Abstract
The age-related mechanisms underlying sarcopenia are largely unknown. We hypothesize that age-related neuromuscular changes depend on brain-derived neurotrophic factor (BDNF) acting through the tropomyosin-related kinase receptor B (TrkB). Maximal specific force and neuromuscular transmission failure were assessed at 6, 18 and 24 months following control, BDNF or phosphoprotein phosphatase 1 derivative (1NMPP1) treatment in male TrkBF616A mice. Phosphoprotein phosphatase-1 derivatives such as 1NMPP1 inhibit TrkB kinase activity as a result of this single amino acid mutation in the ATP binding domain. Maximal twitch and isometric tetanic force were reduced at 24 months compared to 6 and 18 months (P < 0.001). Neuromuscular transmission failure significantly increased at 18 and 24 months compared to 6 months (age × treatment interaction: P < 0.001). Neuromuscular transmission was improved following BDNF at 6 and 18 months and was impaired only at 6 months following 1NMPP1 treatment. Age and inhibition of TrkB kinase activity had similar effects on neuromuscular transmission failure, supporting a critical role for BDNF/TrkB signalling on neuromuscular changes in ageing. These results suggest that an age-related loss of endogenous BDNF precedes reductions in TrkB kinase activity in the diaphragm muscle.
Key points.
Sarcopenia of the diaphragm muscle, i.e. loss of muscle force and size with increasing age, may contribute to respiratory impairment in old age but the exact mechanisms underlying this are currently unknown.
Across the lifespan in mice there is worsening neuromuscular function of the diaphragm muscle, specifically reduced force and impaired neuromuscular transmission.
We tested the hypothesis that age-related changes to the diaphragm muscle depend on brain-derived neurotrophic factor (BDNF), acting through its high affinity receptor.
BDNF improves neuromuscular transmission in the diaphragm muscle into early old age, but not older ages. Inhibition of BDNF signalling impairs neuromuscular transmission only in young adult mice.
Our results suggest that the loss of endogenous BDNF precedes reduced activity of the high affinity receptor tropomyosin-related kinase receptor B in the ageing mouse diaphragm muscle.
Introduction
Impairments in ventilation increase in old age with respiratory complications being a main cause of death in old age (Fein & Niederman, 1994; Houston et al. 1997; Sieck & Mantilla, 2009; Heron, 2011). Recently, we reported that the diaphragm muscle is highly susceptible to sarcopenia, the age-related loss of muscle force and size (Greising et al. 2013a). Throughout life, the diaphragm muscle must generate a large range of forces for both ventilatory and non-ventilatory motor behaviours (e.g. necessary for airway clearance) (Sieck, 1988; Mantilla et al. 2010; Greising et al. 2013b). Importantly, the mechanisms underlying diaphragm muscle sarcopenia remain poorly understood.
Age-related neuromuscular changes may contribute to diaphragm muscle sarcopenia. For instance, morphological changes at the neuromuscular junction (Fahim & Robbins, 1982; Fahim et al. 1983; Prakash & Sieck, 1998; Deschenes et al. 2010; Valdez et al. 2012) may increase the susceptibility to neuromuscular transmission failure (Mantilla & Sieck, 2011). In addition, altered trophic interactions between motor neurons and muscle fibres may also play a role in neuromuscular adaptations including sarcopenia (Mantilla & Sieck, 2008, 2009). In particular, brain-derived neurotrophic factor (BDNF) acting through its high affinity tropomyosin-related kinase receptor subtype B (TrkB) receptor plays an important role in the maintenance of neuromuscular transmission (Funakoshi et al. 1995; Mantilla et al. 2004; Mantilla & Ermilov, 2012). There is a paucity of data regarding the role of BDNF and TrkB signalling in age-related changes to the neuromuscular system. Most of this information is derived from assessment of mRNA expression in limb muscles and motor neuron groups (Johnson et al. 1999; Ming et al. 1999; Kulakowski et al. 2011; Personius & Parker, 2013). Indeed, the role of BDNF/TrkB signalling in the ageing neuromuscular system remains poorly understood. Necessary assessments of BDNF and TrkB protein expression have been hindered by the lack of high quality reagents validated in the neuromuscular system, particularly in skeletal muscles of rodents.
We hypothesized that ageing-related changes in neuromuscular transmission at the diaphragm muscle depend on BDNF acting through TrkB receptors. Ageing effects were evaluated in TrkBF616A knockin mice, which allowed rapid, selective inhibition of TrkB kinase activity (Chen et al. 2005). In these TrkBF616A mice, TrkB kinase activity is sensitive to inhibition by the phosphoprotein phosphatase 1 (PP1) derivative 1NMPP1 due to a phenylalanine-to-alanine mutation in the ATP binding domain of the TrkB receptor. Thus, this chemical–genetic approach permits assessment of the role of BDNF signalling via TrkB kinase activity in the ageing neuromuscular system.
Methods
Ethical approval and animals
Adult male TrkBF616A mice (n = 46) on a C57BL/6×129 background were used in all studies. Mice were bred and maintained in colonies at the Mayo Clinic. All mice used in these studies were genotyped by PCR analysis of DNA isolated from tail snips with primers 5′-GGGCTTGAGAAGAGGGCAAAAGGGTTGCTCAG-3′ and 5′-GTTGGTCACCAGCAGAACACTCGACTCAC-3′, as previously reported (Mantilla et al. 2014). Mice were group housed by genotype until used and were maintained on a 12 h light–dark schedule in specific pathogen-free rooms. Mice had free access to food and water through their lifespan. Mice were examined at three ages, 6, 18 and 24 months, representing survival rates of 100, 90 and 75% based on data from our colony and published estimates (Turturro et al. 1999; Flurkey, 2009; Greising et al. 2013a). All experiments were designed within considerations for animal use in gerontological research (Miller & Nadon, 2000). All protocols and animal care guidelines were approved by the Institutional Animal Care and Use Committee at the Mayo Clinic, in compliance with National Institute of Health Guidelines. At the terminal experiment, all mice were anaesthetized with an i.p. injection of ketamine (90 mg kg−1) and xylazine (10 mg kg−1) and killed by exsanguination. The diaphragm muscle with the phrenic nerve intact was carefully dissected for further evaluation.
