Abstract
Raphé-derived serotonin (5-HT) and thyrotropin-releasing hormone (TRH) play important roles in fundamental, homeostatic control systems such as breathing and specifically the ventilatory CO2 chemoreflex. Brown Norway (BN) rats exhibit an inherent and severe ventilatory insensitivity to hypercapnia but also exhibit relatively normal ventilation at rest and during other conditions, similar to multiple genetic models of 5-HT system dysfunction in mice. Herein, we tested the hypothesis that the ventilatory insensitivity to hypercapnia in BN rats is due to altered raphé gene expression and the consequent deficiencies in raphé-derived neuromodulators such as TRH. Medullary raphé transcriptome comparisons revealed lower expression of multiple 5-HT neuron-specific genes in BN compared to control Dahl salt-sensitive rats, predictive of reduced central nervous system monoamines by bioinformatics analyses and confirmed by high-performance liquid chromatography measurements. In particular, raphé Trh mRNA and peptide levels were significantly reduced in BN rats, and injections of the stable TRH analogue Taltirelin (TAL) stimulated breathing dose-dependently, with greater effects in BN versus control Sprague–Dawley rats. Importantly, TAL also effectively normalized the ventilatory CO2 chemoreflex in BN rats, but TAL did not affect CO2 sensitivity in control Sprague–Dawley rats. These data establish a molecular basis of the neuromodulatory deficiency in BN rats, and further suggest an important functional role for TRH signalling in the mammalian CO2 chemoreflex.
Key points.
Increases in carbon dioxide (CO2) provide a major chemical stimulus to breathe through activation of the ventilatory CO2 chemoreflex, which is heavily influenced by the brainstem serotonergic (5-HT) system.
Brown Norway (BN) rats have an inherent and extremely low ventilatory sensitivity to hypercapnia, which can be augmented with selective serotonin reuptake inhibition.
Using mRNA sequencing, we show that BN rats have reduced medullary raphé expression of multiple 5-HT neuron-specific genes, predictive of lower monoamine levels by informatics pathway analyses and confirmed by high-performance liquid chromatography measurements.
BN rats also showed reduced thyrotropin-releasing hormone (TRH) expression, where injections of the TRH analogue Taltirelin caused greater increases in baseline ventilation, body temperature and the ventilatory CO2 chemoreflex in BN rats compared to control Sprague–Dawley rats.
These data establish a molecular basis of a neuromodulatory deficiency in BN rats, and further suggest an important functional role for TRH signalling in the mammalian CO2 chemoreflex.
Introduction
From birth to death, we continuously breathe to exchange gases with our environment to maintain O2 and CO2 and/or pH homeostasis. The fundamental CNS mechanisms that govern the ventilatory CO2 chemoreflex, which elicits acute changes in ventilation required for CO2/pH homeostasis, are not well understood. Several hindbrain nuclei contain neurons and/or glia that demonstrate cellular CO2/pH chemosensitivity (Dean et al. 1990; Coates et al. 1993; Mulkey et al. 2004; Putnam et al. 2004), including but not limited to the retrotrapezoid nucleus (RTN), nucleus of the solitary tract (NTS) and the medullary raphé (MR) (Wang et al. 1998). A fraction of MR neurons produces serotonin (5-HT) and the co-transmitted neuropeptides substance P and thyrotropin-releasing hormone (TRH) (Wang et al. 2001). These raphé-derived neuromodulators have predominantly excitatory effects within the neural respiratory network (Hodges & Richerson, 2008) and contribute to neural respiratory rhythm/pattern generation particularly during postnatal development (Pena & Ramirez, 2002; Ptak et al. 2009). Sudden infant death syndrome (SIDS) has been linked to multiple defects in the brainstem 5-HT system (Duncan et al. 2010) and hypothesized to result in part from chemoreflex failure (Shannon et al. 1977; Kinney & Thach, 2009). Thus, a complete understanding of the role of raphé-derived neuromodulators and their contributions to the ventilatory CO2 chemoreflex is vital to completing our understanding of SIDS and other respiratory-related disorders in humans involving dysregulation of CO2 homeostasis.
The importance of 5-HT neurons in ventilatory CO2 chemoreception is based in large part on evidence from several mouse models of 5-HT system deficiency. Genetic deletion of most (Hodges et al. 2011) or all (Hodges et al. 2008) 5-HT neurons, or acute and global inhibition of 5-HT neurons using tissue-specific DREADD receptor activation (Ray et al. 2011) each attenuate the CO2 chemoreflex up to 50%, whereas intracerebroventricular infusion of 5-HT in mice lacking all 5-HT neurons normalizes this deficit (Hodges et al. 2008). Remarkably, these models of 5-HT deficiency show a relatively selective deficit in the CO2 chemoreflex, as they otherwise demonstrate normal breathing at rest and robust hypoxic ventilatory responses as adults.
Similarly, the brown Norway (BN; BN/NHsdMcwi) rat is of particular interest because it inherently exhibits a nearly absent ventilatory CO2 chemoreflex (Strohl et al. 1997; Hodges et al. 2002), akin to other rodent models of phenotypic extreme like the substance P-deficient and hypoxia/hypercapnia insensitive naked mole rat (Edrey et al. 2011). Our recent work revealed reduced central nervous system levels of 5-HT, and administration of a selective 5-HT reuptake inhibitor (fluoxetine) modestly augmented the CO2 chemoreflex in BN but not the CO2-sensitive Sprague–Dawley rats (Hodges et al. 2013). These data suggested a role for reduced 5-HT levels and/or altered 5-HT neuronal function in attenuating the ventilatory CO2 chemoreflex in the BN rat, but the molecular basis for the presumed 5-HT system dysfunction is unknown. Furthermore, additional raphé-derived neuromodulators such as TRH may also be deficient in the BN rat. Thus, we tested the hypothesis that the inherent and extreme ventilatory CO2 insensitivity in BN rats was due in part to reduced neuromodulatory gene expression and the consequent reduction in raphé-derived neuromodulators, including TRH.
Methods
Animal models
All procedures and protocols were approved by the Medical College of Wisconsin Institutional Animal Care and Use Committee before studies were initiated. The BN/Mcwi (BN) and Dahl salt-sensitive (SS/JrHsdMcwi; SS) are inbred strains generated and maintained by the Medical College of Wisconsin. Outbred Sprague–Dawley rats were obtained from Harlan Laboratories (Haslett, MI, USA; strain code 002). We used adult (>7 weeks of age), male rats maintained on a 12 h light/dark cycle (06.00–18.00 h lights on) and fed 0.4% NaCl chow (Dyets, Bethlehem, PA, USA) ad libitum from weaning (BN and SS rats) or acquisition (>6 weeks of age). BN (n = 40), SS (n = 27) and Sprague–Dawley (n = 15) rats were used for six experimental purposes: (1) mRNA sequencing (BN, n = 11; SS, n = 11); (2) high-performance liquid chromatography (HPLC) measurements (BN, n = 6; SS, n = 6); (3) metabolic rate studies (BN, n = 7; Sprague–Dawley, n = 5); (4) ventilatory studies (BN, n = 6; Sprague–Dawley, n = 6); (5) immunohistochemical staining and cell counting (BN, n = 6; SS, n = 6; Sprague–Dawley, n = 4); and (6) Western blotting (BN, n = 4; SS, n = 4).
RNA sequencing
Similar to a previous study (Kriegel et al. 2013), 7–8-week-old naïve male BN (n = 11) and SS (n = 11) rats were deeply anaesthetized (20% isoflurane in propylene glycol v/v) before rapid removal of the brain, which was embedded and snap-frozen (2-methybutane) before transfer −80°C for storage. Within 3 weeks of collection, thick slices (∼1 mm) of brainstem tissues were made using an Alto adult rat brain matrix from caudal to rostral beginning with the cervical spinal cord through the pontomedullary junction, and circular tissue punches (blunted 18.5 gauge needle) taken from the ventral midline from the first four slices (slices 1–4) beginning with the one containing obex (−14.0 to −13.0 mm from Bregma; Fig. 1A–C). Punches from slice 1 as caudal MR (cMR), which contains the raphé obscurus (Fig. 1B), and punches from slice 3 as rostral MR (rMR), which contains raphé magnus/pallidus (Fig. 1C). Total RNA was extracted from tissue punches, and quality assessed as described previously (Liu et al. 2014) before cDNA library preparation starting with 264–486 ng total RNA using TruSeq RNA sample prep kit (Illumina, San Diego, CA, USA), cluster generation and sequencing (HiSeq 2000) per vendor instructions, and sequence data analyses performed using an in-house pipeline for read mapping and alignment, transcript construction and quantification (Fig. 1D) and statistical differential expression using Bowtie, Tophat v2 and Cufflinks similar to previous studies (Liu et al. 2014).
Figure 1.
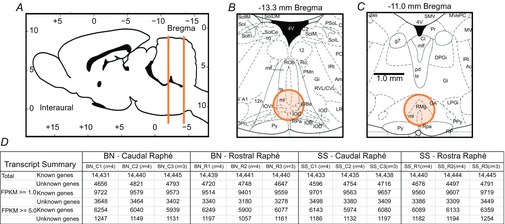
Medullary raphé regions collected and sequence results
A, rat brain (mid-sagittal) with indication of rostrocaudal levels from which medullary raphé tissue punches were obtained. B and C, coronal sections of the medulla at −13.3 mm and −11.0 mm, respectively, with the areas (orange) indicating tissue punch collection sites. D, transcript expression data summarizing total known and unknown genes identified in each cDNA library sequenced (n = number of rats/library) with abundance (FPKM) of more than 1.0 or 5.0 FPKM. Note the similarity among libraries within and across strains in total transcript numbers, indicating reproducibility. BN, brown Norway (rat); SS, Dahl salt-sensitive (rat).
