Abstract
Personality traits may be viable candidates for mediators of the relationship between genetic risk and ADHD. Participants were 578 children (331 boys; 320 children with ADHD) between the ages of six and 18. Parents and teachers completed a comprehensive, multistage diagnostic procedure to assess ADHD and comorbid disorders. Mother completed the California Q-Sort to assess child Big Five personality traits. Children provided buccal samples of DNA which were assayed for selected markers on DRD4, DAT1, and ADRA2A. An additive genetic risk composite was associated with ADHD symptoms and maladaptive personality traits; maladaptive personality traits were associated with ADHD symptoms. Low conscientiousness and high neuroticism partially mediated the relationship between genetic risk and ADHD symptoms. Mediation effects for conscientiousness were specific to inattentive symptoms; effects for neuroticism generalized to all disruptive behaviors. High neuroticism and low conscientiousness may be useful as early markers for children at risk for ADHD.
Keywords: ADHD, Genetics, Personality, Disruptive behaviors
Research on ADHD increasingly focuses on etiological causes of the disorder and associated mechanisms that can be used to better identify the disorder or types of the disorder. ADHD is associated with volumetric reductions in the prefrontal cortices, basal ganglia, cerebellum, and corpus callosum (Durston 2003; Hutchinson et al. 2008; Valera et al. 2007). These structural findings are amplified by consistent functional hypoactivation upon task challenge in dorsolateral and inferior prefrontal cortices, anterior cingulate, and basal ganglia as well as other regions (Bush et al. 2005; Rubia et al. 2009). These regions share some similarities, notably the fact that their activity is heavily modulated by catecholamine activity including dopamine and norepinephrine. Indeed, ADHD has been associated with low extracellular levels of dopamine (Volkow et al. 2005). ADHD is highly heritable, with a heritability estimate of approximately 80% (Faraone et al. 2005; Waldman and Gizer 2006). Although no single gene of major effect has been found, multiple genetic markers of the dopaminergic and adrenergic neurotransmission systems have been associated with ADHD (Gizer et al. 2008).
ADHD comprises two symptom domains (i.e., inattention-disorganization and hyperactivity-impulsivity). These have distinct predictive correlates and so it is possible they also have partially distinct genetic correlates. Indeed, markers in the dopamine D4 receptor gene (DRD4) and the dopamine transporter gene (DAT1) have shown association with both inattentive and hyperactive-impulsive ADHD symptoms (Faraone et al. 2005). In contrast, noradrenergic genetic markers, including those within the adrenergic receptor-2A gene (ADRA2A) have demonstrated specific association with inattentive symptoms only (Park et al. 2005). Importantly, the complexity of ADHD (and its potential genetic associations) extends further than its constituent symptom domains. High comorbidity rates with other disorders, particularly Oppositional-Defiant Disorder (ODD), may complicate the pattern of genetic association, as ODD co-occurs with ADHD approximately half of the time (Jensen et al. 1997).
Due to the extensive literature on determinants of personality, personality traits have been advanced as potential mechanisms that may shed light on the etiology of ADHD, perhaps by being closer to its neurobiological and genotypic underpinnings. Although many temperament and personality models are available for children and adolescents, among the most widely-studied is the Five Factor Model (McCrae and Costa 1987). Long-used in studies of adults, the Five Factors with minor modification are also widely studied in children. In its standard form, the Five Factor Model’s major traits are Neuroticism (i.e., tendency to anxiety, depression, and other negative emotions, as well as difficulty coping with stress), Extraversion (i.e., interpersonal activity directed outward), Openness (i.e., active appreciation of life experiences), Agreeableness (i.e., altruism, trust, compliance, and concern, related to affiliation), and Conscientiousness (i.e., goal-directed behavior, organization, and impulse control). It should be noted that Openness has been less consistently identified than the other four factors, particularly in children (Shiner and Caspi 2003).
Several of the Big Five traits are correlated with ADHD. Compared with typically developing individuals, those with ADHD have been characterized by lower levels of conscientiousness and agreeableness and higher levels of neuroticism (Miller et al. 2008; Nigg et al. 2002). In addition, some studies have found a relationship between ADHD and higher levels of extraversion (Parker et al. 2004), but this has been inconsistent (Nigg et al. 2002). More specific relationships have been found between effortful forms of control like conscientiousness and ADHD inattention and between reactive forms of control like neuroticism and ADHD hyperactivity-impulsivity (Martel and Nigg 2006).
