Abstract
Background
Self-monitoring of blood glucose (SMBG) is used to regulate glucose control. It is unknown whether SMBG can motivate adherence to dietary recommendations. We predicted that participants who used more SMBG would also report lower fat and greater fruit and vegetable consumption.
Methods
The present study was a cross-sectional study of 401 primarily minority individuals living with diabetes in East Harlem, New York. Fat intake and fruit and vegetable consumption were measured with the Block Fruit/Vegetable/Fiber and Fat Screeners.
Results
Greater frequency of SMBG was associated with lower fat intake (rs = −0.15; P < 0.01), but not fruit and vegetable consumption. The effects of SMBG were not moderated by insulin use; thus, the relationship was significant for those individuals both on and not on insulin. A significant interaction was found between frequency of SMBG and changing one’s diet in response to SMBG on total fat intake. The data suggest that participants who use SMBG to guide their diet do not have to monitor multiple times a day to benefit.
Conclusion
The present study found that the frequency of SMBG was associated with lower fat intake. Patients are often taught to use SMBG to guide their self-management. This is one of the first studies to examine whether SMBG is associated with better dietary intake.
Keywords: diabetes, fat intake, fruit and vegetable intake, self-management, self-monitoring of blood glucose
Introduction
Self-management of diabetes is crucial to improving medical outcomes for individuals with diabetes. A large part of diabetic management is performed by the patient outside of any medical encounter.1,2 However, the patient learns the skills, motivation, and self-efficacy to adhere to health regimens in the patient–provider interaction. One skill that may improve self-management is using self-monitoring of blood glucose (SMBG) to improve glucose control. The SMBG provides patients with accurate feedback on how their behaviors affect their immediate glucose control. Providing patients with immediate feedback on the results of their behaviors has been shown to increase adherence to these behaviors.3 Patients with Type 1 diabetes who are taught to self-administer insulin changes in response to their glucose levels can maintain clinically significant improvements in their HbA1c levels for years.4,5 It is not known whether SMBG improves adherence to other self-management behaviors, such as dietary adherence, or whether this leads to improvements in glucose control.6–8
Using SMBG to improve adherence to self-management behaviors is consistent with our theoretical framework, the common-sense model of self-regulation (CSM). The theory underlying the CSM is that individuals actively attempt to understand their health9–12 and will use symptoms and how they feel as a gauge to determine the efficacy of their chosen treatment. Patients with relatively well-controlled diabetes have few symptoms of diabetes and what symptoms do occur are not consistently related to glucose levels.13 Despite this, patients often believe that they can estimate their glucose levels based on how they feel14 and believe that they only have diabetes when they have symptoms.15 This overreliance on symptoms leads to poorer adherence.15 One interventional approach is to teach patients to use objective measures of glucose levels rather than symptoms to guide the management of their diabetes. The SMBG provides an objective measure of glucose levels and makes the patient’s glucose levels explicit and external.
In the present study we examined the relationship between SMBG and dietary behaviors among individuals with diabetes living in an urban community. We hypothesized that monitoring glucose levels provides feedback to the patient about their food choices and subsequently motivates adherence to healthy diet behaviors. Two potential moderators of this relationship were examined, namely insulin use and changing diet in response to SMBG.
Methods
The present survey was part of a series of research studies by a community coalition to facilitate better diabetes care for individuals living in East Harlem.16,17 For the survey, bilingual surveyors contacted 670 English- or Spanish-speaking adults with listed East Harlem zip codes and two or more ambulatory care visits for diabetes (ICD-9 250.xx) at participating clinics. Of the 670 individuals contacted, 401 consented and 334 allowed access to their HbA1c levels through their clinician’s office. The survey was typically completed in 20 min and was written at a sixth grade reading level. The present study was approved by the Institutional Review Board of the Mount Sinai School of Medicine.
