Abstract
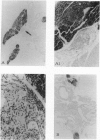
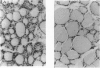
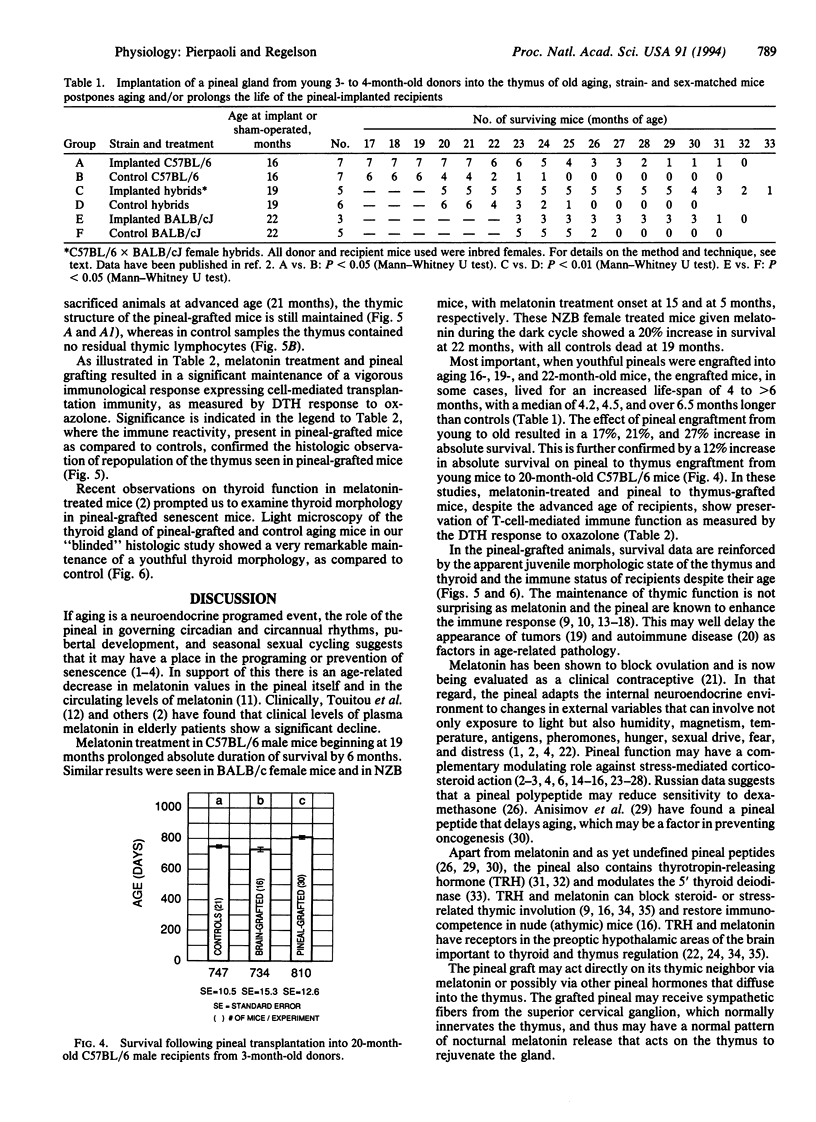
Dark-cycle, night administration of the pineal hormone melatonin in drinking water to aging mice (15 months of age) prolongs survival of BALB/c females from 23.8 to 28.1 months and preserves aspects of their youthful state. Similar results were seen in New Zealand Black females beginning at 5 months and C57BL/6 males beginning at 19 months. As melatonin is produced in circadian fashion from the pineal, we grafted pineals from young 3- to 4-month-old donors into the thymus of 20-month-old syngeneic C57BL/6 male recipients, and a 12% increase in survival was induced. Prolongation of survival was also seen on pineal transplant to the thymus in C57BL/6, BALB/cJ, and hybrid female mice at 16, 19, and 22 months. In all studies, the endogenous pineal of grafted mice was left in situ. Pineal grafted aged mice display a remarkable maintenance of thymic structure and cellularity. Preservation of T-cell-mediated function, despite age, as measured by response to oxazolone is seen. Other evidence suggests that melatonin and/or pineal-related factors could produce their effects through an influence on thyroid function. These data indicate that pineal influences have a place in the physiologic regulation of aging.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartsch H., Bartsch C., Simon W. E., Flehmig B., Ebels I., Lippert T. H. Antitumor activity of the pineal gland: effect of unidentified substances versus the effect of melatonin. Oncology. 1992;49(1):27–30. doi: 10.1159/000227005. [DOI] [PubMed] [Google Scholar]
- Demisch L., Demisch K., Nickelsen T. Influence of dexamethasone on nocturnal melatonin production in healthy adult subjects. J Pineal Res. 1988;5(3):317–322. doi: 10.1111/j.1600-079x.1988.tb00657.x. [DOI] [PubMed] [Google Scholar]
- Erlich S. S., Apuzzo M. L. The pineal gland: anatomy, physiology, and clinical significance. J Neurosurg. 1985 Sep;63(3):321–341. doi: 10.3171/jns.1985.63.3.0321. [DOI] [PubMed] [Google Scholar]
- Fraschini F., Scaglione F., Franco P., Demartini G., Lucini V., Stankov B. Melatonin and immunity. Acta Oncol. 1990;29(6):775–776. doi: 10.3109/02841869009092998. [DOI] [PubMed] [Google Scholar]
- Golikov P. P. Vliianie épifizektomii na sviazyvaiushchuiu sposobnost' transkortina u krys. Probl Endokrinol (Mosk) 1973 Jul-Aug;19(4):100–102. [PubMed] [Google Scholar]
- Guerrero J. M., Reiter R. J. Iodothyronine 5'-deiodinating activity in the pineal gland. Int J Biochem. 1992 Oct;24(10):1513–1523. doi: 10.1016/0020-711x(92)90169-2. [DOI] [PubMed] [Google Scholar]
