This study established a porcine pipeline to evaluate the feasibility and long-term safety of autologous umbilical cord blood mononuclear cells (UCB-MNCs) transplanted into the right ventricle (RV) of juvenile porcine hearts. The results show that autologous UCB-MNCs can be safely collected and surgically delivered in a pediatric setting, establishing the foundation for cell-based therapy directed at the RV of juvenile hearts and aims to accelerate cell-based therapies toward clinical trials for congenital heart disease.
Keywords: Safety, Autologous umbilical cord blood, Congenital heart disease, Porcine, Right ventricle, Intramyocardial delivery
Abstract
Congenital heart diseases (CHDs) requiring surgical palliation mandate new treatment strategies to optimize long-term outcomes. Despite the mounting evidence of cardiac regeneration, there are no long-term safety studies of autologous cell-based transplantation in the pediatric setting. We aimed to establish a porcine pipeline to evaluate the feasibility and long-term safety of autologous umbilical cord blood mononuclear cells (UCB-MNCs) transplanted into the right ventricle (RV) of juvenile porcine hearts. Piglets were born by caesarean section to enable UCB collection. Upon meeting release criteria, 12 animals were randomized in a double-blinded fashion prior to surgical delivery of test article (n = 6) or placebo (n = 6). The UCB-MNC (3 × 106 cells per kilogram) or control (dimethyl sulfoxide, 10%) products were injected intramyocardially into the RV under direct visualization. The cohorts were monitored for 3 months after product delivery with assessments of cardiac performance, rhythm, and serial cardiac biochemical markers, followed by terminal necropsy. No mortalities were associated with intramyocardial delivery of UCB-MNCs or placebo. Two animals from the placebo group developed local skin infection after surgery that responded to antibiotic treatment. Electrophysiological assessments revealed no arrhythmias in either group throughout the 3-month study. Two animals in the cell-therapy group had transient, subclinical dysrhythmia in the perioperative period, likely because of an exaggerated response to anesthesia. Overall, this study demonstrated that autologous UCB-MNCs can be safely collected and surgically delivered in a pediatric setting. The safety profile establishes the foundation for cell-based therapy directed at the RV of juvenile hearts and aims to accelerate cell-based therapies toward clinical trials for CHD.
Introduction
Several types of congenital heart disease (CHD), particularly those with univentricular hearts of right heart ventricular morphology, are characterized by an increased pressure and right ventricular workload. The development of right ventricular dysfunction and cardiac failure is responsible for persistent morbidity in children affected with these cardiac malformations, who require life-long surveillance for declining function that may lead to cardiac transplantation. The study of stem cell strategies to address heart failure has advanced steadily over the last decade, predominantly in the adult population. There is expanding clinical experience with first generation cell-based therapies to mitigate the effects of ischemic and nonischemic heart diseases in adults that involve the administration of both autologous and allogeneic bone marrow-derived stem cells (BMSCs) [1–6]. These studies have provided evidence that validates the overall safety and feasibility of direct injection into the left heart muscle in adult cardiac disease.
However, clinical experience with cell-based therapy for cardiac disease in children is limited to a few case reports [7–9]. To date, no large clinical trials have been reported using any type of stem cells to augment CHD management in the pediatric setting. The most recent publication from Rupp et al. in 2012 [8] reported the safety and feasibility of the intracoronary autologous BMSC administration in nine children with end-stage heart failure following analogous protocols established in the adult experience. Cell-based administration was associated with improved cardiac function and clinical stability with no procedure-related unexpected adverse events.
The ideal source of stem cells for cardiac regenerative purposes is dependent on the individual clinical scenario that includes logistical challenges of collection and processing of the cells, as well as clinical delivery to the cardiac tissue in complex CHD. Umbilical cord blood (UCB) was prioritized herein according to the readily available source of stem cells that contains both hematopoietic and nonhematopoietic tissue precursors. Given the high incidence of prenatal diagnosis of CHD, this source of autologous stem cells was practical to process in the time frame necessary for add-on clinical procedures in early postnatal life. UCB is rich in multipotent stem/progenitor cells with enhanced potency for angiogenic and myogenic differentiation and proliferative characteristics [10–12]. UCB cells have been standardized therapeutic agents in patients suffering from major hematological disorders since 1988 [13]. Subsequently, multiple groups have been pioneering human umbilical cord blood-derived mononuclear cells (UCB-MNCs) in preclinical experimental approaches for myocardial repair and regeneration [14–19]. Collectively, these studies suggest a cell-mediated improvement in the ejection fraction, wall motion, and cardiac contraction, along with a lowered left ventricular end-diastolic pressure. Increases of capillary density were associated with reductions in infarct size, number of apoptotic cells, and left ventricle remodeling [18, 20, 21]. Additionally, intramyocardial delivery of UCB-MNC in an ovine model system suggests a therapeutic benefit cell-based therapy in the right ventricle (RV) [22, 23].
Despite the evidence for efficacy of UCB cells, there are no long-term safety studies that have investigated the comprehensive profile of UCB-MNC transplantation into juvenile cardiac tissue recapitulating the pediatric setting. The experimental approach herein has been conducted to determine the feasibility and long-term safety of autologous cell-based therapy in a double-blinded, large-animal model system with primary outcome focused on electromechanical stability under physiological stress of a juvenile heart. The porcine model system allowed us to develop clinically relevant autologous cell processing of UCB, surgical delivery strategy for the RV, and cardiac imaging modalities calibrated for preclinical validation studies. Collectively, this clinical-grade pipeline has now established the baseline safety profile of autologous UCB-MNCs that demonstrates no measurable toxicity attributable to cell-based cardiac regenerative strategies within the innate physiological challenges of a juvenile/pediatric heart. This pipeline now provides a systematic platform to identify optimal safety profiles of cell-based therapeutics in the pediatric setting and aims to accelerate regenerative strategies for CHD.
MATERIAL AND METHODS
Animals
The care of the experimental animals was in compliance with the Principles of Laboratory Animal Care formulated by the National Society for Medical Research and the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health (NIH publication 86/5-23, National Academy Press, Washington, DC, revised 1996) under the responsibility of Mayo Clinic Department of Comparative Medicine. All procedures were approved by the Institutional Animal Care and Use Committee review board of the Mayo Clinic in Rochester, Minnesota.
Caesarean Delivery and UCB Collection
Synchronized and timed-pregnant sow and gilt pairs were purchased prior to 100 days of gestation. At day 115 of gestation, the gilts were prepared for nonsurvival caesarean section delivery. Midline abdominal incision and electrocautery were used to gain access to the uterus. Individual piglets were delivered through multiple transverse myotomies in the uterus. Sterile umbilical cord clamp (Mabis Healthcare, Waukegan, IL, http://www.mabisdmi.com) was placed within 3–5 cm from the piglet, and the cord was cut. UCB was drained from vein and arteries by gravity into sterile prelabeled 50-ml conical tubes (BD Bioscience, San Jose, CA, http://www.bdbiosciences.com) filled with 10 ml of anticoagulant (citrate phosphate dextrose-adenine) (Sigma-Aldrich, St. Louis, MO, http://www.sigmaaldrich.com). Porcine UCB and anticoagulant contained in the tubes were gently mixed to avoid clot formation.
