Abstract
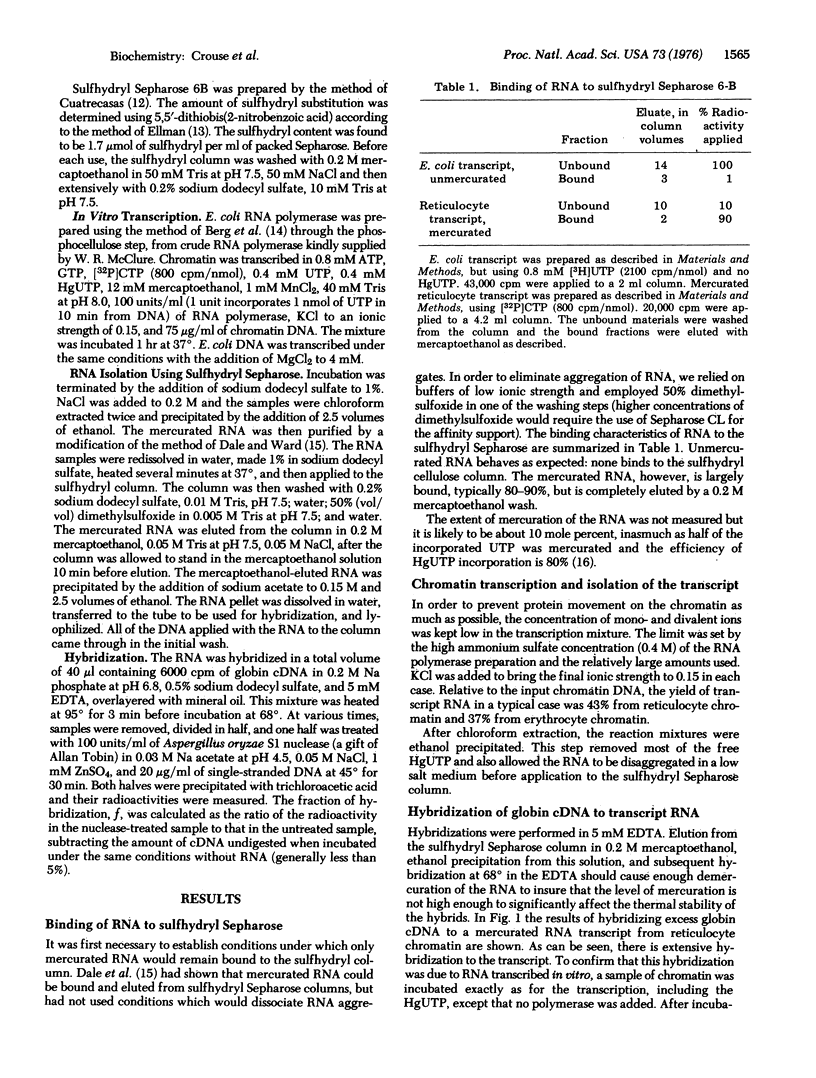
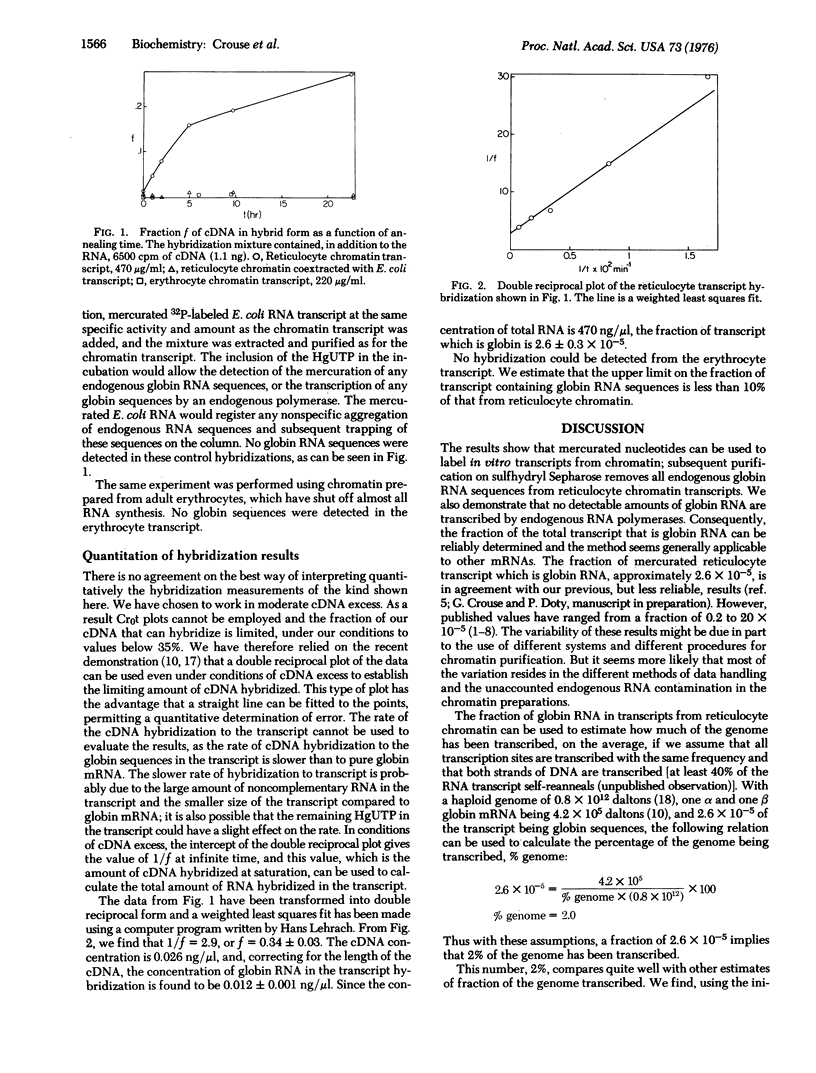
Mercurated uridine triphosphate has been used to label transcripts of chicken reticulocyte chromatin made with Escherichia coli RNA polymerase. The mercury-labeled RNA product can be completely separated from endogenous RNA sequences in the chromatin by passage through a sulfhydryl Sepharose column. Globin cDNA hybridization to the transcript shows that only 2.6 x 10-5 of the transcript is globin RNA. In contrast to this result, erythrocte chromatin transcript contains less than one tenth as many globin RNA sequences.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Axel R., Cedar H., Felsenfeld G. Synthesis of globin ribonucleic acid from duck-reticulocyte chromatin in vitro. Proc Natl Acad Sci U S A. 1973 Jul;70(7):2029–2032. doi: 10.1073/pnas.70.7.2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axel R., Cedar H., Felsenfield G. The structure of the globin genes in chromatin. Biochemistry. 1975 Jun 3;14(11):2489–2495. doi: 10.1021/bi00682a031. [DOI] [PubMed] [Google Scholar]
- Barrett T., Maryanka D., Hamlyn P. H., Gould H. J. Nonhistone proteins control gene expression in reconstituted chromatin. Proc Natl Acad Sci U S A. 1974 Dec;71(12):5057–5061. doi: 10.1073/pnas.71.12.5057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cedar H., Felsenfeld G. Transcription of chromatin in vitro. J Mol Biol. 1973 Jun 25;77(2):237–254. doi: 10.1016/0022-2836(73)90334-3. [DOI] [PubMed] [Google Scholar]
- Cuatrecasas P. Protein purification by affinity chromatography. Derivatizations of agarose and polyacrylamide beads. J Biol Chem. 1970 Jun;245(12):3059–3065. [PubMed] [Google Scholar]
- Dale R. M., Livingston D. C., Ward D. C. The synthesis and enzymatic polymerization of nucleotides containing mercury: potential tools for nucleic acid sequencing and structural analysis. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2238–2242. doi: 10.1073/pnas.70.8.2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale R. M., Martin E., Livingston D. C., Ward D. C. Direct covalent mercuration of nucleotides and polynucleotides. Biochemistry. 1975 Jun 3;14(11):2447–2457. doi: 10.1021/bi00682a027. [DOI] [PubMed] [Google Scholar]