For purposes of this analysis, the dependent variables were fat intake and fruit and vegetable intake. These were assessed using the Block Fruit/Vegetable/Fiber and Fat Screeners.18 The Block Fruit/Vegetable/Fiber Screener asks the frequency of consuming seven foods (fruit juice, fruit, vegetable juice, green salad, potatoes, vegetable soup, and vegetables). An algorithm can be used to estimate total fruit and vegetable servings. The Block Fat Screener asks the frequency of consuming 17 foods (hamburgers, beef or pork, fried chicken, hotdogs or sausage, cold cuts, bacon or breakfast sausage, salad dressing, margarine or butter on bread or potatoes, margarine or butter or oil in cooking, eggs, pizza, cheese, whole milk, French fries, potato chips, doughnuts or pastries, and ice cream). An algorithm can be used to estimate total fat intake. Correlations between dietary behaviors on the Block Screeners and a more comprehensive food questionnaire, the Block 100 item Food Frequency Questionnaire,19,20 are very good (r = 0.69 for total fat and r = 0.71 for fruit and vegetable servings).18 The Block Screeners are valid for White and non-White populations18 and are used in interventional research.21
The three independent variables were frequency of SMBG, changing diet in response to SMBG, and diabetes education. These variables were informed by validated measures.22 In keeping with prior research, the frequency of SMBG and changing the diet in response to SMBG were each captured with a single item using a five-point Likert scale.23–25 Participants were asked seven questions assessing whether they received diabetes education. The sum of these seven questions, with scores ranging from 0 to 7, was used as a measure of diabetes education.
The HbA1c was accessed from patients’ medical records; information regarding height, weight (to compute body mass index (BMI)), age, gender, and insulin use were obtained by self-report.
The variables SMBG, diabetes education, HbA1c, BMI, and total fat intake were found to be skewed. Inverse transformations were used to normalize four variables, namely the frequency of SMBG, diabetes education, HbA1c, and BMI. Total fat intake was normalized using a square root transformation. The frequency of SMBG and changing diet in response to the SMBG were centered to examine interactions. Bivariate relationships among independent and dependent variables were computed using Pearson and Spearman correlations where appropriate, and separate multivariate regression analyses were conducted to determine predictors of total fat intake and servings of fruit and vegetables. Control and independent variables were entered in Step 1, interactions among frequency of SMBG and changing diet in response to SMBG were entered in Step 2 and the interaction between the frequency of SMBG and insulin use (yes/no) were entered in Step 3. Significant interactions were graphed26,27 and probed for regions of significance.28 Regions of significance define the range of the moderator (change in diet in response to SMBG) over which the relationship of the independent variable (SMBG frequency) is significantly related to the dependent variable (total fat intake). For these interaction analyses, only control and independent variables significantly related to the dependent variable were included and the results are reported as raw scores to preserve interpretability.
Results
Participants were primarily non-Hispanic Black (n = 136; 34%) or Hispanic (n = 208; 52%). Most were female (n = 311; 78%) and approximately one-third reported using insulin. The mean (±SD) age was 60.2 ± 11.3 years, mean BMI was obese at 33.8 ± 8.6 kg/m2, and mean HbA1c was 7.9 ± 1.8%. Reported diets were high in fat (mean total fat = 75.2 ± 19.7 g) and low in fruits and vegetables (mean daily servings = 3.3 ± 1.8). Most participants reported frequent checks of blood sugar (twice a day or more, 42%; approximately once a day, 24%; a few times a week, 18%; less than once a week, 12%; never, 6%) and 78% reported changing their diet in response to their glucose readings. Participants responded yes to a mean of 5.4 ± 2.1 of the seven items assessing diabetes education.
Correlation analyses demonstrated that those who used more SMBG reported lower fat intake (rs = −0.15; P < 0.01) and more frequent changes in diet in response to glucose readings (rs = 0.38; P < 0.01). Participants who reported receiving more diabetes education also reported using more SMBG (rs = 0.10; P < 0.05) and changed their diet more frequently in response to the results of the SMBG (rs = 0.18; P < 0.01). Although fruit and vegetable intake was related to lower fat intake (rs = −0.11; P < 0.05), it was not related to any other variables. None of the independent or dependent variables was correlated with HbA1c or BMI.
