This review summarizes recent advances in biomaterials engineering, with a particular focus on hydrogel scaffolds, and their potential applications in the development of in vitro stem cell niche models and for in vivo tissue engineering and regenerative medicine. Engineered hydrogel scaffolds, with their highly tailorable mechanical and biochemical properties, offer great promise in addressing the current clinical challenges associated with translating stem cell-based therapies.
Keywords: Stem cell, Niche, Hydrogel, Scaffold, Tissue engineering, Bioengineering
Abstract
The potential for the clinical application of stem cells in tissue regeneration is clearly significant. However, this potential has remained largely unrealized owing to the persistent challenges in reproducibly, with tight quality criteria, and expanding and controlling the fate of stem cells in vitro and in vivo. Tissue engineering approaches that rely on reformatting traditional Food and Drug Administration-approved biomedical polymers from fixation devices to porous scaffolds have been shown to lack the complexity required for in vitro stem cell culture models or translation to in vivo applications with high efficacy. This realization has spurred the development of advanced mimetic biomaterials and scaffolds to increasingly enhance our ability to control the cellular microenvironment and, consequently, stem cell fate. New insights into the biology of stem cells are expected to eventuate from these advances in material science, in particular, from synthetic hydrogels that display physicochemical properties reminiscent of the natural cell microenvironment and that can be engineered to display or encode essential biological cues. Merging these advanced biomaterials with high-throughput methods to systematically, and in an unbiased manner, probe the role of scaffold biophysical and biochemical elements on stem cell fate will permit the identification of novel key stem cell behavioral effectors, allow improved in vitro replication of requisite in vivo niche functions, and, ultimately, have a profound impact on our understanding of stem cell biology and unlock their clinical potential in tissue engineering and regenerative medicine.
Introduction
Stem cells are defined by their distinctive capability to self-renew and produce differentiated progeny during development and throughout the entire life of an organism. Owing to their unique abilities, stem cells have rapidly been identified as an unprecedented source of clinically relevant differentiated cells for application in tissue engineering and regenerative medicine [1] and as in vitro (disease) models for drug discovery and trials [2]. Despite extensive research and our ever-growing knowledge in stem cell biology, the field is still confronted by a lack of reproducible and reliable methods to control stem cell behavior. Perhaps the greatest challenges that the field is currently facing are (a) to maintain and expand adult stem cells in vitro because of difficulties replicating interactions with the microenvironment that are essential for stem cell function and maintenance [3]; (b) to rationally control stem cell differentiation into defined mature cell types in vitro and/or in vivo that display physiological function [4]; and (c) to engineer multicellular constructs that recapitulate tissue-like (or organ-like) physiological function.
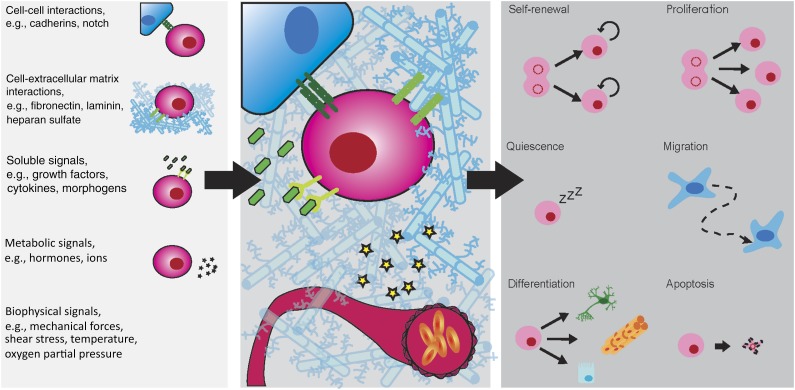
In vivo, stem cells are known to reside in highly specialized microenvironments—termed “niches”—which govern and tightly regulate their fate (Figure 1). A crucial function of the niche is to maintain a constant pool of stem cells and dynamically balance their self-renewal and differentiation to ensure tissue and organ homeostasis or regenerate damaged tissues on injury. The loss of the niche induces the loss of stem cells, which then impairs tissue and organ maintenance and the regenerative capabilities. In their niche, the stem cells are surrounded by supportive cells, the extracellular matrix (ECM) and interstitial fluids. They are thus exposed to a multitude of extrinsic factors such as cell-cell interactions, cell-ECM interactions, physicochemical stimuli (i.e., temperature, partial oxygen pressure), and soluble or ECM-tethered stimuli (i.e., growth factors, cytokines). Moreover, temporally and spatially regulated presentation of these stimuli is known to instruct stem cell fate [5]. Stem cell biology is clearly extremely complex, and stem cells display exquisite sensitivity to microenvironmental signals. To further increase our understanding of the mechanisms that regulate stem cell fate, methods that allow systematic probing of stem cell responses to isolated effectors of a complex and multifaceted system are critical.
Figure 1.
Schematic representation of the stem cell niche and underlying regulatory mechanisms. A large variety of factors (left) present in the stem cell niche are known to tightly regulate stem cell behavior and fate choice. In vivo stem cells reside in anatomically defined location, the stem cell niche (center). The niche is a multifaceted entity (right).
During the past decade, innovative developments in materials science, microfabrication, and associated technologies have enabled in vitro culture systems that allow key properties of the culture environment to be systematically modified. We are now able to manipulate the stem cell microenvironment with greater precision and, further, to monitor effector impacts on stem cells with high resolution in both time and space [6]. Stem cell biology is thus poised to greatly benefit from such advances. Advances in biomaterial science, in particular, the development of synthetic hydrogels, offer significant promise in the field of tissue engineering. The increasing ability to engineer and tailor hydrogel scaffolds provides exciting possibilities to deconstruct the niche and tease out essential elements toward the fabrication of artificial microenvironments capable of controlling stem cell fate in a manner not previously possible [7].
In the present review, we provide a comprehensive synopsis of recent developments in bioengineered hydrogel scaffolds and discuss their emerging applications in probing and directing stem cell biology and tissue regeneration. We emphasize how biomaterials and their potential to emulate the various aspects of the stem cell niche will affect our understanding of the complex mechanisms that regulate stem cell behavior. With the increasing capabilities to engineer advanced biomaterials, we also highlight the recent development of high-throughput methods to generate scaffold microarrays and their application to elucidate the complex interplay that governs stem cell fate. Finally, we present a perspective on future developments in the field and discuss how bioengineering approaches could significantly affect stem cell applications in tissue engineering and regenerative medicine.