Bioinformatics pathway analyses
To aid in a biological interpretation of the gene expression data, bioinformatics pathway analyses were performed by uploading all known genes and their abundance and log-fold change from each comparison (first or second pass significance thresholds) to ingenuity pathway analysis (IPA; http://www.ingenuity.com). A ‘Core analysis’ was then performed, which provided outputs including top canonical pathways, upstream analysis, diseases and functions, regulator effects and networks. Within each of these categories, IPA uses unbiased correlative metrics to rank associated pathways to the gene data, and in some cases, predictions based on the magnitude and direction of gene expression differences.
Immunohistochemistry and cell counts
Rats were anaesthetized, transcardially perfused/fixed and brainstems removed and frozen sectioned (25 μm) coronally from −2.0 to 3.0 mm from obex and affixed to glass slides. Sections were immersed in 0.4% Triton X-100 in phosphate-buffered saline (10 min), blocked with horse serum (5% in 0.2% Triton in phosphate-buffered saline; 1 h), and incubated 1–2 days in primary antibodies targeting: tryptophan hydroxylase (TPH; 1:1000, mouse monoclonal, T0678; Sigma-Aldrich), tyrosine hydroxylase (TH; 1:1000, mouse monoclonal, T2928; Sigma-Aldrich, St. Louis, MO, USA), Phox2b [1:1000, rabbit polyclonal; J.F. Brunet, École Normale Supérieure (IBENS), France] and TRH (1:1000, rabbit polyclonal, HPA035595; Sigma-Aldrich). Secondary antibodies (1:500, biotinylated antimouse or antirabbit, 1 h; Vector Labs, Berlingame, CA, USA) were used for brightfield visualization after avidin–biotin–horseradish peroxidase reaction (Vectastain Elite with DAB or ImmPACT SG chromogens; Vector Labs), or for immunofluorescence (1:500, Alexa 594-conjugated antimouse or Alexa 488-conjugated antirabbit; Invitrogen, Waltham, MA, USA) and standard epifluorescence microscopy. Brainstem cells were considered immunopositive if there was an intense cellular label in a specific subcellular compartment within cells in a region known to express the protein target. TPH-immunopositive (TPH+) cells showed strong immunoreactivity in the cell body and processes but no nuclear labelling, and were confined to midline and ventrolateral medullary regions in the raphé nuclei (Dahlstrom & Fuxe, 1964; Jacobs & Azmitia, 1992). Cellular TH-immunoreactivity (TH+) was similar to TPH, but the cell bodies were found in the classically described adrenergic nuclei ventrolateral (A1/C1) and dorsomedial (A2/C2) brainstem regions (Moore & Bloom, 1979). Phox2b-immunoreactivity was confined to the cellular nucleus as previously described (Stornetta et al. 2006). Specificity of the antibodies for TPH, TH and Phox2b has been previously documented (Weston et al. 2004; Stornetta et al. 2006). Counts of all TPH+ neurons (midline and ventrolateral medullar) were made from each section −2 to 2 mm rostral to obex and averaged each 0.5 mm. Counts of TH+ and Phox2b+ (TH−) cells were made from the solitary complex region from −0.5 to 0.5 mm from obex, and from the RTN region ∼1.5 mm lateral to the midline dorsally to the facial nucleus and to the ventral medullary surface from −12.0 to −10.8 mm from Bregma.
Western blots
BN and SS rat brains were harvested and flash frozen, raphé punches were obtained as described above and punches including the NTS or RTN obtained from frozen slices 1 and 3, respectively. Tissue punches were homogenized by sonication in Lysis Buffer (150 nm NaCl, 50 mm Tris, 1% NP40) and protein concentration assessed with spectrophotometry (Bradford method). Five μg of protein/sample was loaded and run on precast gels (BioRad, Hercules, CA, USA; 10–20% Tris-HCl), and transferred to nitrocellulose paper for incubation with block solution (5% bovine serum albumin in TBS-tween) and then TRH primary antibody (1:200; Sigma HPA035595) or TRH receptor (TRHR) antibody (1:500, ab72179; Abcam, Cambridge, UK) overnight. Nitrocellulose paper was then washed (×3), an antirabbit antibody (1:10,000) added, washed (×3) and developed with SuperSignal West Femto Kit (Thermo Scientific, Waltham, MA, USA), imaged (ChemiDoc Imaging system; BioRad) and processed (Image Lab). The blot was then washed (×3) and incubated with a primary antibody targeting β-actin (sc-47778, 1:10,000; Santa Cruz, Dallas, TX, USA) overnight before additional washes, application of an antimouse secondary antibody (1:10,000) and development using a SuperSignal West Pico Kit (Thermo Scientific).
High-performance liquid chromatography
Tissue collection and HPLC analyses was performed as previously described (Hodges et al. 2013), Briefly, after induction of deep isoflurane anaesthesia the brain was removed and sectioned into 5 regions: the forebrain, hypothalamus, midbrain and pons, cerebellum and medulla. The tissue was frozen (−80°C) until thawed in 0.1 m perchloric acid (0.1 g ml−1), wet weights obtained, tissues sonicated, centrifuged [10,000 rpm (5031 g) for 20 min at 4°C], and supernatant analysed for noradrenaline (NA), adrenaline, dopamine (DA), 3,4-dihydroxyphenylacetic acid, 5-HT, 5-hydroxyindolacetic acid (5-HIAA) and homovanillic acid.
Physiologic measurements and Taltirelin treatment
Surgical catheter implantation, and ventilation and blood gas measurements were obtained as previously described (Mouradian et al. 2012). Resting ventilation (room air breathing) and the CO2 chemoreflex (7% CO2 in room air) was measured in one group of BN (n = 6) and Sprague–Dawley (n = 6) rats before and 1 h after intraperitoneal injections of saline (0.9% NaCl), 0.3 mg kg−1, or 1.0 mg kg−1 Taltirelin (TAL; 2672; Tocris Bioscience, Bristol, UK) on 3 separate days with studies separated by >24 h. These doses for TAL were selected based on the physiologic effects reported in mice (Eto et al. 2011). Metabolic rates were measured in a smaller flow-through chamber (2 l) where O2 and CO2 levels in the outflow (2 l min−1) was measured (Oxigraph, Mountain View, CA, USA) continuously, and 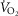 calculated using the Fick equation as previously described (Nattie & Li, 2001).
calculated using the Fick equation as previously described (Nattie & Li, 2001). 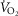 was measured for 30 min before and up to 1 h after injections of saline or TAL.
was measured for 30 min before and up to 1 h after injections of saline or TAL.
Statistics
All data presented are means ± SEM unless otherwise noted. Statistical significance for differential gene expression was obtained as previously described (Liu et al. 2014). All other statistical tests were performed using Sigmaplot v12.3 (which includes a comprehensive statistics package) using the tests and factors as described in the Results section, and considered significant if P < 0.05.
Results
Differential expression analyses point to serotonin neuron-specific differences among strains
To study regional and strain-related differences in MR gene expression we harvested tissue punches (∼1 mm3) from BN (n = 11) and control SS (n = 11) brainstems (Fig. 1A–C) for subsequent RNA extraction, cDNA library preparation and mRNA sequencing. Three biological replicates (n = 3–4/library, n = 11 rats/strain) yielded similar numbers of transcripts detected and mapped (Fig. 1D), most of which were known genes/transcripts (∼14,400) or unknown genes (transcripts that map to a genomic location but have not been identified or characterized as genes; Fig. 1D). Across all cDNA libraries we found high transcript yields (4.97 ± 0.11 Gb), read number (50.4 ± 1.1 million/library), mean quality score (94.6 ± 0.05) and mapping rate (88.8 ± 0.6%). Significant differential expression between raphé regions and strains was initially determined using an adjusted P value of q < 0.05, representing our conservative ‘first pass’ data set. Recognizing the limited statistical power of genome-wide expression profiling studies (Yang et al. 2013), we then relaxed the threshold to P < 0.005 (q < 0.25; ‘second pass’ data set) to broaden the scope of our analysis and enrich the subsequent pathway analyses (see below).
We initially focused on differentially expressed known genes within the rMR (region containing the raphé pallidus and magnus) with 5-HT system-related function that may be deficient in the BN rat. Trh, the gene encoding the excitatory neuromodulator TRH, was among the most significantly differentially expressed genes between strains and was lower in expression in the BN (blue data in Fig. 2A, q = 1.04E−9). However, we found no differences among the strains in MR expression of the TRHR gene. Additional 5-HT neuron-specific genes were also reduced (red data in Fig. 2B; P < 0.005) in the rMR of BN rats, including genes necessary for 5-HT biosynthesis [TPH 2 (Tph2) and amino acid decarboxylase (Ddc)], vesicular packaging of 5-HT [vesicular monoamine transporter 2 (Slc18a2)], 5-HT reuptake [serotonin transporter (Slc6a4)) and metabolic breakdown/inactivation (aldehyde oxidase 1 (Aox1)]. 5-HT neurons also synthesize and co-release substance P, but the gene expression data suggest little or no difference in the rMR in the abundance of Tac1 (substance P) or the substance P receptor (Tacr1). Thus, at first glance the major strain-related differences in gene expression pointed to reduced raphé-derived TRH and 5-HT neuron-specific genes in the BN rat.
Figure 2.
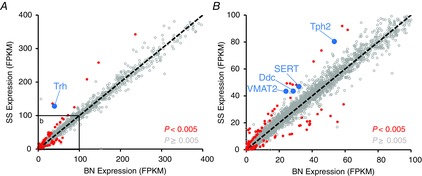
Multiple serotonin neuron-related genes are reduced in BN rats in the rostral medullary raphé
A, gene abundance (FPKM) of all known genes identified in the rostral medullary raphé of BN and SS rats are plotted along a line of identity, including 101 differentially expressed [second pass; q < 0.25 (P < 0.005); red/blue] genes and all other (14,378; grey) known genes. An expansion of the inset in (A) shown in (B). BN, brown Norway (rat); SS, Dahl salt-sensitive (rat).
