The 2014 Regenerative Medicine Foundation Annual Conference had a vision of assisting with translating tissue engineering and regenerative medicine-based technologies by assembling leaders in the field to cover critical areas, including regulatory pathways for regenerative medicine therapies, strategic partnerships, resource coordination, developing standards, government support, industry priorities, biobanking, and new technologies. This article covers lessons learned from the manufacturing sessions and early survey results and provides a road map for future developments in the field.
Summary
The Regenerative Medicine Foundation Annual Conference held on May 6 and 7, 2014, had a vision of assisting with translating tissue engineering and regenerative medicine (TERM)-based technologies closer to the clinic. This vision was achieved by assembling leaders in the field to cover critical areas. Some of these critical areas included regulatory pathways for regenerative medicine therapies, strategic partnerships, coordination of resources, developing standards for the field, government support, priorities for industry, biobanking, and new technologies. The final day of this conference featured focused sessions on manufacturing, during which expert speakers were invited from industry, government, and academia. The speakers identified and accessed roadblocks plaguing the field where improvements in advanced manufacturing offered many solutions. The manufacturing sessions included (a) product development toward commercialization in regenerative medicine, (b) process challenges to scale up manufacturing in regenerative medicine, and (c) infrastructure needs for manufacturing in regenerative medicine. Subsequent to this, industry was invited to participate in a survey to further elucidate the challenges to translation and scale-up. This perspective article will cover the lessons learned from these manufacturing sessions and early results from the survey. We also outline a road map for developing the manufacturing infrastructure, resources, standards, capabilities, education, training, and workforce development to realize the promise of TERM.
Introduction
On May 6 and 7, 2014, in San Francisco, California, the Regenerative Medicine Foundation held a 2-day conference: Translating the Promise of Regenerative Medicine. There were many useful sessions in these 2 days that covered diverse subject areas ranging from enabling commercialization of regenerative medicine (RM)-based products to developing infrastructure and standards for the field to advances in tissue engineering to developing strategic partnerships to U.S. government support in bringing tissue engineering and regenerative medicine (TERM)-based therapies to patients to priorities for industry and much more. The final day of the conference had three manufacturing sessions: (a) product development toward commercialization in RM, (b) process challenges to scale up manufacturing in RM, and (c) infrastructure needs for manufacturing in RM. This perspective article will explain the lessons learned from these manufacturing sessions, early results from a subsequent industry survey, and a road map on how to develop the manufacturing infrastructure, resources, standards, capabilities, education, training, and workforce development to realize the promise of TERM.
State of the Field
TERM is a multidisciplinary field encompassing scientific areas such as biochemistry, biomedical engineering, biomaterial sciences, biomolecules, pharmacology, physiology, genetics, and nanotechnology. Together, these disciplines seek to repair, replace, or regenerate organs and tissue to treat and even cure disease. The current state of the field has been regarded by many reports as being at a crucial point where new developments are being realized each day. Current TERM-based products have focused on skin and cartilage repair. However, therapeutic applications have expanded to include laboratory-grown bladders [1], tracheas [2], blood vessels [3], vaginal organs [4, 5], and urethras [6], which have been implanted in patients. The realization of more of these regenerative medicine-based therapies is being limited, however, by a lack of understanding of advanced manufacturing applied to these products. These roadblocks will be identified below along with lessons learned from experts in the field. Before delving into these topics, however, we will cover the manufacturing workflow for a TERM product.