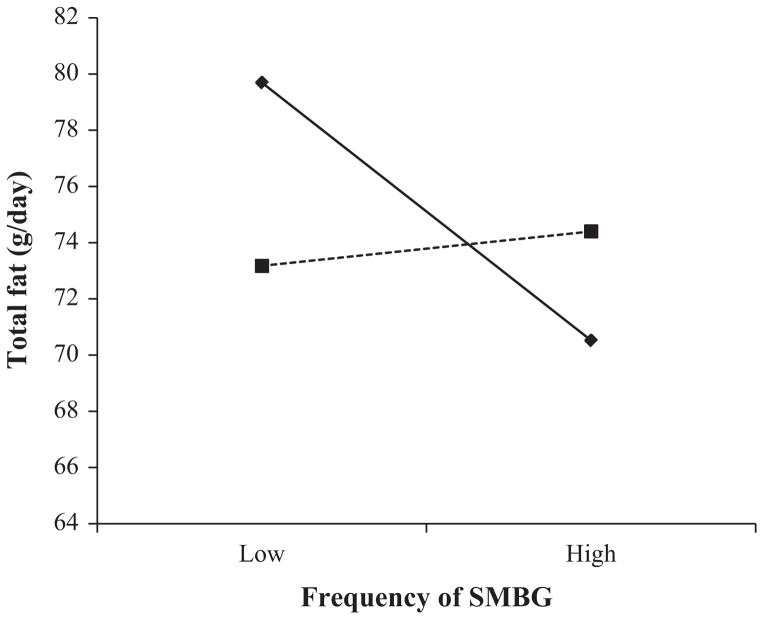
Lower total fat intake was predicted by a higher frequency of SMBG and older age in Step 1 of the regression analysis (see Table 1); the relationship was not moderated by insulin use (Step 3). The interaction between the frequency of SMBG and changing one’s diet in response to the results of the SMBG was significant at Step 2 (see Fig. 1). The upper boundary of the region of significance for the moderator variable (changing diet in response to SMBG) for which the frequency of SMBG was significantly related to fat intake was 2.74 (2 = less than once a week; 3 = a few times a week). Thus, individuals who reported the most frequent changes in diet in response to their SMBG (a few times a week or more) had similar levels of fat intake whether they tested SMBG frequently or infrequently. Fat intake was also low among individuals who made few efforts to change their diet in response to SMBG as long as they performed SMBG frequently. Dietary levels of fat intake were highest among participants who assessed their SMBG infrequently and made few efforts to change their diets in response to the SMBG. The overall model for the regression analysis predicting fruit and vegetable intake was not significant.
Table 1.
Regression analyses predicting total fat
| Total fat (Step 1)
|
Total fat (Step 2)
|
Total fat (Step 3)
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| B | t | P | B | t | P | B | t | P | |
| Gender | 0.04 | 0.25 | NS | 0.04 | 0.24 | NS | 0.03 | 0.23 | NS |
| Age | −0.02 | −3.56 | <0.05 | −0.20 | −3.51 | <0.05 | −0.20 | −0.07 | NS |
| Insulin use | 0.04 | 0.24 | NS | 0.01 | 0.08 | NS | −0.01 | −0.07 | NS |
| SMBG | −0.47 | −2.11 | <0.05 | −0.41 | −1.83 | 0.07 | −0.73 | −1.11 | NS |
| Changing diet in response to SMBG | −0.30 | −0.63 | NS | −0.04 | −0.77 | NS | −0.04 | −0.75 | NS |
| Diabetes education | −0.24 | −1.34 | NS | −0.21 | −1.13 | NS | −0.22 | −1.19 | NS |
| Interactions between SMBG and changing diet in response to SMBG | 0.29 | 2.02 | <0.05 | 0.28 | 1.90 | 0.06 | |||
| Insulin | 0.25 | 0.53 | NS | ||||||
SMBG, self-monitoring of blood glucose.