Diaphragm muscle ex vivo contractility
A strip of midcostal diaphragm muscle (∼3 mm) was examined for isometric contractile properties. As previously described (Lewis et al. 1986; Miyata et al. 1995; Gosselin et al. 1996; Ameredes et al. 2000; Sieck et al. 2012; Greising et al. 2013a), the muscle strip was stabilized by minutien pins (FST # 26002-15) on the rib origin and attached to a force transducer by the central tendon insertion. The muscle segment was maintained at 26°C and incubated in Reese–Simpson buffer (pH 7.4) with 95% O2 and 5% CO2. All measurements were conducted while the muscle was set to optimal length. Silver plate electrodes were used to elicit maximal twitch force (Pt) with a 0.5 ms pulse and isometric tetanic force (Po) with 1000 ms and 120 Hz. Specific force was analysed by normalizing force to physiological muscle strip cross-sectional area determined as diaphragm muscle mass/(optimal length×muscle density). Muscle fatigue was determined by stimulation at 40 Hz in 330 ms trains every second for 2 min. A muscle fatigue index was calculated as the ratio of the force generated after 2 min of repetitive stimulation to the initial force. Accordingly, a greater muscle fatigue index reflects greater muscle fatigue resistance.
Diaphragm muscle neuromuscular transmission failure
A separate diaphragm muscle segment was dissected with the phrenic nerve intact and used for measurements of the contribution of neuromuscular transmission failure to diaphragm muscle fatigue, as previously described (Kuei et al. 1990; Fournier et al. 1991; Johnson & Sieck, 1993; Miyata et al. 1995; Prakash et al. 1999; Mantilla et al. 2004; Ermilov et al. 2010; Mantilla & Ermilov, 2012; Sieck et al. 2012). Briefly, the diaphragm muscle–phrenic nerve preparation was placed on a force transducer setup as described above and the phrenic nerve was maintained in a suction electrode. Stimulation of the phrenic nerve (via the suction electrode) was delivered at 40 Hz in 330 ms trains repeated every second, and direct muscle stimulation (via the plate electrodes) was repeated every 15 s using 330 ms trains of 0.2 ms supramaximal pulses at 40 Hz. Repetitive nerve and intermittent muscle stimulation were delivered for a total of 2 min. This stimulation procedure results in consistent supramaximal stimulation throughout the 2 min protocol (Kuei et al. 1990). The extent of neuromuscular transmission failure was determined by the difference in force generated by nerve and muscle stimulation as: 100×(NF − MF)/(100 − MF) where NF and MF are the percentage decrement in force during nerve and muscle stimulation, respectively.
Ex vivo treatment with BDNF or 1NMPP1 was conducted on the diaphragm muscle–phrenic nerve preparations to assess neuromuscular transmission failure as previously described (Mantilla et al. 2004, 2014; Mantilla & Ermilov, 2012). Briefly, incubation in Reese–Simpson solution was carried out for 30 min with 7.4 nm BDNF (248-BD; R&D Systems, Minneapolis, MN, USA), 25 μm 1NMPP1 (529581; Calbiochem, Billerica, MA, USA), BDNF and 1NMPP1 combined at these same concentrations (30 min for BDNF and 60 min for 1NMPP1; with the addition of BDNF to the bath occurring following the initial 30 min of 1NMPP1 incubation), or vehicle (DMSO, 63 mm). Incubation with vehicle was used to control for any possible effects of DMSO on diaphragm muscle contractility (Reid & Moody, 1994). All treatments occurred prior to and during all neuromuscular transmission failure testing. In most cases, diaphragm muscle–phrenic nerve preparations could be obtained from each hemidiaphragm. Each hemidiaphragm was allocated to a different treatment group such that n reflects the number of mice used in each treatment and age group, but the total number of animals reflects the ability to derive up to two preparations per animal.
The effect of 1NMPP1 on wild-type mice was also assessed in independent studies conducted in adult male mice (C57BL/6 J) untreated or treated with 1NMPP1 (25 μm) (n = 6 per group). Neuromuscular transmission failure was not different between 1NMPP1-treated and untreated diaphragm muscle–phrenic nerve preparations (P = 0.091; 41.9 ± 2.8 vs. 36.5 ± 0.7%, respectively).
Statistical analysis
All data was analysed with JMP (version 9.0.1; SAS Institute Inc., Cary, NC, USA) and are presented as mean ± SEM. One-way ANOVAs comparing across age groups were used for body mass, specific Pt and Po, ratio of Pt/Po, and muscle fatigue index. One-or two-way ANOVAs were also used for comparisons across age groups and treatment for neuromuscular transmission failure, specific Pt and Po, and the ratio of initial nerve and muscle stimulation, as appropriate. Three-way repeated measures ANOVA was used to compare neuromuscular transmission failure across age and treatment over the 2 min period of repetitive stimulation. When appropriate, Tukey–Kramer's honestly significant difference post hoc analyses were conducted. Significance was accepted at P < 0.05.
Results
Mouse characteristics
Mice across the lifespan, at 6, 18 and 24 months of age, were used to examine the role of BDNF/TrkB signalling on ageing effects on neuromuscular transmission in the diaphragm muscle. Older mice had greater body mass than the 6 month group: 31 and 21% greater for the 18 and 24 month groups, respectively (Table1).
Table 1.
Group characteristics of ageing mice
| Age |
P (one-way ANOVA) | |||
|---|---|---|---|---|
| 6 month (n = 14) | 18 months (n = 18) | 24 months (n = 14) | ||
| Age (months) | 6.1±0.1 | 18.0±0.1 | 24.1±0.1 | |
| Body mass (g) | 30.3±1.3 | 39.6±18* | 36.7±1.0* | <0.001 |
Values are shown as mean ± SEM.
Significantly different from 6-month-old mice.