In addition to sharing phenotypic overlap with ADHD (Nigg et al. 2002), personality traits share some of the same neurobiological correlates as those seen in ADHD (Nigg 2006). Control processes, alternatively conceptualized as constraint, effortful control, conscientiousness, or cognitive control (Nigg 2000), rely heavily on the prefrontal cortex, especially the dorsolateral, orbitofrontal, and anterior cingulate cortex (Derryberry and Tucker 2006; Rothbart and Posner 2006) and personality traits related to incentive-based and effortful control are associated with variations in neural structure and neural activation patterns similar to those in ADHD (Gardini et al. 2009; Simon et al. 2009). The neurotransmitters acetylcholine, norephinephrine, dopamine, and serotonin are associated with integrity in control processes (Depue and Lenzenweger 2006; Rothbart and Posner 2006). Negative emotionality, such as fear and negative affect, appears to rely on a neural circuit involving the amygdala, hippocampus, anterior cingulate cortex, and right prefrontal cortex with particular reliance on serotonin neurotransmission (Derryberry and Tucker 2006; Whittle et al. 2006). In contrast, positive emotionality, such as extraversion and positive affect, relies on the amygdala, the nucleus accumbens, the anterior cingulate cortex, and the left prefrontal cortex with particular reliance on dopaminergic neurotransmission (Depue and Lenzenweger 2006). Personality traits also exhibit moderate heritability on the order of approximately 50% which tends to increase somewhat with age (Yamagata et al. 2005). In sum, there appears to be good emerging evidence that personality dimensions (1) involve similar neural circuitry systems as those that underlie measures of psychopathology and (2) evidence moderate heritability.
Thus, one possible explanation for associations between traits and ADHD is common neurobiological and genetic underpinnings. ADHD and key personality traits may share some genetic risk which is manifested in subtle alternations in neurotransmission and subsequently manifested in behavioral tendencies. Based on what is known about the genetic and neurobiological correlates of key personality traits and ADHD, genes which affect dopaminergic neurotransmission in the prefrontal cortex may be a key mechanism that can explain why common genes underlie both personality, particularly conscientiousness, and ADHD symptoms.
In line with this hypothesis, several genes important for dopaminergic neurotransmission have been implicated in both ADHD and personality traits. For example, the DRD4 seven-repeat allele has been associated with high neuroticism and low conscientiousness, as well as ADHD symptoms (Dragan and Oniszczenko 2007). A series of studies by Auerbach and colleagues suggest similar relations. Twelve-month-old infants with the long allele of DRD4 (6–8 repeats) exhibited less sustained attention and less interest during activities (Auerbach et al. 2001). Further, the DRD4 seven-repeat allele appears to be related to temperament traits such as high sensation-seeking (Sheese et al. 2007). Minor allelic variants of the dopamine D2 receptor gene (DRD2) also appear associated with related personality traits (Nyman et al. 2009). Further, initial work in behavioral genetics has suggested a near perfect genetic correlation between ADHD and effortful control in preschoolers (Goldsmith et al. 2004), indicating that nearly all the genetic factors that contribute to ADHD also contribute to effortful control.
The current study explored whether personality traits and ADHD might share common genetic risk, conceptualized as a mediation model in which personality traits mediated genetic effects on ADHD. A candidate gene approach was utilized in order to evaluate the contributions of specific functional genetic risk alleles that have already shown associations with personality traits and ADHD in prior genetic studies, including those taking a genome-wide and dense marker array approach. Genes related to dopaminergic and adrenergic neurotransmission were the focus in the current study, based on their prior association with ADHD, personality traits, and relevant neurobiological structures. The main study hypothesis was that personality traits would mediate the relationship between genetic risk and ADHD via associations with dopaminergic and adrenergic neurotransmission, which are posited to be essential for functioning of neural circuits that include the prefrontal cortex and basal ganglia areas often implicated in ADHD. Specifically, it was predicted that low conscientiousness would mediate the relationship between genetic risk alleles and ADHD inattentive symptoms. To this end, (1) association between personality traits and ADHD, (2) association between genetic risk and ADHD, (3) association between genetic risk and personality traits, and (4) mediation of genetic risk and ADHD association by personality traits were examined sequentially with attention to commonly comorbid disruptive behavior problems and important covariates such as child sex, age, and ethnicity.