Advanced Synthetic Biomaterials to Emulate the Stem Cell Microenvironment
During the past decade, it has been shown that three-dimensional (3D) culture models offer a more physiologically relevant cell culture method, and they have been proposed as intermediate models that can bridge the gap between conventional in vitro culture and in vivo models [8]. Strikingly, 3D culture in naturally derived hydrogels (high-water-content cross-linked proteaceous networks), such as Matrigel (BD Biosciences, San Diego, CA, http://www.bdbiosciences.com) or collagen matrices, has been show to drive cellular self-organization and complex morphogenetic processes to result in sophisticated in vitro models (reviewed in [9–11]). These models offer unprecedented means to study tissue morphogenesis and physiologically relevant in vitro models for drug screening. However, current methods mostly rely on naturally derived materials that fail to enable fine and controlled manipulation of matrix parameters and culture conditions. Thus, synthetic materials, owing to their ability to be engineered to engender the desired biophysical and biochemical properties, can readily be designed to modulate microenvironmental parameters to guide self-assembly of complex tissues from stem cells. Additionally, for clinical translation of such products, all components must be shown to be nontoxic, degradable in or elutable from the body, and manufactured to the highest quality standards, ideally under good manufacturing practice (GMP) conditions, providing advantages for synthetic hydrogels compared with those biologically derived.
Synthetic hydrogels have been engineered to display physicochemical properties reminiscent of the natural cell microenvironment. The ideal 3D culture model must meet several criteria [12]: (a) simple and reproducible fabrication, (b) transparent to allow visualization and imaging, (c) present controlled structural and mechanical properties, (d) potential for presentation of biochemical cues such as tethered adhesion ligands or bioactive molecules (i.e., cytokines, growth factors), (e) cell responsive with regard to cell-mediated degradation or the capture of cell-secreted biomolecules, and (f) allow for modularity and tailoring of scaffold properties independently from each other and for a wide range of physiologically relevant values.
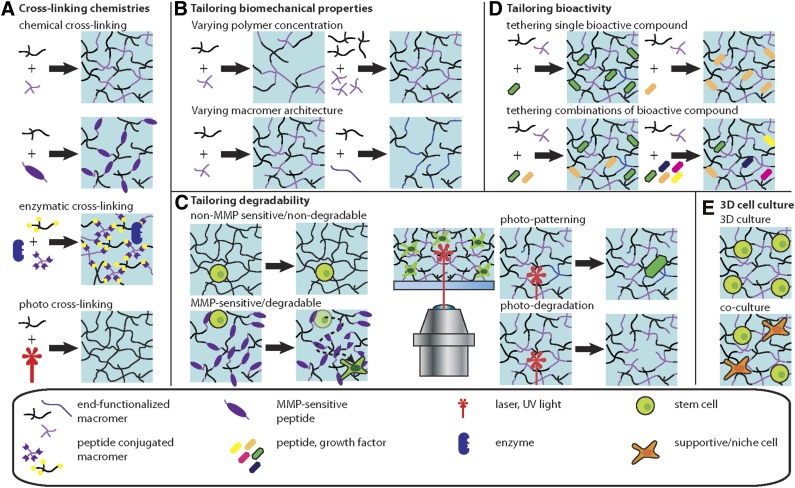
Lessons from developmental biology have significantly contributed to directed differentiation of pluripotent stem cells [13]. Similarly, bridging the gap between tissue engineering and developmental biology offers great promise [14]. We now highlight recent biomaterial developments (Figure 2) and discuss their application in stem cell biology [15–18] (Figure 3); in particular, how they present interesting methods to deconstruct the complex regulatory mechanism of the niche.
Figure 2.
Schematic representation of synthetic hydrogel engineering to emulate the stem cell niche. (A): Cross-linking chemistries. Hydrogel scaffolds are generated from prepolymer solutions, and various cross-linking schemes can be used, such as chemical, enzymatic, or photo-reactions. (B): Tailoring biomechanical properties. Synthetic hydrogels can be tuned to generate hydrogel scaffolds with defined physicochemical properties. Increasing the polymer concentration typically results in hydrogels with increased mechanical properties, including stiffness. Hydrogels formed from macromers of varying architecture (i.e., linear, branched, or multiarm) display different mechanical properties. (C): Tailoring degradability. Depending on the cross-linking reaction, hydrogel networks can display degradable or nondegradable behavior (via hydrolysis). For example, the integration of an MMP-sensitive peptide sequence in the polymer network renders the hydrogel susceptible to cell-mediated degradation via the action of cell-secreted MMP enzymes. Light-triggered reactions have been developed to modify hydrogel scaffolds (photo-patterning of bioactive molecules or local degradation of the polymer networks), enabling alteration of the hydrogel properties at any given point during the course of the cell culture, permitting temporal cues to the stem cells. (D): Tailoring bioactivity. Bioactive compounds, such as adhesion ligands or growth factors, can be covalently tethered to the hydrogel network by consuming reactive groups of the macromer (these will thus not contribute to the network formation). This simple scheme can be readily used to bind single factors or any combination of factors. The introduction of orthogonal chemistries can provide enhanced temporal control over functionalization and cross-linking. (E): 3D cell culture. Hydrogels can be cross-linked in the presence of cells, thus, presenting more appropriate 3D culture models, in terms of mimicking in vivo microenvironments. Abbreviations: 3D, three-dimensional; MMP, matrix metalloproteinase.
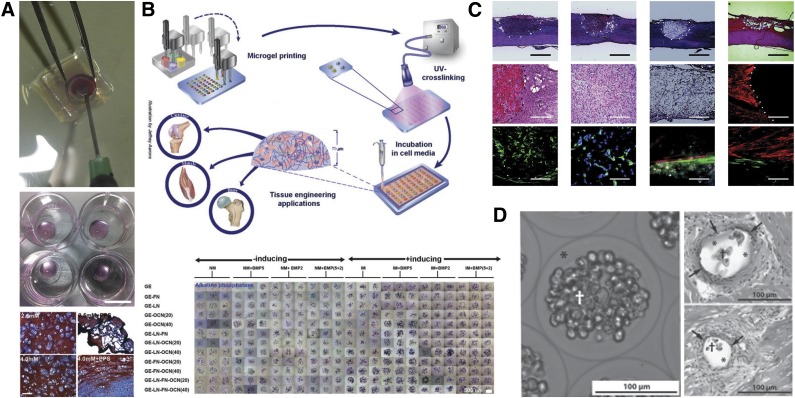
Figure 3.
Examples of applications of hydrogel to stem cell biology and translational medicine. (A): Injectable enzymatically (horseradish peroxidase) cross-linked hydrogel for in vitro and in vivo encapsulation of mesenchymal stem cells (MSCs). Adapted from [15]. (B): High-throughput generation of miniaturized and combinatorial cell-laden microgel arrays. This method was demonstrated to enable the screen of various biomaterials in combination with selected soluble factors, to a total of 96 independent conditions in a single assay, for their MSC osteogenic-inductive potential. Adapted from [16] with permission. (C): Synthetic methacrylate-based hydrogel encapsulating MSC implants to bridge acute spinal cord injury. Adapted from [17] with permission. (D): Enhanced stem cell (MSCs) intracoronary infusion in alginate shells for treatment of acute myocardial infarct. Adapted from [18].
Cross-Linking Chemistries
To produce a hydrogel network from synthetic polymer chains, a method must be applied to create cross-links between functional groups in the polymers (Figure 2A). If encapsulation is required, this reaction must be performed in a solution containing the desired cells. Various cross-linking schemes, such as Michael-type addition [19] or “click” chemistry [20], have been used to fabricate polymeric hydrogel networks. However, in many cases, chemical cross-linking requires harsh conditions or results in the production of toxic side products, which are clearly not ideal for (stem) cell encapsulation. Stem cells are very sensitive to their microenvironment; thus, it is critical to devise mild (cell-friendly) cross-linking chemistries that do not impair cell survival or alter cellular behavior.
