herein we describe the initial implementation of this training program at the Interdisciplinary Stem Cell Institute (ISCI) at the University of Miami Medical School and share the knowledge we have gained with the purpose of promoting the further development of academic fellowship programs in cell-based regenerative medicine.
Physician Training Gap: The Need for Development of Training Programs in Cell-Based Regenerative Medicine
Cell-based regenerative medicine is rapidly expanding in terms of the number of physicians practicing, number of patients being treated, and variety of conditions being treated [4–6]. The lack of formal physician training in regenerative medicine combined with the dramatically increasing use of cell-based therapies for a multitude of diseases creates a potentially dangerous scenario that may result in harm to patients, both physically and psychologically (false promises of cure), and avoidable, punitive conflicts between the governmental regulatory agencies, such as the Food and Drug Administration (FDA), and physicians [2–6, 9–12]. It has been proposed that a more effective approach to promoting compliance and uniformity in this new field is the implementation of physician training programs in an academic setting [5].
To add to the growing concerns regarding the use of cell-based therapies, there is a growing demand to deregulate the use of these therapies [9, 11, 13–15]. For instance, in April 2013, the Italian government amended an already controversial ministerial decree [16] with a clause that would redefine stem cell therapies as tissue transplantation, thereby releasing them from any regulatory oversight [15]. This placed Italy out of step with the rules of the European Medicines Agency and the U.S. FDA, both of which define stem cells modified outside the body as medicines and therefore under their regulatory oversight [2–4]. Although the use of unproven stem cell therapies is not new [17–19], this was the first time that there was some governmental support for the medical use of an unproven therapy in a country where rules set out by regulatory bodies have so far been effective in protecting patients from serious risks associated with their indiscriminate use [13]. However, the strong reaction by the international medical and stem cell communities was successful in arguing against this ruling, which was subsequently overturned.
In summary, we believe it is critical for the medical and scientific community to be the leaders in developing programs to not only educate and train physicians, but also educate patients, the general public, and governmental agencies on the appropriate development, investigation, and clinical use of cell-based therapies [1, 6–8]. To this end, the NIH established the CCTRN to lead this effort in developing training programs that will produce physician-scientists with expertise in all aspects of regenerative medicine as it applies to cardiovascular diseases.
Curriculum and Assessment of Professional Development of the Trainees
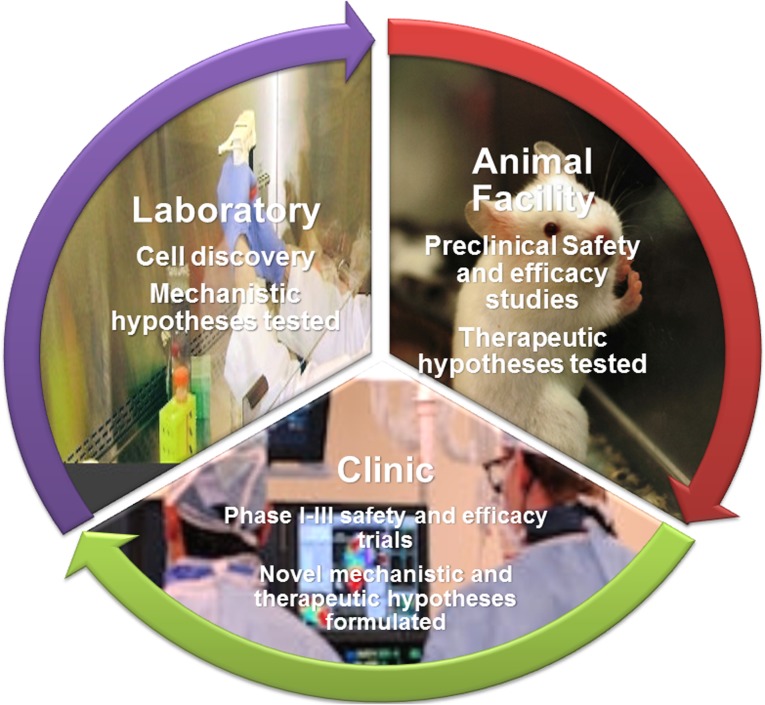
The clinical trainees supported by this CCTRN clinical research training program conduct cell-based clinical and preclinical research projects at the ISCI, a state-of-the-art, multidisciplinary research group under the direction of Dr. Joshua M. Hare. The primary goal of ISCI is to support and develop basic, translational, and clinical research to further understand the use of stem cells and cellular regenerative pathways. Dr. Hare and his group have expertise in cardiovascular stem cell biology and cell-based therapy for cardiac regeneration, having conducted multiple preclinical studies and five phase I clinical trials and a longstanding track record of mentorship in the area of cardiovascular basic and translational research. Through their projects, the trainees participate and become expert in all the main aspects of the conduct and analysis of basic laboratory, preclinical, and clinical stem cell research (Fig. 1), as described herein, as well as data presentations in local, national, and international meetings, manuscript preparation, and grant writing.