Raphé-derived thyrotropin-releasing hormone is reduced in the CO2 insensitive brown Norway rat
In addition to its well-known role in regulating the hypothalamic–pituitary–thyroid axis, TRH is produced in and co-released from MR 5-HT neurons (Dean et al. 1993). However, a major role for TRH in the ventilatory CO2 chemoreflex has not been clearly demonstrated. Based on the reduced TRH gene expression in BN rats, we prepared fixed-frozen brainstem sections for immunofluorescent labelling with antibodies targeting TRH (Fig. 3A and D) and TPH (Fig. 3B and E). Brainstem TRH-immunoreactivity (-ir) appeared nearly exclusively in TPH+ neurons within the raphé (Fig. 3C and F), and ventrolateral medullary regions similar to previous reports (Dean et al. 1993). TRH-ir was not different between BN and SS in the cMR nuclei (raphé obscurus; data not shown), but appeared lower within the rMR regions, including the raphé pallidus (Fig. 3A–F) and magnus (not shown). Consistent with this, TRH peptide levels within tissue punches from the rMR were reduced in BN (n = 4) rats relative to SS (n = 4) rats (Fig. 3G and H; one-way ANOVA with Holm–Sidak post hoc; P = 0.028). A similar Western blot quantitation of TRHRs showed no differences (P > 0.05) between BN (n = 3) and SS (n = 3) rats within two major brainstem sites that receive neuronal projections from the MR, NTS and RTN. However, comparing the known genetic sequences among the BN and SS strains with Variant Visualizer (www.rgd.mcw.edu), we found 60 single nucleotide variants (SNVs) within the TRHR gene. Fifty-eight of these 60 SNVs were located within introns, and two SNVs were within exons of the TRHR. We located one non-synonymous SNV within the coding region of the TRHR gene, suggesting that although TRHR expression may not differ that there may still be functional differences among the strains in TRHR signalling.
Figure 3.
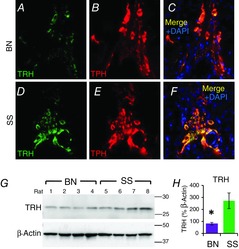
TRH protein expression is reduced in the rostral medullary raphé of BN rats
Representative epifluorescent images of the midline medullary raphé from coronal medullary sections at-11.5 mm Bregma, which are double-labelled with primary antibodies targeting TRH (A and D; green) and TPH (B and E; red), and the merged image with DAPI (blue) counterstaining (C and F) in BN and SS rats. G, TRH peptide levels from rostral medullary raphé appear reduced in BN (n = 4) relative to SS (n = 4) rats, and when expressed as a % of β-actin (H) is significantly lower in BN rats (*one-way ANOVA with Holm–Sidak post hoc; P = 0.028). BN, brown Norway (rat); SS, Dahl salt-sensitive (rat); TPH, tryptophan hydroxylase; TRH, thyrotropin-releasing hormone.
Reduced TRH peptide levels within the rMR of BN rats may be due to fewer numbers of TRH-producing neurons. However, mean counts of TPH-expressing (5-HT) neurons from all (midline and ventrolateral) medullary regions in BN rats were equal to counts from SS and Sprague–Dawley rats (Fig. 4A; one-way ANOVA with Bonferroni post hoc; P = 0.481), suggesting the reduced TRH was due to reduced biosynthesis rather than reduced numbers of 5-HT/TRH neurons. To rule out abnormalities in other brainstem neurons that could contribute to the blunted CO2 chemoreflex in BN rats, counts of catecholaminergic [TH-expressing (TH+)] and/or phox2b-expressing neurons (Dubreuil et al. 2008) were also made in the solitary complex (SC; NTS and dorsal motor nucleus of the vagus) and/or the RTN. TH+ neuron counts were not different among BN and SS rats within the RTN region (Fig. 4B and C; one-way ANOVA with Bonferroni post hoc; P > 0.05) or within the caudal or rostral SC (Fig. 4D–F; one-way ANOVA with Bonferroni post hoc; P > 0.05). We found greater numbers of Phox2b+ TH− neurons in the rostral SC in BN rats (Fig. 4D; one-way ANOVA with Bonferroni post hoc; P < 0.011), but found no differences among the strains in the caudal SC or RTN region (Fig. 4B–F; one-way ANOVA with Bonferroni post hoc; P > 0.05). Thus, the data suggest no gross abnormalities in 5-HT/TRH producing MR neurons and comparable numbers of cell populations that contribute to CO2 chemoreflex function, including TH+ and Phox2b+ brainstem neurons.
Figure 4.
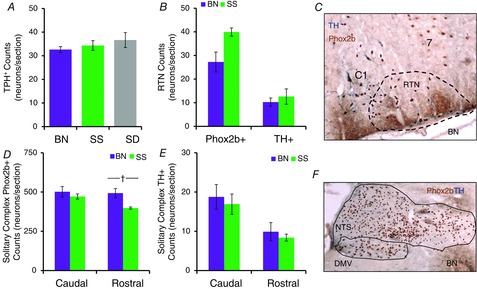
Counts of neurochemically defined medullary neuronal subpopulations are similar among rat strains
A, mean cell counts of 5-HT (TPH+) cells in BN (n = 5), SS (n = 6) and SD (n = 4) rats. B, mean cell counts of Phox2b+/TH− and TH+ cells in the RTN region (representative image from BN shown in C), and in the caudal or rostral solitary complex (NTS and DMV; D and E; representative image from BN rat shown in F). BN, Brown Norway (rat); DMV, dorsal motor nucleus of the vagus; NTS, nucleus of the solitary tract; RTN, retrotrapezoid nucleus; SD, Sprague–Dawley; SS, Dahl salt-sensitive (rat); TH, tyrosine hydroxylase; TPH, tryptophan hydroxylase.
Blunted CO2 chemoreflex in the brown Norway rat is restored with thyrotropin-releasing hormone analogue Taltirelin
TAL is a stable TRH analogue that readily crosses the blood–brain barrier, has up to 100-fold higher CNS activity and 50-fold less endocrine activity than endogenous TRH (Suzuki et al. 1990). Given the reduced rMR expression of TRH in BN rats, we tested if administration of vehicle (saline), or TAL (0.3 mg kg−1 or 1.0 mg kg−1; administered on separate days) would have greater physiologic effects in BN compared to control Sprague–Dawley rats. O2 consumption (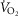 ) increased in a dose-dependent manner in BN and Sprague–Dawley rats after TAL injections (Fig. 5A). TAL increased
) increased in a dose-dependent manner in BN and Sprague–Dawley rats after TAL injections (Fig. 5A). TAL increased 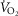 within 15 min, which remained increased ≤1 h postinjection. The increase in
within 15 min, which remained increased ≤1 h postinjection. The increase in 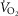 with 0.3 and 1.0 mg kg−1 TAL was greater in Sprague–Dawley rats [Fig. 5A; three-way ANOVA and Bonferroni post hoc; P < 0.001; n = 6–7 (BN), n = 5 (Sprague–Dawley)]. In contrast, body temperature increased with the lower dose of TAL in BN (P = 0.003; n = 12) but not in Sprague–Dawley rats (P = 0.052; n = 12), and body temperature increased more in BN than Sprague–Dawley rats with 1.0 mg kg−1 TAL (Fig. 5B; two-way ANOVA and Bonferroni post hoc; P = 0.021; n = 12/strain). Similarly, there were dose-dependent increases in room air ventilation in BN rats, with the increases in ventilation were greater in BN compared to Sprague–Dawley rats (Fig. 5C and D; two-way repeated measures ANOVA and Bonferroni post hoc; P = 0.019; n = 6/strain) and were due primarily to increased breathing frequency and not tidal volume (data not shown). Saline injections had no effects on breathing, body temperature or
with 0.3 and 1.0 mg kg−1 TAL was greater in Sprague–Dawley rats [Fig. 5A; three-way ANOVA and Bonferroni post hoc; P < 0.001; n = 6–7 (BN), n = 5 (Sprague–Dawley)]. In contrast, body temperature increased with the lower dose of TAL in BN (P = 0.003; n = 12) but not in Sprague–Dawley rats (P = 0.052; n = 12), and body temperature increased more in BN than Sprague–Dawley rats with 1.0 mg kg−1 TAL (Fig. 5B; two-way ANOVA and Bonferroni post hoc; P = 0.021; n = 12/strain). Similarly, there were dose-dependent increases in room air ventilation in BN rats, with the increases in ventilation were greater in BN compared to Sprague–Dawley rats (Fig. 5C and D; two-way repeated measures ANOVA and Bonferroni post hoc; P = 0.019; n = 6/strain) and were due primarily to increased breathing frequency and not tidal volume (data not shown). Saline injections had no effects on breathing, body temperature or 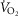 in BN and Sprague–Dawley rats at every time point measured, and there were no chronic effects of TAL injections >24 h post-treatment (Fig. 5A–C and data not shown; two-way repeated measures ANOVA; P > 0.05). It was also notable that TAL injections appeared to increase vigilance and/or general activity in both strains, although we did not quantify potential increases in locomotor activity as others have previously in mice (Asai et al. 2005).
in BN and Sprague–Dawley rats at every time point measured, and there were no chronic effects of TAL injections >24 h post-treatment (Fig. 5A–C and data not shown; two-way repeated measures ANOVA; P > 0.05). It was also notable that TAL injections appeared to increase vigilance and/or general activity in both strains, although we did not quantify potential increases in locomotor activity as others have previously in mice (Asai et al. 2005).
Figure 5.