- Hansson I., Holmdahl R., Mattsson R. Constant darkness enhances autoimmunity to type II collagen and exaggerates development of collagen-induced arthritis in DBA/1 mice. J Neuroimmunol. 1990 Apr;27(1):79–84. doi: 10.1016/0165-5728(90)90139-e. [DOI] [PubMed] [Google Scholar]
- Hastings M. H., Vance G., Maywood E. Some reflections on the phylogeny and function of the pineal. Experientia. 1989 Oct 15;45(10):903–909. [PubMed] [Google Scholar]
- Hoffmann K., Illnerová H., Vanecek J. Comparison of pineal melatonin rhythms in young adult and old Djungarian hamsters (Phodopus sungorus) under long and short photoperiods. Neurosci Lett. 1985 May 1;56(1):39–43. doi: 10.1016/0304-3940(85)90437-9. [DOI] [PubMed] [Google Scholar]
- Khan R., Daya S., Potgieter B. Evidence for a modulation of the stress response by the pineal gland. Experientia. 1990 Aug 15;46(8):860–862. doi: 10.1007/BF01935539. [DOI] [PubMed] [Google Scholar]
- Lew G. M. An immunocytochemical study of thyrotropin releasing hormone in the porcine, ovine and rodent pineal gland. Histochemistry. 1989;91(1):43–46. doi: 10.1007/BF00501909. [DOI] [PubMed] [Google Scholar]
- Maestroni G. J., Conti A., Pierpaoli W. Role of the pineal gland in immunity. Circadian synthesis and release of melatonin modulates the antibody response and antagonizes the immunosuppressive effect of corticosterone. J Neuroimmunol. 1986 Nov;13(1):19–30. doi: 10.1016/0165-5728(86)90047-0. [DOI] [PubMed] [Google Scholar]
- Pierpaoli W., Besedovsky H. O. Role of the thymus in programming of neuroendocrine functions. Clin Exp Immunol. 1975 May;20(2):323–338. [PMC free article] [PubMed] [Google Scholar]
- Pierpaoli W., Dall'Ara A., Pedrinis E., Regelson W. The pineal control of aging. The effects of melatonin and pineal grafting on the survival of older mice. Ann N Y Acad Sci. 1991;621:291–313. doi: 10.1111/j.1749-6632.1991.tb16987.x. [DOI] [PubMed] [Google Scholar]
- Pierpaoli W., Sorkin E. Alterations of adrenal cortex and thyroid in mice with congenital absence of the thymus. Nat New Biol. 1972 Aug 30;238(87):282–285. doi: 10.1038/newbio238282a0. [DOI] [PubMed] [Google Scholar]
- Pierpaoli W. The pineal gland: a circadian or seasonal aging clock? Aging (Milano) 1991 Jun;3(2):99–101. doi: 10.1007/BF03323985. [DOI] [PubMed] [Google Scholar]
- Pierpaoli W., Yi C. The involvement of pineal gland and melatonin in immunity and aging. I. Thymus-mediated, immunoreconstituting and antiviral activity of thyrotropin-releasing hormone. J Neuroimmunol. 1990 May;27(2-3):99–109. doi: 10.1016/0165-5728(90)90059-v. [DOI] [PubMed] [Google Scholar]
- Rebuffat P., Mazzocchi G., Stachowiak A., Belloni A. S., Coi A., Nussdorfer G. G. A morphometric study of the effects of melatonin on the rat adrenal zona glomerulosa. Exp Clin Endocrinol. 1988 Mar;91(1):59–64. doi: 10.1055/s-0029-1210722. [DOI] [PubMed] [Google Scholar]