Figure 1.
Interaction between self-monitoring of blood glucose (SMBG) and changing diet in response to SMBG on total fat intake. (◆), low frequency of changing diet in response to SMBG; (■), high frequency of changing diet in response to SMBG. Slopes for changes in fat intake are estimated 1SD above (high) and 1SD below (low) the mean for SMBG and frequency of changing diet in response to SMBG.
Discussion
As hypothesized, a greater frequency of SMBG was related to greater adherence to diet, specifically lower fat intake, after controlling for diabetes education. This effect was significant for individuals both taking insulin and not taking insulin.
The present study is one of the first to examine whether SMBG is related to adherence to diet. Most studies have either examined the use of SMBG to improve insulin administration or have examined the impact of SMBG on HbA1c. Previous studies for patients not on insulin have primarily examined the relationship between SMBG and long-term glucose control and have reported mixed results.29–36 One reason for the discrepancy may be that SMBG for patients not on insulin will only lead to improvements in HbA1c if SMBG leads to significant long-term improvements in dietary intake. The present study addressed these limitations by examining the impact of SMBG on dietary intake. We found that SMBG is associated with patients making healthier dietary choices. However, low fat intake may not be sufficient to lower BMI or HbA1c. We did not measure other dietary factors, such as carbohydrate intake, that may elevate HbA1c. We also did not examine physical activity. We did find that diabetes education was related to more frequent SMBG. Thus, SMBG may be a tool used by diabetes educators to help patients improve their self-management.
These findings are consistent with our theoretical model, the CSM, which posits that individuals can successfully change their self-care in response to feedback about specific behaviors.35,37 The SMBG provides patients with direct feedback about their success at maintaining glucose control. In the present study, a higher frequency of SMBG was associated with eating foods lower in total fat content. Duran et al.38 recently demonstrated that an SMBG intervention for patients with Type 2 diabetes resulted in lower fat intake. Although fat intake does not affect glucose levels, it is possible that because some of the items on the fat screener are also high in simple carbohydrates (e.g. pastries, French fries) and others may be consumed with foods high in simple carbohydrates (e.g. hamburgers, cold cuts), eating these foods may result in an increase in glucose levels. Future research is needed to understand how SMBG may lead to reductions in fat intake.
The frequency of SMBG was not related to fruit and vegetable consumption among our participants. Previous studies have found a lower availability of fruits and vegetables in East Harlem,39 which may lead to lower intake and difficulty showing associations with SMBG as a result of low variability. Participants with diabetes may also limit their fruit intake because it is high in carbohydrates.
The present study found a significant interaction between the frequency of SMBG and changing one’s diet in response to SMBG on total fat intake. Examination of this interaction showed that total fat intake was lowest either when individuals used the SMBG more frequently, or when they changed their diet in response to even relatively infrequent assessments of SMBG. Those who did not perform SMBG frequently and who did not change their diet in response to SMBG had the highest level of total fat intake. Our theoretical model suggests that the benefit of SMBG is derived from the patients’ use of SMBG as feedback to evaluate meals and not from the frequency of SMBG per se. The data suggest that participants who use SMBG to guide their diet do not have to monitor multiple times a day to benefit.
The limitations of the present study include its cross-sectional design and homogeneous population. Confounding variables, such as dedication to a healthy lifestyle, may account for both more frequent SMBG and lower fat intake. In addition, the frequency of SMBG and changing one’s diet in response to SMBG were measured using single items and our measures of diet intake were screening instruments that do not assess total energy or carbohydrate intake and have not yet been validated among immigrant populations.