Method
Participants
Overview
Participants were 578 children (331 boys) between the ages of six and 18 years. Children were initially included in one of two groups: those diagnosed with ADHD (n=320) and non-ADHD comparison youth (n=208). Fifty additional children who were classified as having situational or sub-threshold ADHD (did not meet criteria for either ADHD or non-ADHD comparison group as explained below), were included to provide more complete coverage of the dimensional trait space of both personality and ADHD (Levy et al. 1997). Using a DSM-IV perspective, the ADHD group included 116 ADHD-Predominantly Inattentive type (ADHD-PI; i.e., met criteria for six or more inattentive symptoms, plus impairment, onset, and duration, and never in the past met criteria for combined type) and 204 ADHD-Combined type (ADHD-C; i.e., met criteria for six or more inattentive symptoms and six or more hyperactive-impulsive symptoms, plus impairment, onset, and duration). The current sample included no children with the hyperactive-impulsive ADHD subtype, similar to other clinical samples of children with ADHD (e.g., Shaw et al. 2007). In the total sample, 191 children met DSM-IV criteria for Oppositional-Defiant Disorder (ODD), and 46 were diagnosed with Conduct Disorder (CD); of these, 80% of children with ODD and 89% of those with CD were also diagnosed with ADHD (see Table 1). Children came from 497 families; 416 families had one child in the study, and 81 families had two children in the study. All parents and children completed informed consent, in conformity with local IRB, NIH, and APA ethical guidelines. Descriptive statistics for the entire sample are presented in Table 1. It should be noted that children with subthreshold ADHD were not included in ADHD versus control between-group comparisons, but were included in dimensional symptom count analyses.
Table 1.
Descriptive Statistics on Sample
| M(SD) | ADHD n=320 |
Control n=208 |
Total N=578a |
|---|---|---|---|
| Boys n(%) | 211(65.9) | 100(48.1) | 331(57.3)** |
| Girls n(%) | 109(34.1) | 108(51.9) | 247(42.7) |
| Ethnic minority n(%) | 84(26.3) | 55(26.4) | 155(26.8) |
| Alaska native | 1(0.3) | 0(0) | 1(0.2) |
| American Indian | 3(0.9) | 3(1.4) | 6(1) |
| Asian | 1(0.3) | 2(1) | 3(0.5) |
| Black | 29(9.1) | 24(11.5) | 59(10) |
| Latino | 17(5.3) | 11(5.3) | 31(5.3) |
| Middle Eastern | 1(0.3) | 0(0) | 1(0.2) |
| Pacific Islander | 0(0) | 1(0.5) | 1(0.2) |
| White | 233(72.8) | 152(73.1) | 418(71.5) |
| Other | 17(5.3) | 5(2.4) | 26(4.4) |
| Mixed | 15(4.7) | 9(4.3) | 27(4.6) |
| Age | 11.31(2.95) | 12.5(3.25) | 11.67(3.07)** |
| IQ | 103.83(13.98) | 110.81(14.88) | 106.14(14.73)** |
| Family income | 62250.32(66785.37) | 74890.45(51032.16) | 66302(59617.18)* |
| ADHD-C n(%) | 204(63.8) | – | 204 (35.3)** |
| ADHD-PI n(%) | 116(36.3) | – | 116(20.1)** |
| ODD n(%) | 153(47.8) | 18(8.7) | 191(33)** |
| CD n(%) | 41(12.8) | 3(1.4) | 46(8)** |
| Neuroticism | 4.62(1.18) | 3.74(1.05) | 4.29(1.22)** |
| Extraversion | 5.97(1.44) | 5.47(1.32) | 5.75(1.41)** |
| Openness | 6.04(1.21) | 5.94(1.06) | 5.97(1.18) |
| Agreeableness | 5.85(1.31) | 6.74(1.03) | 6.19(1.29)** |
| Conscientiousness | 3.99(1.26) | 6.41(1.16) | 4.94(1.69)** |
| DRD4 risk allele n(%) | 206(64.4) | 124(59.6) | 362(62.6) |
| DAT1 risk allele n(%) | 167(52.2) | 106(51) | 290(50.2) |
| ADRA2A risk allele n(%) | 112(35) | 54(26) | 182(31.5)* |
| Composite risk n(%) | 171(53) | 85(41) | 276(47) |
| Inattentive symptoms | 7.83(2.21) | 2.96(3.11) | 5.87(3.51)** |
| Hyperactive symptoms | 5.79(3.29) | 2.11(2.64) | 4.28(3.52)** |
ADHD-C ADHD combined subtype, ADHD-PI ADHD, predominantly inattentive subtype, ODD oppositional-defiant disorder, CD conduct disorder
Fifty children were identified as having situational ADHD or were screened out of the study at a later point in time, but were included in study analyses because they had data on traits and genetics
p<0.05.
p<0.01, via t-tests or chi-squares
Recruitment and Identification
A broad community-based recruitment strategy was used, with mass mailings to parents in local school districts, public advertisements, and fliers at local clinics, to mimic the recruitment strategy of the MTA study (Arnold et al. 1997). Families initially recruited then passed through a standard multi-gate screening process to establish diagnostic groupings. At Stage 1, all families were screened by phone to rule out youth prescribed long-acting psychotropic medication (e.g. antidepressants), neurological impairments, seizure history, head injury with loss of consciousness, other major medical conditions, or a prior diagnosis of mental retardation or autistic disorder, as reported by the parent.