Manufacturing Workflows for a TERM Product
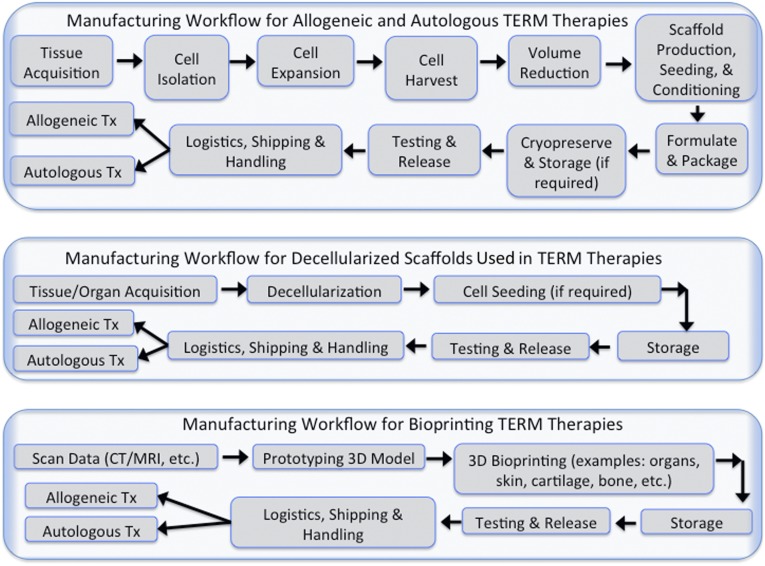
There are four main manufacturing approaches to TERM therapy: (a) manufacturing allogeneic (universal donor) TERM therapies, (b) manufacturing autologous (patient-specific) TERM products, (c) manufacturing decellularized scaffolds for TERM therapies, and (d) bioprinting for TERM therapies. Products from all four manufacturing approach categories are available at various clinical stage trials. and some are even in commercial level production. All of these approaches are illustrated in Figure 1.
Figure 1.
Manufacturing workflow for allogeneic and autologous TERM products. Illustrated is a workflow that details the manufacturing process in the top panel for allogeneic or “off-the-shelf” and autologous TERM therapies. The middle panel shows the workflow for decellularized scaffolds. The bottom panel depicts the workflow for bioprinting. Abbreviations: CT, computed tomography; MRI, magnetic resonance imaging; TERM, tissue engineering and regenerative medicine; Tx, treatments.
Allogeneic and Autologous TERM Therapies
Allogeneic based products are amenable to a scale-up-based manufacturing approach, suitable for an “off-the-shelf” or “on-demand” product. The manufacturing system for these products can be viewed as a “push” system, wherein both information and process goods flow in the same direction. These products are produced in large quantities, stored, released, and shipped as needed. Manufacturing strategies for this approach can borrow extensively from similar strategies used in biologics and the medical device industry involving the production of biopharmaceutical therapeutics such as viruses, antibodies, and other recombinant proteins. As shown in Figure 1, cells are obtained from tissue biopsies of individual donors, stored in master cell banks, expanded to form working cell banks, and then expanded to production level large quantities. Unlike with production of biopharmaceuticals, cells are living products and are carried forward through every processing stage. Challenges involve actively monitoring cellular yields and maintaining the product’s critical quality parameters such as purity, potency, and viability during the large-scale production run. Products that involve the use of scaffolds (required in musculoskeletal and other whole organ replacement) will involve an additional layer of complexity because of the need to maintain its own quality attributes prior to seeding them with the therapeutic cell product. Such complex therapeutic products will require conditioning, real-time monitoring, and noninvasive release testing procedures before delivery to the patient.
Autologous based products are patient-specific because of the need for immunologic compatibility. These products are suitable for a scaled-out approach, wherein ideally a facility accommodates the parallel production of multiple, separate, and automated closed systems from multiple patients. Production is generally seen as a “pull” system, wherein customer requirements drive the processing cycles, and hence information flows in the opposite direction to product flow. Cells from individual donors are obtained, expanded, and processed to meet clinical specifications and then finally delivered back to the same patient. Economies of scale can only be obtained by using the same infrastructure repeatedly while still maintaining distinct, parallel product flows with near-zero chances of cross-contamination. Systems used at each processing stage should be automated, closed, and tracked in real time and often involve single-use disposable equipment. If scaffolds are used in combination with the therapeutic cells, the scaffold’s structural architecture and mechanical characteristics will be driven by clinical specifications for the patient’s defect site. In-process control and release testing at each processing stage will be critical to maintain product integrity and safety. It is possible for such therapies to be decentralized to be produced at the hospital care site. This will then simplify issues related to shipping and logistics of transporting a live product. The challenge would be to sufficiently understand the autologous cell therapy processing steps to embed them into an easy-to-use production machine platform that can be housed within a hospital setting.