Diaphragm muscle ex vivo contractility
Specific Pt and Po at 24 months of age was significantly lower than at both 6 and 18 months (P ≤ 0.001; Fig.1). Importantly, no difference in the Pt/Po ratio of the diaphragm muscle existed across age groups (0.32 ± 0.02, 0.31 ± 0.02 and 0.32 ± 0.02 respectively for the 6, 18 and 24 month groups; P = 0.325). Muscle fatigue index (the ratio of force after 2 min of stimulation to initial) was different across age groups (P = 0.024), being greater at 24 months than at both 6 and 18 months (Fig. 2).
Figure 1.
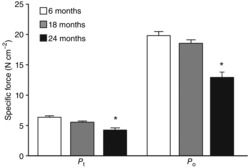
Maximal isometric twitch force (Pt) and maximal tetanic force (Po), normalized to physiological cross-sectional area, of midcostal diaphragm muscles of mice across the lifespan (aged 6, 18 and 24 months)
Data were analysed by one-way ANOVA (P < 0.001 for both Pt and Po). *Significantly different from 6-and 18-month-old mice.
Figure 2.
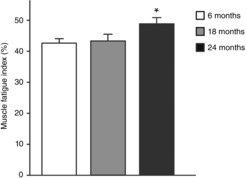
Muscle fatigue index of diaphragm muscle following 2 min of repetitive muscle stimulation across the lifespan in mice (aged 6, 18 and 24 months)
Muscle fatigue index is calculated as the ratio of the force at 2 min to the initial force. Data were analysed by one-way ANOVA (P = 0.024). *Significantly different from 6-and 18-month-old mice.
In additional experiments using diaphragm muscle–phrenic nerve preparations, contractile properties were essentially identical to those presented above (Table2). Of note, treatment with BDNF or 1NMPP1 had minor effects on the contractile properties of the diaphragm muscle across age groups when directly stimulated in the muscle alone. Both specific Pt and specific Po were decreased at 24 months compared to both 6 and 18 months. There was a slight reduction in specific Pt with 1NMPP1 compared to control (vehicle, DMSO-treated), but not BDNF. Neither BDNF nor 1NMPP1 treatment changed specific Po for the respective age.
Table 2.
Diaphragm muscle contractile properties across the lifespan in mice (6, 18 and 24 months)
|
P (two-way ANOVA) |
||||||
|---|---|---|---|---|---|---|
| Control | BDNF | 1NMPP1 | Age effect | Treatment effect | Interaction | |
| Pt (N cm−2) | <0.001 | 0.015 | 0.759 | |||
| 6 months | 6.1 ± 0.5 | 6.0 ± 0.3 | 5.3 ± 0.3 | |||
| 18 months | 5.8 ± 0.2 | 5.6 ± 0.2 | 4.9 ± 0.3 | |||
| 24 months | 4.7 ± 0.3* | 3.9 ± 0.4* | 3.8 ± 0.4* | |||
| Po (N cm−2) | <0.001 | 0.897 | 0.412 | |||
| 6 months | 18.8 ± 1.0 | 19.7 ± 1.1 | 19.5 ± 1.1 | |||
| 18 months | 18.6 ± 0.8 | 18.8 ± 0.6 | 17.5 ± 0.7 | |||
| 24 months | 14.7 ± 0.3* | 12.9 ± 1.0* | 14.5 ± 1.0* | |||
Maximal twitch force (Pt) and isometric tetanic force (Po) are normalized to physiological cross-sectional area (mean ± SEM). Data were obtained using diaphragm muscle–phrenic nerve preparations in control, or BDNF-or 1NMPP1-treated groups (n = 7–9 per group) prior to neuromuscular transmission failure testing. Diaphragm muscle contractile properties were assessed only with direct muscle stimulation and were thus independent of the data presented in Fig. 1, but were necessary to verify preparation quality and possible effects of treatment. Post hoc comparisons show both Pt and Po at 24 months significantly different from at 6 and 18 months(*) and Pt in the control is significantly different from in the 1NMPP1 treatment group.
Diaphragm muscle neuromuscular transmission failure
The contribution of neuromuscular transmission failure to muscle fatigue was determined over a 2 min period of repetitive nerve (via suction electrode) and intermittent muscle stimulation (via plate electrodes). A representative tracing for control (vehicle-treated) diaphragm muscle–phrenic nerve preparations of each age group is presented in Fig. 3. There was no effect of age or treatment on the ratio of the initial nerve stimulation-derived to initial muscle stimulation-derived force (overall: 0.84 ± 0.01 across all groups; age effect, P = 0.166; treatment effect, P = 0.653; interaction, P = 0.872). With repetitive phrenic nerve stimulation, diaphragm muscle force decreased over time, and this reduced force generation reflects neuromuscular transmission failure. Forces generated by direct muscle stimulation also decreased over time, but it is important to highlight that the level of fidelity of neuromuscular transmission in activating diaphragm muscle fibres would also contribute to the resulting muscle fatigue. Accordingly, the decline in diaphragm muscle force resulting from nerve and muscle stimulation (muscle fatigue) must be considered concurrently as an assessment of neuromuscular transmission failure, which was expressed as the percentage decline in muscle force. As such, at time 0 there was no neuromuscular transmission failure, and neuromuscular transmission failure increased with time. Note that all components of neural excitation–contraction coupling are reflected in this global measurement of neuromuscular transmission.
Figure 3.