At Stage 2, parents and teachers of remaining eligible youth completed the following standardized rating scales: Child Behavior Checklist/Teacher Report Form (CBCL/TRF; Achenbach 1991), Conners Rating Scales-Revised, (Conners 1997), and the ADHD Rating Scale (ADHD-RS; DuPaul et al. 1998). In addition, parents completed a structured clinical interview to ascertain symptom presence, duration, and impairment. Parents and teachers were instructed to rate children’s behavior off medication. At this visit, children completed IQ and achievement testing. A four-subtest short form of the WISC-III (Wechsler 1991; data collection was begun prior to publication of the WISC-IV) was administered; estimated full scale IQ over 75 was required for inclusion. Families were screened out here if they failed to attend the diagnostic visit.
The choice of diagnostic interview depended on the year of data collection due to monetary constraints related to grant funding and resulting limitations in number and type of study staff available to conduct diagnostic interviews in earlier years of data collection. For participants recruited between 1997 and 2001 (N=218), the Diagnostic Interview Schedule for Children (DISC-IV; Shaffer et al. 2000) was completed with the parent by telephone or during on-campus visits. A trained interviewer (a graduate student or advanced undergraduate with at least 10 h of training) administered the DISC-IV. Fidelity to interview procedure was checked by having the interview recorded with 5% reviewed by a certified trainer. Children who met duration, onset, and impairment criteria, as well as exhibited six or more symptoms within one or both ADHD symptom domains, were diagnosed with ADHD. Teacher-reported symptoms on the ADHD Rating Scale (i.e., items rated as a “2” or “3” on the 0 to 3 scale) could be added to the parent-endorsed symptom total, up to a maximum of three additional symptoms, to obtain the total number of symptoms, in line with the recommendations from the DSM-IV field trials regarding the implementation of the “or” algorithm (Lahey et al. 1994). Children failing to meet cut-offs for all parent and teacher ADHD rating scales at the 80th percentile and having four or fewer symptoms of ADHD with the “or” algorithm were considered non-ADHD comparison children.
For participants recruited between 2002 and 2008, youth and their primary caregiver completed the Kiddie Schedule for Affective Disorders and Schizophrenia (KSADS-E; Puig-Antich and Ryan 1986). The data from the interviews and parent and teacher rating scales were then presented to a clinical diagnostic team consisting of a board certified child psychiatrist and licensed clinical child psychologist. They were allowed to use the same “or” algorithm in their diagnostic decision making. Their agreement rates were acceptable for ADHD diagnosis, subtypes, and current ODD and CD (all kappas ≥ 0.89). Pooling the data across families that received the KSADS and the DISC was justified based on our analysis of agreement between the two methods in 430 youth for whom a parent completed both a KSAD and a DISC-IV. The two interviews agreed adequately for total number of symptoms (inattention, ICC = 0.88; hyperactivity, ICC = 0.86), presence of six or more symptoms of ADHD (kappa = 0.79), presence of impairment (kappa = 0.64), and presence of ADHD (defined as six or more symptoms + cross situational impairment in each interview for purposes of computing agreement; kappa = 0.79).
Comorbid Child Diagnoses
The structured diagnostic interview was used for establishing the presence of ODD, CD, anxiety disorders, and depressive disorders based on DSM-IV criteria.
ADHD Symptoms
Three different indices of ADHD symptoms were evaluated. An “or” algorithm of parent- and teacher-rated ADHD symptoms was examined (described above). In addition, parent and teacher ratings were examined separately via ratings on the ADHD Rating Scale (DuPaul et al. 1998).
Measures
All parents and youth attended a second laboratory visit a few weeks later during which time parents completed Q-sort personality ratings and children completed neuropsychological testing not included in this report.
