Abstract
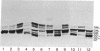
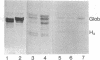
The patterns of long-chain neutral glycolipids and gangliosides of established, L-cell-derived murine fibroblast lines and their low- and high-tumorigenic hybrids were studied.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brady R. O., Fishman P. H. Biosynthesis of glycolipids in virus-transformed cells. Biochim Biophys Acta. 1974 Sep 9;355(2):121–148. doi: 10.1016/0304-419x(74)90001-8. [DOI] [PubMed] [Google Scholar]
- Burger M. M. Surface changes in transformed cells detected by lectins. Fed Proc. 1973 Jan;32(1):91–101. [PubMed] [Google Scholar]
- Chatterjee S., Sweeley C. C., Velicer L. F. Glycosphingolipids of human KB cells grown in monolayer, suspension, and synchronized cultures. J Biol Chem. 1975 Jan 10;250(1):61–66. [PubMed] [Google Scholar]
- Critchley D. R., Macpherson I. Cell density dependent glycolipids in NILz hamster cells, derived malignant and transformed cell lines. Biochim Biophys Acta. 1973 Jan 19;296(1):145–159. doi: 10.1016/0005-2760(73)90054-4. [DOI] [PubMed] [Google Scholar]
- Den H., Sela B. A., Roseman S., Sachs L. Blocks in ganglioside synthesis in transformed hamster cells and their revertants. J Biol Chem. 1974 Jan 25;249(2):659–661. [PubMed] [Google Scholar]
- Diringer H., Ströbel G., Koch M. A. Glycolipids of mouse fibroblasts and virus transformed mouse cell lines. Hoppe Seylers Z Physiol Chem. 1972 Nov;353(11):1769–1774. doi: 10.1515/bchm2.1972.353.2.1769. [DOI] [PubMed] [Google Scholar]
- Dod B. J., Gray G. M. The lipid composition of rat-liver plasma membranes. Biochim Biophys Acta. 1968 Apr 29;150(3):397–404. doi: 10.1016/0005-2736(68)90138-7. [DOI] [PubMed] [Google Scholar]
- Gahmberg C. G., Hakomori S. I. Altered growth behavior of malignant cells associated with changes in externally labeled glycoprotein and glycolipid. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3329–3333. doi: 10.1073/pnas.70.12.3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gahmberg C. G., Hakomori S. Surface carbohydrates of hamster fibroblasts. I. Chemical characterization of surface-labeled glycosphingolipids and aspecific ceramide tetrasaccharide for transformants. J Biol Chem. 1975 Apr 10;250(7):2438–2446. [PubMed] [Google Scholar]
- Hakomori S. Cell density-dependent changes of glycolipid concentrations in fibroblasts, and loss of this response in virus-transformed cells. Proc Natl Acad Sci U S A. 1970 Dec;67(4):1741–1747. doi: 10.1073/pnas.67.4.1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakomori S. Glycolipids of tumor cell membrane. Adv Cancer Res. 1973;18:265–315. doi: 10.1016/s0065-230x(08)60755-1. [DOI] [PubMed] [Google Scholar]
- Hakomori S., Stellner K., Watanabe K. Four antigenic variants of blood group A glycolipid: examples of highly complex, branched chain glycolipid of animal cell membrane. Biochem Biophys Res Commun. 1972 Nov 15;49(4):1061–1068. doi: 10.1016/0006-291x(72)90320-8. [DOI] [PubMed] [Google Scholar]
- Harris H., Bregula U., Klein G. The analysis of malignancy by cell fusion. II. Hybrids between Ehrlich cells and normal diploid cells. J Cell Sci. 1971 May;8(3):673–680. doi: 10.1242/jcs.8.3.673. [DOI] [PubMed] [Google Scholar]
- Hynes R. O. Alteration of cell-surface proteins by viral transformation and by proteolysis. Proc Natl Acad Sci U S A. 1973 Nov;70(11):3170–3174. doi: 10.1073/pnas.70.11.3170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein G., Bregula U., Wiener F., Harris H. The analysis of malignancy by cell fusion. I. Hybrids between tumour cells and L cell derivatives. J Cell Sci. 1971 May;8(3):659–672. doi: 10.1242/jcs.8.3.659. [DOI] [PubMed] [Google Scholar]