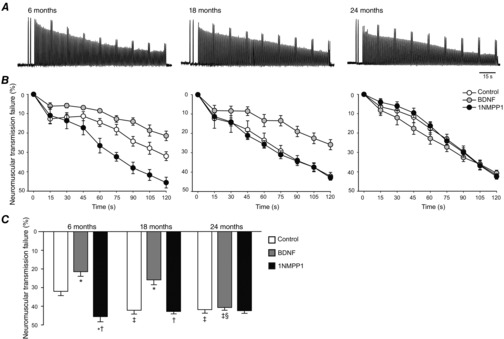
The contribution of neuromuscular transmission failure to diaphragm muscle fatigue over a 2 min period of repetitive nerve stimulation and superimposed intermittent muscle stimulation across the lifespan in mice (aged 6, 18 and 24 months)
A, representative tracings for control (vehicle-treated) diaphragm muscle–phrenic nerve preparations of each age group. B, time course of neuromuscular transmission failure during repetitive stimulation in control, and BDNF-or 1NMPP1-treated preparations at each age group. In all age and treatment groups, there is progressively greater neuromuscular transmission failure over time. Data were analysed by three-way repeated measures ANOVA (age × treatment × time – repeated; P < 0.001). C, neuromuscular transmission failure following 2 min of repetitive stimulation in control, and BDNF-or 1NMPP1-treated groups. Summary data are shown to facilitate comparisons across treatment and age groups. Data were analysed by two-way ANOVA (age × treatment; interaction P < 0.001). Post hoc comparisons: *significantly different from control at the same age; †significantly different from BDNF at the same age; ‡significantly different from 6 months within the same treatment; §significantly different from 18 months within the same treatment.
Neuromuscular transmission failure varied significantly based on time of stimulation, treatment and age groups (three-way repeated measures ANOVA; P < 0.001). Across treatment groups at 6, 18 and 24 months, repetitive stimulation resulted in a progressive decline in diaphragm muscle force generation reflecting neuromuscular transmission failure, which became significant by 15–45 s of stimulation (Fig. 3). In the control group, differences across age became significant by 2 min of repetitive stimulation. In the BDNF-treated group, differences across age were evident after 45 s of stimulation and were present consistently thereafter. Following treatment with 1NMPP1, differences across age were evident by 45–75 s of stimulation, with greater initial neuromuscular transmission failure at 6 and 18 months.
There was a significant interaction between age and treatment on neuromuscular transmission failure measured following 2 min of repetitive stimulation (P < 0.001; Fig. 3). Neuromuscular transmission failure was greater at 18 and 24 months compared to 6 months in control diaphragm muscle–phrenic nerve preparations. Treatment with BDNF reduced neuromuscular transmission failure at 6 and 18 months, but not at 24 months. Inhibiting TrkB kinase activity via 1NMPP1 treatment increased neuromuscular transmission failure at 6 months compared to control, but did not change neuromuscular failure at 18 and 24 months. The impairment in neuromuscular transmission induced by 1NMPP1 treatment at 6 months was not different from that present in diaphragm muscle–phrenic nerve preparation from older mice (either 18 or 24 months). To verify that the effects of BDNF on neuromuscular transmission were mediated by TrkB kinase activity, an additional treatment group (BDNF and 1NMPP1 combined) was included at 18 months of age. Combined BDNF and 1NMPP1 treatment was not different from same age control or 1NMPP1 treatment (43.1 ± 4.1%) but was significantly different from BDNF alone (P < 0.001). These results suggest a time course of changes in neuromuscular transmission in old age, with reduced endogenous BDNF availability at diaphragm neuromuscular junctions by 18 months of age and subsequent reduction in TrkB signalling by 24 months of age.
Discussion
Declines in neuromuscular system performance may be evident by examining muscle fibre properties and neuromuscular transmission failure. Decreases in neurotrophic signalling may lead to altered neuromuscular function in old age. We hypothesized that ageing-related changes in neuromuscular transmission at the diaphragm muscle depend on BDNF acting through TrkB receptors. As such, we examined the role of BDNF/TrkB signalling in the diaphragm muscle by using a global measure of neuromuscular transmission. The results of the present study collectively identify neurotrophic signalling via BDNF/TrkB as a significant contributor to the ageing-related changes in diaphragm neuromuscular function and provide novel, mechanistic targets that may help mitigate sarcopenia.
Sarcopenia was previously examined in the diaphragm muscle of young and old mice, substantiating a difference in specific force of 34% at comparable ages, between 5 and 23 months of age (Greising et al. 2013a). The results of the present study show a nearly identical loss of diaphragm muscle force (35%) between 6 and 24 months of age. Of note, the present examination of an intermediate age (18 months) indicates no reduction in specific force between 6 and 18 months of age. Thus, a significant change in force-generating capacity occurs within a relatively short time window of 6 months. The present study examined the possible role of BDNF/TrkB signalling in the age-related decline in neuromuscular system performance.
Neuromuscular transmission failure was used as a global method to evaluate force generated by the diaphragm muscle in response to nerve activation. Clearly, this global measurement is affected by changes in contractile properties in the diaphragm muscle. In this sense, it is important to realize that alterations in diaphragm muscle neuromuscular transmission failure did not follow the same pattern into old age as did changes in diaphragm muscle force or fatigue resistance. Increased failure in diaphragm muscle neuromuscular transmission was evident by 18 months of age with no further changes at 24 months. This mismatch in the time course of functional decline within the neuromuscular system suggests primary alterations in neuromuscular transmission occurring around 18 months of age. Indeed, functional changes in neuromuscular transmission apparently precede loss of diaphragm muscle force.
It is important to realize that all components of neural excitation–contraction coupling are reflected in the global measurement of neuromuscular transmission used in the present study (Sieck & Prakash, 1995; Mantilla & Sieck, 2009). Thus, changes in axonal conduction, branch-point failure, synaptic vesicle release and cycling as well as structural changes within pre-or postsynaptic structures could contribute to impaired neuromuscular transmission in old age. A previous study in the diaphragm muscle of aged rats (at 25–50% survival) reported increased synaptic depression with repetitive stimulation as well as increased action potential blockade at lower frequencies of stimulation compared to adult rats (at ∼100% survival) (Smith, 1979). Although the age groups used in the present study included animals at 100% (6 months old), 90% (18 months old) and 75% (24 months old) survival in mice, it is possible that deficits in axonal conduction, increased branch-point failure and synaptic transmission contribute to the age-related impairment in neuromuscular transmission.