- Dale R. M., Ward D. C. Mercurated polynucleotides: new probes for hybridization and selective polymer fractionation. Biochemistry. 1975 Jun 3;14(11):2458–2469. doi: 10.1021/bi00682a028. [DOI] [PubMed] [Google Scholar]
- ELLMAN G. L. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959 May;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- Gilmour R. S., Paul J. Tissue-specific transcription of the globin gene in isolated chromatin. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3440–3442. doi: 10.1073/pnas.70.12.3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmour R. S., Windass J. D., Affara N., Paul J. Control of transcription of the globin gene. J Cell Physiol. 1975 Apr;85(2 Pt 2 Suppl 1):449–458. doi: 10.1002/jcp.1040850411. [DOI] [PubMed] [Google Scholar]
- Imaizumi T., Diggelmann H., Scherrer K. Demonstration of globin messenger sequences in giant nuclear precursors of messenger RNA of avian erythroblasts. Proc Natl Acad Sci U S A. 1973 Apr;70(4):1122–1126. doi: 10.1073/pnas.70.4.1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConaughy B. L., McCarthy B. J. Fractionation of chromatin by thermal chromatography. Biochemistry. 1972 Mar 14;11(6):998–1003. doi: 10.1021/bi00756a008. [DOI] [PubMed] [Google Scholar]
- Steggles A. W., Wilson G. N., Kantor J. A., Picciano D. J., Falvey A. K., Anderson W. F. Cell-free transcription of mammalian chromatin: transcription of globin messenger RNA sequences from bone-marrow chromatin with mammalian RNA polymerase. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1219–1223. doi: 10.1073/pnas.71.4.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma I. M., Baltimore D. Purification of the RNA-directed DNA polymerase from avian myeloblastosis virus and its assay with polynucleotide templates. Methods Enzymol. 1974;29:125–130. doi: 10.1016/0076-6879(74)29015-3. [DOI] [PubMed] [Google Scholar]
- Wilson G. N., Steggles A. W., Kantor J. A., Nienhuis A. W., Anderson W. F. Cell-free transcription of mammalian chromatin. Quantitative measurement of newly synthesized globin messenger RNA sequences. J Biol Chem. 1975 Nov 25;250(22):8604–8613. [PubMed] [Google Scholar]