Figure 1.
Bench-to-bedside training in the development of cell-based therapies.
Basic Laboratory Techniques
Under the supervision of ISCI research faculty and staff, the trainees have the opportunity to engage in basic science research and learn various basic laboratory skills, including techniques in molecular biology, cardiovascular physiology, stem cell culture and biology, histology, and emerging technologies. In addition, exposure to and/or training in a current good manufacturing practice (GMP) laboratory for the preparation of stem cells for human clinical trials is an important component of the training program in regenerative medicine. At the cell production facility, the trainees have the opportunity to learn about the GMP regulations and get trained in the isolation, expansion, storage, and preparation for injection of stem cells, as well as perform biomarker and molecular analyses.
Imaging Modalities
Faculty mentors from the Department of Radiology provide training in imaging acquisition and analysis, which provides a valuable tool for evaluation of patients receiving cell-based therapy. Imaging modalities such as cardiovascular magnetic resonance, multidetector computer tomography (MDCT), and single-photon emission computed tomography have been the focus of cardiac function assessment after stem cell therapy at our institute [20–22]. The institute has a state-of-the-art biplane angiography system for the cardiac catheterization laboratory where all the large animal experiments are conducted by the trainees and mentors. The institute’s 3.0 T magnetic resonance imaging (MRI) offers sophisticated imaging abilities that mimic hospital-based facilities.
Preclinical Animal Models
The development of preclinical animal models permits trainees to understand cellular therapy from the time of obtaining the cells from the donor to the administration of the cells to the patient, using minimally invasive imaging techniques. The animal model provides an opportunity to obtain extensive hemodynamic data that can be correlated with biochemical assessment of cardiac tissue. The trainees get extensive training in the assessment of cardiovascular hemodynamics and myocardial energetics in the porcine model of ischemic cardiomyopathy, as well as with coronary catheterization procedures, creation of novel models of cardiovascular disease, and catheter-based delivery of molecular therapies to the myocardium [23]. These experiences are ideal for the translation of this research to human application. The choice of the porcine model was made because the porcine cardiac anatomy is similar to that of humans. As with humans, swine have few collateral coronary vessels, thus enabling the reliable creation of transmural infarction by the temporary occlusion of a single coronary artery. The size of swine and adult human hearts is comparable, allowing the use of human catheters and other cardiovascular devices, and the techniques developed in swine are readily translatable to human clinical application.
Transendocardial stem cell injection is performed after induction of myocardial infarction in the porcine animal model. The use of catheters with retrograde access across the aortic valve into the left ventricle permits minimal invasive delivery of cell products. Trainees work with the University of Miami Hospital (UMH) interventional cardiologists and receive hands-on experience.
Clinical Trials
Experience in the conduct of clinical trials is another key component of the training program. UMH, which is within walking distance of the institute laboratory, is a major tertiary care center that includes state-of-the-art Siemens cardiac catheterization laboratories equipped with biplane imaging. Attending physicians in the catheterization laboratory participate in a number of multicenter trials of new devices, techniques, and drug treatments used in conjunction with cardiac interventions. Experienced interventional cardiologists skilled at using catheter delivery systems for stem cells mentor and supervise trainees.