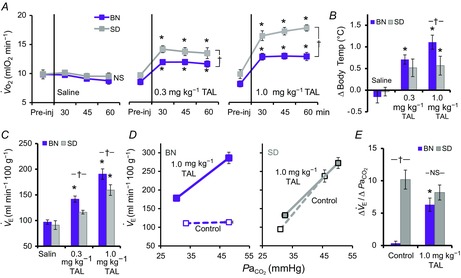
Thyrotropin-releasing hormone analogue injections normalize the CO2 chemoreflex in BN rats
A, oxygen consumption (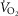 ), changes in body (rectal) temperature (B), and eupneic (room air) ventilation (C) increased in a dose-dependent manner in BN and SS rats ≤60 min after 0.3 mg kg−1 or 1.0 mg kg−1 injections of the thyrotropin-releasing hormone analogue TAL [two-way (Δbody temp and minute ventilation (
), changes in body (rectal) temperature (B), and eupneic (room air) ventilation (C) increased in a dose-dependent manner in BN and SS rats ≤60 min after 0.3 mg kg−1 or 1.0 mg kg−1 injections of the thyrotropin-releasing hormone analogue TAL [two-way (Δbody temp and minute ventilation (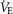 ) or three-way (
) or three-way (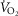 ) ANOVA with Bonferroni post hoc; P < 0.05 (*vs. Pre-injection control or †between strains)]. D, relationship between ventilation and arterial CO2 (
) ANOVA with Bonferroni post hoc; P < 0.05 (*vs. Pre-injection control or †between strains)]. D, relationship between ventilation and arterial CO2 (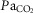 ) breathing room air or 7% CO2 was dramatically lower in BN relative to SD rats. However, treatment with 1.0 mg kg−1 TAL injection increased this relationship in BN but not SD rats, where the Δ
) breathing room air or 7% CO2 was dramatically lower in BN relative to SD rats. However, treatment with 1.0 mg kg−1 TAL injection increased this relationship in BN but not SD rats, where the Δ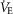 /Δ
/Δ (E) was equal among SD and BN rats after TAL treatment [two-way ANOVA with Bonferroni post hoc; P < 0.05 (*vs. Pre-injection control or †between strains)]. BN, Brown Norway (rat); SD, Sprague–Dawley; TAL, Taltirelin.
(E) was equal among SD and BN rats after TAL treatment [two-way ANOVA with Bonferroni post hoc; P < 0.05 (*vs. Pre-injection control or †between strains)]. BN, Brown Norway (rat); SD, Sprague–Dawley; TAL, Taltirelin.
As TRH analogue injections had greater effects on ventilation in BN rats, we determined if TAL injections had strain-specific effects on the ventilatory CO2 chemoreflex (Fig. 5D and E). Measurements of ventilation and arterial blood gases while breathing room air or 7% CO2 (to increase arterial  ) were obtained before and ∼1 h after 1.0 mg kg−1 TAL injections in BN and Sprague–Dawley rats. Before injection, Sprague–Dawley rats demonstrated a robust increase in ventilation with increased
) were obtained before and ∼1 h after 1.0 mg kg−1 TAL injections in BN and Sprague–Dawley rats. Before injection, Sprague–Dawley rats demonstrated a robust increase in ventilation with increased  , whereas BN rats had little or no ventilatory sensitivity to increased inspired CO2 as previously shown (Hodges et al. 2002) (Fig. 5D, dotted lines). However, TAL elicited an increase in the ventilatory sensitivity to hypercapnia in BN rats but not Sprague–Dawley rats. The TAL-induced increase in the ventilatory CO2 chemoreflex in BN rats was not different from that in Sprague–Dawley rats [Fig. 5D and E; two-way repeated measures ANOVA and Bonferroni post hoc; P < 0.001 (BN pre-vs. post-TAL), P = 0.231 (Sprague–Dawley pre-vs. post-TAL); n = 6/strain]. These data highlight a major functional role for TRH signalling in the CO2 chemoreflex, and further support the concept that raphé-derived neuromodulation is reduced in the BN rat.
, whereas BN rats had little or no ventilatory sensitivity to increased inspired CO2 as previously shown (Hodges et al. 2002) (Fig. 5D, dotted lines). However, TAL elicited an increase in the ventilatory sensitivity to hypercapnia in BN rats but not Sprague–Dawley rats. The TAL-induced increase in the ventilatory CO2 chemoreflex in BN rats was not different from that in Sprague–Dawley rats [Fig. 5D and E; two-way repeated measures ANOVA and Bonferroni post hoc; P < 0.001 (BN pre-vs. post-TAL), P = 0.231 (Sprague–Dawley pre-vs. post-TAL); n = 6/strain]. These data highlight a major functional role for TRH signalling in the CO2 chemoreflex, and further support the concept that raphé-derived neuromodulation is reduced in the BN rat.
Regionally distinct transcriptomes in the medullary raphé suggest differential contributions to physiologic function
Previous studies suggest potential regional differences in the contributions to ventilatory control of caudal and rostral raphé nuclei, including an excitatory neuromodulatory role for the cMR compared to a CO2 chemoreflex-associated function of the rMR (Dias et al. 2007, 2008). To gain further insights into this hypothesis, we made additional transcriptome comparisons across MR regions within each strain (with an emphasis on differentially expressed genes common to both strains) to determine if there was an expression ‘fingerprint’ that might allude to such a regional difference in MR function. The statistically stringent ‘first pass’ data set identified 40 commonly differentially expressed genes, which increased to 54 using second pass criteria comparing the cMR and rMR (Fig. 6A), and all 54 genes demonstrated the same relative expression pattern (Fig. 6B and C, bold genes), where genes that were increased in the cMR in one strain were also increased in the cMR in the other strain. Genes associated with neuronal inhibition (Gad1, Gad2, Gabrb2, Gabra1, galr1 and Slc32a1; GABA vesicular transporter) were consistently lower in the cMR relative to the rMR (Fig. 6B, red/pink), whereas genes broadly related to excitation and/or G protein-coupled receptors [Hrt5b, Gpr88, Slc17a7 and Slc17a6 (VGlut1 and VGlut2, respectively), Crh, Trh, Gpr26, Pgr15l, Rgs4, Tac1 (substance P), Tph2 (TPH 2) and Slc6a4 (SERT)] were higher in the cMR compared to the rMR in both strains (Fig. 6C, green/yellow). These data reinforce the general concept that the cMR is a major source of excitatory neuromodulators, and suggest that the rostral raphé appears to contain genes predominantly related to neuronal inhibition.
Figure 6.
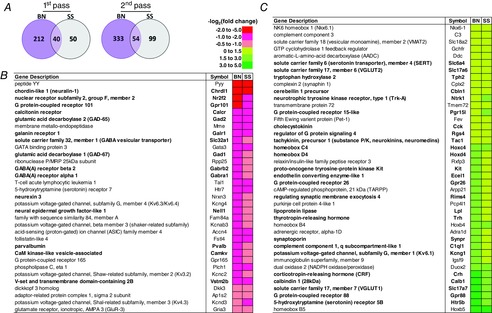
Differentially expressed genes between cMR and rMR and common to both strains highlight potential functional heterogeneity among raphé nuclei
A, Venn diagrams displaying known genes in BN (purple) or SS (grey) rats differentially expressed between the cMR and rMR, and those common to both strains (overlap) using first pass (q < 0.05; left) or second pass (q < 0.25; P < 0.005) statistical criteria. B and C, known genes differentially expressed among the raphé regions within each strain, where relative gene abundance [−log2(fold change)] in the cMR is lower (b; red/pink) or higher (c; yellow/green) compared to the rMR. First pass genes are bold in (B) and (C). BN, brown Norway (rat); cMR, caudal medullary raphé; rMR, rostral medullary raphé; SS, Dahl salt-sensitive (rat).
In contrast, there were fewer commonly differentially expressed genes comparing strains within a particular raphé region (Fig. 7A and B). Only 17 first pass and 42 second pass genes were differentially expressed (q < 0.05) between the BN and SS strains and were represented in both MR regions, although the expression pattern was consistent (Fig. 7B, bold genes). In addition, there appeared to be no clear categorization of function of the commonly differentially expressed genes among strains, although one gene in particular, Acaa2 (acetyl-coenzyme A acetyltransferase), was reduced in BN vs. SS in both raphé regions. This gene has been previously found to be downregulated in the SS rat kidney after chromosomal substitution of chromosome 13 from the BN rat on to the genetic background of the SS strain (Liang et al. 2008). In general, the strain-related differences in gene expression were not consistent between the cMR and rMR.
Figure 7.
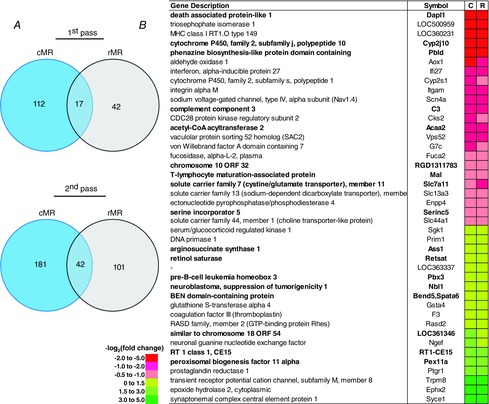
Fewer genes are differentially expressed between strains and common to the cMR and rMR
A, Venn diagrams displaying known genes in cMR (blue) or rMR (grey) regions differentially expressed between the BN and SS strains, and those common to both regions (overlap) using first pass (q < 0.05; left) or second pass (q < 0.25; P < 0.005) statistical criteria. B, list of known genes differentially expressed among strains common to both regions, where relative abundance [−log2(fold change)] in the BN is lower (red/pink) or higher (yellow/green) compared to the SS. First pass genes are bold in (B). cMR, caudal medullary raphé; rMR, rostral medullary raphé.