Scaffolds for TERM Therapies
Manufacturing of decellularized scaffolds will be relatively less complex than their cell-based counterparts. They are often developed as an off-the-shelf product item with modifications done only at the clinical site of use. Because the starting raw materials can be from both human and animal sources, issues related to contamination and disease transmission are critical. Once living cellular material is completely removed, these naturally derived scaffolds are then processed downstream like any other medical device product. Issues related to quality control, release testing, packaging, shipping, and logistics are fairly well-determined and established.
Bioprinting for TERM Therapies
Bioprinting includes the design, prototyping, and fabrication of three-dimensional (3D) anatomical structures (e.g., organs, skin, cartilage, bone) that can be used in TERM therapeutic approaches. This approach would be developed either as an “off-the-shelf” product used by many patients or a product customized for each end user. In the latter circumstance, developing a 3D bioprinter that could be housed in a patient facility setting would enable real-time biofabrication of a product that could be brought into the surgical suite. Issues related to quality control, release testing, packaging, shipping, and logistics would need to be determined and established for each “off-the-shelf” product.
Manufacturing Sessions: Identify Roadblocks
Collectively, the four approaches identified for delivering TERM therapies have many roadblocks that were identified during the conference. The roadblocks ranged from equipment design and regulation to product development economics. The major roadblocks, although not surprising, were remarkably consistent in that several common threads were noted. Some of the roadblocks and critical needs include (a) the need to identify a common constituent component that will be part of many TERM products and then produce it to well-defined quality standards, (b) the need to better define product standardization and characterization so that assays (or other quality assessments) can be developed and product quality can be ensured with respect to both the product and the production processes used for the product, (c) the need to develop efficient scaled-up or scaled-out manufacturing processes and systems prior to Food and Drug Administration (FDA) approval, (d) the need to define and develop appropriate supply chain and logistics models so that gaps between research and product translation can be realized through well-thought-out product development and well-engineered production systems, and (e) the need to develop flexible modular manufacturing systems for biologics.
One of the common constituents mentioned as a candidate for high-volume production for TERM-based applications was induced pluripotent stem cells (iPSCs). Creating common resources such as setting up standardized, well-characterized, and well-defined research and clinical grade iPSC lines that both academia and industry could use that have little variability between production batches would be a major step forward. The second key identified was that of “standards” for both products and processes. Defining critical-to-quality (CTQ) attributes and placing acceptable tolerance limits on products is a requirement for any manufactured product. Unfortunately, most current standards in the TERM product manufacturing space are qualitative and difficult to enforce. In addition, attention must be given to the design of efficient manufacturing processes and systems prior to FDA approval. The final roadblock noted was that of a well-defined supply chain for TERM. The supply chain source nodes are still poorly defined for these products. Similarly, the vocabulary for supplier’s components and their CTQs and tolerances are not well-defined or evolved.
Overcoming these roadblocks will enable the translation of TERM products into common practice. There are many analogs from other consumer products. One example is the automobile, for which advances in materials and fundamental science of combustion made their design and evolution possible. However, it was not until the concept of interchangeable parts, just-in-time production, and the moving assembly line reduced the cost of cars by half that automobiles became commonplace. Similarly, in the semiconductor industry, the physics of materials ushered in a new era of advanced products, but it was not until the development and improvement of manufacturing science that the cost of integrated circuits fell to levels where they became commonplace in many commercial products. Although the science of TERM is not complete, the challenge of developing the manufacturing science to support these regenerative medical developments so that cost-effective, high-quality products can be brought to the consumer market is just beginning. The successful implementation of the road map illustrated in this perspective will enable the field of TERM to develop cost-effective manufacturing processes that permit the successful commercialization of these next-generation medical products. Before considering this road map, we will focus on the lessons learned during the manufacturing sessions.