Neurotrophins, such as BDNF, acting via Trk receptors regulate synaptic transmission in the CNS (Huang & Reichardt, 2003; Lu, 2004) and acutely enhance neuromuscular transmission. Indeed, BDNF and neurotrophin-4 reduce neuromuscular transmission failure at diaphragm muscle–phrenic nerve preparations (Mantilla et al. 2004). The effect of BDNF on neuromuscular transmission is probably mediated through activation of the TrkB receptor given that enhanced neuromuscular transmission is also evident with small molecule TrkB agonists such as 7,8-dihydroxyflavone (Mantilla & Ermilov, 2012), whereas neuromuscular transmission failure increases following treatment with the tyrosine kinase inhibitor K252a (Mantilla et al. 2004) and with inhibition of TrkB kinase activity by 1NMPP1 in TrkBF616A mice (present study). These rapid effects of BDNF/TrkB signalling on neuromuscular transmission probably involve interaction with pre-synaptic adenosine A2A receptors (Pousinha et al. 2006) and muscarinic receptors (Garcia et al. 2010a) involved in modulation of acetylcholine release, supporting a role for neurotrophin-induced enhancements in synaptic transmission across the lifespan. Importantly, the effects of BDNF/TrkB signalling on neuromuscular transmission vary with age. In the present study, BDNF treatment enhanced neuromuscular transmission at both 6 and 18 months of age, consistent with previous results in 3-to 4-month-old rats (Mantilla et al. 2004). However, at 24 months of age, there was no longer an effect of BDNF on neuromuscular transmission failure. Inhibition of TrkB kinase activity by 1NMPP1 worsened neuromuscular transmission at 6 months of age, resulting in similar levels of neuromuscular transmission failure to those in 18-and 24-month-old controls (vehicle-treated). Furthermore, in mice at both 18 and 24 months of age, 1NMPP1 treatment exerts no additional effect on neuromuscular transmission failure compared to controls of the same age. These results suggest that there is reduced TrkB kinase activity in the older age groups. Given that at 18 months of age, addition of exogenous BDNF could still enhance neuromuscular transmission, these results suggest that the lack of an effect of 1NMPP1 at 18 months of age may simply reflect the lack of endogenous neurotrophin activity at diaphragm neuromuscular junctions in this age group. By 24 months of age, the lack of effects of both BDNF and 1NMPP1 suggests reduced expression of the full-length TrkB receptor capable of kinase activity and/or increased expression of truncated TrkB receptor isoforms lacking the intracellular kinase domain.
Studies addressing the expression of BDNF/TrkB signalling pathway components should specifically address motor neuron and muscle fibre expression of full-length and truncated forms of the high affinity TrkB receptor, expression of the low affinity p75 receptor as well as expression and release of BDNF (mature and immature forms) at both the neuromuscular junction and, possibly, extrasynaptic sites. Unfortunately, necessary assessments of BDNF and TrkB protein expression have been hindered by the lack of high quality reagents validated in the neuromuscular system. Indeed, Dieni et al. (2012) recently completed a study demonstrating related challenges in determining BDNF signalling in the hippocampus where commercially available antibodies could not be validated. We have attempted to quantify the protein expression of BDNF and TrkB receptor in the diaphragm muscle of rodents, and have been unable to obtain reliable results using a wide assortment of commercially available anti-BDNF and anti-TrkB antibodies (unpublished observations). Regardless, interpretation of the novel role of BDNF/TrkB signalling in the regulation of neuromuscular transmission across the lifespan is not dependent on measurements of the expression levels of either BDNF or TrkB receptors within the neuromuscular system. The present study provides direct, mechanistic insight into the varying effects of BDNF/TrkB signalling throughout the lifespan resulting from the age-related change in BDNF effects and TrkB kinase inhibition. Furthermore, the results of the present study identify a critical period of changes in BDNF/TrkB effects in the neuromuscular system that informs the conduct of future comprehensive studies examining the possible changes in expression at specific components of diaphragm motor units (i.e. phrenic motor neurons and diaphragm muscle fibres) as well as perisynaptic Schwann cells.
Previous reports document changes in BDNF/TrkB signalling across the lifespan across various organ systems. An age-related loss of BDNF was reported in the hippocampus and prefrontal cortex (Calabrese et al. 2013). Unfortunately, limited research has examined the neuromuscular system and available studies have not conducted detailed analyses of cellular expression and signalling within diaphragm motor units. A trend for reduced BDNF mRNA was reported in the triceps surae muscle of aged rats (Ming et al. 1999). Studies examining TrkB receptor expression have yielded conflicting results, but have not consistently reported critical antibody validation steps. In aged mice (24 months of age), immunohistochemical staining for TrkB receptor reportedly decreased at soleus muscle neuromuscular junctions compared to 3-and 12-month-old animals (Personius & Parker, 2013). However, in this same report, there was a concurrent increase in the full-length TrkB copy number and no change in truncated TrkB mRNA. Immunohistochemical analyses are most useful in providing information regarding the localization of specific proteins but are distinctly limited in providing quantitative results. Garcia et al. (2010a,b) reported expression of the BDNF and TrkB receptors at both pre-and postsynaptic sites of neuromuscular junctions at the levator auris longus muscles of adult mice. Based on the results of previous studies in rodents examining the effect of BDNF and other TrkB ligands (e.g. neurotrophin-4 and 7,8-dihydroxyflavone) on neuromuscular transmission, the full-length TrkB receptor would be expected to be present presynaptically (Mantilla et al. 2004; Garcia et al. 2010a; Mantilla & Ermilov, 2012). Studies examining age-related changes in TrkB receptor expression must explore expression at the motor neuron, including the balance between the expression of full-length (kinase active) and truncated isoforms (Dorsey et al. 2012).