Of the available cross-linking methods that meet these requirements, light-triggered hydrogel cross-linking shows much promise, because it enables manipulation and controlled in situ gelation, a method that is also very appealing from a clinical perspective (reviewed in 21]). However, the method has raised concerns regarding the toxicity of the photo-initiators and UV light itself, and also affects the genetic integrity of cells under extended UV exposure. A water-soluble and nontoxic photo-initiator recently introduced by Fairbanks et al. [22], promises to overcome some limitations (solubility and cytotoxicity) and concerns and has been demonstrated to support encapsulated human mesenchymal stem cells (hMSCs) [23].
Enzymatic reactions have also been proposed as a cell-friendly method to cross-link hydrogels, because they can be performed under physiological conditions and often display a high degree of specificity [12]. To this end, Ehrbar et al. [24] devised a “fibrin-clotting analog” hydrogel cross-linking scheme. Complimentary short peptide sequences acting as a substrate for the activated coagulation factor XIII (FXIIIa) (and comprising a short matrix metalloproteinase [MMP]-sensitive sequence) were conjugated to multiarm poly(ethylene glycol) (PEG) macromers and biomolecules (e.g., the cell-adherent RGD peptide and recombinant truncated vascular endothelial growth factor). This system could be readily cross-linked in situ and in near physiological conditions and was shown to promote angiogenesis in an embryonic chick chorioallantoic membrane assay [24]. The same system has shown utility in a flexible and versatile layer-by-layer deposition and patterning method that generated artificial vascularized bone-like structures [25].
Our group and others have used the enzymatic reaction of horseradish peroxidase (HRP) to cross-link hydrogels [26, 27]. HRP is advantageous compared with the FXIIIa system owing to its smaller size (better diffusive properties) and ease of production. The HRP reaction is triggered by the addition of H2O2 and covalently cross-links tyrosine residues, which requires careful titration of the HRP/H2O2 ratio to minimize cell toxicity and could raise issues with the specificity of the reaction [26]. HRP was demonstrated to enable the fabrication of hybrid gelatin/PEG hydrogels [27] or directly incorporate any tyrosine containing biomolecules, such as fibronectin, into the gel without the need for prefunctionalization, a substantial advantage for clinical translation [26]. These hydrogels were shown to support hMSC survival, proliferation, and differentiation in vitro and in vivo [15, 26] (Figure 3A).
Another advantage of enzyme-mediated cross-linking schemes is that they are capable of initiating covalent integration of the injected hydrogel into host tissue. Enzymes that have been derived from nature are active at physiological conditions and highly biocompatible. These enzyme-mediated reactions can thus be used to cross-link the hydrogel to the local ECM in the host tissue site, permitting rapid integration of the construct and immediately improving its contribution to the structure and mechanical properties of the damaged tissue site [15, 27]. Furthermore, owing to the ability to mechanically match the injected material with the mechanical properties of the host tissue, at the same time permitting mechanical integration with the surrounding tissue, this cross-linking method thus offers significant advantages compared with many others.
Tailoring Biomechanical Properties
A significant body of evidence has now suggested that the mechanical properties of biomaterials, such as Young’s moduli [28] and viscoelastic properties [29], can elicit significant effects on stem cell behavior. ECM-derived hydrogels, such as fibrin, collagen gels, or Matrigel offer no (or minimal) possibilities to alter their mechanical properties in a controlled fashion. Synthetic biomaterials, such as PEG macromers, are rapidly becoming the preferred candidates as primary structural units, because their architecture and molecular weight are modular and can be tightly controlled. By selecting the polymer network building components, it is possible to generate hydrogels with distinct bulk mechanical properties or even transition from homogeneous to phase-separated networks at the nanoscale [30] (Figure 2B).
Tailoring Degradability
The rate and mechanism by which synthetic hydrogels degrade has seen significant evolution during the past decade, from uncontrolled (and potentially catalyzed) degradation through, for example, hydrolysis of ester bonds throughout the hydrogels, to highly specific degradation of cross-links via the incorporation of MMP-sensitive cross-linkers (Figure 2C). When coupled with photochemistry, such systems have been shown to enable patterning and alteration of synthetic polymer networks in a very controlled fashion. Using “click” chemistry, Singh et al. [31] synthesized a peptide functionalized PEG hydrogel in which mechanical modulus and cell-adhesive properties were tuned to assess their effect on tumor cell growth. Integration of an MMP-sensitive cross-linker to complement a multiarm PEG macromer conjugated with norbornene end-group moieties was used to study tumor cell migration [32]. In that study, the architecture of the polymer network was intentionally designed to yield pore sizes much smaller than the cell diameter, such that migration would be limited to only proteolytic mechanisms. Similar chemistry was used by Anderson et al. to encapsulate hMSCs in cell-responsive (MMP-mediated degradation) PEG-based hydrogels [33]. A significant correlation between MMP-mediated degradation of the hydrogel with enhanced differentiation of hMSCs was demonstrated. A similar photochemistry-based method was also shown to enable site-specific degradation of hydrogels [34], which offers a method to manipulate mechanical properties of the matrix at any given point during 3D cell culture.
Tailoring Bioactivity
Biomolecular signaling is of the upmost importance in governing many biological and cellular processes and plays a key role in directing stem cell fate. In the context of the stem cell niche, biomolecular signaling comprises interactions of the stem cells with the surrounding ECM as soluble or ECM-bound factors. The binding of proteins to the ECM is a mechanism to sequestrate soluble proteins via electrostatic interactions, to present them to cells on demand, and to protect them from (enzymatic) degradation and increase their bioactivity [35].
Synthetic approaches, such as the use of manufactured peptides, are often preferred to the direct bioconjugation of native macromolecules, owing to concerns with production costs and preservation of bioactivity (Figure 2D). The use of engineered peptides as a component of engineered biomaterials is now a standard technique to confer these materials with cell-instructive and -responsive properties. Typical examples include the presentation of cell-adhesive domains derived from collagen (DGEA, RGD), laminin (IKVAV, RGD, YIGSR), and fibronectin (REDV, RGDS), as well as growth factor-binding domains to enable specific tethering and the incorporation of MMP-sensitive sequences in the polymer network [19].
In many cases, providing the necessary cues to direct a given biological process as a bulk condition is not sufficient. The temporal and spatial presentation of biomolecules such as morphogen gradients is known to play a key role in morphogenesis. The recent introduction of photo-patterning techniques offers an elegant means to overcome this limitation and provide an exquisite method to manipulate, in time and space, the presentation of tethered biomolecular cues to encapsulated cells [20, 36] (reviewed in [21, 37]).