The institute houses a clinical coordinating center with extensive experience in cell-based therapy for heart disease and a regulatory office that interacts with the FDA and the University of Miami Institutional Review Board (IRB). The clinical coordinating center is staffed by research coordinators who oversee patient enrollment and follow-up and actively participate in the training program. The skills taught include regulatory documentation; investigational new drug application submission to the FDA, IRB, and institutional animal care and use committee (IACUC) documentation; patient enrollment and follow-up; noninvasive and invasive cardiovascular hemodynamic evaluation, monitoring, and data analysis; and cardiac MDCT and MRI imaging data acquisition and analysis. All trainees are required to complete courses on the responsible conduct of research, which consists of online training via the Collaborative Institutional Training Initiative Program, as well as class options at the University’s Bioethics Program, prior to initiation of any project.
Mentoring and Assessment
During the initial phase of training program, the program leaders, Drs. Hare, Balkan, and Schulman, work closely with the trainees, ensuring that they are thoroughly trained in the technical aspects of clinical and preclinical protocol preparation for the FDA, IRB, and/or IACUC, preparation and delivery of stem cell injections, performance and analysis of cardiac hemodynamic studies, analysis of MRI and MDCT imaging software, tissue preparation for histology, and cell culture. Over time, the trainees are expected to independently design and perform their own projects, as well as to write the initial drafts of their manuscripts. The trainees are encouraged and expected to apply for independent funding, such as career development awards or other investigator-initiated research funding.
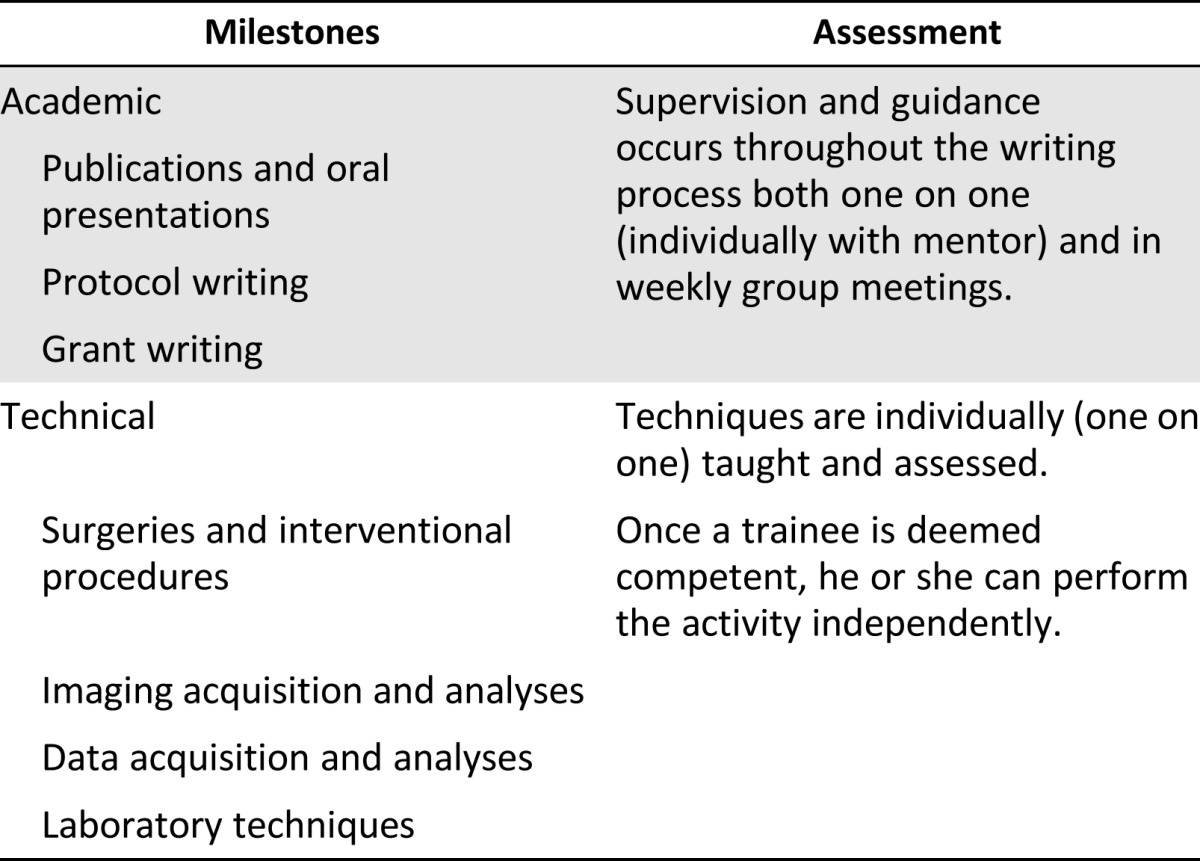
Progress is evaluated on a daily, ongoing basis through discussion with research team members and principal investigators. In addition, more formal evaluation of progress is conducted during weekly laboratory meeting presentations, weekly meetings with the clinical trial investigators and clinical coordinators for patient recruitment and follow-up, and weekly meetings with the large animal surgical team for protocol development, experimental planning, and progress of studies (Table 1).
