The Irish statesman and philosopher, Edmund Burke, aptly stated, ‘Those who don't know history are destined to repeat it.’ While this also applies to scientific research, it is common and often worthwhile to repeat published experiments in one's own laboratory. However, dismissing the earlier studies (history) in order to accentuate one's own findings does not lead to advancement in the field. Such is the case in a recent publication (Liu et al. 2013). The difference in the Seow and colleagues’ (Liu et al. 2013) conclusions and our data (Ashton et al. 1975) centres on differences in the length, variability in lengths, stability of myosin filaments and the architecture of the contractile apparatus in smooth muscle tissues. Why does this matter? Because these properties contribute to the fundamental contractile phenotype characteristic of smooth muscle (SM), such as the sliding filament basis of the length–tension curve and the ability of smooth muscles to develop equivalent amounts of force (2.2 × 106 dyn cm−2) compared with vertebrate striated muscle fibres in spite of the approximately fivefold lower concentration of myosin in SM.
In our earlier study (Ashton et al. 1975), the length of the thick filaments in rabbit portal mesenteric vein was measured in longitudinal sections (Fig. 1) using stereo views to establish that the filaments were completely within the plain of the section and that overlapping portions of two filaments did not introduce an error (see Plates II, III and IV in Ashton et al. 1975). To allow clear visualization of filaments, a section thickness of 0.16–0.18 μm was chosen because this thickness was expected to contain just two to three filaments at different levels based on the 60–80 nm thick filament lattice measured in this muscle (Rice et al. 1971). As discussed below, intermediate high voltage microscopy was used to allow precise visualization of myosin filaments in sections of this thickness. The length of such ‘complete’ filaments in longitudinal sections, not transverse sections as stated by Dr Seow, was 2.2 ± 0.14 μm (mean ± SD) (n = 20; mode 2.2–2.4 μm). Shorter filaments were incomplete as they were always seen to exit the plane of the section. These results were supported by serial reconstructions of myosin filaments from 0.47 μm-thick transverse sections, although precision of the measurements is less than in the longitudinal sections as the extent of the protrusion of the tapered filament ends into the ‘terminal’ sections could not be accurately measured. However, of the 255 filaments followed, they all were present in 4–6 sections, with the majority complete in 5 sections, consistent with the longitudinal filament length measurements.
Figure 1.
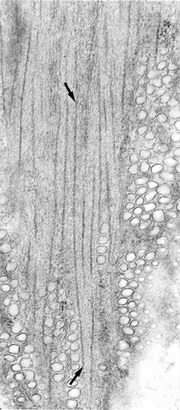
Myosin filaments in portal vein are 2.2 ± 0.14 μm long
Single view from a stereo pair of electron micrographs of 160 nm-thick longitudinal section cut near the surface of a smooth muscle cell in the rabbit portal mesenteric vein. A 2.3 μm-long myosin filament (ends marked by arrows) is completely included in the section when viewed in stereo. Magnification ×50,000.
Seow et al. addressed the question of myosin filament length using thinner 50–60 nm-thick transverse sections of sheep trachealis, sheep pulmonary artery and rabbit carotid artery smooth muscle cells. A distribution of filament lengths was measured in all cases, with 24% of filaments being 50 nm or less in length in the sheep trachea that increased to 38% upon stimulation with acetylcholine. In sheep pulmonary artery, 40% of filaments were 100 nm or less in length. Distribution of lengths fell rapidly, with the majority of filaments being <500 nm. The authors interpret their findings as being due to a dynamic equilibrium of linear myosin polymerization and depolymerization with myosin dimers as substrates for filament formation (Liu et al. 2013). Myosin monomers, dimers, tetramers and hexamers cannot be imaged in these sections. Lengths measured in longitudinal sections of trachealis were similar but as these are made on thin 50 nm-thick sections in the absence of stereo tilt views, it is highly unlikely that complete myosin filaments would be captured. This fundamental difference from our study is dismissed by Dr Seow and colleagues as reflecting the reduced resolution in our 0.47 μm-thick transverse sections suggesting that in our study we could not resolve gaps between longitudinally aligned short filaments. I would like to correct that misinterpretation. The whole point of using intermediate (200 kV) high voltage imaging is the greater penetration of electrons allowing the use of thicker sections than conventional electron microscopy with no loss of resolution. Thicker sections increase the sample depth through the smooth muscle cells. Furthermore, the goniometer stage on this instrument allowed tilting of the specimen to obtain stereo views, such that in one view the transverse filaments are seen in cross-section and the other tilted view shows the side of the filament revealing any gaps or discontinuities (see Plates VI and VII in Ashton et al. 1975). Thus, this argument does not account for the differences in the two studies. Interestingly, Dr Seow and colleagues have also reported the longer form of myosin (1.8–2.2 μm) in pig trachealis (Herrera et al. 2005). If so, one must conclude that in the trachealis differences in myosin filament lengths and distribution of lengths must occur between species. The special mechanical properties of tracheal smooth muscle has been attributed to plasticity of the filaments, as suggested by Dr Seow. If true, then one would expect to observe these differences in the sheep, but not pig, trachealis, a possible direction for new studies. As pointed out in our Discussion, ‘It should also be emphasized that the myosin filaments of vertebrate smooth muscle other than portal-mesenteric vein may have significantly different lengths from that found in this study.’ Indeed there are small variations in myosin filament length in some striated muscles (Franzini-Armstrong, 1970) but nothing within the extreme range of mini-filament lengths as reported by Seow et al.