Genetic models, such as the TrkBF616A mouse (Chen et al. 2005; Mantilla & Ermilov, 2012; Mantilla et al. 2014), provide important information that is not subject to technical issues related to reagent quality and validation. In mice heterozygous for TrkB (TrkB+/−), with an expected 50% reduction of full-length TrkB, detrimental morphological changes at the neuromuscular junction of the soleus muscle were reported by 6 months of age, which were similar to those of old mice at 24 months of age (Kulakowski et al. 2011). In addition, neuromuscular transmission failure was exacerbated in the soleus muscle of both 24-month-old wild-type and 6-month-old TrkB+/− mice. Reducing full-length TrkB receptor activity either by adenovirus-mediated overexpression of truncated TrkB receptors or genetically (in TrkB+/− mice) resulted in postsynaptic disassembly of the neuromuscular junction (Gonzalez et al. 1999). In addition, nerve-evoked muscle tension was reported to increase in mice lacking truncated TrkB receptor (Dorsey et al. 2012). Importantly, the present study provides direct evidence for an age-related change in BDNF/TrkB signalling that is not influenced by possible developmental effects related to genetically induced low levels of TrkB receptor expression (TrkB+/−) or high levels of truncated TrkB receptor isoforms. Thus, the time course of age-related effects reported in the present study provides important novel information to guide future studies of the ageing neuromuscular system.
To confirm that into old age BDNF signalling is mediated via full-length TrkB receptors, we also examined the combined treatment effect of BDNF and 1NMPP1. Neuromuscular transmission failure was specifically studied in TrkBF616A mice at 18 months of age as BDNF effects were still present in this age group. Importantly, there was no difference between 1NMPP1 treatment groups in the presence or absence of BDNF. Thus, inhibiting TrkB kinase activity was sufficient to block BDNF effects on neuromuscular transmission. Elucidating whether reduced BDNF signalling in old age contributes directly to subsequent changes in TrkB (full-length or truncated) receptor expression in the neuromuscular system is an important next step for investigation. The exact time line for these hypothesized changes is unknown, but the results of the present study suggest that age-related reductions in BDNF occur first and are reversible (by exogenous supplementation), and precede persistent reductions in TrkB kinase activity in the diaphragm muscle. Regardless, based on the results of the present study, therapies that restore TrkB signalling either by increasing BDNF availability or by small molecule TrkB agonists such as 7,8-dihydroxyflavone may help mitigate the age-related decline in neuromuscular transmission. Whether such therapies can also mitigate sarcopenia remains to be determined.
Contractile properties were essentially unchanged by neurotrophic treatment, regardless of age. There was no difference in specific Po of the diaphragm muscle during BDNF or 1NMPP1 treatment. There was, however, a slight decrease in specific Pt of diaphragm muscles incubated with 1NMPP1 compared to vehicle treatment (main treatment effect). The physiological relevance of this slight change is probably minimal. Diaphragm muscle fatigue resistance was only different at 24 months of age, representing a slight improvement in fatigue resistance of ∼12% compared to younger age groups. Fatigue resistance in a muscle such as the diaphragm reflects the relative proportion of various muscle fibre types (usually classified based on myosin heavy chain isoform composition). With age there is change in the proportion of fibre types, such that the diaphragm muscle has more type IIa and fewer type IIx and/or IIb fibres than younger mice (Greising et al. 2013a). Thus, it is reasonable to hypothesize that the apparent (and relatively modest) improvement in diaphragm muscle fatigue resistance at 24 months of age (and associated reduced overall force generation) reflects age-related changes in the proportion of muscle fibre types with greater contribution of fatigue-resistant fibres. Thus, there are probably minimal effects of BDNF or 1NMPP1 directly on diaphragm muscle fibres.
Collectively, BDNF/TrkB signalling plays a critical role in the regulation of neuromuscular function during ageing. Future studies should be designed to specifically quantify and examine the mechanisms of reduced neurotrophic signalling in old age. Furthermore, examination of motor neurons is necessary to inform age-related decline in neuromuscular function and sarcopenia.
Glossary
- 1NMPP1
phosphoprotein phosphatase 1 inhibitor derivative
- BDNF
brain-derived neurotrophic factor
- Pt
maximal twitch force
- Po
isometric tetanic force
- TrkB
tropomyosin-related kinase receptor B
Additional information
Competing interests
The authors declare no actual or potential conflicts of interest.
Author contributions
Conception and design of the experiments: S.M.G., G.C.S., C.B.M. Collection, analysis and interpretation of data: S.M.G., L.G.E., C.B.M. Drafting the article or revising it critically for intellectual content: S.M.G., G.C.S., C.B.M. All experiments were carried out at the Mayo Clinic. All authors have read and approved the final submission.
Funding
This research was supported by grants from National Institute of Health R01-AG044615 (C.B.M. & G.C.S.) and T32-HL105355 (S.M.G.), and the Mayo Clinic.
References
- Ameredes BT, Zhan W-Z, Vanderboom R, Prakash YS. Sieck GC. Power fatigue of the rat diaphragm muscle. J Appl Physiol. 2000;89:2215–2219. doi: 10.1152/jappl.2000.89.6.2215. [DOI] [PubMed] [Google Scholar]