Manufacturing Sessions: Lessons Learned
Product Development Toward Commercialization in RM
The first manufacturing session focused on enablers of product development that are critical for the commercialization of TERM products. These enablers included crowd sourcing, early translational experiences, developing common standards, and quality by design. A crowd-sourcing model has been used as a strategy for iPSC technology to advance both screening and cell therapy efforts. This model has many benefits including developing common resources that could advance efforts underway by both academia and industry by reducing investment and minimizing translational risk. In addition to cost sharing cells, developing shared operating protocols and methods that can be further developed into standardized best practices, which leads to cost reduction, standardized operating procedure (SOP) standardization, and ultimately advancing translation, was proposed. Early translational experiences of the California Institute for Regenerative Medicine (CIRM) were reviewed; in 2009 they started with 63 early translation awards to establish preclinical proof of concept and to identify lead candidates. It is anticipated that by December 2014, there will be 10 clinical trials initiated that will be enrolling patients in treatments ranging from HIV/AIDS, to congestive heart failure, to cancer, to degenerative eye diseases, to diabetes, and other indications as well. Two salient themes were covered: the importance of working with the FDA and other agencies on regulatory pathways for regenerative medicine and the benefits of engaging industry where CIRM has successfully leveraged industry investments by more than 5.4 times.
Developing common standards for regenerative medicine was covered in this session by medical director of the Foundation for the Accreditation of Cellular Therapy (FACT), a voluntary organization for setting standards and awarding accreditation for cellular therapy. Highlighted were FACT standards that are evidence-based and developed by experts in the field and intended to serve as minimal requirements for achieving quality. Incidentally, earlier in the conference, we also had an entire session devoted to leveraging standards to expedite clinical product delivery and biobanking. AABB (not an acronym, formerly known as American Association of Blood Banks) is another organization that is focusing on advancing the practice and standards of transfusion medicine and cellular therapies that will translate into better patient care and safety while also lowering cost. The product development session concluded with considering quality by design to ensure successful commercialization. These principles seek to solve such problems as quality, cost, scale, and sustainability by using a cross-functional approach to design and achieve consistent high quality product at a reasonable cost to meet the demand over the commercial life of the product. With quality by design, SOP and process plans are essential for the success of TERM product translation.
Process Challenges to Manufacturing in RM
Four speakers representing the government, industry, and academia shared their insight and experiences on how much the process can affect the product. Two main challenges were identified as part of this session: (a) measurement challenges for process standardization (including defining the criteria to measure in-process product characteristics through standardized assays) and (b) scale-up and scale-out cell expansion using bioreactors. The research community has agreed that standards must be developed to lower the cost of research and development efforts needed to bring a therapy to market. A key component of standardization is the ability to measure key product and process quality attributes in a reliable and reproducible manner. The importance of the process metrics is highlighted by the fact that the process can change the product. Without quantitative measures for the metrics to track quality attributes in TERM products, it is infeasible to achieve commercial translation. Identifying measures for clinical effectiveness and safety are needed and would be achieved through assays of several types targeted for the mechanism of action for a particular product. For reproducibility and scalability of process, assays must be fully understood. All variables that can contribute to variation of product quality must be understood.
The second process challenge is scale-up and scale-out expansion. Currently millions of cells are required for an effective therapy for a single patient. From an industry perspective, iPSC-based allogeneic therapy takes approximately 3–4 months to generate economically viable quantities. With this time frame, it leads to challenges of developing processes to reduce large quantities of cells to appropriate doses in vials, storage of these vials, and shipment of these vials to point of use (Fig. 1). For autologous therapy, the costs for therapy are significantly higher. There must be newer methods to process therapies in a parallel manner by using the same infrastructure over and over again. Flat plate culture is simply not feasible. Single-use bioreactors capable of expanding and harvesting the culture of adherent cells are the only way this can be done. Different cells being developed for therapy require differing media conditions, oxygen gradients, pH levels, and cell seeding density and in general require varying optimal cell culture conditions. In addition, integrating these cells into the appropriate scaffolds and biomaterials for effective TERM-based applications is another consideration that leads to a significant amount of time that must be invested by stakeholders of a TERM-based therapy. It is imperative that lessons learned by the current biomanufacturing industry for the production of recombinant proteins and viral vectors are assimilated into TERM-based manufacturing workflows to accelerate the development and commercialization of these next-generation therapies. We believe that these developments, combined with adequate in-process monitoring technologies and process-modeling algorithms, will lead to economical manufacturing of TERM-based technologies and therapies.