More recently, the incorporation of ECM-binding peptides has been proposed to recruit and retain in situ ECM proteins secreted by cells encapsulated within the scaffold. A collagen-binding peptide sequence was integrated in alginate hydrogels by Lee et al. [38]. The high affinity of the peptide sequence derived from the collagen-binding domain of osteopontin was shown to promote osteogenesis in vitro and in vivo. A similar approach by Robert et al. [39] described a hyaluronic acid binding PEG-based hydrogel that was shown to improve neocartilage formation from encapsulated chondrocytes in vitro. These novel approaches offer great promise for application in tissue engineering, because they elegantly provide a simple and generic method for encapsulated cells to remodel their microenvironment.
An extensive review of the multitude of bioconjugation schemes and their use within hydrogel scaffolds is beyond the scope of the present review. Additional detailed information is given in [7, 40].
High-Throughput Methods to Deconstruct the Stem Cell Niche and Enhance Differentiation Outcomes In Vitro
Cellular and ECM microarray approaches offer high-throughput and combinatorial methods to screen biomaterials and probe their biological effect on stem cell maintenance and differentiation [41]. Robotic spotting of polymer precursor libraries on a glass substrate followed by in situ UV polymerization is an efficient and reproducible method to generate combinatorial and miniaturized arrays of unique cell culture substrates [42]. This method was applied to probe the maintenance and expansion of human pluripotent stem cells (hPSCs) [43, 44]. The importance of tackling the issue of current state-of-the-art culture of hPSCs, which still relies on less-than-optimal feeder cells or Matrigel, has been extensively discussed previously [45, 46].
A similar robotic microarray spotting technology (Figure 3B) was developed to screen for 3D hydrogel microenvironments [16]. In that report, deposition of nanoliter droplets containing cells suspended in methacrylated gelatin and various ECM proteins followed by in situ UV polymerization enabled the fabrication of a combinatorial microarray of cell-laden hydrogels. These arrays were then cultured in various differentiation media, which enabled the investigators to probe their approach to define optimal osteogenic culture conditions for encapsulated human mesenchymal stem cells. This simple, rapid, and cost-effective method has been demonstrated to enable high-throughput screening for multiplexed 3D culture conditions and thus should be readily applicable to other stem cells. However, it is important to note that, depending on the material selection, rapid sedimentation of cells through the hydrogel precursor solution to the underlying solid interface can occur, in particular, if using low-viscosity precursor solutions. Thus, the cells might not necessarily be homogenously embedded in the hydrogel after cross-linking.
More recently, Ranga et al. [47] reported an automated nanoliter liquid-dispensing technology that enabled them to simultaneously generate more than 1,000 unique hydrogel microenvironments. Their 3D niche microarray was shown to allow probing individually or in complex combinations of the effect of biomolecular, biochemical, and mechanical signals. To demonstrate its relevance, the investigators used their method to draw a comprehensive map of the complex interplay of the effect of matrix elasticity, degradability, and selected soluble and tethered biomolecules on mouse embryonic stem cell (mESC) self-renewal. Their analysis underscored the prominent role of leukemia inhibitory factor in promoting mESC self-renewal; nonetheless, their analysis also elucidated novel and interesting synergistic effects of the other variables, highlighting the value of their system-level approach.
Moving Toward Clinical Translation
The Need for Vascularization
Although neovascularization is essential for the success of any implanted cellularized construct, it is now becoming clear that it could be particularly important for the survival of stem cells, in particular MSCs, after implantation [48]. With or without a scaffold, the invasion of host endothelial cells and potentially also the differentiation of the implanted stem cells into vascular phenotypes has unfortunately been insufficient to encourage neovascularization to support these exogenous cells [49]. In vitro, it has been shown that patterned encapsulation of MSCs with endothelial cells in a fibrin hydrogel matrix indicated that bone marrow-derived MSCs migrated toward endothelial cells and encouraged vessel formation, acting as “supporting cells” for the endothelial cells in forming the vasculature [50–53]. In addition, a recent in vivo study that used stem cells (without a supporting scaffold) has shown the complementary effect, in which MSC survival was enhanced when injected in conjunction with endothelial cells [54]. That study used endothelial progenitors isolated from blood, which has obvious harvesting advantages when considered as an addition to MSC-based therapies. Taken together, these two studies suggest that the translational success of stem cells encapsulated in hydrogel implants can be improved with the addition of endothelial cells.
Tissue-engineered constructs targeted for in vivo applications are typically restricted to a thickness of only a few hundreds of microns, owing to the diffusion limitations of oxygen and nutrients. To overcome this hurdle, and as an alternative to adding endothelial cells and waiting for the formation of self-organized vascular structures, Cabodi et al. recently devised microfluidic biomaterials [55]. Microfabrication was applied to various hydrogels to construct micron-size microchannels embedded within the scaffold to mimic the function of the vasculature in native tissues. Perfusion through the microchannel facilitated encapsulated cell survival in large tissue-engineered constructs, along with the ability to generate soluble factor gradients [56]. This approach also enables the generation of microvasculature systems of a defined size and shape to study critical developmental and regeneration processes, such as angiogenesis, vasculogenesis, and thrombosis in vitro [57, 58]. Similar approaches have been used to design vascularized tissue-engineered solid polymeric scaffolds (reviewed in [58–61]). Despite interest in the application of complex engineered constructs in developing in vitro models for drug discovery or ex vivo artificial tissue maturation, in its present form, the concept of a microfluidic scaffold has limited potential in clinical applications, principally owing to the difficulty in connecting the host and artificial vasculature. However, future developments could certainly overcome such constraints.
Engineering Tissue Level Complexity in 3D Coculture Models
The ability to create tissue-like or even organ-like constructs consisting of multiple cell types is important for both the application of stem cells as an in vitro model (Figure 2E) and the most distant goal of driving tissue and organ regeneration in vivo from stem cell starting points. This can be realized through the combination of stem cells with differentiated cells or by encouraging stem cell differentiation down concurrent lineages. However, heterotypic cell-cell interactions can have a large impact on stem cell differentiation. This has been illustrated particularly well in the process of bone formation through endochondral ossification, in which signaling from chondrocytes drives the invasion and differentiation of mesenchymal progenitors to osteoblasts. Among other targets, the interactions between MSCs and chondrocytes in 3D matrices have been investigated [62–68]. Ideally, it would be preferable to elicit a level of control over such interactions to probe this complex interactome. Hydrogel systems provide both the ability to generate large numbers of coculture microtissues and precisely control the location of, and hence the interactions between, different cell types. Tumarkin et al. applied a microfluidic system for generating cell-laden hydrogel microbeads to establish coculture assays, thus creating a robust and controlled method for identifying the support roles between different cell types, in particular, blood progenitors [69]. Others have used photo-patterning [70], layer-by-layer deposition [71], and consecutive [72] seeding techniques to investigate coculture in a low-throughput but still highly controlled manner. The application of these techniques to stem cells will provide valuable insights into the role of cell-cell interactions in the stem cell niche. Moreover, with improved control over micropatterning and seeding methods, this approach could ultimately allow for the creation of prestructured tissue-engineered constructs that, after implantation, could thereafter accelerate and enhance tissue formation and maturation.