- Regelson W., Pierpaoli W. Melatonin: a rediscovered antitumor hormone? Its relation to surface receptors; sex steroid metabolism, immunologic response, and chronobiologic factors in tumor growth and therapy. Cancer Invest. 1987;5(4):379–385. [PubMed] [Google Scholar]
- Sharma M., Palacios-Bois J., Schwartz G., Iskandar H., Thakur M., Quirion R., Nair N. P. Circadian rhythms of melatonin and cortisol in aging. Biol Psychiatry. 1989 Feb 1;25(3):305–319. doi: 10.1016/0006-3223(89)90178-9. [DOI] [PubMed] [Google Scholar]
- Thomas D. R., Miles A. Melatonin secretion and age. Biol Psychiatry. 1989 Feb 1;25(3):365–367. doi: 10.1016/0006-3223(89)90187-x. [DOI] [PubMed] [Google Scholar]
- Touitou Y., Fevre-Montange M., Proust J., Klinger E., Nakache J. P. Age- and sex-associated modification of plasma melatonin concentrations in man. Relationship to pathology, malignant or not, and autopsy findings. Acta Endocrinol (Copenh) 1985 Jan;108(1):135–144. doi: 10.1530/acta.0.1080135. [DOI] [PubMed] [Google Scholar]
- Trentini G. P., Genazzani A. R., Criscuolo M., Petraglia F., De Gaetani C., Ficarra G., Bidzinska B., Migaldi M., Genazzani A. D. Melatonin treatment delays reproductive aging of female rat via the opiatergic system. Neuroendocrinology. 1992 Sep;56(3):364–370. doi: 10.1159/000126250. [DOI] [PubMed] [Google Scholar]
- Uede T., Ishii Y., Matsuura A., Shimogawara I., Kikuchi K. Immunohistochemical study of lymphocytes in rat pineal gland: selective accumulation of T lymphocytes. Anat Rec. 1981 Feb;199(2):239–247. doi: 10.1002/ar.1091990208. [DOI] [PubMed] [Google Scholar]
- Voordouw B. C., Euser R., Verdonk R. E., Alberda B. T., de Jong F. H., Drogendijk A. C., Fauser B. C., Cohen M. Melatonin and melatonin-progestin combinations alter pituitary-ovarian function in women and can inhibit ovulation. J Clin Endocrinol Metab. 1992 Jan;74(1):108–117. doi: 10.1210/jcem.74.1.1727807. [DOI] [PubMed] [Google Scholar]
- Vriend J. Testing the TRH hypothesis of pineal function. Med Hypotheses. 1978 Jul-Aug;4(4):376–387. doi: 10.1016/0306-9877(78)90073-7. [DOI] [PubMed] [Google Scholar]
- Yuwiler A. Neonatal steroid treatment reduces catecholamine-induced increases in pineal serotonin N-acetyltransferase activity. J Neurochem. 1985 Apr;44(4):1185–1193. doi: 10.1111/j.1471-4159.1985.tb08742.x. [DOI] [PubMed] [Google Scholar]
- del Gobbo V., Libri V., Villani N., Caliò R., Nisticò G. Pinealectomy inhibits interleukin-2 production and natural killer activity in mice. Int J Immunopharmacol. 1989;11(5):567–573. doi: 10.1016/0192-0561(89)90187-2. [DOI] [PubMed] [Google Scholar]