Table 1.
Measurable milestones
Program Success
ISCI joined the CCTRN in March 2012, and this training program has thus far supported three postdoctoral fellows. The three fellows supported have medical degrees: two are foreign medical graduates, and the most recent one to join the program is an American graduate. They each have presented several posters and oral presentations at national and international meetings and have published multiple original research articles and review articles in peer-reviewed journals [23–34]. Our first fellow has successfully completed the training program and joined a radiology residency program. The other two fellows are expected to finish the program this academic year and return to internal medicine and surgery residency programs, respectively, for completion of their medical training with the goal of becoming independent physician-scientists.
Conclusion
With the advent of cell-based therapy as a potential therapeutic strategy for tissue regeneration in various chronic conditions, it is imperative that academic programs be instituted to formally train physicians and scientists in the conduct of translational and clinical research, as well as in the technical procedures used in the regenerative medicine field. With this goal in mind, the NIH CCTRN has been instrumental in supporting training programs that will produce physician-scientists with expertise in all aspects of regenerative medicine. The mission of the CCTRN is to “achieve public health advances for the treatment of cardiovascular diseases, through the conduct and dissemination of collaborative research leading to evidence-based treatment options and improved outcome for patients with heart disease” [7, 8]. Trained personnel are vital for a successful research mission. The structured training programs supported by the CCTRN provide a high quality scientific educational value and directly address one of the most pertinent problems in the field of cardiovascular cell therapy: the lack of qualified personnel to support the growing portfolio of basic, translational, and clinical research. The goal is that these scholars will bring novel ideas related to cardiovascular cell therapy to early-phase clinical trials, with the ultimate goal of completing research studies that will lead to more effective treatments for patients with cardiovascular disease.
Acknowledgments
This work was supported by NIH Grants UM1-HL113460, R01-HL084275, and R01-HL110737 and Cooperative Agreement 5 UM1-HL087318-08 (J.M.H.), an American Heart Association Grant (V.K.), and the Starr Foundation.
Author Contributions
I.H.S.: conception and design, financial support, administrative support, collection and/or assembly of data, manuscript writing, final approval of manuscript; V.S., V.K., and W.B.: collection and/or assembly of data, manuscript writing, final approval of manuscript; J.M.H.: conception and design, financial support, manuscript writing, final approval of manuscript.
Disclosure of Potential Conflicts of Interest
J.M.H. has compensated employment, consultancy, and stock options with Vestion; is a compensated patent holder; and has compensated research funding. The other authors indicated no potential conflicts of interest.