In conclusion, the frequency of SMBG was related to lower fat intake for minority individuals with diabetes regardless of insulin use. Changing diet in response to SMBG was associated with lower fat intake even in those who performed SMBG less frequently. SMBG was not associated with fruit and vegetable intake, BMI, or HbA1c. The present study addressed limitations of previous research on SMBG by specifically examining whether SMBG is associated with a healthier diet. Future studies should continue to explore associations between SMBG and dietary behaviors, physical activity, and glucose control to determine when and what targets patients should use to make changes to their diabetes self-care.
Acknowledgments
This study was funded by the New York State Department of Health Diabetes Prevention and Control Program and the National Center on Minority Health and Health Disparities (R24 MD 001691).
Footnotes
Aspects of this material are the result of work supported with resources and the use of facilities at the War Related Illness and Injury Study Center (WRIISC), Department of Veterans Affairs, New Jersey Health Care System. The views expressed are those of the authors and do not reflect the official policy or position of the US Government.
Disclosure
There are no conflicts of interest for any of the authors.
References
- 1.Russell LB, Suh DC, Safford MA. Time requirements for diabetes self-management: Too much for many? J Fam Pract. 2005;54:52–6. [PubMed] [Google Scholar]
- 2.Bodenheimer T, Lorig K, Holman H, Grumbach K. Patient self-management of chronic disease in primary care. JAMA. 2002;288:2469–75. doi: 10.1001/jama.288.19.2469. [DOI] [PubMed] [Google Scholar]
- 3.Michie S, Abraham C, Whittington C, McAteer J, Gupta S. Effective techniques in healthy eating and physical activity interventions: A meta-regression. Health Psychol. 2009;28:690–701. doi: 10.1037/a0016136. [DOI] [PubMed] [Google Scholar]
- 4.Reichard P. To be a teacher, a tutor and a friend: The physician’s role according to the Stockholm Diabetes Intervention Study (SDIS) Patient Educ Couns. 1996;29:231–5. doi: 10.1016/s0738-3991(96)00967-6. [DOI] [PubMed] [Google Scholar]
- 5.DAFNE Study Group. Training in flexible, intensive insulin management to enable dietary freedom in people with Type 1 diabetes: Dose adjustment for normal eating (DAFNE) randomised controlled trial. BMJ. 2002;325:746–9. doi: 10.1136/bmj.325.7367.746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.American Diabetes Association. Standards of medical care in diabetes: 2009. Diabetes Care. 2009;32 (Suppl 1):S13–61. doi: 10.2337/dc09-S013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Farmer AJ, Wade AN, French DP, et al. Blood glucose self-monitoring in Type 2 diabetes: A randomised controlled trial. Health Technol Assess. 2009;13:iii–45. doi: 10.3310/hta13150. [DOI] [PubMed] [Google Scholar]
- 8.McAndrew L, Schneider SH, Burns E, Leventhal H. Does patient blood glucose monitoring improve diabetes control? A systematic review of the literature. Diabetes Educ. 2007;33:991–1011. doi: 10.1177/0145721707309807. [DOI] [PubMed] [Google Scholar]
- 9.Leventhal H, Benyamini Y, Brownlee S, et al. Illness representations: Theoretical foundations. In: Petrie KJ, Weinman JA, editors. Perceptions of Health and Illness. Harwood; Amsterdam: 1997. pp. 19–46. [Google Scholar]
- 10.Leventhal H, Weinman J, Leventhal EA, Phillips LA. Health psychology: The search for pathways between behavior and health. Annu Rev Psychol. 2008;59:477–505. doi: 10.1146/annurev.psych.59.103006.093643. [DOI] [PubMed] [Google Scholar]