Isolation and Cryopreservation of UCB-MNCs
UCB was processed within 24 hours by diluting with phosphate-buffered saline (PBS) at a ratio of 1:1 and slowly overlaid onto 15 ml of Ficoll density medium (1.077 g/ml; GE Healthcare, Little Chalfont, U.K., http://www.gehealthcare.com) in 50-ml conical polypropylene tubes (BD Bioscience), followed by centrifugation for 30 minutes at 400g. The interface buffy layer was carefully collected and pooled into new 50-ml conical tubes. The cells were washed twice with PBS and, after the second wash, were resuspended in 20 ml of PBS. Two-hundred microliters of the cell solution was taken for total nucleated cells (TNCs) and mononuclear cells (MNCs) counting, viability by flow cytometer, and 100 μl for endotoxin testing. The remaining cells were centrifuged for 10 minutes at 500g at room temperature. After centrifugation, the supernatant was removed to a cell pellet and was used for sterility testing. The final cell-based product was cryopreserved in sterile 2-ml cryovials with 10% dimethyl sulfoxide (DMSO) (Cryostor CS-10; Biolife Solutions, Bothell, WA, http://biolifesolutions.com) at a desired cell concentration of 30 × 106 MNCs/ml. Equivalent numbers of cryovials were produced at the same time with vehicle control solution (DMSO 10%) plus 10 µl of autologous red blood cells to recapitulate the external appearance of the cell-based product. All vials were stored long term in liquid nitrogen in a dedicated box.
Postprocessing Quality Control Assays
The absolute TNC count and MNC fraction were calculated by the microsphere-based technique using a single-platform flow cytometry. Aerobic and anaerobic microbiologic culture analysis (BD Bactec Peds Plus and BD Bactec Lytic/10 Anaerobic) (BD Biosciences, San Jose, CA, http://www.bd.com) was completed in the Mayo Clinic microbiology laboratory. Endotoxin was monitored using the Endosafe PTS (Charles River Laboratories, Wilmington, MA, http://www.criver.com), which uses Limulus amebocyte lysate kinetic chromogenic methodology to measure color intensity directly related to the endotoxin concentration in a sample (supplemental online data).
Surgical Procedures
All surgery was conducted in a specialized operating room designated for large-animal cardiothoracic surgical procedures. Each animal was sedated with Telazol (tiletamine HCl and zolazepam HCl; Zoetis, Florham Park, NJ, https://www.zoetisus.com) (5 mg/kg intramyocardially [IM]) and xylazine (2 mg/kg IM) and premedicated including antibiotics and analgesics (cefazolin [40 mg/kg i.v.] with or without Excede [ceftiofur; Zoetis] [5 mg/kg IM], and buprenorphine SR 0.15 mg/kg s.c.). The experimental animals were endotracheally intubated to protect the airway and allow administration of inhaled 1%–2% isoflurane as needed. During surgery, the animals were monitored by heart rate, blood pressure, respiratory rate, temperature, and continuous electrocardiography (ECG).
Telemetry Device Implantation for Continuous Cardiac Monitoring
Piglets that were predetermined to be eligible for the randomization arms of the study had an implantable loop recorder (ILR) (Medtronic, Minneapolis, MN, http://www.medtronic.com) placed 3–5 days prior to thoracotomy. The ILR device ID was linked to the individual piglet. The animals were placed in a sternal position to expose the posterior left paraspinal area, and skin incision at T4 was made 1–2 cm lateral to the spinal process. The ILR device was placed in the desired location subcutaneously, positioned until good signal was achieved, and secured using the sutured tab. ILR data were interpreted by a clinically trained pediatric cardiologist in a blinded fashion, using data directly uploaded into CareLink, a clinically approved device and reporting system by Medtronic. Tachycardia was defined as ≥250 beats per minute for more than 30 seconds, bradycardia was defined as <60 beats per minute for more than 30 seconds, and asystole was defined as a lack of electrical activity for more than 6 seconds. One minute before and after, each episode was recorded and analyzed to determine artifact or clinically meaningful episode of arrhythmia.
Intramyocardial Delivery of Cell-Based Product versus Placebo
Starting with the oldest animals in each cohort eligible for randomization, a lateral right thoracotomy was performed at the T4–T5 intercostal space. The pericardium was reflected to expose the epicardial surface of the RV. Immediately prior to product delivery, each case was randomized in a double-blinded fashion to receive either UCB-MNCs (3 × 106 cells per kilogram of body weight) or a comparable volume (0.1 ml/kg of body weight) of a 10% DMSO-based final solution as a vehicle control. The selected product was thawed at 37°C for approximately 5 minutes or until all solids were eliminated within the tube with careful mixing of the vial contents. The product was loaded into 1-ml syringes with an 18-gauge needle and then connected to the delivery device with a 27-gauge needle on flexible tubing. Avoiding major blood vessels and the conduction system, a 2–4-cm-diameter target area was identified on the surface of the RV. Epicardial injections delivered over 20 seconds to 5–15 sites calculated as 0.1 ml per site with a total dose of 0.1 ml/kg of body weight.
Cardiovascular Parameters Assessment
Surface electrocardiograms and echocardiography images were evaluated by an independent contract research organization (QTest Laboratories, Columbus, OH, http://www.qtestlabs.com) in a blinded (treatment vs. placebo) fashion (supplemental online data).
Veterinarian Medical Examination
In addition to research staff measuring and documenting vital signs and cage-side observations (daily observation for adverse events), formal veterinary medical examinations were performed on a predetermined schedule of baseline, 4 weeks post-thoracotomy, and 12 weeks post-thoracotomy beyond standard care.
Pathology and Necropsy
All laboratory samples were analyzed by Mayo Medical Laboratory at defined time intervals. Animals were euthanized 12 weeks after cell/placebo infusion. Necropsy was then performed, and tissues were placed in 10% buffered formalin. Heart tissue was collected from three independent sites within the free wall of the RV (around the injection sites) and the left ventricle, and cardiac weight was recorded. The samples were stained with hematoxylin and eosin, and sections of heart were also stained with Masson’s trichrome. Samples were analyzed in a blinded fashion by a certified veterinary pathologist (Vet Path Services, Inc., Mason, OH, http://www.vetpathservicesinc.com).
CFSE Labeling and Cell Tracking
In a nonrandomized fashion, the cell-based product was thawed at 37°C, washed, and then labeled with 5- (and-6)-carboxyfluorescein diacetate succinimidyl ester (CFSE) (Invitrogen Molecular Probes, Grand Island, NY, http://www.invitrogen.com) following the manufacturer’s recommendations (supplemental online data).
Statistical Analysis
Comparisons between groups were made using Student’s t test. Probability values of less than .05 were considered significant. The data are presented as means ± SD. A customized Medidata Rave database was built to track the health of individual piglets throughout the protocol and ensure strict clinical-grade procedures to certify compliance with double-blinded, randomized study design.