Personality Traits
Parents completed the California Child Q-Sort (CCQ), specifically the common language version (Caspi et al. 1992). The CCQ is a typical Q-Sort consisting of 100 cards which must be placed in a forced-choice, nine-category, rectangular distribution. The rater (in this case, the mother) describes the child by placing descriptive cards in one of the categories, ranging from one (least descriptive) to nine (most descriptive). Instructions were derived from the standard instruction set provided by Jack Block (personal communication to J. Nigg 1996). To measure the Big Five Factors, scales developed by John et al. (1994) were used. A composite score was generated by reverse-scoring selected items and computing the average. Scale reliabilities were all above 0.70 with the exception of the scale for openness (α=0.56). Intercorrelations between the Big Five Factors ranged from −0.04 (p>0.05) to 0.45 (p<0.01).
Since item overlap between personality and symptom scales could affect results, potential overlap among these measures was evaluated by independent judges based on conceptual similarity of items (see Martel and Nigg 2006 for more information). Their agreement was 95%. In order to avoid the potential confound that could result from inclusion of overlapping items (i.e., artificially inflated correlations between symptoms and personality traits), these items were removed from the personality trait scales; two items were removed from the extraversion scale, and three items were removed from the conscientiousness scale. Reliability for these scales remained acceptable (α=0.74 for extraversion and 0.77 for conscientiousness).
Catecholamine Genetic Markers
Buccal and salivary DNA samples were obtained from participating children. Markers in three genes (DRD4, DAT1, and ADRA2A) were chosen for genotyping based on prior findings in the literature (e.g., Faraone et al. 2005). Specifically, these included a 120 bp promoter Insertion/Deletion polymorphism of DRD4 (McCracken et al. 2000), variable number of tandem repeat polymorphism in the 3′ untranslated region of DAT1 (Vandenbergh et al. 1992), and rs553668 a DraI polymorphism (C>T) in ADRA2A (Park et al. 2005). DNA samples were purified using a method by Meulenbelt et al. (1995). Genomic DNA (40 to 60 ng) was amplified using 0.5U of Taq polymerase (Invitrogen Corp., Carlsbad, CA) in standard PCR buffer consisting of 20 mM Tris HCl and 50 mM KCl, 1.5 mM MgCl2, 0.2 mM dNTPs. Reaction conditions and specific primers were as previously described except the DAT1 which was assayed as follows: PCR primers (forward 5′-CCTTGAAACCAGCTCAG-3′ and reverse 5′-TATTGATGTGGCACGCACCT-3′) were used under amplification conditions described by Vandenbergh et al. (1992) with the addition of a 1:5 dilution of Q solution (Qiagen Inc., Valencia, CA) due to the high GC content of the amplicon. The 10 repeat allele is 581 bp while the 9 repeat allele is 541 bp.
The following genotypes for DRD4, DAT1, and ADRA2A were associated with ADHD diagnostic status in our sample and were therefore labeled “risk”: DRD4 insertion/insertion, DAT1 10/10-repeat genotype, and genotypes involving the T allele of the ADRA2A DraI polymorphism (i.e., C/T and T/T genotypes), respectively based on prior case-control association work in a portion of this sample (Park et al. 2005) and in the literature (see Faraone et al. 2005; Kustanovich et al. 2004). Based on these results, a binary composite catecholamine risk score was generated as an index of additive genetic risk (see Nigg et al. 2007). The composite was created by summing the number of “risk” genotypes across DRD4, DAT1, and ADRA2A; those with two or three risk alleles were coded as high risk; those with 0 or 1 were coded as low risk. The generation of an additive composite genetic risk score was considered justifiable based on the evidence of at least partial influences from additive genetic risk factors on ADHD which would not be captured as well with single genetic markers. In addition, the genetic composite was viewed as increasing power to detect relatively small effects.
Data Analysis
Data analysis was conducted using the Mplus software package (Muthen and Muthen 1998–2008). The Mplus software package allows for the statistical control of non-normality and outliers through the use of robust maximum likelihood estimation (Curran et al. 1996). Missingness was minimal in the current study; 5% of clinical data were missing. Therefore, full information likelihood estimation (i.e., FIML or direct fitting), a method of directly fitting models to raw data without imputing values (McCartney et al. 2006), was utilized to address this missingness. The presence of siblings and the resulting non-independence of data points were addressed using the clustering feature of Mplus. This clustering feature takes into account the non-independence of the data when computing test statistics and significance tests for all models reported.
Group differences in descriptive statistics were examined using t-tests and chi-square statistics. Bivariate correlations and multivariate regressions were conducted in order to assess associations between variables. Lastly, mediation analyses were conducting using the Sobel test with delta method standard errors since this method allowed for correction for clustered data, whereas bootstrapped standard errors could not be computed while also accounting for clustered data.