Natural Versus Synthetic Hydrogels for Stem Cell Therapy
When wishing to use hydrogels in vitro to mimic niche or differentiation conditions, the analysis of cells (via in situ visualization or cell recovery) remains a primary concern. In contrast, the main properties required for their use in vivo depend primarily on the intended purpose of the hydrogel. Because of their limited mechanical property envelope (Young’s modulus ∼0.1–100 kPa), hydrogels are better suited for the engineering of soft tissues, such as neuronal tissue and cartilage, which have both been studied in animal models using adult stem cells in synthetic and naturally derived scaffolds [17, 62, 73]. The current applications of hydrogels in stem cell-based therapy in clinical trials has been mostly limited to naturally derived systems, such as alginate and fibrin, presumably because of reduced concerns over biocompatibility, higher costs of production of synthetic materials owing to the requirement to meet manufacturing (GMP) standards, and their approval by regulatory agencies [74]. In terms of synthetic hydrogels, as an example, in their recent publication, Hejčl et al. compared different methacrylate-based gels loaded with MSCs in a rat model of spinal cord injury [17]. They found that the inclusion of a synthetic peptide was required to encourage cell adhesion (RGD) to improve ingrowth of blood vessels and the use of a solid porogen increased neuronal regeneration [17] (Figure 3C). Regeneration of hard tissues, such as bone, has been studied in vivo using MSCs and hydrogels [73, 75], the mechanical properties of hydrogels are generally not suited for the immediate replacement of mechanically loaded bone function. The evolution of in vitro culture models that use synthetic hydrogels in terms of their ability to modulate various critical microenvironmental parameters (independently or in combination) will ultimately lead to improved overall performance in vivo.
Aside from the use of stem cells to regenerate tissue in vitro and in vivo, stem cells have also been proposed as implantable in vivo production units for therapeutic biomolecules. However, stem cells that have been genetically modified to produce a certain factor need to be protected in the body and localized to the therapeutically relevant area. Encapsulating stem cells in hydrogel microbeads (Figure 3D) has enabled progress in the treatment of ischemia [18, 76, 77], cancer [78], and traumatic brain injury [79]. Again, most applications used naturally derived hydrogel materials; however, it is apparent that once synthetic materials begin showing signs of success in clinical trials and the body of knowledge around their optimization continues to increase, they will ultimately become a desirable option for this type of stem cell therapy.
Future Perspectives
The modularity and precision provided by synthetic scaffolds allows control and manipulation of their physicochemical and biochemical properties. With the growing capability to control the characteristics of in vitro models, it becomes impractical to manually test all the possible combinations; therefore, high-throughput and combinatorial methods to generate arrays of 3D cell-laden scaffolds is key to deconstruct the complexity of the stem cell niche. Along these lines, microfluidic technologies offer alluring experimental paradigms to manipulate stem cells and their microenvironment. The enhanced capabilities to process small amounts of liquid within these miniaturized devices allows the control of stem cell culture conditions with unprecedented levels of precision. These capabilities have, during the past decade, spurred the development of innovative technologies to address a wealth of biological phenomena [80]. Water-in-oil droplet generators have been extensively used to fabricate cell-laden hydrogels at very high frequencies [81, 82]. These methods naturally avail themselves to being applied to the parallel generation of cell-laden 3D scaffolds, providing powerful tools to screen biomaterials and assess their effect on stem cell fate. Such high-throughput combinatorial methods to biologically assess and validate modular polymeric hydrogel systems biologically have the potential, in the near future, to profoundly affect stem cell biology.
The ability to deconstruct the complex interplay of the interactions of stem cells with their instructive microenvironment is essential to expand our understanding of underlying mechanisms that control stem cell fate and therefore greatly contribute to advance the field toward clinical applications. The direct application of the outcomes from such novel, investigative in vitro methods, as described, has the potential to yield robust and reliable culture methods for stem cell expansion and their directed differentiation toward clinically relevant endpoints.
With continued development, tailored synthetic hydrogel substrates and scaffolds that display the desired biological and physicochemical properties have the potential to overcome the current difficulties associated with the culture of stem cells in vitro, enabling optimal maintenance and expansion of stem cells in a context of affordable, GMP-compliant and xeno-free procedures, providing a clearer path for their clinical use in regenerative medicine applications. For example, the development of synthetic microcarriers presenting such tailored biomaterial surfaces offers the possibility of large-scale production of stem cells or their differentiated progeny at clinically relevant quantities [82–84].
The differentiation outcomes have been shown to be greatly enhanced by synthetic biomimetic biomaterial systems [15, 16] and, ultimately, with additional advances, we believe that stem cells, when incorporated into future generation biomimetic hydrogel scaffolds, will be actively driven to maturation and the development of true physiological function. Engineering multicellular constructs, such as stem cell-derived organoids, using synthetic hydrogels presents the opportunity to construct compositionally tailored in vitro tissue models in a high-throughput manner to discover such optimal hydrogel systems. Furthermore, it opens the door for drug screening and discovery applications, inclusive of toxicological screening and the possibility for drug stratification at a personalized level (when combined with patient-derived induced pluripotent stem cells).
With the recent advent of mild cross-linking schemes and the now relative ease of coupling bioactive signaling molecules into synthetic hydrogels without the need for premodification, we foresee that after the injection and in situ gelation of these stem cell instructive biomaterials within host diseased or injured tissue, they will enable either directed, efficient differentiation of injected stem cells into defined cell types or the recruitment of host endogenous stem cells and, thereafter, their directed differentiation as they colonize the hydrogel scaffold or, ideally, both.
Conclusion
In writing the present review, we aimed to provide a synopsis of recent advances in biomaterials engineering, with a particular focus on hydrogel scaffolds. We have presented perspectives on their potential applications in the development of in vitro stem cell niche models and for in vivo tissue engineering and regenerative medicine. Engineered hydrogel scaffolds, with their highly tailorable mechanical and biochemical properties, offer great promise in addressing the current clinical challenges associated with translating stem cell-based therapies.
Author Contributions
S.C.: conception and design, collection and/or assembly of data, manuscript writing; E.A.O. and H.H.: collection and/or assembly of data, manuscript writing; J.J.C.-W.: conception and design, manuscript writing, financial support, administrative support, final approval of manuscript.
Disclosure of Potential Conflicts of Interest
The authors indicated no potential conflicts of interest.