Results
Experimental Design
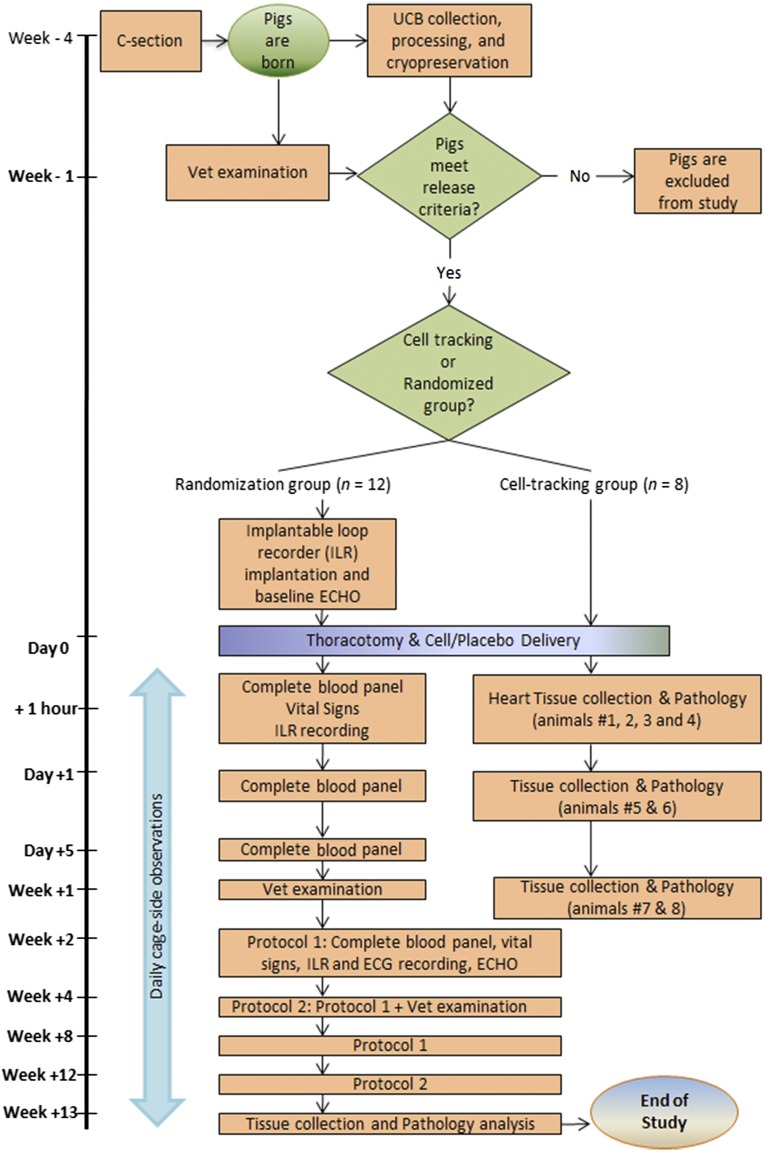
Piglets were born at Mayo Clinic from two litters and had autologous UCB collected, processed, and analyzed to achieve predetermined release criteria. When the piglets were ready to be weaned, a trained veterinarian completed a baseline medical exam. The piglets were eligible for this study if they had no active medical conditions, had autologous UCB-MNCs meeting all release criteria, and were 3–4 weeks of age. The four oldest animals (defined by the time of birth) plus four more animals that did not meet cellular release criteria were identified as the nonrandomized satellite group that contained labeled cells to allow for cell-tracking and biodistribution data in parallel to the randomized cohort. The next 13 oldest animals received ILR and had baseline vital signs and echocardiography performed 3–5 days prior to planned thoracotomy. At the time of thoracotomy, planned according to the sequential order of the oldest to youngest animals, 12 healthy piglets were enrolled and randomized once they were successfully intubated; they were monitored and were stable after exposing the cardiac surface via thoracotomy. Preprinted envelopes numbered 1–12 determined, in a double-blinded procedure, the selection into either vehicle control (n = 6) or cell-therapy (n = 6) arms of the study. Following thoracotomy procedure, physiological parameters were monitored until the termination of the study (Fig. 1).
Figure 1.
Study design and time-course data of the porcine safety study. Abbreviations: C-section, caesarean section; ECG, electrocardiography; ECHO, echocardiography; ILR, implantable loop recorder; UCB, umbilical cord blood.
Feasibility of Porcine UCB Collection and Processing
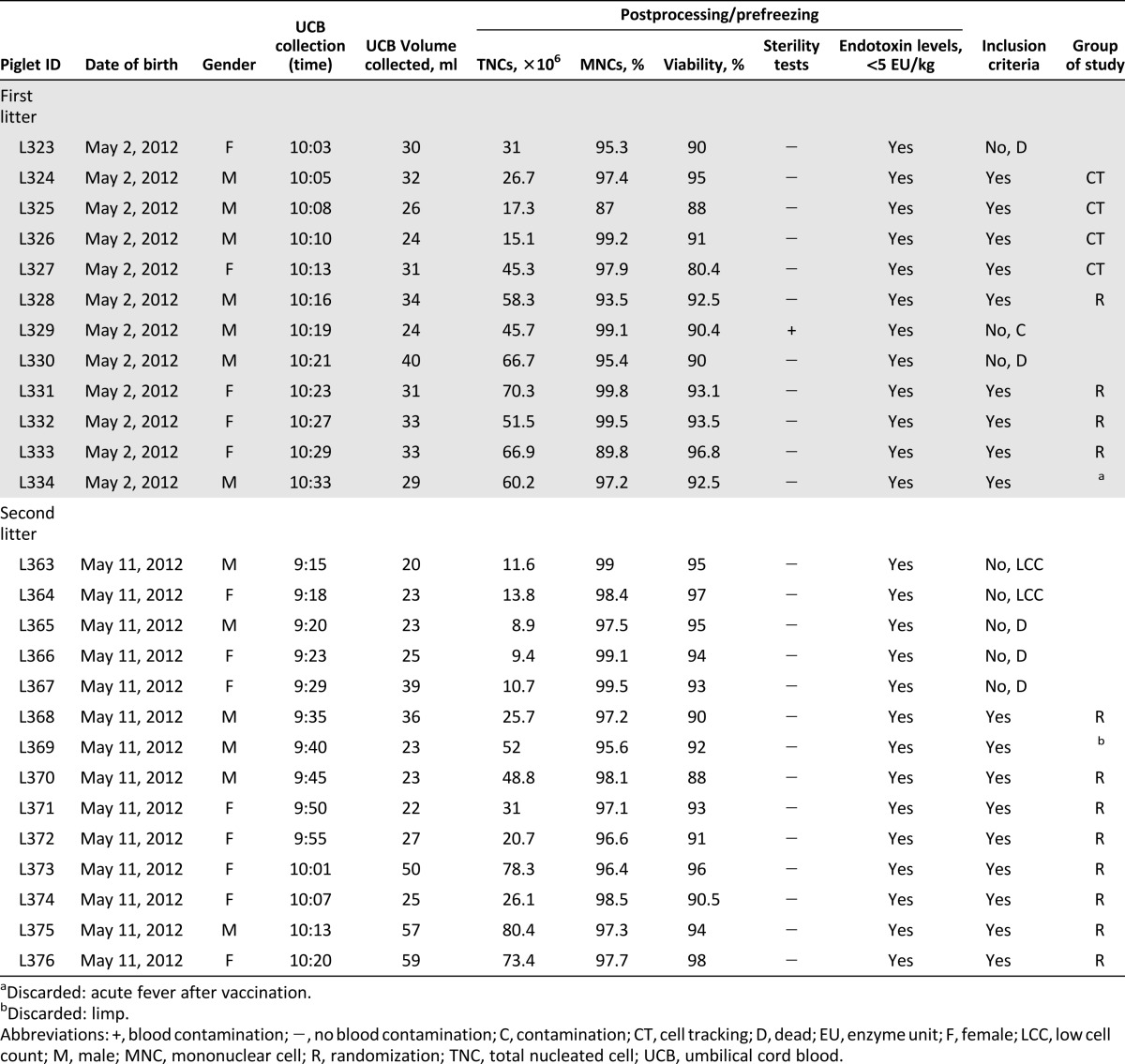
UCB was collected from 26 piglets while the placenta was still in utero. UCB was drained by gravity until there was no further blood flow, between 2 and 7 minutes. UCB volume collected was 20–59 ml (mean = 31.5 ml, SE = 2.03 ml), and none of the samples had signs of coagulation or hemolysis. Transport time was within 2 hours at ambient temperature (∼20°C) (Fig. 2A, 2B). No complications were encountered during UCB collection, and all piglets recovered within hours of delivery. Release criteria were documented with human analogous procedures for cellular composition and viability, microbiologic testing, and endotoxin levels (Fig. 2C, 2D). The resulting products for all piglets born from the two litters are noted in Table 1. Of the 26 piglets born by caesarean delivery, none demonstrated insufficient viability (mean = 92.3%, SE = 0.71%) or endotoxin contamination. TNC counts ranged from 8.9 × 106 cells to 80.4 × 106 cells (mean = 40.2 × 106 cells, SE = 4.68 × 106 cells) and all animals met the minimum mononuclear cell percentage required in the release criteria (mean = 96.9%, SE = 0.58%) (supplemental online Fig. 1A). Pearson’s correlation shows a positive correlation between porcine UCB volume and postprocessing TNC counts (R2 = 0.42, p = .0003) (supplemental online Fig. 1B). Table 1 notes a single UCB unit that was contaminated in the cohort of 26 piglets. Furthermore, two piglets were excluded from randomization because of low TNC count secondary to low UCB collection volumes. Thus, 92% of piglets achieve predefined cellular release criteria.