References
- 1.Hare JM, Bolli R, Cooke JP, et al. Phase II clinical research design in cardiology: Learning the right lessons too well: Observations and recommendations from the Cardiovascular Cell Therapy Research Network (CCTRN) Circulation. 2013;127:1630–1635. doi: 10.1161/CIRCULATIONAHA.112.000779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ancans J. Cell therapy medicinal product regulatory framework in Europe and its application for MSC-based therapy development. Front Immunol. 2012;3:253. doi: 10.3389/fimmu.2012.00253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.U.S. Department of Health and Human Services, Food and Drug Administration, Center for Biologics Evaluation and Research. Guidance for human somatic cell therapy and gene therapy. Hum Gene Ther. 2001;12:303–314. doi: 10.1089/10430340150218431. [DOI] [PubMed] [Google Scholar]
- 4.Martín PG, Martinez AR, Lara VG, et al. Regulatory considerations in production of a cell therapy medicinal product in Europe to clinical research. Clin Exp Med. 2014;14:25–33. doi: 10.1007/s10238-012-0213-6. [DOI] [PubMed] [Google Scholar]
- 5.Knoepfler PS. Call for fellowship programs in stem cell-based regenerative and cellular medicine: New stem cell training is essential for physicians. Regen Med. 2013;8:223–225. doi: 10.2217/rme.13.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Knoepfler PS. Key action items for the stem cell field: Looking ahead to 2014. Stem Cells Dev. 2013;22(suppl 1):10–12. doi: 10.1089/scd.2013.0322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Simari RD, Moyé LA, Skarlatos SI, et al. Development of a network to test strategies in cardiovascular cell delivery: The NHLBI-sponsored Cardiovascular Cell Therapy Research Network (CCTRN) J Cardiovasc Transl Res. 2010;3:30–36. doi: 10.1007/s12265-009-9160-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Park KE, Moyé LA, Henry TD, et al. Implementation of cardiovascular cell therapy network trials: Challenges, innovation and lessons learned from experience in the CCTRN. Expert Rev Cardiovasc Ther. 2013;11:1495–1502. doi: 10.1586/14779072.2013.839943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chirba-Martin MA, Noble A. Our bodies, our cell: FDA regulation of autologous adult stem cell therapies. Bill of Health 2013.
- 10.Yuan BZ, Wang J. The regulatory sciences for stem cell-based medicinal products. Front Med. 2014;8:190–200. doi: 10.1007/s11684-014-0323-5. [DOI] [PubMed] [Google Scholar]
- 11.Preventive therapy. Nature. 2013;494:147–148. doi: 10.1038/494147b. [DOI] [PubMed] [Google Scholar]
- 12.Unknown territory. Nature. 2013;494:5. doi: 10.1038/494005a. [DOI] [PubMed] [Google Scholar]
- 13.Bianco P, Barker R, Brüstle O, et al. Regulation of stem cell therapies under attack in Europe: For whom the bell tolls. EMBO J. 2013;32:1489–1495. doi: 10.1038/emboj.2013.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cyranoski D. Stem cells in Texas: Cowboy culture. Nature. 2013;494:166–168. doi: 10.1038/494166a. [DOI] [PubMed] [Google Scholar]
- 15.Smoke and mirrors. Nature. 2013;496:269–270. doi: 10.1038/496269b. [DOI] [PubMed] [Google Scholar]
- 16.Abbott A. Stem-cell ruling riles researchers. Nature. 2013;495:418–419. doi: 10.1038/495418a. [DOI] [PubMed] [Google Scholar]
- 17.Regenberg AC, Hutchinson LA, Schanker B, et al. Medicine on the fringe: Stem cell-based interventions in advance of evidence. Stem Cells. 2009;27:2312–2319. doi: 10.1002/stem.132. [DOI] [PubMed] [Google Scholar]
- 18.Enserink M. Biomedicine. Selling the stem cell dream. Science. 2006;313:160–163. doi: 10.1126/science.313.5784.160. [DOI] [PubMed] [Google Scholar]
- 19.Hyun I, Lindvall O, Ahrlund-Richter L, et al. New ISSCR guidelines underscore major principles for responsible translational stem cell research. Cell Stem Cell. 2008;3:607–609. doi: 10.1016/j.stem.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 20.Schuleri KH, Centola M, George RT, et al. Characterization of peri-infarct zone heterogeneity by contrast-enhanced multidetector computed tomography: A comparison with magnetic resonance imaging. J Am Coll Cardiol. 2009;53:1699–1707. doi: 10.1016/j.jacc.2009.01.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schuleri KH, Boyle AJ, Centola M, et al. The adult Göttingen minipig as a model for chronic heart failure after myocardial infarction: Focus on cardiovascular imaging and regenerative therapies. Comp Med. 2008;58:568–579. [PMC free article] [PubMed] [Google Scholar]