Results
As shown in Table 1, children with ADHD were younger (t[520]=4.63, p<0.01) and more likely to be male than non-ADHD comparison children (Χ2[1]=16.99, p<0.01). Therefore, secondary checks (described below) were conducted in which sex and age were covaried. Children with ADHD exhibited significantly higher neuroticism and extraversion, lower agreeableness and conscientiousness, and increased risk allele frequency for the catecholaminergic genetic risk composite (MANOVA F[6,495]=71.68, p<0.01).
All available children were used in subsequent analyses (N=578). Bivariate correlations among genetic risk, personality traits, and ADHD symptoms are shown in Table 2. Higher neuroticism, higher extraversion, lower agreeableness, and lower conscientiousness were significantly related to increased inattentive and hyperactive-impulsive ADHD symptoms (all p<0.01). Increased catecholaminergic genetic risk was related to more inattentive and hyperactive-impulsive ADHD symptoms (p<0.05) with ADRA2A appearing to carry most of this effect.
Table 2.
Bivariate Correlations Among Genetic Risk, Personality Traits, and Parent- and Teacher-Rated “or” Algorithm ADHD Symptoms
| Inattentive symptoms |
Hyperactive-impulsive symptoms |
|
|---|---|---|
| DRD4 risk | 0.02 | 0.05 |
| DAT1 risk | −0.02 | 0.01 |
| ADRA2A risk | 0.10* | 0.09* |
| Composite risk | 0.09* | 0.10* |
| Neuroticism | 0.35** | 0.22** |
| Extraversion | 0.14** | 0.34** |
| Openness | 0.03 | 0.01 |
| Agreeableness | −0.35** | −0.44** |
| Conscientiousness | −0.71** | −0.58** |
p<0.05.
p<0.01
Question 1: Are Personality Traits Associated with ADHD Symptoms?
In order to evaluate the relationship between personality traits and ADHD symptoms, two multivariate analyses of variance (MANOVA) were conducted. The Big Five personality traits significantly predicated inattentive (F[5,499]= 16.80, p<0.01) and hyperactive-impulsive (F[5,499]=13.42, p<0.01) ADHD symptoms. Next, inattentive and hyperactive ADHD symptoms were individually regressed on all Big Five personality traits in two separate multivariate regression analyses, shown in Table 3. Decreased conscientiousness (β=−0.64) and increased neuroticism (β=0.12) and openness (β=0.07) significantly predicted inattentive ADHD symptoms (all p<0.05). High extraversion was marginally related to inattentive symptoms (β=0.05; p=0.09). Increased extraversion (β=0.27) and decreased agreeableness (β=−0.24) and conscientiousness (β=−0.41) significantly predicted hyperactive-impulsive ADHD symptoms (all p<0.01). Higher neuroticism was marginally related to hyperactive-impulsive ADHD symptoms (β=0.07; p=0.06). Results held even after the elimination of item overlap between ADHD symptoms and extraversion and conscientiousness.
Table 3.
Inattentive and Hyperactive-Impulsive ADHD Symptom Regression on Big Five Personality Traits
| Inattention | Hyperactivity-impulsivity | |
|---|---|---|
| Neuroticism | 0.12** | 0.07+ |
| Extraversion | 0.05+ | 0.27** |
| Openness | 0.07* | 0.03 |
| Agreeableness | −0.04 | −0.24** |
| Conscientiousness | −0.64** | −0.41** |
p<0.05.
p<0.01.
p<0.10
Question 2: Are Genes Associated with ADHD Diagnostic Status and ADHD Symptoms?
The binary composite catecholamine risk score was significantly associated with ADHD diagnostic status (χ2=8.74, p<0.01). To further evaluate this association, chi-square analyses were conducted to assess relations between specific genetic risk alleles and ADHD diagnostic status and a MANOVA was conducted to assess multivariate associations between specific risk genotypes and ADHD symptoms. As shown in Table 1, although DRD4 and DAT1 were not significantly associated with ADHD diagnostic status (p>0.05), ADRA2A was significantly associated with ADHD diagnostic status (p<0.05).
The multivariate association between the binary catecholamine genetic risk composite and ADHD inattention and hyperactivity-impulsivity was significant (F[2,540]= 3.05, p<0.05). Catecholamine genetic risk was related to both inattention (F[1]=4.51, p<0.05; η2=0.01) and hyperactivity/ impulsivity (F[1]=5.84, p<0.05; η2=0.01), such that those with two or three risk genotypes exhibited significantly higher ADHD symptoms. Yet multivariate associations between ADHD inattentive and hyperactive-impulsive symptom domains (entered together as dependent variables) and DRD4, DAT1, and ADRA2A were not significant (F[2,509]=0.57, p>0.05; F[2,509]=0.17, p> 0.05; F[2,250]=0.14, p>0.05 respectively), although the between-subjects test for ADRA2A was marginal and approached significance (F[1]=3.69, p<0.06). Thus, the catecholaminergic genetic risk composite appears to capture something more than the single risk alleles.