Bioinformatics pathway analyses identify a molecular basis for serotonin system deficiencies in brown Norway rats
To gain greater insights into within-and cross-strain differences in gene expression, we analysed all known genes commonly differentially expressed between the cMR and rMR (all genes listed in Fig. 6B and C) using IPA to provide an unbiased approach to understanding the broader implications of the gene expression differences. ‘Core’ analyses of differences in gene expression between the cMR and rMR identified top ‘networks’ such as nervous system development and function (Score = 40; 18 of 74 genes, 24.3%) and top ‘functions’ such as neurotransmission, emotional behaviour and depressive disorder, with the most significant score being anxiety (P = 2.16E-15; 15 of 74 genes, 20.2%). Three of the top seven ‘canonical pathways’ identified were related to the 5-HT system, including serotonin and melatonin biosynthesis and tryptophan degradation X and the most significantly related top overall canonical pathway, serotonin receptor signalling (P = 6.53E-07), and five of the top six ‘upstream regulators’ identified by IPA were transcription factors, including (in order of decreasing significance) REST, Bdnf, Fev (Pet-1), Htt (huntingtin), Pou4f1 (also known as Brn-3) and Tlx3 (also known as Hox11l2). In total, these identified biological functions, networks, canonical pathways and upstream regulators highlight an expected enrichment in ‘neural’ genes and functions, and further support the concept of regional differences in MR function.
Fewer genes (42) were commonly differentially expressed between strains within each MR region (Fig. 7B), warranting pathway analyses between strains within individual regions. IPA analyses of all differentially expressed genes between strains within the cMR genes yielded top ‘networks’ such as nervous system development and function (score = 44; 21 genes), and multiple associated ‘functions’ such as Huntington's disease, synaptic transmission, neurotransmission, neuromuscular disease, movement disorder and learning. ‘Canonical pathways’ identified included synaptic long-term potentiation, and the top canonical pathway neuropathic pain signalling in dorsal horn neurons. ‘Upstream regulators’ identified included multiple receptors Grm3 (mGluR3), Ppara, REST, Grin1 (NMDA receptor subunit 1), MECP2 (methyl CpG binding protein 2; Rett syndrome), and mGluR1 and mGluR5. Thus, it appears that the major strain differences in gene expression within the cMR are broadly related to synaptic neurotransmission, but more specifically glutamate receptor activity and genes associated with synaptic or other forms of neuronal plasticity.
Likewise, differential expression of all known genes among the strains specifically within the rMR was analysed with IPA, yielding top ‘networks’ such as cell-to-cell signalling, and three of the five ‘functions’ identified were related to the quantity of monoamines (P = 2.06E-06), with a predicted activation state of ‘decreased’. In addition, serotonin receptor signalling and serotonin and melatonin biosynthesis were two of the top five ‘canonical pathways’ identified, and the top ‘upstream regulator’ predicted by IPA was FEV, which is the human homolog of Pet-1 and is the major transcriptional regulator of the expression of multiple 5-HT neuron-specific genes, including (but not limited to) Tph2, Ddc, slc6a4, slc18a2 and Aox1, which are reduced in BN rats in this region. Thus, using this unbiased approach to understanding the broader implications of the differentially expressed genes between strains pointed to differences in the 5-HT system, and specifically predicted lower levels of monoamines in the BN strain.
Monoamines are reduced in multiple central nervous system regions in brown Norway rats
We previously demonstrated that the BN rat had reduced tissue levels of 5-HT, 5-HIAA and NA in multiple CNS regions compared to Sprague–Dawley rats (Hodges et al. 2013), consistent with the predicted reductions in monoamines from the pathway analysis herein. To test this further, we collected the medulla, pons/midbrain, hypothalamus, cerebellum and forebrain tissues from BN and SS rats for HPLC analyses of tissue monoamine levels. 5-HT was lower in the medulla and hypothalamus, and the 5-HT metabolite 5-HIAA reduced in all brain regions in BN rats (Table1; one-way ANOVA and Bonferroni post hoc; P < 0.05). NA was also reduced in the medulla, pons/midbrain and hypothalamus, and DA lower in the medulla of BN rats (Table1; one-way ANOVA and Bonferroni post hoc; P < 0.05). Thus, the HPLC data were consistent with the bioinformatics predictions based on gene expression in the rMR, demonstrating reduced monoamines in multiple CNS regions in BN rats.
Table 1.
CNS monoamines are reduced in BN rats
| Strain | n | Region | NA | P | DA | P | 5-HT | P | 5-HIAA | P |
|---|---|---|---|---|---|---|---|---|---|---|
| SS | 6 | Forebrain | 348.7 ± 44.4 | 1250 ± 470.5 | 300.2 ± 32.6 | 328.8 ± 36 | ||||
| 6 | Hypothalamus | 1617.3 ± 95.4 | 207 ± 11.9 | 391.8 ± 21.6 | 622.4 ± 35 | |||||
| 5 | Midbrain/pons | 752.5 ± 37.2 | 187.9 ± 11.4 | 502.7 ± 15.7 | 611.6 ± 10.1 | |||||
| 6 | Cerebellum | 211.3 ± 11 | ND | 39.9 ± 2.6 | ND | |||||
| 5 | Medulla | 726 ± 43.4 | 56.5 ± | 512.6 ± 14.2 | 486.4 ± 21.8 | |||||
| BN | 4–6 | Forebrain | 236.9 ± 29.9 | 1427.2 ± 281 | 243.2 ± 20 | 196.7 ± 12.5* | 0.006 | |||
| 6 | Hypothalamus | 1073.3 ± 76.4* | 0.001 | 235 ± 19.3 | 288.5 ± 10.4 * | 0.002 | 411.7 ± 21.1 * | <0.001 | ||
| 6 | Midbrain/pons | 601.5 ± 41.7 * | 0.027 | 198.6 ± 12.2 | 534.8 ± 50.5 | 456.9 ± 45.4 * | 0.014 | |||
| 6 | Cerebellum | 189.6 ± 20.2 | ND | 25.7 ± 1.3 * | <0.001 | ND | ||||
| 6 | Medulla | 564.2 ± 47.5 * | 0.036 | 39.9 ± * | 0.021 | 400.6 ± 24.5 * | 0.005 | 333.8 ± 23.8 * | 0.001 |
Abbreviations: 5-HIAA, 5-hydroxyindolacetic acid; 5-HT, serotonin; BN, Brown Norway (rat); DA, dopamine; NA, noradrenaline; ND, not detectable; SS, Dahl salt-sensitive (rat). High-performance liquid chromatography measurements of NA, DA, 5-HT and its metabolite, 5-HIAA, were reduced in reduced in BN (n = 4–6) rats compared to SS (n = 6) rats (two-way ANOVA (strain and CNS region as factors) with Bonferroni post hoc;
P < 0.05).
Differential expression of candidate pH-sensitive ion channel expression across strains within a region and across regions within a strain
Raphé 5-HT neurons are important determinants of the ventilatory CO2 chemoreflex in part through their neuromodulation of the respiratory network, but also as potential CO2/pH ‘sensors’. Respiratory CO2/pH chemoreceptors are thought to uniquely express one or more pH-sensitive ion channels, including but not limited to two pore ‘leak’ (TASK), inward rectifying (Kir), voltage-gated (Kv) and TWIK-related (TREK) potassium (K+) channels and/or L-type calcium channels (Putnam et al. 2004). Thus, we analysed potential strain-related differences in MR expression of pH-sensitive ion channels that might account for the major differences in ventilatory CO2 sensitivity among the strains. However, there were no differences among the strains within the cMR or rMR in all ion channels using the first or second pass significance thresholds. We noted that Kir5.1 (Kcnj16) was slightly greater (P < 0.05) in abundance in SS vs. BN rats in the rMR, but this difference failed to reach significance. In addition, we found no differences in the expression of all known pH-sensitive ion channel genes between the cMR and rMR. Thus, the major phenotypic differences in the ventilatory CO2 chemoreflex among BN and other strains is probably not determined by expression differences in pH-sensitive ion channels in the MR, although differences in K+ channel expression have been shown in the NTS (Martino et al. 2014). Furthermore, a lack of differential expression of these pH-sensitive ion channels between the cMR and rMR does not support the concept of differences in the functional contributions of each to CO2 chemoreception.
Discussion
5-HT and other excitatory neuromodulators can be found at nearly every level of the mammalian neuraxis, where they heavily influence numerous brain functions (Jacobs & Azmitia, 1992). Here, we aimed to gain insights into the potential importance of raphé-derived neuromodulators in mechanisms that regulate ventilation by studying a rat model of ventilatory phenotypic extreme: the CO2-insensitive BN rat. In addition to identifying reduced expression of multiple 5-HT neuron-specific genes in the BN rat, one of the largest and most significant strain-related differences in MR gene expression was Trh, an excitatory neuromodulator and well-established regulator of thyroid function, feeding behaviours and arousal state (Zhang & van den Pol, 2012). However, TRH is also produced in and co-released with 5-HT in a fraction of MR neurons (Dean et al. 1993). TRH peptide levels were also reduced within the MR of BN rats, consistent with the gene expression data. TRHR levels at sites receiving inputs from MR 5-HT neurons were not different among BN and Sprague–Dawley rats, but there we also identified a known non-synonymous SNV within a coding region of the TRHR gene. Overall, these data are consistent with the conclusion that endogenous raphé TRH signalling is altered in the BN rat. The TRH deficiency in BN rats is probably a major determinant of the inherently attenuated CO2 chemoreflex in the BN rat given that the TRH analogue TAL essentially normalized the ventilatory CO2 chemoreflex in the BN rat without affecting Sprague–Dawley rats. However, the extreme respiratory phenotype of the BN rat is probably not solely due to a TRH-specific deficit. Instead, the data collectively suggest a broader dysregulation of raphé-derived excitatory neuromodulation based on the differences in 5-HT-related gene expression and the HPLC data showing relatively lower levels of 5-HT and other monoamines in the BN rat. Furthermore, although the central and peripheral mechanisms of the TAL-induced physiologic effects are not well understood, others have shown that TAL/TRH administration can increases release and turnover of DA and stimulates the monoamine system (Fukuchi et al. 1998). Thus, the effects of TAL may include direct and indirect increases in CNS excitatory neuromodulation through activation of TRHRs and increasing monoaminergic activity, respectively.