Infrastructure Need for Manufacturing in RM
Infrastructure needs for manufacturing in RM formed the subject of the final manufacturing session. The requisite infrastructure for TERM manufacturing is predicated on the development of a well-defined, tiered supply chain that enables consistent and reliable access to high-quality, well-defined, clinical-grade raw materials produced in certified facilities. Although we are moving critical TERM products from laboratory bench tops to clinical applications, there are a number of critical, yet poorly understood and poorly defined needs necessary for the evolution of such a supply chain. This supply chain will consist of multiple tiers of suppliers and manufacturers and a transportation network that will deliver products to critical need customers. Many of these principles have already been considered in the manufacturing workflow shown in Figure 1. A functional TERM supply chain should contain well-defined (a) products and product definitions, (b) resources and resource classifications necessary to wisely choose suppliers and sources, (c) a delivery network to move the TERM product from resource to resource, and (d) a transaction management system that will track products throughout the network, providing real-time information on the products in the TERM supply chain. This session focused on these issues as they relate to new product development and the translation of products from the development laboratories to the clinics. Speakers in the session focused on models, methods, and techniques being used to promote these advances.
TERM Industry Survey
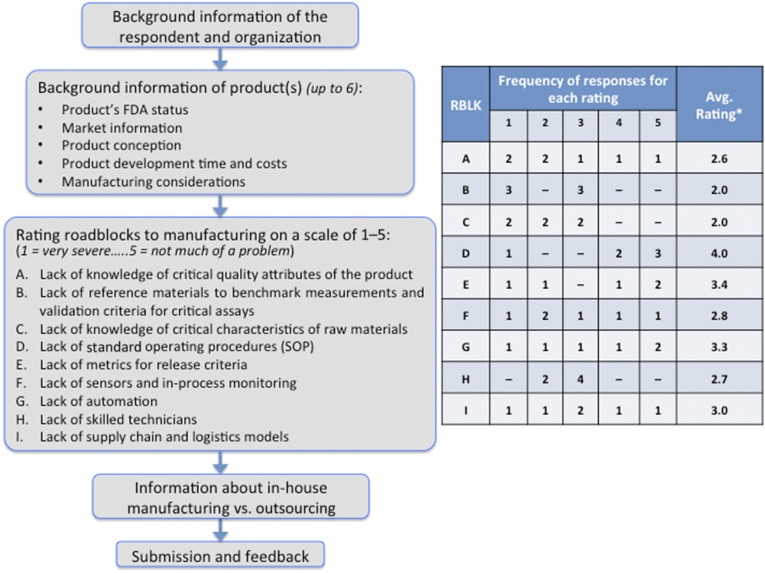
Based on the talks and discussions during the conference, we developed a survey to obtain a broader industry perspective on the translation issues in TERM. The primary objectives of this survey are to identify the time, effort, and challenges involved in the concept-to-market translation of TERM products from the perspective of product development and manufacturing. The survey is available at http://www.ise.ncsu.edu/RMsurvey/, and its outline is presented in Figure 2. Our industry readers are encouraged to complete the survey, the results of which will be updated regularly and will be made available to respondents at the end of the survey. Once we have a significant number of responses, we will publish a summary of the data and its analysis in a future publication. Currently, we have received responses from seven companies, of which three companies had two or more TERM products under development or on the market. Overall, the responses covered eleven TERM products, of which five are in phase I clinical trials, four are in phase II or III clinical trials, and two are FDA-approved. Of the eleven products, conception of nine products occurred via industry research, whereas one evolved out of university research. Based on eight responses, critical consideration was given to SOP development before the investigational new drug/investigational device exemption filing for six products, during phase I clinical trials for one product, and during phase II clinical trials for another one. The responses to the question regarding roadblocks to manufacturing are summarized in Figure 2 (labeled A–I). Among the nine roadblocks, the average severity of roadblocks B (lack of reference materials to benchmark measurements and validation criteria for critical assays) and C (lack of knowledge of critical characteristics of raw materials) was higher than rest of the roadblocks, whereas roadblock D (lack of SOP) was rated to be the least severe on average. We will continue to incorporate the industry perspectives gained from this survey into our manufacturing TERM road map, which we outline below.
Figure 2.