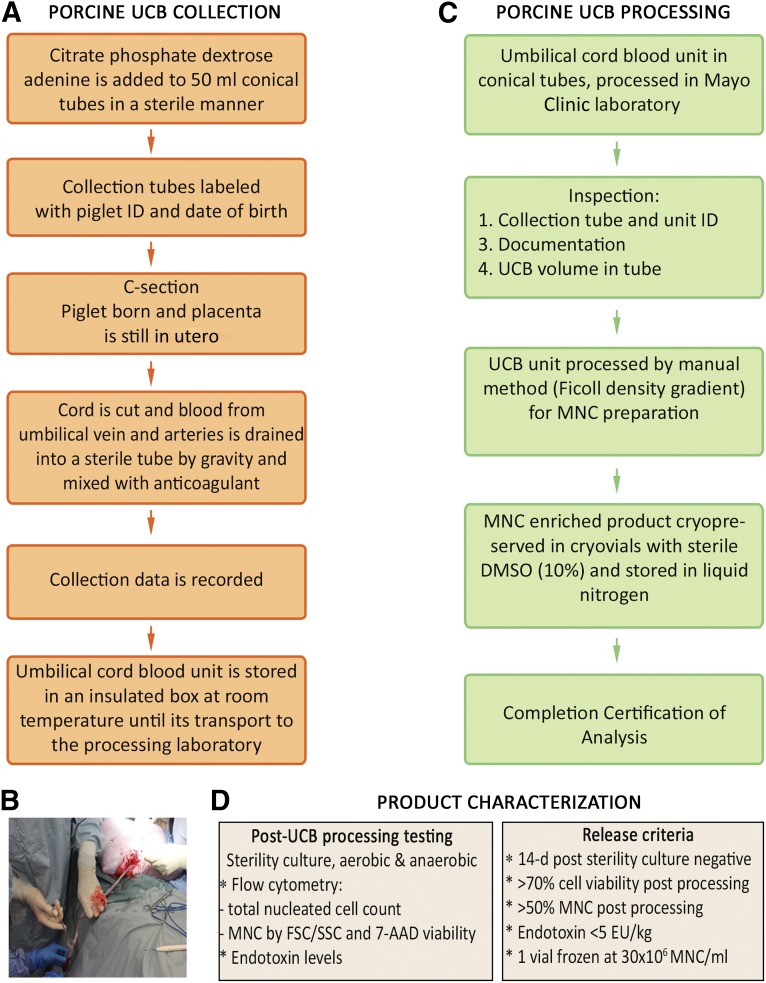
Figure 2.
UCB collection and MNCs isolation. (A): Scheme of autologous porcine UCB collection process at Mayo Clinic. (B): Porcine UCB collection photo. (C): Scheme of isolation of MNCs and cryopreservation process from porcine UCB by Ficoll density gradient (manual method). (D): Autologous product characterization carried out in autologous porcine UCB-derived MNCs after Ficoll density gradient processing and before cryopreservation and release criteria to be achieved by every porcine UCB unit collected. Abbreviations: C-section, caesarean section; 7-AAD, 7-aminoactinomycin D; d, day; DMSO, dimethyl sulfoxide; EU, enzyme unit; FSC, forward scatter; MNC, mononuclear cell; SSC, side scatter; UCB, umbilical cord blood.
Table 1.
Piglet characteristics and release criteria
Randomization Procedure, Thoracotomy, and Quality Assurance
Six of the original twenty-six animals were not included in the randomization schedule because of failure of meeting health status release criteria (inadvertent injuries inflicted by the surrogate sow resulted in two dead animals, three animals were sacrificed because of large litter size for surrogate mother, and one piglet had an unexplained fever during routine vaccination). Randomization was achieved according to a defined procedure. Immediately after randomization, animals received either UCB cells (3 × 106 cells per kilogram) or the same volume of vehicle solution (DMSO, 10%) intramyocardially into 5–15 sites of a 2–4-cm-diameter target area of the RV under visualization by open-heart surgery. No complications including mortality were registered caused by the surgical procedure in any of the groups. Identity of each animal assigned to each group was confirmed with postprocedural quality assurance testing of the vials that were not used in the surgical delivery of randomized product. There were no discrepancies noted between the real-time Medidata Rave documentation at the time of procedure randomization and the postprocedural quality assurance testing of the unused frozen products after delivery (Fig. 3).
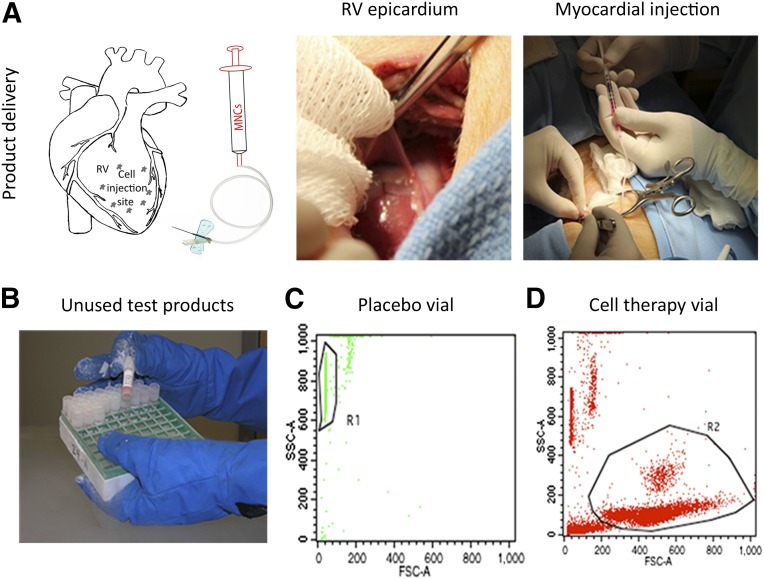
Figure 3.
Autologous umbilical cord blood-derived mononuclear cells versus placebo transplantation and quality assurance testing of unused frozen products. (A): Scheme of the injection target area. Avoiding major blood vessels and the conduction system, a 2–4-cm diameter target area was identified on the surface of the right ventricle. Epicardial injections of cell product and placebo were delivered to 5–15 sites calculated as 0.1 ml per site with a total dose of 0.1 ml/kg of body weight (left). The pericardium was reflected to expose the epicardial surface of the right ventricle through a lateral thoracotomy at T4–T5 intercostal space (middle). The cell/placebo injection into the porcine right ventricle myocardium in a 4-week-old piglet was carried out using a 1-ml syringe connected to the delivery device with a 27-gauge needle on flexible tubing over 20 seconds (right). (B): Frozen cells and placebo vials that were not used for myocardial injection after randomization were kept in liquid nitrogen for quality assurance purposes. (C): Flowchart showing lack of cells from an unused vial labeled as placebo. R1 gate shows beads from Trucount tubes (BD Biosciences, Franklin Lakes, NJ, http://www.bdbiosciences.com) used for counting the absolute number of cells. (D): Flowchart showing presence of leukocytes by FSC/SSC (R2 gate) after a vial containing umbilical cord blood mononuclear cells was analyzed to confirm its content vial. Abbreviations: FSC, forward scatter; MNCs, mononuclear cells; RV, right ventricle; SSC, side scatter.