- 22.Schuleri KH, Amado LC, Boyle AJ, et al. Early improvement in cardiac tissue perfusion due to mesenchymal stem cells. Am J Physiol Heart Circ Physiol. 2008;294:H2002–H2011. doi: 10.1152/ajpheart.00762.2007. [DOI] [PubMed] [Google Scholar]
- 23.McCall FC, Telukuntla KS, Karantalis V, et al. Myocardial infarction and intramyocardial injection models in swine. Nat Protoc. 2012;7:1479–1496. doi: 10.1038/nprot.2012.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karantalis V, Balkan W, Schulman IH, et al. Cell-based therapy for prevention and reversal of myocardial remodeling. Am J Physiol Heart Circ Physiol. 2012;303:H256–H270. doi: 10.1152/ajpheart.00221.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hare JM, Fishman JE, Gerstenblith G, et al. Comparison of allogeneic vs autologous bone marrow–derived mesenchymal stem cells delivered by transendocardial injection in patients with ischemic cardiomyopathy: The POSEIDON randomized trial [published correction appears in JAMA 2013;310:750] JAMA. 2012;308:2369–2379. doi: 10.1001/jama.2012.25321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Karantalis V, DiFede DL, Gerstenblith G, et al. Autologous mesenchymal stem cells produce concordant improvements in regional function, tissue perfusion, and fibrotic burden when administered to patients undergoing coronary artery bypass grafting: The Prospective Randomized Study of Mesenchymal Stem Cell Therapy in Patients Undergoing Cardiac Surgery (PROMETHEUS) trial. Circ Res. 2014;114:1302–1310. doi: 10.1161/CIRCRESAHA.114.303180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Suncion VY, Ghersin E, Fishman JE, et al. Does transendocardial injection of mesenchymal stem cells improve myocardial function locally or globally?: An analysis from the Percutaneous Stem Cell Injection Delivery Effects on Neomyogenesis (POSEIDON) randomized trial. Circ Res. 2014;114:1292–1301. doi: 10.1161/CIRCRESAHA.114.302854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heldman AW, DiFede DL, Fishman JE, et al. Transendocardial mesenchymal stem cells and mononuclear bone marrow cells for ischemic cardiomyopathy: The TAC-HFT randomized trial. JAMA. 2014;311:62–73. doi: 10.1001/jama.2013.282909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Telukuntla KS, Suncion VY, Schulman IH, et al. The advancing field of cell-based therapy: Insights and lessons from clinical trials. J Am Heart Assoc. 2013;2:e000338. doi: 10.1161/JAHA.113.000338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Williams AR, Suncion VY, McCall F, et al. Durable scar size reduction due to allogeneic mesenchymal stem cell therapy regulates whole-chamber remodeling. J Am Heart Assoc. 2013;2:e000140. doi: 10.1161/JAHA.113.000140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Williams AR, Hatzistergos KE, Addicott B, et al. Enhanced effect of combining human cardiac stem cells and bone marrow mesenchymal stem cells to reduce infarct size and to restore cardiac function after myocardial infarction. Circulation. 2013;127:213–223. doi: 10.1161/CIRCULATIONAHA.112.131110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Suncion VY, Schulman IH, Hare JM. Concise review: The role of clinical trials in deciphering mechanisms of action of cardiac cell-based therapy. Stem Cells Translational Medicine. 2012;1:29–35. doi: 10.5966/sctm.2011-0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Karantalis V, Schulman IH, Hare JM. Nitroso-redox imbalance affects cardiac structure and function. J Am Coll Cardiol. 2013;61:933–935. doi: 10.1016/j.jacc.2012.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Golpanian S, El-Khorazaty J, Mendizabal A, et al. Effect of aging on human mesenchymal stem cell therapy in ischemic cardiomyopathy patients. JACC. 2014 doi: 10.1016/j.jacc.2014.10.040. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]