An outline of the tissue engineering and regenerative medicine (TERM) industry survey (left). We conducted a survey to gain insight into TERM products that are either already on the market or are in development and what roadblocks companies faced in developing these products with a particular focus on manufacturing criteria as shown (A–I). The table (right) summarizes these preliminary results from the TERM survey (based on 7 industry responses about 11 products, received before the submission of this manuscript). The frequency of responses for each rating is shown (where 1 indicates very severe, 2 indicates severe, 3 indicates somewhat of a problem, 4 indicates mild problem, and 5 indicates not much of a problem). The asterisk indicates that the average is weighted. The lower the value, the higher the severity. Abbreviations: Avg., average; FDA, Food and Drug Administration; RBLK, roadblock; SOP, standard operating procedure.
Developing a Manufacturing Road Map for TERM Products
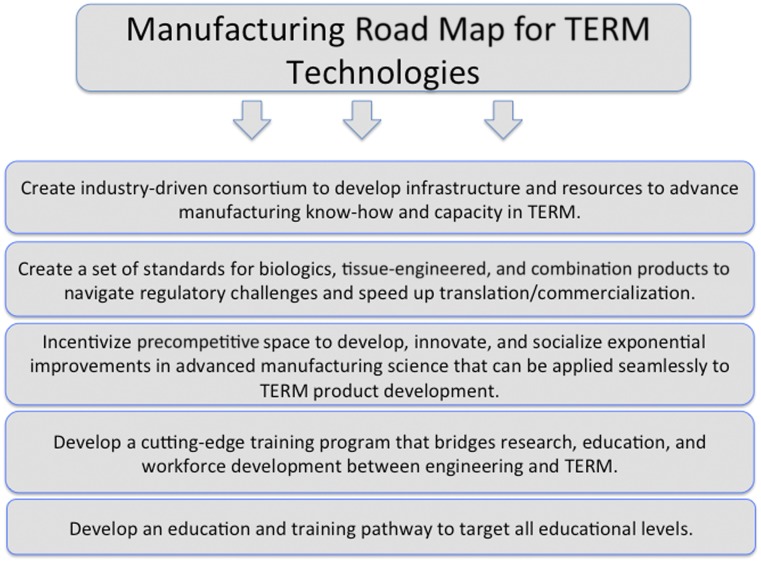
To address the need to advance manufacturing science and engineering to ensure commercial success of TERM products, we propose a road map of national scale that will capitalize upon the current administration’s efforts to promote both TERM and advanced manufacturing in the U.S. This road map will consist of five elements that are depicted in Figure 3, and we describe these elements below in detail.
Figure 3.
Manufacturing road map for TERM technologies. Depicted is an envisioned road map that will help address current manufacturing challenges plaguing the field. Abbreviation: TERM, tissue engineering and regenerative medicine.
Elements of the Road Map
Create an industry-driven consortium to develop infrastructure and resources to advance manufacturing know-how and capacity in TERM
Create a set of standards for biologics, tissue-engineered, and combination products to navigate regulatory challenges and speed up translation/commercialization
Incentivize precompetitive space to develop, innovate, and socialize exponential improvements in advanced manufacturing science (design of production processes, production equipment, and control infrastructures) that can be applied seamlessly to TERM product development
Develop a cutting-edge training program that bridges research, education, and workforce development between engineering science and regenerative medicine
Develop an education and training pathway to target all educational levels
Industry-Driven Consortium
Creating an industry-driven consortium that is built based on consortium member needs to develop infrastructure and resources to advance manufacturing know-how and capacity in TERM that can have national impact is paramount. This consortium will be focused on industry needs and be able to collectively and efficiently partner all stakeholders (industry, academia, government, nonprofit foundations, investors, etc.) to achieve common goals.
Developing Standards for TERM
Developing standards for biologics, tissue-engineered, and combination products to navigate regulatory challenges and speed up translation/commercialization is a necessity for the field. The industry-driven consortium will have a shared goal to devote resources to develop standards in conjunction with regulatory authorities. The incentives for consortium members are that they will have developed the models and expertise both within the consortium and internally to effectively define a process that enables efficient progress and a clearly defined path forward toward translation and commercialization. These standards will simplify the regulatory process and clearly set the milestones and deliverables required to successfully bring a product to market.