Safety of UCB-MNC Intramyocardial Transplantation Throughout 3-Month Follow-Up
Clinical Exam
Vital signs did not show sustained differences between placebo and cell-based groups (p < .05) (supplemental online Fig. 2). Daily cage-side observations revealed five events for two animals, both in the control group. Animal L331 had a necrotic tail the day after thoracotomy that required surgical excision. This was likely because of the routine trauma of living in a pen of littermates and not attributable to any procedure-related activities. The same animal had a pocket abscess noted 7 days after ILR implantation. Antibiotics were given, and draining and cleaning was applied daily. Eventually, the device was displaced from the animal by day 28, and 2 weeks later the device was reimplanted when infection resolved. Finally, animal L375 developed swelling at the thoracotomy site 14 days after surgery that was resolved following standard veterinarian medical care and intramuscular antibiotics. Notably, there were no observations in the cell-therapy cohort noted, after scheduled independent clinical veterinarian examinations. There were no additional medical issues or concerns discovered outside of the daily cage-side observations according to predetermined veterinarian examinations.
Clinical Biochemistry, Hematology, and Coagulation
Blood samples were obtained according to predefined schedule to document the natural trends in the placebo control group and compare with the cell-based treatment group. There were insignificant sporadic values that were different between the two groups, yet no clinical chemistry values represented any evidence of systemic toxicity (Fig. 4A; supplemental online Table 1). Because the animals received either cell-based therapy or placebo injection, all randomized pigs were also monitored for cardiac damage caused by the 10–15 injections of 0.1-ml volume each by measuring plasma levels of cardiac enzymes. Troponin-T levels <0.01 ng/ml were considered below detectable levels as expected if no significant injury was caused by the injections. A single measurement was reported as being mildly elevated (0.03 ng/ml) in an animal within the control group at 2 weeks with CK-MB isoenzyme concurrently monitored not being elevated. This likely represented an inflammatory signal caused by a documented skin incision infection at this time within this animal. Furthermore, no differences were detected in the hematology and coagulation analyses between the placebo control and cell-based treatment groups throughout the 12-week follow-up study (Fig. 4B; supplemental online Table 2). Also, no abnormalities were observed on the hematology blood smears for the placebo control and cell-based treatment groups. Overall, there was no evidence of cardiac injury caused by the injections or biochemical deviation between the two groups as a result of cell-based treatment during 3 months of follow-up.
Figure 4.
Porcine blood tests at baseline and 2, 4, 8, and 12 weeks after thoracotomy. (A): Clinical biochemistry did not show any difference (p > .05) between groups after placebo/cell injection. (B): Umbilical cord blood-derived mononuclear cells transplanted into the porcine right ventricle did not alter either hematological or coagulation parameters. Control and cell-therapy group values are statistically not different (p > .05). We established the age-dependent baseline clinical chemistry and hematology values without clinically relevant differences between the groups. Blue curves belong to the cell-therapy group, and orange curves represent the placebo group. Abbreviations: ALP, alkaline phosphatase; ALT, alanine transaminase; AST, aspartate aminotransferase; Bb, bilirubin; BUN, blood urea nitrogen; GGT, γ-glutamyl transferase; LDH, lactate dehydrogenase; MCV, mean corpuscular volume; RBCC, red blood cell count; TG, triglycerides.
Cardiac Electrophysiology Showed No Differences Between Placebo and Cell-Therapy Groups
As expected, there was wide variation in heart rate and in component deflections and intervals in the serial recordings of 60 ECG tracings evaluated qualitatively over this study period, likely significantly influenced by the body size and lead positions. Notably, all pigs were in normal sinus rhythm at all times throughout the 3-month study procedures, without differences between the placebo and cell-therapy groups. Additionally, there were a total of four episodes of abnormal cardiac rhythm, occurring in 2 of the 12 animals monitored using ILR over 3 months that were determined by a blinded pediatric cardiologist specializing in electrophysiology. Animal L376 had two episodes of transient asystole with the first being recorded 30 minutes prior to thoracotomy at the time of anesthesia being induced and intubation and the second episode occurring 1 hour after cell injection at the time of extubation. This animal also had a 9-second tachycardia at day 29 after cell injection, recorded after sedation for a routine blood draw that was uneventful and self-limiting. A second animal, L372, also demonstrated a transient asystole episode 12 hours after intramyocardial injection with medical record noting normal behavior. No evidence of any morbidity or mortality was noted for the remainder of the 12-week follow-up.
Echocardiography Revealed No Cardiac Structure and Function Changes Between Groups
More than 4,000 echocardiography images from a total of 12 juvenile pigs were evaluated both qualitatively and quantitatively. The quality of the data collection was limited because of the body size variations range from 5 kg of body weight to 50 kg of body weight in the study period. Despite the technical limitations of data collection, data analysis was sufficient to determine that there were no specific changes in echocardiography images at any times compared with baseline recordings or between cohorts. The blinded echocardiography analysis did not reveal any evidence of structural abnormalities in the cell-based treatment group compared with placebo control group in this study (Fig. 5). Biventricular wall motion appeared vigorous and uncompromised throughout the study. Animal L372 had an apparent symmetrically thickened left ventricle at baseline (prior to receive the randomized cell therapy). This was the same animal that had an asystole episode 12 hours after cell delivery that was self-limiting and went unnoticed until the study was unblinded at the conclusion of data collection. Overall, cardiac imaging demonstrated normal cardiac function in the absence of structural heart disease and provided an age-dependent baseline for future studies.
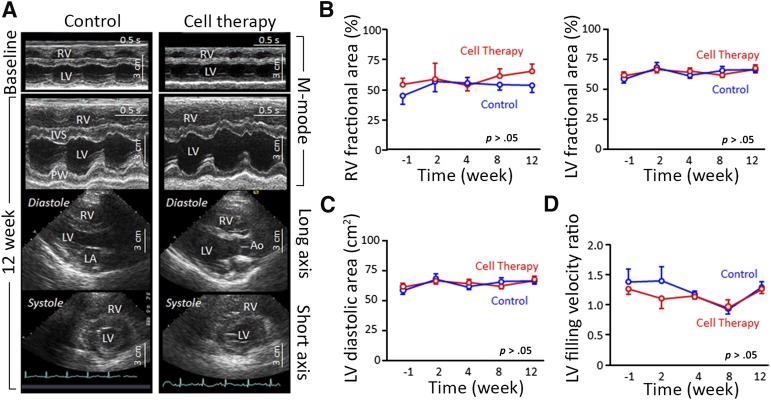
Figure 5.
Echocardiography studies show that autologous cell-therapy injection into the porcine right ventricle is safe. (A): Echocardiography images at baseline (M-mode) and at 12-week follow-up [M-mode and two-dimensional echocardiography views (long axis and short axis for the systolic phase)], with a normal electrocardiography record in both study groups. The left column represents the control animal group, and the right column represents the cell-therapy group. The images did not differ between groups. (B–D): There were no significant statistical differences (p > .05) between control (blue curves) and cell-therapy group (red curves) at −1, 2, 4, 8, and 12 weeks of follow-up of thoracotomy on the right and left ventricle fractional area change (B), on the left ventricle diastolic area (C), and on the early to late left ventricle filling velocity ratio (D). Abbreviations: Ao, aortic artery; IVS, interventricular septum; LA, left atrium; LV, left ventricle; PW, posterior wall; RV, right ventricle.