References
- 1.Smith AG. Embryo-derived stem cells: Of mice and men. Annu Rev Cell Dev Biol. 2001;17:435–462. doi: 10.1146/annurev.cellbio.17.1.435. [DOI] [PubMed] [Google Scholar]
- 2.Alison MR. Stem cells in pathobiology and regenerative medicine. J Pathol. 2009;217:141–143. doi: 10.1002/path.2497. [DOI] [PubMed] [Google Scholar]
- 3.Morrison SJ, Spradling AC. Stem cells and niches: Mechanisms that promote stem cell maintenance throughout life. Cell. 2008;132:598–611. doi: 10.1016/j.cell.2008.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rossant J. The impact of developmental biology on pluripotent stem cell research: Successes and challenges. Dev Cell. 2011;21:20–23. doi: 10.1016/j.devcel.2011.06.010. [DOI] [PubMed] [Google Scholar]
- 5.Gurdon JB, Bourillot PY. Morphogen gradient interpretation. Nature. 2001;413:797–803. doi: 10.1038/35101500. [DOI] [PubMed] [Google Scholar]
- 6.Kobel S, Lutolf M. High-throughput methods to define complex stem cell niches. Biotechniques. 2010;48:ix–xxii. doi: 10.2144/000113401. [DOI] [PubMed] [Google Scholar]
- 7.Lutolf MP, Gilbert PM, Blau HM. Designing materials to direct stem-cell fate. Nature. 2009;462:433–441. doi: 10.1038/nature08602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yamada KM, Cukierman E. Modeling tissue morphogenesis and cancer in 3D. Cell. 2007;130:601–610. doi: 10.1016/j.cell.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 9.Sasai Y. Next-generation regenerative medicine: Organogenesis from stem cells in 3D culture. Cell Stem Cell. 2013;12:520–530. doi: 10.1016/j.stem.2013.04.009. [DOI] [PubMed] [Google Scholar]
- 10.Sasai Y, Eiraku M, Suga H. In vitro organogenesis in three dimensions: Self-organising stem cells. Development. 2012;139:4111–4121. doi: 10.1242/dev.079590. [DOI] [PubMed] [Google Scholar]
- 11.Gjorevski N, Ranga A, Lutolf MP. Bioengineering approaches to guide stem cell-based organogenesis. Development. 2014;141:1794–1804. doi: 10.1242/dev.101048. [DOI] [PubMed] [Google Scholar]
- 12.Lutolf MP. Integration column: Artificial ECM: Expanding the cell biology toolbox in 3D. Integr Biol (Camb) 2009;1:235–241. doi: 10.1039/b902243k. [DOI] [PubMed] [Google Scholar]
- 13.Murry CE, Keller G. Differentiation of embryonic stem cells to clinically relevant populations: Lessons from embryonic development. Cell. 2008;132:661–680. doi: 10.1016/j.cell.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 14.Ingber DE, Mow VC, Butler D, et al. Tissue engineering and developmental biology: Going biomimetic. Tissue Eng. 2006;12:3265–3283. doi: 10.1089/ten.2006.12.3265. [DOI] [PubMed] [Google Scholar]
- 15.Frith JE, Cameron AR, Menzies DJ, et al. An injectable hydrogel incorporating mesenchymal precursor cells and pentosan polysulphate for intervertebral disc regeneration. Biomaterials. 2013;34:9430–9440. doi: 10.1016/j.biomaterials.2013.08.072. [DOI] [PubMed] [Google Scholar]
- 16.Dolatshahi-Pirouz A, Nikkhah M, Gaharwar AK, et al. A combinatorial cell-laden gel microarray for inducing osteogenic differentiation of human mesenchymal stem cells. Sci Rep. 2014:4. doi: 10.1038/srep03896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hejčl A, Růžička J, Kapcalová M, et al. Adjusting the chemical and physical properties of hydrogels leads to improved stem cell survival and tissue ingrowth in spinal cord injury reconstruction: A comparative study of four methacrylate hydrogels. Stem Cells Dev. 2013;22:2794–2805. doi: 10.1089/scd.2012.0616. [DOI] [PubMed] [Google Scholar]
- 18.Houtgraaf JH, de Jong R, Monkhorst K, et al. Feasibility of intracoronary GLP-1 eluting CellBead™ infusion in acute myocardial infarction. Cell Transplant. 2013;22:535–543. doi: 10.3727/096368912X638973. [DOI] [PubMed] [Google Scholar]
- 19.Patterson J, Hubbell JA. Enhanced proteolytic degradation of molecularly engineered PEG hydrogels in response to MMP-1 and MMP-2. Biomaterials. 2010;31:7836–7845. doi: 10.1016/j.biomaterials.2010.06.061. [DOI] [PubMed] [Google Scholar]
- 20.DeForest CA, Polizzotti BD, Anseth KS. Sequential click reactions for synthesizing and patterning three-dimensional cell microenvironments. Nat Mater. 2009;8:659–664. doi: 10.1038/nmat2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nimmo CM, Shoichet MS. Regenerative biomaterials that “click”: Simple, aqueous-based protocols for hydrogel synthesis, surface immobilization, and 3D patterning. Bioconjug Chem. 2011;22:2199–2209. doi: 10.1021/bc200281k. [DOI] [PubMed] [Google Scholar]
- 22.Fairbanks BD, Schwartz MP, Bowman CN, et al. Photoinitiated polymerization of PEG-diacrylate with lithium phenyl-2,4,6-trimethylbenzoylphosphinate: Polymerization rate and cytocompatibility. Biomaterials. 2009;30:6702–6707. doi: 10.1016/j.biomaterials.2009.08.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fairbanks BD, Schwartz MP, Halevi AE, et al. A versatile synthetic extracellular matrix mimic via thiol-norbornene photopolymerization. Adv Mater. 2009;21:5005–5010. doi: 10.1002/adma.200901808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ehrbar M, Rizzi SC, Hlushchuk R, et al. Enzymatic formation of modular cell-instructive fibrin analogs for tissue engineering. Biomaterials. 2007;28:3856–3866. doi: 10.1016/j.biomaterials.2007.03.027. [DOI] [PubMed] [Google Scholar]
- 25.Sala A, Hänseler P, Ranga A, et al. Engineering 3D cell instructive microenvironments by rational assembly of artificial extracellular matrices and cell patterning. Integr Biol (Camb) 2011;3:1102–1111. doi: 10.1039/c1ib00045d. [DOI] [PubMed] [Google Scholar]
- 26.Menzies DJ, Cameron A, Munro T, et al. Tailorable cell culture platforms from enzymatically cross-linked multifunctional poly(ethylene glycol)-based hydrogels. Biomacromolecules. 2013;14:413–423. doi: 10.1021/bm301652q. [DOI] [PubMed] [Google Scholar]
- 27.Park KM, Ko KS, Joung YK, et al. In situ cross-linkable gelatin-poly(ethylene glycol)-tyramine hydrogel via enzyme-mediated reaction for tissue regenerative medicine. J Mater Chem. 2011;21:13180–13187. [Google Scholar]
- 28.Discher DE, Janmey P, Wang YL. Tissue cells feel and respond to the stiffness of their substrate. Science. 2005;310:1139–1143. doi: 10.1126/science.1116995. [DOI] [PubMed] [Google Scholar]
- 29.Cameron AR, Frith JE, Gomez GA, et al. The effect of time-dependent deformation of viscoelastic hydrogels on myogenic induction and Rac1 activity in mesenchymal stem cells. Biomaterials. 2014;35:1857–1868. doi: 10.1016/j.biomaterials.2013.11.023. [DOI] [PubMed] [Google Scholar]