Incentivize Precompetitive Space
We envision developing an intellectual property (IP) landscape that fosters a vibrant ecosystem for innovation, automation, and development of exponential improvements in advanced manufacturing science. This will include design of production processes, production equipment, and control infrastructures that can be applied seamlessly to regenerative medicine product development.
Bridging Engineering and TERM
To foster further advances in regenerative medicine, there is even greater need for creating cutting-edge training programs that bridge research, education, and workforce development between engineering and regenerative medicine. Although TERM includes numerous disciplines in addition to engineering, engineering is fundamental and a core capability.
Creating an Education and Training Pathway
Although it takes industry decades to develop novel and transformative TERM products, it is essential to grow, train, and develop the next-generation workforce to support, sustain, and invigorate new technological breakthroughs. We envision developing technology-specific teams within this industry-driven consortium that can rapidly build and expand current expertise within the consortium and internally within each member’s institution while also cultivating an ecosystem that nurtures training programs spanning kindergarten through high school and at the undergraduate, graduate, postgraduate, and professional levels.
Conclusion
The translation of TERM products into common practice is in its infancy. There are many analogs from current consumer products that can be applied to the advancement of TERM manufacturing. Although the science of TERM is not complete, the challenge of developing the manufacturing science to support these regenerative medical developments so that cost-effective, high-quality products can be brought to the consumer market is just beginning. This perspective article has provided a focused summary on the manufacturing challenges and identified solutions that arose from the 2014 Regenerative Medicine Foundation Conference in San Francisco. The authors have extended these findings and delivered a manufacturing road map for regenerative medicine that consists of five components: an industry-driven consortium, developing standards for TERM, incentivizing precompetitive space, bridging engineering and TERM, and creating an education and training pathway. The successful implementation of these components, we believe, will provide a manufacturing road map for TERM therapies that will overcome current manufacturing challenges and establish the infrastructure, expertise, resources, training, education, and workforce development to accelerate a process pipeline of cost-effective TERM-based therapies.
Acknowledgments
We acknowledge the Regenerative Medicine Foundation for putting on this conference along with the conference organizers. In addition, we thank Robert Lasson (North Carolina State Industrial and Systems Engineering Department), who assisted with making the industry survey website.
Author Contributions
J.H., O.H., R.S., B.S., R.W., P.C., J.A., and J.Y.: conception and design, manuscript writing; A.A.: conception and design, financial support, manuscript writing.
Disclosure of Potential Conflicts of Interest
The authors indicated no potential conflicts of interest.
References
- 1.Atala A. Tissue engineering of human bladder. Br Med Bull. 2011;97:81–104. doi: 10.1093/bmb/ldr003. [DOI] [PubMed] [Google Scholar]
- 2.Elliott MJ, De Coppi P, Speggiorin S, et al. Stem-cell-based, tissue engineered tracheal replacement in a child: A 2-year follow-up study. Lancet. 2012;380:994–1000. doi: 10.1016/S0140-6736(12)60737-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Samuel R, Daheron L, Liao S, et al. Generation of functionally competent and durable engineered blood vessels from human induced pluripotent stem cells. Proc Natl Acad Sci USA. 2013;110:12774–12779. doi: 10.1073/pnas.1310675110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Filippo RE, Bishop CE, Filho LF, et al. Tissue engineering a complete vaginal replacement from a small biopsy of autologous tissue. Transplantation. 2008;86:208–214. doi: 10.1097/TP.0b013e31817f1686. [DOI] [PubMed] [Google Scholar]
- 5.Raya-Rivera AM, Esquiliano D, Fierro-Pastrana R, et al. Tissue-engineered autologous vaginal organs in patients: A pilot cohort study. Lancet. 2014;384:329–336. doi: 10.1016/S0140-6736(14)60542-0. [DOI] [PubMed] [Google Scholar]
- 6.Raya-Rivera A, Esquiliano DR, Yoo JJ, et al. Tissue-engineered autologous urethras for patients who need reconstruction: An observational study. Lancet. 2011;377:1175–1182. doi: 10.1016/S0140-6736(10)62354-9. [DOI] [PMC free article] [PubMed] [Google Scholar]