Histopathology Findings
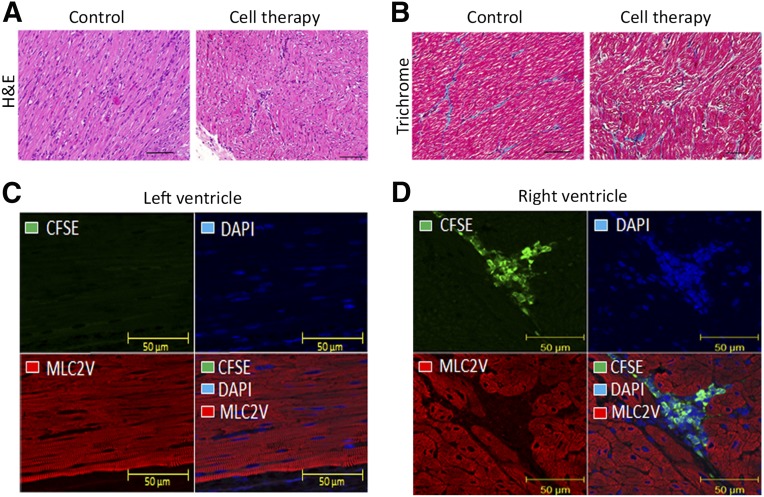
Microscopic histopathological findings correlated with the gross necropsy findings. The occurrence of adhesions of the epicardium, heart surface, right heart wall, pericardium, and thoracotomy site, thickened pericardium, pleural cavity fluid, and thoracotomy site swelling and fibrosis were considered to be consequences of the surgical procedure, because these findings occurred in the randomization and cell-tracking groups. Given this, no significant changes were noted in control or cell-treated animals at necropsy, and none were associated with either the cell or control arms of the experiment (Fig. 6A, 6B). The cell tracking group had the heart, lung, liver, spleen, aorta, and bone marrow evaluated for fluorescence of CFSE-labeled injected cells. No staining for injected cells were observed in any of the animals in any tissues examined, including the step-sectioned blocks from the RV at 24 hours (n = 2) and 1 week (n = 2) postinjection. However, the RV was positive for CFSE-labeled UCB-MNCs 1 hour after cell injection in the 4 animals evaluated. On the contrary, the left ventricle was negative for green cells at all time points (Fig. 6C, 6D).
Figure 6.
Microscopic images of the porcine right ventricle (RV) postmortem 3 months after placebo and cell infusion and autologous umbilical cord blood mononuclear cells (UCB-MNCs) tracking images in the porcine heart after CFSE labeling. (A): Light photomicrograph of RV cardiac tissue area of placebo/cell injection stained with H&E. (B): Trichrome staining 12 week post-thoracotomy (magnification, ×50). H&E staining did not show any lesions either in the control or in the cell-therapy group. Trichrome staining did not show an increase of tissue fibrosis. Sections of each group did not show any pathological changes in the RV region of the cells transplantation, being comparable with the control group. (C, D): Immunohistochemistry of cardiac tissue after 1 hour of CFSE-labeled autologous porcine UCB-MNCs injected into the RV of a 4-week-old piglet. Heart tissues were stained with CFSE in green, DAPI for cell nuclei are in blue, and MLC2V are in red. The left and right ventricles were analyzed by confocal laser scanning microscopy (magnification, ×40). (C): Photographs of left ventricle show no CFSE-labeled UCB-MNCs. MLC2V staining demonstrates that processed tissue is heart (ventricle). (D): The RV, the site of cell injection, is positive for CFSE-labeled UCB-MNCs (green fluorescence). CFSE staining with DAPI and MLC2V (bottom right panels) illustrates the density of UCB-MNCs in the location of cell administration. Abbreviations: CFSE, 5- (and -6)-carboxyfluorescein diacetate succinimidyl ester; DAPI: 4′,6-diamidino-2-phenylindole; H&E, hematoxylin and eosin; MLC2V, myosin light chain 2 ventricular antibody.
Discussion
The delivery of stem cells directly to the myocardium has safely been performed in a large number of experimental and clinical trials in the left heart ventricle of adult populations [24, 25]. However, congenital cardiac anomalies have not been the target of intramyocardial cell-based cardiac regenerative approaches. Given the critical importance of safety using a novel cell-based product and delivery strategy in a pediatric heart, this long-term safety study was designed and executed as a double-blinded, randomized controlled study in a porcine model system. The specific focus in this study was to evaluate at the pediatric stage of cardiac development the procedural feasibility and long-term risk of adverse effects on cardiovascular parameters, in addition to systemic effects following intramyocardial delivery of an autologous cell-based product. We selected to initially test UCB-MNCs because of the many advantages from an autologous yet available source in CHD. UCB is a well-established source of hematopoietic stem cells for bone marrow transplantation and is emerging as a vital source of nonhematopoietic cells [10] with an increased use in preclinical settings. UCB is a rich source of naïve progenitor cells and has demonstrated a higher proliferation capacity than the adult cell sources [26–28].
To simulate the autologous application of UCB for intramyocardial delivery in the pediatric setting, we established an analogous process that mirrored the essential clinical attributes in a large animal model system. Piglets were born by cesarean delivery to allow UCB collection of each individual piglet. The piglet weight is generally 2–4 times lower (0.5–1.5 kg) than a human newborn; therefore, cell recovery was anticipated to be lower than human cord blood units. We drained blood from arteries and vein to maximize the UCB volume collected, because of the small size of the animals. The porcine UCB was processed using the Ficoll density gradient separation and cryopreserved in 10% DMSO according to an analogous procedure tested and validated for human UCB in the GMP facility at Mayo Clinic. Every banked UCB unit was tested at the end of processing and met viability, MNC-percentage, and endotoxin-level release criteria, without showing significant differences with the human UCB units processed in parallel at Mayo Clinic facilities. Collection and processing of UCB is at risk of microbiological contamination. In this study, a single UCB unit of 26 (3.8%) had an anaerobic contamination of Corynebacterium spp. after 48 hours of culture and was excluded from the safety study. Thus, the feasibility of a porcine model for autologous UCB was established and recapitulated the anticipated quality control metrics for an equivalent birth weight in a pediatric setting.
The primary endpoint of this preclinical large animal study was to determine the safety profile of autologous UCB-MNCs with intramyocardial delivery during an open chest procedure. The cell-based and placebo products were thawed at 37°C at the surgery room and directly injected into the myocardium without a washing step leaving the 10% vol/vol DMSO cryoprotectant in the product as it was delivered into the myocardium. The justification for this procedure was to allow the most consistent and reproducible delivery at the time of anticipated clinical trials. A post-thaw washing step involves potential for technical errors in handling procedures and risks of cell loss and contamination [29]. However, DMSO can be toxic and damage progenitor cells at prolonged periods at room temperature. Huang et al. [30] recently published that the optimal length of time of cryopreserved UCB infusion should be no more than 20 minutes after thawing. Therefore, the UCB-MNCs were thawed as quickly as possible and delivered within 5 minutes. The intramyocardial route for delivery of UCB-MNCs required careful consideration for the thin myocardium of the RV. For the open chest delivery, we used a delivery device that allowed the needle track to be within the myocardial tissue and parallel to the epicardial/luminal tissue plane sufficient to inject 0.1 ml of volume with minimal leakage of the delivered product. In a preclinical study by Borenstein et al. [31], myogenic cells were implanted into the RV myocardium in a setting of pulmonary artery banding, and two animals were lost as a result of the several injections that presumably led to RV edema and failure. Because the surgeries done within our study were at the hands of an experienced pediatric cardiovascular surgeon, we effectively avoided bleeding or electrical disturbances caused by procedure-related complications and demonstrated proficiency within the team to safely deliver the cell-based product during an open chest procedure.