Question 3: Are Genes Associated with Personality Traits?
Overall catecholamine risk was associated with personality traits (F[5,542]=2.74, p<0.05). Higher catecholaminergic risk was associated with higher neuroticism (F[1]=4.74, p<0.05; η2=0.01) and lower conscientiousness (F[1]=9.77, p<0.01; η2=0.02). Multivariate associations between personality traits (entered together as dependent variables) and the individual genetic risk alleles of DRD4, DAT1, and ADRA2A (entered separately) were not significant, although associations between DAT1 and personality traits approached significance (F[5,511]=2.2, p<0.06).
Question 4: Do Personality Traits Mediate Genetic Effects on ADHD Symptoms?
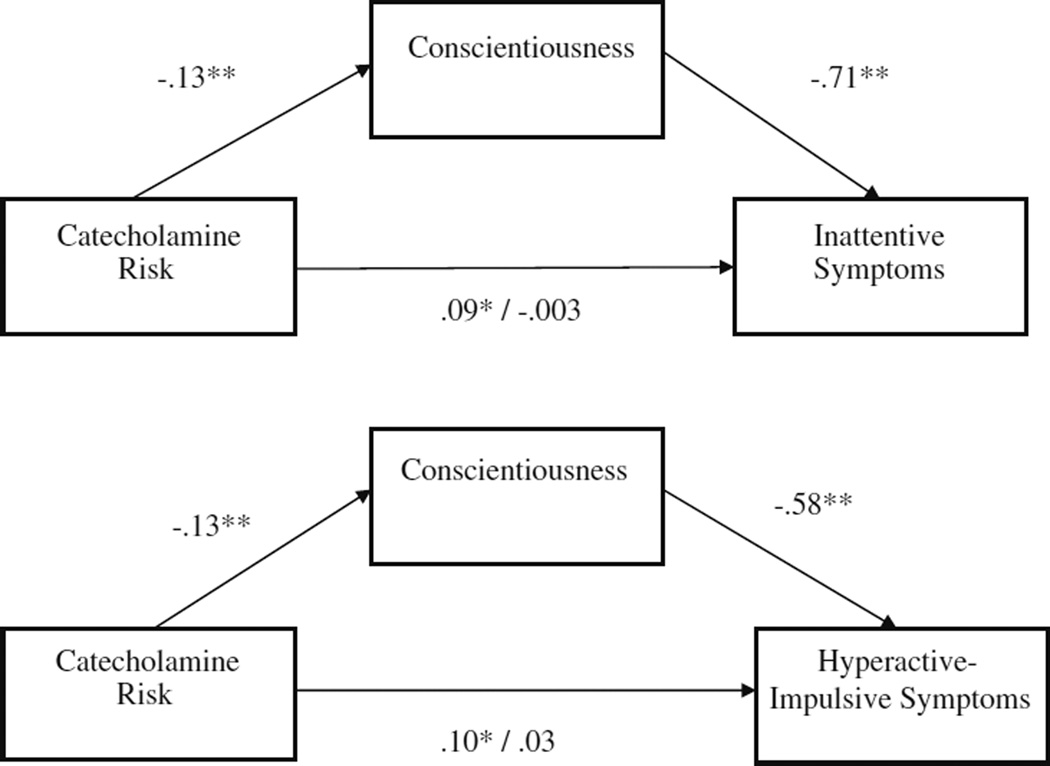
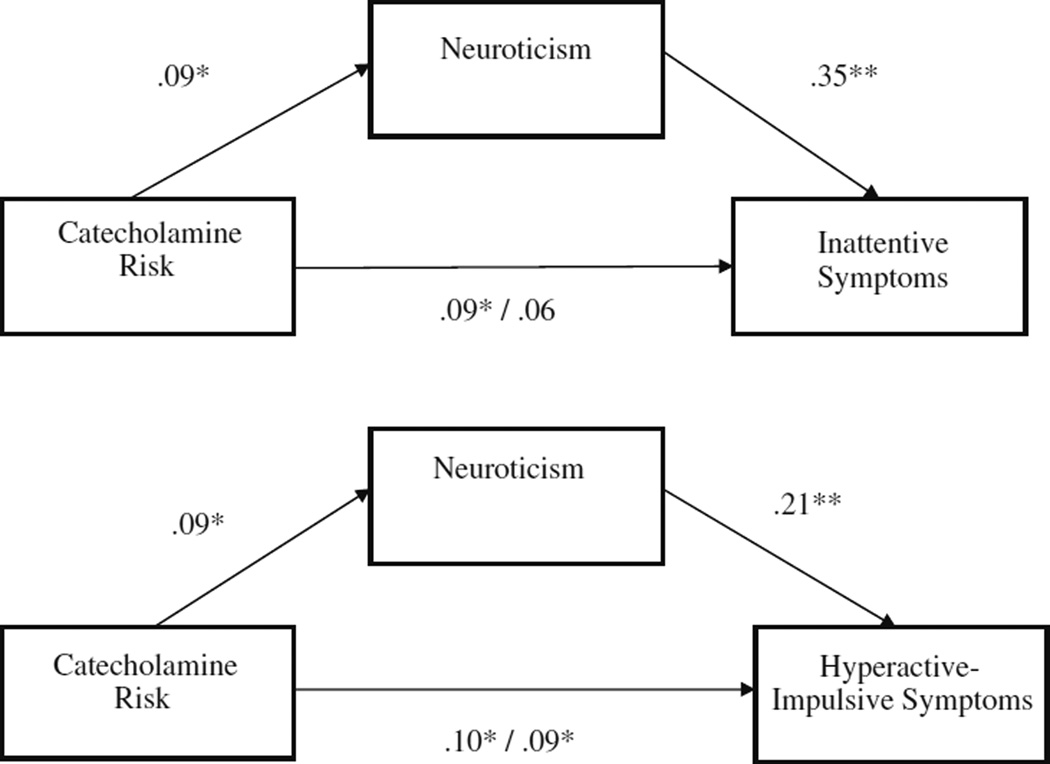
Based on the prior analyses, there was preliminary support to test the proposed mediation effects between the catecholaminergic genetic risk composite, the personality traits of conscientiousness and neuroticism, and ADHD. As shown in Fig. 1, conscientiousness significantly partially mediated the association between catecholamine genetic risk and ADHD symptoms (indirect effect = 0.09, 0.08; p<0.01 for inattentive and hyperactive-impulsive symptoms respectively). As shown in Fig. 2, neuroticism also significantly partially mediated the association between catecholamine genetic risk and ADHD symptoms (indirect effect = 0.03, 0.02; p<0.05 for inattentive and hyperactive-impulsive symptoms respectively). No other personality variables mediated relations between catecholamine genetic risk and ADHD symptoms (all p>0.05).
Fig. 1.
Conscientiousness mediates association between catecholamine risk and parent- and teacher-rated “or” algorithm ADHD
Fig. 2.
Neuroticism mediates association between catecholamine risk and parent- and teacher-rated “or” algorithm ADHD symptoms