The regulation of non-muscle and smooth muscle myosin monomeric and polymeric states has been studied extensively in vitro and depends on salt composition, ionic strength, ATP and phosphorylation of the regulatory myosin light chain (RLC20). Bipolar, side polar, short and long filaments can be formed and it has been difficult to translate these in vitro experiments to the in situ myosin filament structure. This is compounded by the well-known difficulties of achieving good fixation of smooth muscle myosin especially in tissues with a great deal of connective tissue such as arteries. On the other hand, polymerization and depolymerization of myosin filaments readily occurs and is necessary in cultured cells, cells undergoing mitosis, proliferation and migration such as during development, angiogenesis and tissue remodelling. Phosphorylation of RLC20 promotes filament assembly as originally shown in thymus cells (Scholey et al. 1980) and since reported in many non-muscle cells (Tan et al. 1992). Thus, in terms of smooth muscle it is important to discriminate between cultured cells and tissues. This is illustrated in a recent study demonstrating a large population of total myosin existing in the folded 10S monomeric state in cultured airway smooth muscle cells, using antibodies specific for the 10S form (Milton et al. 2011). In contrast, earlier studies found no evidence for any 10S molecules in smooth muscle cells in chicken gizzard tissue, in either the relaxed or contracted state, also using antibodies to probe for the 10S species (Horowitz et al. 1994). Altogether it would seem that myosin filament conformation in the sheep trachealis, sheep pulmonary artery and rabbit carotid artery smooth muscle cells, reported by Dr Seow, resemble non-muscle, proliferating or cultured smooth muscle cells.
Overall, the intent of this commentary is to clarify the erroneous interpretation of our data on myosin filament length in the rabbit portal mesenteric vein and to add a cautionary note concerning the complexity of determination of the smooth muscle myosin filament characteristics. While it is difficult to envision short filaments, including myosin dimers and multimers of eight molecules, positioning themselves in an orderly fashion between oppositely polarized long actin filaments in such a way that they can account for the well-established mechanical properties of smooth muscle, this model raised by Dr Seow and colleagues remains open for rigorous testing. No information is provided for the kinetics of myosin turnover in filaments. If the proposed increased turnover of myosin with acetylcholine stimulation resulting in longer filaments contributes to the acetylcholine-induced contraction of the sheep trachealis, the kinetics must be sufficiently rapid to account for the rapid rise in tension and stiffness (latencies of 500 ms) upon electrical stimulation of bovine trachea (Kamm & Stull, 1986).
Additional information
Competing interests
None declared.
References
- Ashton FT, Somlyo AV. Somlyo AP. The contractile apparatus of vascular smooth muscle: Intermediate high voltage stereo electron microscopy. J Mol Biol. 1975;98:17–24. doi: 10.1016/s0022-2836(75)80098-2. [DOI] [PubMed] [Google Scholar]
- Franzini-Armstrong C. Natural variability in the length of thin and thick filaments in single fibres from a crab, Portunus depurator. J Cell Sci. 1970;6:559–592. doi: 10.1242/jcs.6.2.559. [DOI] [PubMed] [Google Scholar]
- Herrera AM, McParland BE, Bienkowska A, Tait R, Pare PD. Seow CY. ‘Sarcomeres’ of smooth muscle: functional characteristics and ultrastructural evidence. J Cell Sci. 2005;118:2381–2392. doi: 10.1242/jcs.02368. [DOI] [PubMed] [Google Scholar]
- Horowitz A, Trybus KM, Bowman DS. Fay FS. Antibodies probe for folded monomeric myosin in relaxed and contracted smooth muscle. J Cell Biol. 1994;126:1195–1200. doi: 10.1083/jcb.126.5.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamm KE. Stull JT. Activation of smooth muscle contraction: relation between myosin phosphorylation and stiffness. Science. 1986;232:80–82. doi: 10.1126/science.3754063. [DOI] [PubMed] [Google Scholar]
- Liu JC, Rottler J, Wang L, Zhang J, Pascoe CD, Lan B, Norris BA, Herrera AM, Paré PD. Seow CY. Myosin filaments in smooth muscle cells do not have a constant length. J Physiol. 2013;591:5867–5878. doi: 10.1113/jphysiol.2013.264168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milton DL, Schneck AN, Ziech DA, Ba M, Facemyer KC, Halayko AJ, Baker JE, Gerthoffer WT. Cremo CR. Direct evidence for functional smooth muscle myosin II in the 10S self-inhibited monomeric conformation in airway smooth muscle cells. Proc Natl Acad Sci U S A. 2011;108:1421–1426. doi: 10.1073/pnas.1011784108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice RV, McManus GM, Devine OF. Somlyo AP. Regular organization of thick filaments in mammalian smooth muscle. Nat New Biol. 1971;231:242–243. doi: 10.1038/newbio231242a0. [DOI] [PubMed] [Google Scholar]
- Scholey JM, Taylor KA. Kendrick-Jones J. Regulation of non-muscle myosin assembly by calmodulin-dependent light chain kinase. Nature. 1980;287:233–235. doi: 10.1038/287233a0. [DOI] [PubMed] [Google Scholar]
- Tan JL, Ravid S. Spudich JA. Control of nonmuscle myosins by phosphorylation. Annu Rev Biochem. 1992;61:721–759. doi: 10.1146/annurev.bi.61.070192.003445. [DOI] [PubMed] [Google Scholar]