The porcine model system is commonly used for preclinical cardiovascular studies because of the anatomical and physiological similarities with humans. However, this animal model system has some challenges, such as the fact that swine hearts are relatively difficult to interrogate by cardiac echocardiography. These challenges were addressed with the addition of continuous loop records and clinical team members from pediatric electrophysiology and echocardiography. During the 3-month follow-up, the data showed no significant acute or chronic cardiac injury pattern caused by the intramyocardial delivery as measured by cardiac enzyme levels of Troponin-T, unlike previous reports that involved an ischemic cardiac porcine model system [32].
Continuous cardiac monitoring revealed four events in two animals of cell-therapy group. Three of the four events were transient and subclinical asystole in the perioperative period that likely were due to hypersensitivity of anesthesia and vagal stimulation during endotracheal intubation/extubation.
Based on time-stamped recordings and medical records, these events were not attributed to cell delivery, and clinically the animals never displayed any health concerns with veterinarian care teams monitoring daily. Furthermore, clinical echocardiology data indicated that there were no specific changes in echocardiography images at any time compared with baseline recordings or between the cell-based and placebo test subjects, despite the challenges of echocardiology data collection. One animal in the cell-based treatment group had an apparent symmetrically thickened left ventricle at baseline, prior to randomization and receiving the cell-based product. Interestingly, this was the same animal that had a self-limiting asystole episode 12 hours after surgery and went unnoticed until the study was unblinded. It is not clear whether cardiac hypertrophy could be a risk factor for cardiac ectopy during cell delivery with a single episode; however, this will be continuously monitored in ongoing studies. Supporting the evidence of a lack of adverse outcomes, macroscopic changes noted at the time of scheduled necropsy were consistent with surgical procedure of right thoracotomy causing scaring and fibrosis in the epicardium across all test subjects. The cell-based or placebo injections were not associated with any detectable evidence of cardiac or noncardiac toxicity upon microscopic analysis. Consistent with these findings, Yerebakan et al. [23] evaluated the efficacy of intramyocardial autologous UCB-MNC transplantation on RV function in a 4-month-old sheep model of chronic RV volume overload. They reported that the area of fibrosis did not show any significant difference between the placebo and stem cell groups, although a detailed analysis of toxicology was not completed in this reported study, and no evidence of acute physiological, biochemical, or electromechanical complications was encountered during the procedure.
Histology analysis of a nonrandomized cohort used to track CFSE-labeled cell product did not reveal significant signal beyond 1 week of intramyocardial delivery. The lack of long-term cellular engraftment was not surprising, because multiple studies have failed to demonstrate long-term engraftment of MNCs in adult studies and a modest ratio of transplanted cells in preclinical studies [22, 33]. Future studies will benefit from optimized protocols for tracking the labeled cells in the setting of autologous cells not requiring immunosuppression therapy. Furthermore, protocols that improve the cellular retention and engraftment may offer significant advantages in the field of CHD regenerative strategies.
Conclusion
To our knowledge, this is the first double-blinded, randomized safety study using autologous UCB-MNCs in a large animal model system focused on long-term RV outcomes emulating the pediatric setting. Injection of autologous UCB-MNC into the RV was not associated with any detectable adverse events following this clinical-grade procedure. The final analysis revealed no statistical differences between the cohorts receiving the autologous cell-based product and placebo control injections, and all animals survived the procedures without serious medical complications. This safety study demonstrates that porcine autologous UCB-MNCs can be safely collected and surgically delivered in early stages of development and should establish the foundation to advance new therapeutic modalities aiming toward clinical trials in those affected with CHD. Future dedicated studies will be required to establish a diseased model system to properly monitor efficacy of cell-based therapy in enhancing right ventricular function in a setting to recapitulate CHD.
Supplementary Material
Acknowledgments
We thank Adam Armstrong for assistance with endotoxin testing and Diane M. Jech for help with data acquisition of transthoracic echocardiography. This work was supported by the Todd and Karen Wanek Program for Hypoplastic Left Heart Syndrome and the Mayo Clinic Foundation. Wanek Program Porcine Pipeline Group: Sarah L. Edgerton, Scott H. Suddendorf, Steve Krage, Mindy Rice, Joseph A. Rysavy, Joanna M. Powers, Boyd W. Rasmussen, Jennifer M. Miller, Traci L. Paulson, Rebecca K. Lindquist, Chelsea L. Reece, Angela R. Miller, Douglas J. Padley, Mark A. Wentworth, Alexander C. Greene, and Amy G. Andrews with direction from the Wanek Program leadership of Timothy J. Nelson, Patrick W. O’Leary, Timothy M. Olson, and Andre Terzic.
Author Contributions
S.C.P. and H.M.B.: conception and design, provision of study material or patients, collection and/or assembly of data, data analysis and interpretation, manuscript writing, final approval of manuscript; S.O.: conception and design, collection and/or assembly of data, data analysis and interpretation; S.Y. and B.C.C.: collection and/or assembly of data, data analysis and interpretation; S.L.N.: provision of study material or patients; X.L.: data analysis and interpretation; P.W.O. and A.T.: conception and design; T.J.N.: conception and design, financial support, provision of study material or patients, collection and/or assembly of data, data analysis and interpretation, manuscript writing, final approval of manuscript.
Disclosure of Potential Conflicts of Interest
B.C.C. has a compensated consultancy with Medtronic. The other authors indicated no potential conflicts of interest.