While many data suggest a prominent role of 5-HT and TRH in the mammalian CO2 chemoreflex, the mechanisms by which these neuromodulators contribute to the CO2 chemoreflex remain unclear. For example, knockout mice lacking all CNS 5-HT neurons have a blunted hypercapnic ventilatory response (∼50% of controls), and this deficit could be restored with increased exogenous intracerebroventricular 5-HT (Hodges et al. 2008). Similarly, we previously showed reduced CNS 5-HT in BN rats compared to Sprague–Dawley rats, and reuptake inhibition with fluoxetine augmented the CO2 chemoreflex in BN but not Sprague–Dawley rats (Hodges et al. 2013). Herein, we showed TRHR stimulation in the TRH-deficient BN rat restored ventilatory CO2 sensitivity, but failed to significantly alter CO2 sensitivity in Sprague–Dawley rats. Finally, although thyroid dysfunction has been linked to reduced CO2 sensitivity in humans, TRH infusions in healthy humans have no effect on the CO2 chemoreflex (Nink et al. 1991; Schulz et al. 1996). Thus, it appears that data implicating a major role for neuromodulators in facilitating the mammalian CO2 chemoreflex has been in the context of neuromodulatory deficiency, as is the case in the CO2 insensitive BN rat. Given that 7% inspired CO2 increased 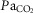 to ∼47–50 mmHg in both strains, it could be that the BN rat has a lower CO2 chemoreflex recruitment threshold and a robust hypercapnic ventilatory response above those levels. In this case, increased neuromodulation such as that observed with TAL or fluoxetine could simply reduce the recruitment threshold and shift the
to ∼47–50 mmHg in both strains, it could be that the BN rat has a lower CO2 chemoreflex recruitment threshold and a robust hypercapnic ventilatory response above those levels. In this case, increased neuromodulation such as that observed with TAL or fluoxetine could simply reduce the recruitment threshold and shift the 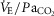 relationship leftward. Irrespective of the potential mechanisms, we suggest that the appropriate levels of raphé-derived neuromodulators are required for full expression of the CO2 chemoreflex, and may be viable therapeutic targets for disorders in humans that have altered ventilatory chemoreflex control. Perhaps the most beneficial application of therapies directed to augment raphé-derived neuromodulators include infants at high risk for SIDS or those born with a mutation in the transcription factor phox2b (central hypoventilation syndrome), which are associated with or defined by reduced ventilatory CO2 chemoreflexes. Reduced thyroid function has also been linked to attenuation of the ventilatory CO2 chemoreflex in adult humans (Zwillich et al. 1975; Ladenson et al. 1988) and rats (Olea et al. 2007), although our data and those reported previously (Kim et al. 2002) do not support overt hypothyroidism in BN rats. TRH expression and receptor binding are low at birth in rats, but dramatically increase until reaching a plateau ∼12–21 days of age (Bayliss et al. 1994). This time-dependent expression of the medullary TRH throughout development when combined with its major role in the CO2 chemoreflex may have further implications for developmental 5-HT system defects and potential chemoreflex failure in SIDS, which to our knowledge has not been studied.
relationship leftward. Irrespective of the potential mechanisms, we suggest that the appropriate levels of raphé-derived neuromodulators are required for full expression of the CO2 chemoreflex, and may be viable therapeutic targets for disorders in humans that have altered ventilatory chemoreflex control. Perhaps the most beneficial application of therapies directed to augment raphé-derived neuromodulators include infants at high risk for SIDS or those born with a mutation in the transcription factor phox2b (central hypoventilation syndrome), which are associated with or defined by reduced ventilatory CO2 chemoreflexes. Reduced thyroid function has also been linked to attenuation of the ventilatory CO2 chemoreflex in adult humans (Zwillich et al. 1975; Ladenson et al. 1988) and rats (Olea et al. 2007), although our data and those reported previously (Kim et al. 2002) do not support overt hypothyroidism in BN rats. TRH expression and receptor binding are low at birth in rats, but dramatically increase until reaching a plateau ∼12–21 days of age (Bayliss et al. 1994). This time-dependent expression of the medullary TRH throughout development when combined with its major role in the CO2 chemoreflex may have further implications for developmental 5-HT system defects and potential chemoreflex failure in SIDS, which to our knowledge has not been studied.
While peripheral (non-CNS) effects of the TRH analogue cannot be excluded, there are brainstem sites that could mediate the observed ventilatory effects. The solitary complex (NTS and dorsal motor nucleus of the vagus) and RTN both receive major projections from MR 5-HT neurons (Mulkey et al. 2007), express neuromodulatory receptors (Hodges & Richerson, 2008), and are thought to contribute to ventilatory CO2/pH chemoreception (Dean et al. 1990; Mulkey et al. 2004). For example, Phox2b+ RTN chemoreceptors are directly stimulated by increased exogenous 5-HT, substance P and TRH in vitro, independent of the effects of CO2/pH (Mulkey et al. 2007), and RTN microdialysis of TRH increases breathing in conscious adult rats (Cream et al. 1999). The data herein provide a molecular basis for the inherently reduced ventilatory CO2 chemoreflex in BN rats; reduced endogenous expression/production of MR TRH (and 5-HT), which probably manifests at the projection sites of MR 5-HT neurons (NTS/RTN), which express TRHRs in equal quantity but have predicted differences in their function.
Many of the 5-HT neuron-specific genes that were in lower abundance in the BN rat have a common upstream transcriptional regulator, Pet-1 (Fev), which suggests it is a candidate gene that could drive the strain-related differences in 5-HT neuron function. Pet-1 is an ETS domain transcription factor that regulates expression of 5-HT biosynthetic enzymes (Tph2, Ddc) and transporters (slc6a4, slc18a2) and the 5-HT1A autoreceptor specifically within 5-HT neurons (Liu et al. 2010). The RNASeq-derived abundance profile for Pet-1 was similar to Tph2, Ddc, Slc6a4 and slc18a2, but failed to reach significance in the first and second pass data sets. In addition, there are no known single nucleotide polymorphisms within or 5 kb up-or downstream of Pet-1 comparing the published SS and BN sequences, and the expression of other Pet-1-regulated genes (hrt1a and Gata3) were not differentially expressed among the strains. There are few known transcriptional regulators of Pet-1, but there is an upstream consensus binding site for Gata-1, Gata-2 and Gata-3 (Krueger & Deneris, 2008), and BN rats did show a marginally reduced MR expression of Gata-3. Thus, additional studies are necessary to determine the key factor(s) driving the strain-specific differences in gene expression profiles, including identifying potential Pet-1-dependent and-independent transcriptional regulators of 5-HT neuron-specific genes, and/or additional factors that regulate Pet-1 expression.
Transcriptome-level comparisons represent a broad approach to understanding neurophysiology, but our comparisons among rat strains with phenotypic extremes (CO2 insensitive and sensitive) also suggest fundamentally distinct properties of regional MR function. Multiple physiologic studies suggest the cMR and rMR play functionally distinct roles in respiratory control (Taylor et al. 2005; Dias et al. 2007, 2008), which we noted was reflected in regional differences in their transcriptional profiles in both the BN and SS strains. Wylie et al. (2010) recently provided data using microarray to identify commonality among genes expressed in postmitotic hindbrain and midbrain 5-HT neuron populations, revealing hundreds of 5-HT neuron-specific genes contributing to a potentially universal transcriptional 5-HT neuron ‘fingerprint’, but also discovered multiple transcriptional and other elements that differentiate hindbrain and midbrain 5-HT neuron populations. There were 74 differentially expressed genes between the cMR and rMR common to both rat strains, 93.2% of which were represented in previous raphé transcriptome data sets in mice (Wylie et al. 2010). Most of these commonly differentially expressed genes pointed to relatively greater excitatory neuromodulation in the cMR compared to the rMR. This assumption fits with functional data demonstrating the importance of endogenously released, raphé-derived neuromodulators in respiratory rhythm and/or pattern generating brainstem slices that include the caudal raphé nuclei (Doi & Ramirez, 2007), further strengthening the concept of a transcriptional fingerprint for differential functional contributions to respiratory control in the cMR and rMR. However, we did not find strain-or MR region-specific differences in pH-sensitive ion channels, suggesting that the differences among the cMR and rMR were not due to known molecules that underlie cellular chemoreception per se. Greater insights into functional heterogeneity in the contributions of MR 5-HT neurons to ventilatory control are needed, and may be enhanced by an expression analysis of select 5-HT neuron subpopulations from distinct anatomic locations (Wylie et al. 2010) or from specific developmental origins (Jensen et al. 2008; Bang et al. 2012).
It is worth nothing that several aspects of our study were linked to, or predictive of altered behaviours in the BN rat, including anxiety and/or depression. Generally, clinical depression in humans is correlated with reduced levels of 5-HT and NA and/or increased levels of corticotrophin-releasing hormone, consistent with the antidepressant effects of 5-HT and/or NA reuptake inhibitors (Blier & El Mansari, 2013). We found herein reductions in transcript levels of multiple 5-HT-specific genes and tissue levels of 5-HT and NA in multiple brain regions of BN rats, consistent with previous findings (Hodges et al. 2013) and a broadly neuromodulator-deficient BN rat. There were fewer strain-related differences in CNS DA, but Crh expression was >4-fold greater in the cMR in BN rats, which collectively contribute to potential depression-like phenotypes in the BN rat. BN rats have been shown to have an increased immobility (45% of test time) in the forced swim test when compared to other inbred (SS, August × Copenhagen Irish and fawn-hooded hypertensive) rat strains (C. Moreno-Quinn, personal communication). Although more investigation is warranted, the data suggest that the BN rat exhibits multiple deficits in monoaminergic and TRH signalling systems and may be a potentially useful model for the study of affective disorders such as depression and anxiety.