- Calabrese F, Guidotti G, Racagni G. Riva MA. Reduced neuroplasticity in aged rats: a role for the neurotrophin brain-derived neurotrophic factor. Neurobiol Aging. 2013;34:2768–2776. doi: 10.1016/j.neurobiolaging.2013.06.014. [DOI] [PubMed] [Google Scholar]
- Chen X, Ye H, Kuruvilla R, Ramanan N, Scangos KW, Zhang C, Johnson NM, England PM, Shokat KM. Ginty DD. A chemical–genetic approach to studying neurotrophin signaling. Neuron. 2005;46:13–21. doi: 10.1016/j.neuron.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Deschenes MR, Roby MA, Eason MK. Harris MB. Remodeling of the neuromuscular junction precedes sarcopenia related alterations in myofibers. Exp Gerontol. 2010;45:389–393. doi: 10.1016/j.exger.2010.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieni S, Matsumoto T, Dekkers M, Rauskolb S, Ionescu MS, Deogracias R, Gundelfinger ED, Kojima M, Nestel S, Frotscher M. Barde YA. BDNF and its pro-peptide are stored in presynaptic dense core vesicles in brain neurons. J Cell Biol. 2012;196:775–788. doi: 10.1083/jcb.201201038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorsey SG, Lovering RM, Renn CL, Leitch CC, Liu X, Tallon LJ, Sadzewicz LD, Pratap A, Ott S, Sengamalay N, Jones KM, Barrick C, Fulgenzi G, Becker J, Voelker K, Talmadge R, Harvey BK, Wyatt RM, Vernon-Pitts E, Zhang C, Shokat K, Fraser-Liggett C, Balice-Gordon RJ, Tessarollo L. Ward CW. Genetic deletion of trkB.T1 increases neuromuscular function. Am J Physiol Cell Physiol. 2012;302:C141–153. doi: 10.1152/ajpcell.00469.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ermilov LG, Pulido JN, Atchison FW, Zhan WZ, Ereth MH, Sieck GC. Mantilla CB. Impairment of diaphragm muscle force and neuromuscular transmission after normothermic cardiopulmonary bypass: effect of low dose inhaled CO. Am J Physiol Regul Integr Comp Physiol. 2010;298:R784–789. doi: 10.1152/ajpregu.00737.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahim MA, Holley JA. Robbins N. Scanning and light microscopic study of age changes at a neuromuscular junction in the mouse. J Neurocytol. 1983;12:13–25. doi: 10.1007/BF01148085. [DOI] [PubMed] [Google Scholar]
- Fahim MA. Robbins N. Ultrastructural studies of young and old mouse neuromuscular junctions. J Neurocytol. 1982;11:641–656. doi: 10.1007/BF01262429. [DOI] [PubMed] [Google Scholar]
- Fein AM. Niederman MS. Severe pneumonia in the elderly. Clin Geriatr Med. 1994;10:121–143. [PubMed] [Google Scholar]
- Flurkey K, editor. The Jackson Laboratory Handbook on Genetically Standardized Mice. Bar Harbor, ME: Jackson Laboratory; 2009. [Google Scholar]
- Fournier M, Alula M. Sieck GC. Neuromuscular transmission failure during postnatal development. Neurosci Lett. 1991;125:34–36. doi: 10.1016/0304-3940(91)90124-c. [DOI] [PubMed] [Google Scholar]
- Funakoshi H, Belluardo N, Arenasa E, Yamamoto Y, Casabona A, Persson H. Ibanez CF. Muscle-derived neurotrophin-4 as an activity-dependent trophic signal for adult motor neurons. Science. 1995;268:1495–1499. doi: 10.1126/science.7770776. [DOI] [PubMed] [Google Scholar]
- Garcia N, Tomas M, Santafe MM, Besalduch N, Lanuza MA. Tomas J. The interaction between tropomyosin-related kinase B receptors and presynaptic muscarinic receptors modulates transmitter release in adult rodent motor nerve terminals. J Neurosci. 2010a;30:16514–16522. doi: 10.1523/JNEUROSCI.2676-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia N, Tomas M, Santafe MM, Lanuza MA, Besalduch N. Tomas J. Localization of brain-derived neurotrophic factor, neurotrophin-4, tropomyosin-related kinase b receptor, and p75 NTR receptor by high-resolution immunohistochemistry on the adult mouse neuromuscular junction. J Peripher Nerv Syst. 2010b;15:40–49. doi: 10.1111/j.1529-8027.2010.00250.x. [DOI] [PubMed] [Google Scholar]
- Gonzalez M, Ruggiero FP, Chang Q, Shi YJ, Rich MM, Kraner S. Balice-Gordon RJ. Disruption of Trkb-mediated signaling induces disassembly of postsynaptic receptor clusters at neuromuscular junctions. Neuron. 1999;24:567–583. doi: 10.1016/s0896-6273(00)81113-7. [DOI] [PubMed] [Google Scholar]
- Gosselin LE, Zhan WZ. Sieck GC. Hypothyroid-mediated changes in adult rat diaphragm muscle contractile properties and MHC isoform expression. J Appl Physiol. 1996;80:1934–1939. doi: 10.1152/jappl.1996.80.6.1934. [DOI] [PubMed] [Google Scholar]
- Greising SM, Mantilla CB, Gorman BA, Ermilov LG. Sieck GC. Diaphragm muscle sarcopenia in aging mice. Exp Gerontol. 2013a;48:881–887. doi: 10.1016/j.exger.2013.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greising SM, Sieck DC, Sieck GC. Mantilla CB. Novel method for transdiaphragmatic pressure measurements in mice. Respir Physiol Neurobiol. 2013b;188:56–59. doi: 10.1016/j.resp.2013.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heron M. Deaths: leading causes for 2007. Natl Vital Stat Rep. 2011;59:1–95. [PubMed] [Google Scholar]
- Houston MS, Silverstein MD. Suman VJ. Risk factors for 30-day mortality in elderly patients with lower respiratory tract infection. Community-based study. Arch Intern Med. 1997;157:2190–2195. [PubMed] [Google Scholar]
- Huang EJ. Reichardt LF. Trk receptors: roles in neuronal signal transduction. Annu Rev Biochem. 2003;72:609–642. doi: 10.1146/annurev.biochem.72.121801.161629. [DOI] [PubMed] [Google Scholar]
- Johnson H, Hokfelt T. Ulfhake B. Expression of p75(NTR), trkB and trkC in nonmanipulated and axotomized motoneurons of aged rats. Brain Res Mol Brain Res. 1999;69:21–34. doi: 10.1016/s0169-328x(99)00068-6. [DOI] [PubMed] [Google Scholar]
- Johnson BD. Sieck GC. Differential susceptibility of diaphragm muscle fibers to neuromuscular transmission failure. J Appl Physiol. 1993;75:341–348. doi: 10.1152/jappl.1993.75.1.341. [DOI] [PubMed] [Google Scholar]