References
- 1.Stamm C, Westphal B, Kleine H-D, et al. Autologous bone-marrow stem-cell transplantation for myocardial regeneration. Lancet. 2003;361:45–46. doi: 10.1016/S0140-6736(03)12110-1. [DOI] [PubMed] [Google Scholar]
- 2.Hendrikx M, Hensen K, Clijsters C, et al. Recovery of regional but not global contractile function by the direct intramyocardial autologous bone marrow transplantation: results from a randomized controlled clinical trial. Circulation. 2006;114(suppl):I101–I107. doi: 10.1161/CIRCULATIONAHA.105.000505. [DOI] [PubMed] [Google Scholar]
- 3.Pokushalov E, Romanov A, Chernyavsky A, et al. Efficiency of intramyocardial injections of autologous bone marrow mononuclear cells in patients with ischemic heart failure: A randomized study. J Cardiovasc Transl Res. 2010;3:160–168. doi: 10.1007/s12265-009-9123-8. [DOI] [PubMed] [Google Scholar]
- 4.Ang K-L, Chin D, Leyva F, et al. Randomized, controlled trial of intramuscular or intracoronary injection of autologous bone marrow cells into scarred myocardium during CABG versus CABG alone. Nat Clin Pract Cardiovasc Med. 2008;5:663–670. doi: 10.1038/ncpcardio1321. [DOI] [PubMed] [Google Scholar]
- 5.van Ramshorst J, Bax JJ, Beeres SL, et al. Intramyocardial bone marrow cell injection for chronic myocardial ischemia: A randomized controlled trial. JAMA. 2009;301:1997–2004. doi: 10.1001/jama.2009.685. [DOI] [PubMed] [Google Scholar]
- 6.Zhao Q, Sun Y, Xia L, et al. Randomized study of mononuclear bone marrow cell transplantation in patients with coronary surgery. Ann Thorac Surg. 2008;86:1833–1840. doi: 10.1016/j.athoracsur.2008.08.068. [DOI] [PubMed] [Google Scholar]
- 7.Rupp S, Bauer J, Tonn T, et al. Intracoronary administration of autologous bone marrow-derived progenitor cells in a critically ill two-yr-old child with dilated cardiomyopathy. Pediatr Transplant. 2009;13:620–623. doi: 10.1111/j.1399-3046.2008.01024.x. [DOI] [PubMed] [Google Scholar]
- 8.Rupp S, Jux C, Bönig H, et al. Intracoronary bone marrow cell application for terminal heart failure in children. Cardiol Young. 2012;22:558–563. doi: 10.1017/S1047951112000066. [DOI] [PubMed] [Google Scholar]
- 9.Rupp S, Zeiher AM, Dimmeler S, et al. A regenerative strategy for heart failure in hypoplastic left heart syndrome: Intracoronary administration of autologous bone marrow-derived progenitor cells. J Heart Lung Transplant. 2010;29:574–577. doi: 10.1016/j.healun.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 10.Harris DT, Rogers I. Umbilical cord blood: A unique source of pluripotent stem cells for regenerative medicine. Curr Stem Cell Res Ther. 2007;2:301–309. doi: 10.2174/157488807782793790. [DOI] [PubMed] [Google Scholar]
- 11.Nieda M, Nicol A, Denning-Kendall P, et al. Endothelial cell precursors are normal components of human umbilical cord blood. Br J Haematol. 1997;98:775–777. doi: 10.1046/j.1365-2141.1997.2583074.x. [DOI] [PubMed] [Google Scholar]
- 12.Pelosi E, Castelli G, Martin-Padura I, et al. Human haemato-endothelial precursors: Cord blood CD34+ cells produce haemogenic endothelium. PLoS One. 2012;7:e51109. doi: 10.1371/journal.pone.0051109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gluckman E, Broxmeyer HA, Auerbach AD, et al. Hematopoietic reconstitution in a patient with Fanconi’s anemia by means of umbilical-cord blood from an HLA-identical sibling. N Engl J Med. 1989;321:1174–1178. doi: 10.1056/NEJM198910263211707. [DOI] [PubMed] [Google Scholar]
- 14.Henning RJ, Abu-Ali H, Balis JU, et al. Human umbilical cord blood mononuclear cells for the treatment of acute myocardial infarction. Cell Transplant. 2004;13:729–739. doi: 10.3727/000000004783983477. [DOI] [PubMed] [Google Scholar]
- 15.Henning RJ, Burgos JD, Ondrovic L, et al. Human umbilical cord blood progenitor cells are attracted to infarcted myocardium and significantly reduce myocardial infarction size. Cell Transplant. 2006;15:647–658. doi: 10.3727/000000006783981611. [DOI] [PubMed] [Google Scholar]
- 16.Henning RJ, Burgos JD, Vasko M, et al. Human cord blood cells and myocardial infarction: Effect of dose and route of administration on infarct size. Cell Transplant. 2007;16:907–917. doi: 10.3727/096368907783338299. [DOI] [PubMed] [Google Scholar]
- 17.Hirata Y, Sata M, Motomura N, et al. Human umbilical cord blood cells improve cardiac function after myocardial infarction. Biochem Biophys Res Commun. 2005;327:609–614. doi: 10.1016/j.bbrc.2004.12.044. [DOI] [PubMed] [Google Scholar]
- 18.Ma N, Stamm C, Kaminski A, et al. Human cord blood cells induce angiogenesis following myocardial infarction in NOD/scid-mice. Cardiovasc Res. 2005;66:45–54. doi: 10.1016/j.cardiores.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 19.Pinho-Ribeiro V, Maia AC, Werneck-de-Castro JP, et al. Human umbilical cord blood cells in infarcted rats. Braz J Med Biol Res. 2010;43:290–296. doi: 10.1590/s0100-879x2010007500007. [DOI] [PubMed] [Google Scholar]
- 20.Hu CH, Wu GF, Wang XQ, et al. Transplanted human umbilical cord blood mononuclear cells improve left ventricular function through angiogenesis in myocardial infarction. Chin Med J (Engl) 2006;119:1499–1506. [PubMed] [Google Scholar]
- 21.Wu KH, Zhou B, Yu CT, et al. Therapeutic potential of human umbilical cord derived stem cells in a rat myocardial infarction model. Ann Thorac Surg. 2007;83:1491–1498. doi: 10.1016/j.athoracsur.2006.10.066. [DOI] [PubMed] [Google Scholar]
- 22.Davies B, Elwood NJ, Li S, et al. Human cord blood stem cells enhance neonatal right ventricular function in an ovine model of right ventricular training. Ann Thorac Surg. 2010;89:585–593. doi: 10.1016/j.athoracsur.2009.10.035. [DOI] [PubMed] [Google Scholar]
- 23.Yerebakan C, Sandica E, Prietz S, et al. Autologous umbilical cord blood mononuclear cell transplantation preserves right ventricular function in a novel model of chronic right ventricular volume overload. Cell Transplant. 2009;18:855–868. doi: 10.3727/096368909X471170. [DOI] [PubMed] [Google Scholar]
- 24.Donndorf P, Kundt G, Kaminski A, et al. Intramyocardial bone marrow stem cell transplantation during coronary artery bypass surgery: A meta-analysis. J Thorac Cardiovasc Surg. 2011;142:911–920. doi: 10.1016/j.jtcvs.2010.12.013. [DOI] [PubMed] [Google Scholar]
- 25.Wen Y, Meng L, Xie J, et al. Direct autologous bone marrow-derived stem cell transplantation for ischemic heart disease: A meta-analysis. Expert Opin Biol Ther. 2011;11:559–567. doi: 10.1517/14712598.2011.560567. [DOI] [PubMed] [Google Scholar]
- 26.Lansdorp PM, Dragowska W, Mayani H. Ontogeny-related changes in proliferative potential of human hematopoietic cells. J Exp Med. 1993;178:787–791. doi: 10.1084/jem.178.3.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lu L, Xiao M, Shen RN, et al. Enrichment, characterization, and responsiveness of single primitive CD34 human umbilical cord blood hematopoietic progenitors with high proliferative and replating potential. Blood. 1993;81:41–48. [PubMed] [Google Scholar]
- 28.Traycoff CM, Abboud MR, Laver J, et al. Evaluation of the in vitro behavior of phenotypically defined populations of umbilical cord blood hematopoietic progenitor cells. Exp Hematol. 1994;22:215–222. [PubMed] [Google Scholar]
- 29.Laroche V, McKenna DH, Moroff G, et al. Cell loss and recovery in umbilical cord blood processing: A comparison of postthaw and postwash samples. Transfusion. 2005;45:1909–1916. doi: 10.1111/j.1537-2995.2005.00638.x. [DOI] [PubMed] [Google Scholar]
- 30.Huang L, Song G-Q, Wu Y, et al. Optimal length of time of cryopreserved umbilical cord blood infusion after thawing. Hematology. 2014;19:73–79. doi: 10.1179/1607845413Y.0000000101. [DOI] [PubMed] [Google Scholar]
- 31.Borenstein N, Jian Z, Fromont G, et al. Noncultured cell transplantation in an ovine model of right ventricular preparation. J Thorac Cardiovasc Surg. 2005;129:1119–1127. doi: 10.1016/j.jtcvs.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 32.Goodchild T, Pang W, Tondato F, et al. Safety of intramyocardial injection of autologous bone marrow cells to treat myocardial ischemia in pigs. Cardiovasc Revasc Med. 2006;7:136–145. doi: 10.1016/j.carrev.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 33.Bhakta S, Greco NJ, Finney MR, et al. The safety of autologous intracoronary stem cell injections in a porcine model of chronic myocardialischemia. [published correction appears in J Invasive Cardiol 2006;18:297] J Invasive Cardiol. 2006;18:212–218. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.