- 30.Saffer EM, Lackey MA, Griffin DM, et al. SANS study of highly resilient poly(ethylene glycol) hydrogels. Soft Matter. 2014;10:1905–1916. doi: 10.1039/C3SM52395K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Singh SP, Schwartz MP, Lee JY, et al. A peptide functionalized poly(ethylene glycol) (PEG) hydrogel for investigating the influence of biochemical and biophysical matrix properties on tumor cell migration. Biomater Sci. 2014;2:1024–1034. doi: 10.1039/C4BM00022F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schwartz MP, Fairbanks BD, Rogers RE, et al. A synthetic strategy for mimicking the extracellular matrix provides new insight about tumor cell migration. Integr Biol (Camb) 2010;2:32–40. doi: 10.1039/b912438a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Anderson SB, Lin CC, Kuntzler DV, et al. The performance of human mesenchymal stem cells encapsulated in cell-degradable polymer-peptide hydrogels. Biomaterials. 2011;32:3564–3574. doi: 10.1016/j.biomaterials.2011.01.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kloxin AM, Kasko AM, Salinas CN, et al. Photodegradable hydrogels for dynamic tuning of physical and chemical properties. Science. 2009;324:59–63. doi: 10.1126/science.1169494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ramirez F, Rifkin DB. Cell signaling events: A view from the matrix. Matrix Biol. 2003;22:101–107. doi: 10.1016/s0945-053x(03)00002-7. [DOI] [PubMed] [Google Scholar]
- 36.Lutolf MP. Biomaterials: Spotlight on hydrogels. Nat Mater. 2009;8:451–453. doi: 10.1038/nmat2458. [DOI] [PubMed] [Google Scholar]
- 37.Khetan S, Burdick JA. Patterning hydrogels in three dimensions towards controlling cellular interactions. Soft Matter. 2011;7:830–838. [Google Scholar]
- 38.Lee JY, Choo JE, Park HJ, et al. Injectable gel with synthetic collagen-binding peptide for enhanced osteogenesis in vitro and in vivo. Biochem Biophys Res Commun. 2007;357:68–74. doi: 10.1016/j.bbrc.2007.03.106. [DOI] [PubMed] [Google Scholar]
- 39.Roberts JJ, Elder RM, Neumann AJ, et al. Interaction of hyaluronan binding peptides with glycosaminoglycans in poly(ethylene glycol) hydrogels. Biomacromolecules. 2014;15:1132–1141. doi: 10.1021/bm401524h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lampe KJ, Heilshorn SC. Building stem cell niches from the molecule up through engineered peptide materials. Neurosci Lett. 2012;519:138–146. doi: 10.1016/j.neulet.2012.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang F, Mei Y, Langer R, et al. High throughput optimization of stem cell microenvironments. Comb Chem High Throughput Screen. 2009;12:554–561. doi: 10.2174/138620709788681916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Anderson DG, Levenberg S, Langer R. Nanoliter-scale synthesis of arrayed biomaterials and application to human embryonic stem cells. Nat Biotechnol. 2004;22:863–866. doi: 10.1038/nbt981. [DOI] [PubMed] [Google Scholar]
- 43.Mei Y, Saha K, Bogatyrev SR, et al. Combinatorial development of biomaterials for clonal growth of human pluripotent stem cells. Nat Mater. 2010;9:768–778. doi: 10.1038/nmat2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zonca MR, Jr, Yune PS, Heldt CL, et al. High-throughput screening of substrate chemistry for embryonic stem cell attachment, expansion, and maintaining pluripotency. Macromol Biosci. 2013;13:177–190. doi: 10.1002/mabi.201200315. [DOI] [PubMed] [Google Scholar]
- 45.Villa-Diaz LG, Ross AM, Lahann J, et al. Concise review: The evolution of human pluripotent stem cell culture: From feeder cells to synthetic coatings. Stem Cells. 2013;31:1–7. doi: 10.1002/stem.1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Higuchi A, Ling Q-D, Kumar SS, et al. Design of polymeric materials for culturing human pluripotent stem cells: Progress toward feeder-free and xeno-free culturing. Prog Polym Sci. 2014;39:1348–1374. [Google Scholar]
- 47.Ranga A, Gobaa S, Okawa Y, et al. 3D niche microarrays for systems-level analyses of cell fate. Nat Commun. 2014;5:4324. doi: 10.1038/ncomms5324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Frenette PS, Pinho S, Lucas D, et al. Mesenchymal stem cell: Keystone of the hematopoietic stem cell niche and a stepping-stone for regenerative medicine. Annu Rev Immunol. 2013;31:285–316. doi: 10.1146/annurev-immunol-032712-095919. [DOI] [PubMed] [Google Scholar]
- 49.Rouwkema J, Rivron NC, van Blitterswijk CA. Vascularization in tissue engineering. Trends Biotechnol. 2008;26:434–441. doi: 10.1016/j.tibtech.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 50.Trkov S, Eng G, Di Liddo R, et al. Micropatterned three-dimensional hydrogel system to study human endothelial-mesenchymal stem cell interactions. J Tissue Eng Regen Med. 2010;4:205–215. doi: 10.1002/term.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sorrell JM, Baber MA, Caplan AI. Influence of adult mesenchymal stem cells on in vitro vascular formation. Tissue Eng Part A. 2009;15:1751–1761. doi: 10.1089/ten.tea.2008.0254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lin R-Z, Moreno-Luna R, Zhou B, et al. Equal modulation of endothelial cell function by four distinct tissue-specific mesenchymal stem cells. Angiogenesis. 2012;15:443–455. doi: 10.1007/s10456-012-9272-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Traphagen SB, Titushkin I, Sun S, et al. Endothelial invasive response in a co-culture model with physically-induced osteodifferentiation. J Tissue Eng Regen Med. 2013;7:621–630. doi: 10.1002/term.554. [DOI] [PubMed] [Google Scholar]
- 54.Lin R-Z, Moreno-Luna R, Li D, et al. Human endothelial colony-forming cells serve as trophic mediators for mesenchymal stem cell engraftment via paracrine signaling. Proc Natl Acad Sci USA. 2014;111:10137–10142. doi: 10.1073/pnas.1405388111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cabodi M, Choi NW, Gleghorn JP, et al. A microfluidic biomaterial. J Am Chem Soc. 2005;127:13788–13789. doi: 10.1021/ja054820t. [DOI] [PubMed] [Google Scholar]
- 56.Choi NW, Cabodi M, Held B, et al. Microfluidic scaffolds for tissue engineering. Nat Mater. 2007;6:908–915. doi: 10.1038/nmat2022. [DOI] [PubMed] [Google Scholar]
- 57.Cross VL, Zheng Y, Won Choi N, et al. Dense type I collagen matrices that support cellular remodeling and microfabrication for studies of tumor angiogenesis and vasculogenesis in vitro. Biomaterials. 2010;31:8596–8607. doi: 10.1016/j.biomaterials.2010.07.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zheng Y, Chen J, Craven M, et al. In vitro microvessels for the study of angiogenesis and thrombosis. Proc Natl Acad Sci USA. 2012;109:9342–9347. doi: 10.1073/pnas.1201240109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Huang GY, Zhou LH, Zhang QC, et al. Microfluidic hydrogels for tissue engineering. Biofabrication. 2011;3:012001. doi: 10.1088/1758-5082/3/1/012001. [DOI] [PubMed] [Google Scholar]