- Kuei JH, Shadmehr R. Sieck GC. Relative contribution of neurotransmission failure to diaphragm fatigue. J Appl Physiol. 1990;68:174–180. doi: 10.1152/jappl.1990.68.1.174. [DOI] [PubMed] [Google Scholar]
- Kulakowski SA, Parker SD. Personius KE. Reduced TrkB expression results in precocious age-like changes in neuromuscular structure, neurotransmission, and muscle function. J Appl Physiol. 2011;111:844–852. doi: 10.1152/japplphysiol.00070.2011. [DOI] [PubMed] [Google Scholar]
- Lewis MI, Sieck GC, Fournier M. Belman MJ. Effect of nutritional deprivation on diaphragm contractility and muscle fiber size. J Appl Physiol. 1986;60:596–603. doi: 10.1152/jappl.1986.60.2.596. [DOI] [PubMed] [Google Scholar]
- Lu B. Acute and long-term synaptic modulation by neurotrophins. Prog Brain Res. 2004;146:137–150. doi: 10.1016/s0079-6123(03)46010-x. [DOI] [PubMed] [Google Scholar]
- Mantilla CB. Ermilov LG. The novel TrkB receptor agonist 7,8-dihydroxyflavone enhances neuromuscular transmission. Muscle Nerve. 2012;45:274–276. doi: 10.1002/mus.22295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantilla CB, Greising SM, Stowe JM, Zhan WZ. Sieck GC. TrkB kinase activity is critical for recovery of respiratory function after cervical spinal cord hemisection. Exp Neurol. 2014;261:190–195. doi: 10.1016/j.expneurol.2014.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantilla CB, Seven YB, Zhan WZ. Sieck GC. Diaphragm motor unit recruitment in rats. Respir Physiol Neurobiol. 2010;173:101–106. doi: 10.1016/j.resp.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantilla CB. Sieck GC. Trophic factor expression in phrenic motor neurons. Respir Physiol Neurobiol. 2008;164:252–262. doi: 10.1016/j.resp.2008.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantilla CB. Sieck GC. Neuromuscular adaptations to respiratory muscle inactivity. Respir Physiol Neurobiol. 2009;169:133–140. doi: 10.1016/j.resp.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantilla CB. Sieck GC. Age-related remodeling of neuromuscular junctions. In: Lynch GS, editor; Sarcopenia-Age-Related Muscle Wasting and Weakness. Dordrecht: Springer; 2011. pp. 37–54. [Google Scholar]
- Mantilla CB, Zhan WZ. Sieck GC. Neurotrophins improve neuromuscular transmission in the adult rat diaphragm. Muscle Nerve. 2004;29:381–386. doi: 10.1002/mus.10558. [DOI] [PubMed] [Google Scholar]
- Miller RA. Nadon NL. Principles of animal use for gerontological research. J Gerontol A Biol Sci Med Sci. 2000;55:B117–123. doi: 10.1093/gerona/55.3.B117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ming Y, Bergman E, Edstrom E. Ulfhake B. Reciprocal changes in the expression of neurotrophin mRNAs in target tissues and peripheral nerves of aged rats. Neurosci Lett. 1999;273:187–190. doi: 10.1016/s0304-3940(99)00655-2. [DOI] [PubMed] [Google Scholar]
- Miyata H, Zhan WZ, Prakash YS. Sieck GC. Influence of myoneural interactions on contractile properties of rat diaphragm. Jpn J Exerc Physiol. 1995;2:167–176. [Google Scholar]
- Personius KE. Parker SD. TrkB expression at the neuromuscular junction is reduced during aging. Muscle Nerve. 2013;47:532–538. doi: 10.1002/mus.23616. [DOI] [PubMed] [Google Scholar]
- Pousinha PA, Diogenes MJ, Ribeiro JA. Sebastiao AM. Triggering of BDNF facilitatory action on neuromuscular transmission by adenosine A2A receptors. Neurosci Lett. 2006;404:143–147. doi: 10.1016/j.neulet.2006.05.036. [DOI] [PubMed] [Google Scholar]
- Prakash YS, Miyata H, Zhan WZ. Sieck GC. Inactivity-induced remodeling of neuromuscular junctions in rat diaphragmatic muscle. Muscle Nerve. 1999;22:307–319. doi: 10.1002/(sici)1097-4598(199903)22:3<307::aid-mus3>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Prakash YS. Sieck GC. Age-related remodeling of neuromuscular junctions on type-identified diaphragm fibers. Muscle Nerve. 1998;21:887–895. doi: 10.1002/(sici)1097-4598(199807)21:7<887::aid-mus6>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Reid MB. Moody MR. Dimethyl sulfoxide depresses skeletal muscle contractility. J Appl Physiol. 1994;76:2186–2190. doi: 10.1152/jappl.1994.76.5.2186. [DOI] [PubMed] [Google Scholar]
- Sieck GC. Diaphragm muscle: structural and functional organization. Clin Chest Med. 1988;9:195–210. [PubMed] [Google Scholar]
- Sieck GC. Mantilla CB. Neuromuscular junction (NMJ): aging. In: Mobbs CV, editor; Hof PR, editor. Handbook of the Neuroscience of Aging. London: Elsevier Academic Press; 2009. pp. 223–228. [Google Scholar]
- Sieck GC. Prakash YS. Fatigue at the neuromuscular junction. Branch point vs. presynaptic vs. postsynaptic mechanisms. Adv Exp Med Biol. 1995;384:83–100. [PubMed] [Google Scholar]
- Sieck DC, Zhan WZ, Fang YH, Ermilov LG, Sieck GC. Mantilla CB. Structure–activity relationships in rodent diaphragm muscle fibers vs. neuromuscular junctions. Respir Physiol Neurobiol. 2012;180:88–96. doi: 10.1016/j.resp.2011.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DO. Reduced capabilities of synaptic transmission in aged rats. Exp Neurol. 1979;66:650–666. doi: 10.1016/0014-4886(79)90210-3. [DOI] [PubMed] [Google Scholar]
- Turturro A, Witt WW, Lewis S, Hass BS, Lipman RD. Hart RW. Growth curves and survival characteristics of the animals used in the Biomarkers of Aging Program. J Gerontol A Biol Sci Med Sci. 1999;54:B492–501. doi: 10.1093/gerona/54.11.b492. [DOI] [PubMed] [Google Scholar]
- Valdez G, Tapia JC, Lichtman JW, Fox MA. Sanes JR. Shared resistance to aging and ALS in neuromuscular junctions of specific muscles. PloS One. 2012;7:e34640. doi: 10.1371/journal.pone.0034640. [DOI] [PMC free article] [PubMed] [Google Scholar]