- 11.Meyer D, Leventhal H, Gutmann M. Common-sense models of illness: The example of hypertension. Health Psychol. 1985;4:115–35. doi: 10.1037//0278-6133.4.2.115. [DOI] [PubMed] [Google Scholar]
- 12.Rabin C, Ward S, Leventhal H, Schmitz M. Explaining retrospective reports of symptoms in patients undergoing chemotherapy: Anxiety, initial symptom experience, and posttreatment symptoms. Health Psychol. 2001;20:91–8. [PubMed] [Google Scholar]
- 13.Halm EA, Mora P, Leventhal H. No symptoms, no asthma: The acute episodic disease belief is associated with poor self-management among inner-city adults with persistent asthma. Chest. 2006;129:573–80. doi: 10.1378/chest.129.3.573. [DOI] [PubMed] [Google Scholar]
- 14.Mann DM, Ponieman D, Leventhal H, Halm EA. Misconceptions about diabetes and its management among low-income minorities with diabetes. Diabetes Care. 2009;32:591–3. doi: 10.2337/dc08-1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mann DM, Ponieman D, Leventhal H, Halm EA. Predictors of adherence to diabetes medications: The role of disease and medication beliefs. J Behav Med. 2009;32:278–84. doi: 10.1007/s10865-009-9202-y. [DOI] [PubMed] [Google Scholar]
- 16.Horowitz CR, Williams L, Bickell NA. A community-centered approach to diabetes in East Harlem. J Gen Intern Med. 2003;18:542–8. doi: 10.1046/j.1525-1497.2003.21028.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Horowitz CR, Goldfinger JZ, Muller SE, et al. A model for using community-based participatory research to address the diabetes epidemic in East Harlem. Mt Sinai J Med. 2008;75:13–21. doi: 10.1002/msj.20017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Block G, Gillespie C, Rosenbaum EH, Jenson C. A rapid food screener to assess fat and fruit and vegetable intake. Am J Prev Med. 2000;18:284–8. doi: 10.1016/s0749-3797(00)00119-7. [DOI] [PubMed] [Google Scholar]
- 19.Block G, Hartman AM, Dresser CM, Carroll MD, Gannon J, Gardner L. A data-based approach to diet questionnaire design and testing. Am J Epidemiol. 1986;124:453–69. doi: 10.1093/oxfordjournals.aje.a114416. [DOI] [PubMed] [Google Scholar]
- 20.Block G, Woods M, Potosky A, Clifford C. Validation of a self-administered diet history questionnaire using multiple diet records. J Clin Epidemiol. 1990;43:1327–35. doi: 10.1016/0895-4356(90)90099-b. [DOI] [PubMed] [Google Scholar]
- 21.Campbell MK, Carbone E, Honess-Morreale L, Heisler-Mackinnon J, Demissie S, Farrell D. Randomized trial of a tailored nutrition education CD-ROM program for women receiving food assistance. J Nutr Educ Behav. 2004;36:58–66. doi: 10.1016/s1499-4046(06)60134-6. [DOI] [PubMed] [Google Scholar]
- 22.Fitzgerald JT, Davis WK, Connell CM, Hess GE, Funnell MM, Hiss RG. Development and validation of the Diabetes Care Profile. Eval Health Prof. 1996;19:208–30. doi: 10.1177/016327879601900205. [DOI] [PubMed] [Google Scholar]
- 23.Franciosi M, Pellegrini F, De Berardis G, et al. The impact of blood glucose self-monitoring on metabolic control and quality of life in Type 2 diabetic patients: An urgent need for better educational strategies. Diabetes Care. 2001;24:1870–7. doi: 10.2337/diacare.24.11.1870. [DOI] [PubMed] [Google Scholar]
- 24.Levine DA, Allison JJ, Cherrington A, Richman J, Scarinci IC, Houston TK. Disparities in self-monitoring of blood glucose among low-income ethnic minority populations with diabetes, United States. Ethn Dis. 2009;19:97–103. [PubMed] [Google Scholar]
- 25.Harris MI. Frequency of blood glucose monitoring in relation to glycemic control in patients with Type 2 diabetes. Diabetes Care. 2001;24:979–82. doi: 10.2337/diacare.24.6.979. [DOI] [PubMed] [Google Scholar]