- 60.Forgacs G. Tissue engineering: Perfusable vascular networks. Nat Mater. 2012;11:746–747. doi: 10.1038/nmat3412. [DOI] [PubMed] [Google Scholar]
- 61.Bettinger CJ, Borenstein JT. Biomaterials-based microfluidics for engineered tissue constructs. Soft Matter. 2010;6:4999–5015. [Google Scholar]
- 62.Dahlin RL, Kinard LA, Lam J, et al. Articular chondrocytes and mesenchymal stem cells seeded on biodegradable scaffolds for the repair of cartilage in a rat osteochondral defect model. Biomaterials. 2014;35:7460–7469. doi: 10.1016/j.biomaterials.2014.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Diao HJ, Yeung CW, Yan CH, et al. Bidirectional and mutually beneficial interactions between human mesenchymal stem cells and osteoarthritic chondrocytes in micromass co-cultures. Regen Med. 2013;8:257–269. doi: 10.2217/rme.13.22. [DOI] [PubMed] [Google Scholar]
- 64.Lai JH, Kajiyama G, Smith RL, et al. Stem cells catalyze cartilage formation by neonatal articular chondrocytes in 3D biomimetic hydrogels. Sci Rep. 2013;3:3553. doi: 10.1038/srep03553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Levorson EJ, Mountziaris PM, Hu O, et al. Cell-derived polymer/extracellular matrix composite scaffolds for cartilage regeneration. Part 1: Investigation of cocultures and seeding densities for improved extracellular matrix deposition. Tissue Eng Part C Methods. 2014;20:340–357. doi: 10.1089/ten.tec.2013.0286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Levorson EJ, Santoro M, Kasper FK, et al. Direct and indirect co-culture of chondrocytes and mesenchymal stem cells for the generation of polymer/extracellular matrix hybrid constructs. Acta Biomater. 2014;10:1824–1835. doi: 10.1016/j.actbio.2013.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang L, He A, Yin Z, et al. Regeneration of human-ear-shaped cartilage by co-culturing human microtia chondrocytes with BMSCs. Biomaterials. 2014;35:4878–4887. doi: 10.1016/j.biomaterials.2014.02.043. [DOI] [PubMed] [Google Scholar]
- 68.Thompson AD, Betz MW, Yoon DM, et al. Osteogenic differentiation of bone marrow stromal cells induced by coculture with chondrocytes encapsulated in three-dimensional matrices. Tissue Eng Part A. 2009;15:1181–1190. doi: 10.1089/ten.tea.2007.0275. [DOI] [PubMed] [Google Scholar]
- 69.Tumarkin E, Tzadu L, Csaszar E, et al. High-throughput combinatorial cell co-culture using microfluidics. Integr Biol (Camb) 2011;3:653–662. doi: 10.1039/c1ib00002k. [DOI] [PubMed] [Google Scholar]
- 70.Liu Tsang V, Chen AA, Cho LM, et al. Fabrication of 3D hepatic tissues by additive photopatterning of cellular hydrogels. FASEB J. 2007;21:790–801. doi: 10.1096/fj.06-7117com. [DOI] [PubMed] [Google Scholar]
- 71.Rinker TE, Hammoudi TM, Kemp ML, et al. Interactions between mesenchymal stem cells, adipocytes, and osteoblasts in a 3D tri-culture model of hyperglycemic conditions in the bone marrow microenvironment. Integr Biol (Camb) 2014;6:324–337. doi: 10.1039/c3ib40194d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Campbell JJ, Davidenko N, Caffarel MM, et al. A multifunctional 3D co-culture system for studies of mammary tissue morphogenesis and stem cell biology. PLoS One. 2011;6:e25661. doi: 10.1371/journal.pone.0025661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kim J, Bae WG, Choung HW, et al. Multiscale patterned transplantable stem cell patches for bone tissue regeneration. Biomaterials. 2014;35:9058–9067. doi: 10.1016/j.biomaterials.2014.07.036. [DOI] [PubMed] [Google Scholar]
- 74.Drury JL, Mooney DJ. Hydrogels for tissue engineering: Scaffold design variables and applications. Biomaterials. 2003;24:4337–4351. doi: 10.1016/s0142-9612(03)00340-5. [DOI] [PubMed] [Google Scholar]
- 75.Kwon JS, Kim SW, Kwon DY, et al. In vivo osteogenic differentiation of human turbinate mesenchymal stem cells in an injectable in situ-forming hydrogel. Biomaterials. 2014;35:5337–5346. doi: 10.1016/j.biomaterials.2014.03.045. [DOI] [PubMed] [Google Scholar]
- 76.Levit RD, Landázuri N, Phelps EA, et al. Cellular encapsulation enhances cardiac repair. J Am Heart Assoc. 2013;2:e000367. doi: 10.1161/JAHA.113.000367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Panda NC, Zuckerman ST, Mesubi OO, et al. Improved conduction and increased cell retention in healed MI using mesenchymal stem cells suspended in alginate hydrogel. J Interv Card Electrophysiol. 2014;41:117–127. doi: 10.1007/s10840-014-9940-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Duebgen M, Martinez-Quintanilla J, Tamura K, et al. Stem cells loaded with multimechanistic oncolytic herpes simplex virus variants for brain tumor therapy. J Natl Cancer Inst. 2014;106:dju090. doi: 10.1093/jnci/dju090. [DOI] [PubMed] [Google Scholar]
- 79.Heile A, Brinker T. Clinical translation of stem cell therapy in traumatic brain injury: The potential of encapsulated mesenchymal cell biodelivery of glucagon-like peptide-1. Dialogues Clin Neurosci. 2011;13:279–286. doi: 10.31887/DCNS.2011.13.2/aheile. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Titmarsh DM, Chen H, Glass NR, et al. Concise review: Microfluidic technology platforms: Poised to accelerate development and translation of stem cell-derived therapies. Stem Cells Translational Medicine. 2014;3:81–90. doi: 10.5966/sctm.2013-0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mazzitelli S, Capretto L, Quinci F, et al. Preparation of cell-encapsulation devices in confined microenvironment. Adv Drug Deliv Rev. 2013;65:1533–1555. doi: 10.1016/j.addr.2013.07.021. [DOI] [PubMed] [Google Scholar]
- 82.Velasco D, Tumarkin E, Kumacheva E. Microfluidic encapsulation of cells in polymer microgels. Small. 2012;8:1633–1642. doi: 10.1002/smll.201102464. [DOI] [PubMed] [Google Scholar]
- 83.Allazetta S, Hausherr TC, Lutolf MP. Microfluidic synthesis of cell-type-specific artificial extracellular matrix hydrogels. Biomacromolecules. 2013;14:1122–1131. doi: 10.1021/bm4000162. [DOI] [PubMed] [Google Scholar]
- 84.Tabata Y, Horiguchi I, Lutolf MP, et al. Development of bioactive hydrogel capsules for the 3D expansion of pluripotent stem cells in bioreactors. Biomater Sci. 2014;2:176–183. doi: 10.1039/c3bm60183h. [DOI] [PubMed] [Google Scholar]