- Klenk H. D., Choppin P. W. Glycosphingolipids of plasma membranes of cultured cells and an enveloped virus (SV5) grown in these cells. Proc Natl Acad Sci U S A. 1970 May;66(1):57–64. doi: 10.1073/pnas.66.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenblith J. Z., Ukena T. E., Yin H. H., Berlin R. D., Karnovsky M. J. A comparative evaluation of the distribution of concanavalin A-binding sites on the surfaces of normal, virally-transformed, and protease-treated fibroblasts. Proc Natl Acad Sci U S A. 1973 Jun;70(6):1625–1629. doi: 10.1073/pnas.70.6.1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SVENNERHOLM L. CHROMATOGRAPHIC SEPARATION OF HUMAN BRAIN GANGLIOSIDES. J Neurochem. 1963 Sep;10:613–623. doi: 10.1111/j.1471-4159.1963.tb08933.x. [DOI] [PubMed] [Google Scholar]
- Saito T., Hakomori S. I. Quantitative isolation of total glycosphingolipids from animal cells. J Lipid Res. 1971 Mar;12(2):257–259. [PubMed] [Google Scholar]
- Sakiyama H., Terasima T. The synthesis of Forssman glycolipid in clones of nil 2 hamsters fibroblasts grown in monolayer or spinner culture. Cancer Res. 1975 Jul;35(7):1723–1726. [PubMed] [Google Scholar]
- Steiner S. M., Melnick J. L., Kit S., Somers K. D. Fucosylglycolipids in cells transformed by a temperature-sensitive mutant of murine sarcoma virus. Nature. 1974 Apr 19;248(5450):682–684. doi: 10.1038/248682a0. [DOI] [PubMed] [Google Scholar]
- Steiner S., Brennan P. J., Melnick J. L. Fucosylglycolipid metabolism in oncornavirus-transformed cell lines. Nat New Biol. 1973 Sep 5;245(140):19–21. doi: 10.1038/newbio245019a0. [DOI] [PubMed] [Google Scholar]
- Stellner K., Hakomori S., Warner G. S. Enzymic conversion of "H1-glycolipid" to A or B-glycolipid and deficiency of these enzyme activities in adenocarcinoma. Biochem Biophys Res Commun. 1973 Nov 16;55(2):439–445. doi: 10.1016/0006-291x(73)91106-6. [DOI] [PubMed] [Google Scholar]
- Stellner K., Watanabe K., Hakomori S. Isolation and characterization of glycosphingolipids with blood group H specificity from membranes of human erythrocytes. Biochemistry. 1973 Feb;12(4):656–661. doi: 10.1021/bi00728a014. [DOI] [PubMed] [Google Scholar]
- Suzuki K. The pattern of mammalian brain gangliosides. II. Evaluation of the extraction procedures, postmortem changes and the effect of formalin preservation. J Neurochem. 1965 Jul;12(7):629–638. doi: 10.1111/j.1471-4159.1965.tb04256.x. [DOI] [PubMed] [Google Scholar]
- Warren L., Fuhrer J. P., Buck C. A. Surface glycoproteins of cells before and after transformation by oncogenic viruses. Fed Proc. 1973 Jan;32(1):80–85. [PubMed] [Google Scholar]
- Weinstein D. B., Marsh J. B., Glick M. C., Warren L. Membranes of animal cells. VI. The glycolipids of the L cell and its surface membrane. J Biol Chem. 1970 Aug 10;245(15):3928–3937. [PubMed] [Google Scholar]
- Wiener F., Klein G., Harris H. The analysis of malignancy by cell fusion. IV. Hybrid between tumour cells and a malignant L cell derivative. J Cell Sci. 1973 Jan;12(1):253–261. doi: 10.1242/jcs.12.1.253. [DOI] [PubMed] [Google Scholar]
- Wiener F., Klein G., Harris H. The analysis of malignancy by cell fusion. V. Further evidence of the ability of normal diploid cells to suppress malignancy. J Cell Sci. 1974 Jun;15(1):177–183. doi: 10.1242/jcs.15.1.177. [DOI] [PubMed] [Google Scholar]
- Yogeeswaran G., Sheinin R., Wherrett J. R., Murray R. K. Studies on the glycosphingolipids of normal and virally transformed 3T3 mouse fibroblasts. J Biol Chem. 1972 Aug 25;247(16):5146–5148. [PubMed] [Google Scholar]
- Yu R. K., Ledeen R. W. Gangliosides of human, bovine, and rabbit plasma. J Lipid Res. 1972 Sep;13(5):680–686. [PubMed] [Google Scholar]