- 26.Aiken LS, West SG. Multiple Regression: Testing and Interpreting Interactions. Sage; London: 1991. [Google Scholar]
- 27.Dawson JF, Richter AW. Probing three-way interactions in moderated multiple regression: Development and application of a slope difference test. J Appl Psychol. 2006;91:917–26. doi: 10.1037/0021-9010.91.4.917. [DOI] [PubMed] [Google Scholar]
- 28.Preacher KJ, Curran PJ, Bauer DJ. Computational tools for proving interaction effects in multiple linear regression, multilevel modeling, and latent curve analysis. J Educ Behav Stat. 2006;31:437–48. [Google Scholar]
- 29.St John A, Davis WA, Price CP, Davis TM. The value of self-monitoring of blood glucose: A review of recent evidence. J Diabetes Complications. 2010;24:129–41. doi: 10.1016/j.jdiacomp.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 30.Poolsup N, Suksomboon N, Rattanasookchit S. Metaanalysis of the benefits of self-monitoring of blood glucose on glycemic control in Type 2 diabetes patients: An update. Diabetes Technol Ther. 2009;11:775–84. doi: 10.1089/dia.2009.0091. [DOI] [PubMed] [Google Scholar]
- 31.Allemann S, Houriet C, Diem P, Stettler C. Self-monitoring of blood glucose in non-insulin treated patients with Type 2 diabetes: A systematic review and metaanalysis. Curr Med Res Opin. 2009;25:2903–13. doi: 10.1185/03007990903364665. [DOI] [PubMed] [Google Scholar]
- 32.Sarol JN, Jr, Nicodemus NA, Jr, Tan KM, Grava MB. Self-monitoring of blood glucose as part of a multicomponent therapy among non-insulin requiring Type 2 diabetes patients: A meta-analysis (1966–2004) Curr Med Res Opin. 2005;21:173–84. doi: 10.1185/030079904X20286. [DOI] [PubMed] [Google Scholar]
- 33.Welschen LM, Bloemendal E, Nijpels G, et al. Self-monitoring of blood glucose in patients with Type 2 diabetes who are not using insulin: A systematic review. Diabetes Care. 2005;28:1510–7. doi: 10.2337/diacare.28.6.1510. [DOI] [PubMed] [Google Scholar]
- 34.Palmer AJ, Dinneen S, Gavin JR, III, Gray A, Herman WH, Karter AJ. Cost-utility analysis in a UK setting of self-monitoring of blood glucose in patients with Type 2 diabetes. Curr Med Res Opin. 2006;22:861–72. doi: 10.1185/030079906X104669. [DOI] [PubMed] [Google Scholar]
- 35.Klonoff DC. Benefits and limitations of self-monitoring of blood glucose. J Diabetes Sci Technol. 2007;1:130–2. doi: 10.1177/193229680700100121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Neeser K, Erny-Albrecht K, Weber C. Cost-effectiveness of self-monitoring of blood glucose in Type 2 diabetic patients not receiving insulin. Diabetes Care. 2006;29:480–1. doi: 10.2337/diacare.29.02.06.dc05-1857. [DOI] [PubMed] [Google Scholar]
- 37.McAndrew LM, Musumeci-Szabo TJ, Mora PA, et al. Using the common sense model to design interventions for the prevention and management of chronic illness threats: From description to process. Br J Health Psychol. 2008;13 (Pt 2):195–204. doi: 10.1348/135910708X295604. [DOI] [PubMed] [Google Scholar]
- 38.Duran A, Martin P, Runkle I, et al. Benefits of self-monitoring blood glucose in the management of new-onset Type 2 diabetes mellitus: The St Carlos Study, a prospective randomized clinic-based interventional study with parallel groups. J Diabetes. 2010;2:203–11. doi: 10.1111/j.1753-0407.2010.00081.x. [DOI] [PubMed] [Google Scholar]
- 39.Horowitz CR, Colson KA, Hebert PL, Lancaster K. Barriers to buying healthy foods for people with diabetes: Evidence of environmental disparities. Am J Public Health. 2004;94:1549–54. doi: 10.2105/ajph.94.9.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]