Our interpretations of these data are tempered by the limitations of the data set, such as the use of raphé tissue punches rather than purified cell populations and the use of a more inclusive significance threshold for differential expression and subsequent pathway analyses. Our first pass data set was derived from an adjusted P value (q < 0.05) to determine significance in expression levels, providing an ‘unbiased’ screen for altered gene expression to limit type II error. However, genome-wide expression studies are probably extremely underpowered (Yang et al. 2013), and restricting the data set limited the power of subsequent pathway analyses. This inclusionary approach resulted in highly enriched pathway analyses and predictive value of the sequence data, which was subsequently supported by the protein, neurochemical and physiologic measurements in the BN and two control strains. However, another limitation of our approach was the inability to gain insights into the relevance of the unknown differentially expressed transcripts. Further coverage and genome annotation may introduce a bevy of novel transcripts that may provide further insights into the strain-related phenotypes, but were essentially excluded from our transcriptome and pathway analyses.
We conclude that deficits in TRH contribute to the ventilatory CO2 insensitivity observed in the BN rat, highlighting a major role for raphé-derived neuromodulators in the regulation of the ventilatory CO2 chemoreflex. A greater understanding of how raphé-derived neuromodulators contribute to mechanisms that govern the ventilatory CO2 chemoreflex may be paramount to furthering our understanding of disorders in humans that are marked by 5-HT system dysfunction, including but not limited to SIDS.
Glossary
- BN
brown Norway (rat)
- cMR
caudal medullary raphé
- DA
dopamine
- 5-HIAA
5-hydroxyindolacetic acid
- HPLC
high-performance liquid chromatography
- 5-HT
serotonin
- -ir
immunoreactivity
- MR
medullary raphé
- NA
noradrenaline
- NTS
nucleus of the solitary tract
- rMR
rostral medullary raphé
- RTN
retrotrapezoid nucleus
- SNV
single nucleotide variant
- SS
Dahl salt-sensitive (rat)
- TAL
Taltirelin
- TH
tyrosine hydroxylase
- TPH
tryptophan hydroxylase
- TRH
thyrotropin-releasing hormone
- TRHR
thyrotropin-releasing hormone receptor
Additional information
Competing interests
None.
Funding
This work was supported by NIH HL097033 (M.R.H., A.E.E. and M.M.P.), Advancing a Healthier Wisconsin Fund FP1701 and FP1703 (P.L.).
Acknowledgements
The authors thank the following for their specific contributions to this work: Dr Bert Forster for his insightful guidance and comments in the preparation of this manuscript, Dr J.F. Brunet, for generously providing the Phox2b primary antibody, Dr Carol Moreno-Quinn for the forced swim data summary, Danielle Twaroski for tissue staining assistance and the MCW Sequencing and Biochemistry Core labs.
References
- Asai H, Asahi T, Yamamura M, Yamauchi-Kohno R. Saito A. Lack of behavioral tolerance by repeated treatment with taltirelin hydrate, a thyrotropin-releasing hormone analog, in rats. Pharmacol Biochem Behav. 2005;82:646–651. doi: 10.1016/j.pbb.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Bang SJ, Jensen P, Dymecki SM. Commons KG. Projections and interconnections of genetically defined serotonin neurons in mice. Eur J Neurosci. 2012;35:85–96. doi: 10.1111/j.1460-9568.2011.07936.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayliss DA, Viana F, Kanter RK, Szymeczek-Seay CL, Berger AJ. Millhorn DE. Early postnatal development of thyrotropin-releasing hormone (TRH) expression, TRH receptor binding, and TRH responses in neurons of rat brainstem. J Neurosci. 1994;14:821–833. doi: 10.1523/JNEUROSCI.14-02-00821.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blier P. El Mansari M. Serotonin and beyond: therapeutics for major depression. Philos Trans R Soc Lond B Biol Sci. 2013;368:20120536. doi: 10.1098/rstb.2012.0536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coates EL, Li A. Nattie EE. Widespread sites of brain stem ventilatory chemoreceptors. J Appl Physiol. 1993;75:5–14. doi: 10.1152/jappl.1993.75.1.5. [DOI] [PubMed] [Google Scholar]
- Cream C, Nattie E. Li A. TRH microdialysis into the RTN of the conscious rat increases breathing, metabolism, and temperature. J Appl Physiol. 1999;87:673–682. doi: 10.1152/jappl.1999.87.2.673. [DOI] [PubMed] [Google Scholar]
- Dahlstrom A. Fuxe K. Localization of monoamines in the lower brain stem. Experientia. 1964;20:398–399. doi: 10.1007/BF02147990. [DOI] [PubMed] [Google Scholar]
- Dean JB, Bayliss DA, Erickson JT, Lawing WL. Millhorn DE. Depolarization and stimulation of neurons in nucleus tractus solitarii by carbon dioxide does not require chemical synaptic input. Neuroscience. 1990;36:207–216. doi: 10.1016/0306-4522(90)90363-9. [DOI] [PubMed] [Google Scholar]
- Dean C, Marson L. Kampine JP. Distribution and co-localization of 5-hydroxytryptamine, thyrotropin-releasing hormone and substance P in the cat medulla. Neuroscience. 1993;57:811–822. doi: 10.1016/0306-4522(93)90026-c. [DOI] [PubMed] [Google Scholar]
- Dias MB, Li A. Nattie E. Focal CO2 dialysis in raphe obscurus does not stimulate ventilation but enhances the response to focal CO2 dialysis in the retrotrapezoid nucleus. J Appl Physiol. 2008;105:83–90. doi: 10.1152/japplphysiol.00120.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias MB, Nucci TB, Margatho LO, Antunes-Rodrigues J, Gargaglioni LH. Branco LG. Raphe magnus nucleus is involved in ventilatory but not hypothermic response to CO2. J Appl Physiol. 2007;103:1780–1788. doi: 10.1152/japplphysiol.00424.2007. [DOI] [PubMed] [Google Scholar]
- Doi A, Ramirez JM. State-dependent interactions between excitatory neuromodulators in the neuronal control of breathing. J Neurosci. 2010;30:8251–8262. doi: 10.1523/JNEUROSCI.5361-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubreuil V, Ramanantsoa N, Trochet D, Vaubourg V, Amiel J, Gallego J, Brunet JF. Goridis C. A human mutation in Phox2b causes lack of CO2 chemosensitivity, fatal central apnea, and specific loss of parafacial neurons. Proc Natl Acad Sci U S A. 2008;105:1067–1072. doi: 10.1073/pnas.0709115105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan JR, Paterson DS, Hoffman JM, Mokler DJ, Borenstein NS, Belliveau RA, Krous HF, Haas EA, Stanley C, Nattie EE, Trachtenberg FL. Kinney HC. Brainstem serotonergic deficiency in sudden infant death syndrome. JAMA. 2010;303:430–437. doi: 10.1001/jama.2010.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edrey YH, Park TJ, Kang H, Biney A. Buffenstein R. Endocrine function and neurobiology of the longest-living rodent, the naked mole-rat. Exp Gerontol. 2011;46:116–123. doi: 10.1016/j.exger.2010.09.005. [DOI] [PubMed] [Google Scholar]
- Eto K, Kim SK, Nabekura J. Ishibashi H. Taltirelin, a thyrotropin-releasing hormone analog, alleviates mechanical allodynia through activation of descending monoaminergic neurons in persistent inflammatory pain. Brain Res. 2011;1414:50–57. doi: 10.1016/j.brainres.2011.07.065. [DOI] [PubMed] [Google Scholar]
- Fukuchi I, Asahi T, Kawashima K, Kawashima Y, Yamamura M, Matsuoka Y. Kinoshita K. Effects of taltirelin hydrate (TA-0910), a novel thyrotropin-releasing hormone analog, on in vivo dopamine release and turnover in rat brain. Arzneimittelforschung. 1998;48:353–359. [PubMed] [Google Scholar]
- Hodges MR, Best S. Richerson GB. Altered ventilatory and thermoregulatory control in male and female adult Pet-1 null mice. Respir Physiol Neurobiol. 2011;177:133–140. doi: 10.1016/j.resp.2011.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges MR, Echert AE, Puissant MM. Mouradian GC., Jr Fluoxetine augments ventilatory CO2 sensitivity in Brown Norway but not Sprague Dawley rats. Respir Physiol Neurobiol. 2013;186(2):221–228. doi: 10.1016/j.resp.2013.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges MR, Forster HV, Papanek PE, Dwinell MR. Hogan GE. Ventilatory phenotypes among four strains of adult rats. J Appl Physiol. 2002;93:974–983. doi: 10.1152/japplphysiol.00019.2002. [DOI] [PubMed] [Google Scholar]
- Hodges MR. Richerson GB. Contributions of 5-HT neurons to respiratory control: Neuromodulatory and trophic effects. Respir Physiol Neurobiol. 2008;164:222–232. doi: 10.1016/j.resp.2008.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges MR, Tattersall G, Harris MB, McEvoy S, Richerson D, Deneris ES, Johnson RL, Chen ZF. Richerson GB. Defects in breathing and thermoregulation in mice with near-complete absence of central serotonin neurons. J Neurosci. 2008;28:2495–2505. doi: 10.1523/JNEUROSCI.4729-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs BL. Azmitia EC. Structure and function of the brain serotonin system. Physiol Rev. 1992;72:165–229. doi: 10.1152/physrev.1992.72.1.165. [DOI] [PubMed] [Google Scholar]
- Jensen P, Farago AF, Awatramani RB, Scott MM, Deneris ES. Dymecki SM. Redefining the serotonergic system by genetic lineage. Nat Neurosci. 2008;11:417–419. doi: 10.1038/nn2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim IS, Ariyaratne HB. Mendis-Handagama SM. Changes in the testis interstitium of Brown Norway rats with aging and effects of luteinizing and thyroid hormones on the aged testes in enhancing the steroidogenic potential. Biol Reprod. 2002;66:1359–1366. doi: 10.1095/biolreprod66.5.1359. [DOI] [PubMed] [Google Scholar]
- Kinney HC. Thach BT. The sudden infant death syndrome. N Engl J Med. 2009;361:795–805. doi: 10.1056/NEJMra0803836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegel AJ, Liu Y, Liu P, Baker MA, Hodges MR, Hua X. Liang M. Characteristics of microRNAs enriched in specific cell types and primary tissue types in solid organs. Physiol Genomics. 2013;45:1144–1156. doi: 10.1152/physiolgenomics.00090.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger KC. Deneris ES. Serotonergic transcription of human FEV reveals direct GATA factor interactions and fate of Pet-1-deficient serotonin neuron precursors. J Neurosci. 2008;28:12748–12758. doi: 10.1523/JNEUROSCI.4349-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladenson PW, Goldenheim PD. Ridgway EC. Prediction and reversal of blunted ventilatory responsiveness in patients with hypothyroidism. Am J Med. 1988;84:877–883. doi: 10.1016/0002-9343(88)90066-6. [DOI] [PubMed] [Google Scholar]
- Liang M, Lee NH, Wang H, Greene AS, Kwitek AE, Kaldunski ML, Luu TV, Frank BC, Bugenhagen S, Jacob HJ. Cowley AW., Jr Molecular networks in Dahl salt-sensitive hypertension based on transcriptome analysis of a panel of consomic rats. Physiol Genomics. 2008;34:54–64. doi: 10.1152/physiolgenomics.00031.2008. [DOI] [PubMed] [Google Scholar]
- Liu Y, Liu P, Yang C, Cowley AW., Jr Liang M. Base-resolution maps of 5-methylcytosine and 5-hydroxymethylcytosine in Dahl S rats: effect of salt and genomic sequence. Hypertension. 2014;63:827–838. doi: 10.1161/HYPERTENSIONAHA.113.02637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Maejima T, Wyler SC, Casadesus G, Herlitze S. Deneris ES. Pet-1 is required across different stages of life to regulate serotonergic function. Nat Neurosci. 2010;13:1190–1198. doi: 10.1038/nn.2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martino PF, Olesiak S, Batuuka D, Riley D, Neumueller S, Forster HV. Hodges MR. Strain differences in pH-sensitive K+ channel-expressing cells win chemosensory and nonchemosensory brainstem nuclei. J Appl Physiol. 2014;117(8):848–856. doi: 10.1152/japplphysiol.00439.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore RY. Bloom FE. Central catecholamine neuron systems: anatomy and physiology of the norepinephrine and epinephrine systems. Annu Rev Neurosci. 1979;2:113–168. doi: 10.1146/annurev.ne.02.030179.000553. [DOI] [PubMed] [Google Scholar]
- Mouradian GC, Forster HV. Hodges MR. Acute and chronic effects of carotid body denervation (CBD) on ventilation and chemoreflexes in three rat strains. J Physiol. 2012;590:3335–3347. doi: 10.1113/jphysiol.2012.234658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulkey DK, Rosin DL, West G, Takakura AC, Moreira TS, Bayliss DA. Guyenet PG. Serotonergic neurons activate chemosensitive retrotrapezoid nucleus neurons by a pH-independent mechanism. J Neurosci. 2007;27:14128–14138. doi: 10.1523/JNEUROSCI.4167-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulkey DK, Stornetta RL, Weston MC, Simmons JR, Parker A, Bayliss DA. Guyenet PG. Respiratory control by ventral surface chemoreceptor neurons in rats. Nat Neurosci. 2004;7:1360–1369. doi: 10.1038/nn1357. [DOI] [PubMed] [Google Scholar]
- Nattie EE. Li A. CO2 dialysis in the medullary raphe of the rat increases ventilation in sleep. J Appl Physiol. 2001;90:1247–1257. doi: 10.1152/jappl.2001.90.4.1247. [DOI] [PubMed] [Google Scholar]
- Nink M, Krause U, Lehnert H, Heuberger W, Huber I, Schulz R, Hommel G. Beyer J. Thyrotropin-releasing hormone has stimulatory effects on ventilation in humans. Acta Physiol Scand. 1991;141:309–318. doi: 10.1111/j.1748-1716.1991.tb09086.x. [DOI] [PubMed] [Google Scholar]
- Olea E, Gonzales C, Gallego R. Giejo-Barrientos ELA. PTU-induced hypothyrodism reduces hypoxic ventilatory drive by impairing carotid body chemosensitivity in adult rats. Acta Physiol. 2007;190(Suppl. 655):51. [Google Scholar]
- Pena F. Ramirez JM. Endogenous activation of serotonin-2A receptors is required for respiratory rhythm generation in vitro. J Neurosci. 2002;22:11055–11064. doi: 10.1523/JNEUROSCI.22-24-11055.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ptak K, Yamanishi T, Aungst J, Milescu LS, Zhang R, Richerson GB. Smith JC. Raphé neurons stimulate respiratory circuit activity by multiple mechanisms via endogenously released serotonin and substance P. J Neurosci. 2009;29(12):3720–3737. doi: 10.1523/JNEUROSCI.5271-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putnam RW, Filosa JA. Ritucci NA. Cellular mechanisms involved in CO2 and acid signaling in chemosensitive neurons. Am J Physiol Cell Physiol. 2004;287:C1493–C1526. doi: 10.1152/ajpcell.00282.2004. [DOI] [PubMed] [Google Scholar]
- Ray RS, Corcoran AE, Brust RD, Kim JC, Richerson GB, Nattie E. Dymecki SM. Impaired respiratory and body temperature control upon acute serotonergic neuron inhibition. Science. 2011;333:637–642. doi: 10.1126/science.1205295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz R, Nink M, Werner GS, Andreas S, Kreuzer H, Beyer J. Lehnert H. Human corticotropin-releasing hormone and thyrotropin-releasing hormone modulate the hypercapnic ventilatory response in humans. Eur J Clin Invest. 1996;26:989–995. doi: 10.1046/j.1365-2362.1996.2130573.x. [DOI] [PubMed] [Google Scholar]
- Shannon DC, Kelly DH. O'Connell K. Abnormal regulation of ventilation in infants at risk for sudden-infant-death syndrome. N Engl J Med. 1977;297:747–750. doi: 10.1056/NEJM197710062971403. [DOI] [PubMed] [Google Scholar]
- Stornetta RL, Moreira TS, Takakura AC, Kang BJ, Chang DA, West GH, Brunet JF, Mulkey DK, Bayliss DA. Guyenet PG. Expression of Phox2b by brainstem neurons involved in chemosensory integration in the adult rat. J Neurosci. 2006;26:10305–10314. doi: 10.1523/JNEUROSCI.2917-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strohl KP, Thomas AJ, St JP, Schlenker EH, Koletsky RJ. Schork NJ. Ventilation and metabolism among rat strains. J Appl Physiol. 1997;82:317–323. doi: 10.1152/jappl.1997.82.1.317. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Sugano H, Matsumoto K, Yamamura M. Ishida R. Synthesis and central nervous system actions of thyrotropin-releasing hormone analogues containing a dihydroorotic acid moiety. J Med Chem. 1990;33:2130–2137. doi: 10.1021/jm00170a014. [DOI] [PubMed] [Google Scholar]
- Taylor NC, Li A. Nattie EE. Medullary serotonergic neurones modulate the ventilatory response to hypercapnia, but not hypoxia in conscious rats. J Physiol. 2005;566:543–557. doi: 10.1113/jphysiol.2005.083873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Pizzonia JH. Richerson GB. Chemosensitivity of rat medullary raphe neurones in primary tissue culture. J Physiol. 1998;511(Pt 2):433–450. doi: 10.1111/j.1469-7793.1998.433bh.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Tiwari JK, Bradley SR, Zaykin RV. Richerson GB. Acidosis-stimulated neurons of the medullary raphe are serotonergic. J Neurophysiol. 2001;85:2224–2235. doi: 10.1152/jn.2001.85.5.2224. [DOI] [PubMed] [Google Scholar]
- Weston MC, Stornetta RL. Guyenet PG. Glutamatergic neuronal projections from the marginal layer of the rostral ventral medulla to the respiratory centers in rats. J Comp Neurol. 2004;473:73–85. doi: 10.1002/cne.20076. [DOI] [PubMed] [Google Scholar]
- Wylie CJ, Hendricks TJ, Zhang B, Wang L, Lu P, Leahy P, Fox S, Maeno H. Deneris ES. Distinct transcriptomes define rostral and caudal serotonin neurons. J Neurosci. 2010;30:670–684. doi: 10.1523/JNEUROSCI.4656-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C, Stingo FC, Ahn KW, Liu P, Vannucci M, Laud PW, Skelton M, O'Connor P, Kurth T, Ryan RP, Moreno C, Tsaih SW, Patone G, Hummel O, Jacob HJ, Liang M. Cowley AW., Jr Increased proliferative cells in the medullary thick ascending limb of the loop of Henle in the Dahl salt-sensitive rat. Hypertension. 2013;61:208–215. doi: 10.1161/HYPERTENSIONAHA.112.199380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X. van den Pol AN. Thyrotropin-releasing hormone (TRH) inhibits melanin-concentrating hormone neurons: implications for TRH-mediated anorexic and arousal actions. J Neurosci. 2012;32:3032–3043. doi: 10.1523/JNEUROSCI.5966-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwillich CW, Pierson DJ, Hofeldt FD, Lufkin EG. Weil JV. Ventilatory control in myxedema and hypothyroidism. N Engl J Med. 1975;292:662–665. doi: 10.1056/NEJM197503272921302. [DOI] [PubMed] [Google Scholar]