These mediation effects might be influenced by child characteristics, differences between parent and teacher report of symptoms, or item overlap between personality and symptoms. Therefore, mediation analyses were checked controlling for these possible confounds. All mediation effects remained significant after controlling for child sex, age, and ethnicity. In order to test for possible effects of population stratification based on child ethnicity, possible group (i.e., ethnic) differences in the distribution of genotypes and in levels of ADHD symptoms were evaluated, per Hutchison et al. (2004). The genetic risk composite used in the current study was not significantly related to ethnicity (Χ2[9]=6.32, p>0.05), and total ADHD symptoms were also not significantly related to ethnicity (F[9,553]=0.86, p>0.05). Therefore, population stratification seems unlikely. Mediation effects were also significant whether ADHD symptoms were measured using maternal or teacher report (all indirect effects p<0.05). Mediation effects remained significant when controlling for item overlap between personality traits and ADHD symptoms (p<0.01).
In order to examine possible specificity of mediation effects on ADHD and disruptive behavior symptom domains, controlling for the covariance between symptom domains, mediation analyses were conducted controlling for these other commonly comorbid symptom domains. Mediation effects for conscientiousness appeared most specific to inattentive ADHD symptoms since this effect remained significant controlling for hyperactive-impulsive and ODD symptoms (p<0.05), but effects of conscientiousness on hyperactive-impulsive ADHD symptoms did not survive correction for the other disruptive behavior symptom domains (p>0.05). Mediation effects for neuroticism appeared specific to both inattention and oppositional-defiance since these effects survived correction for the other symptom domains (p<0.05), but the effects of neuroticism on hyperactivity-impulsivity did not (p>0.05).
Discussion
ADHD is a highly heritable disorder (Faraone et al. 2005; Waldman and Gizer 2006), but the psychological mechanisms via which genetic variations influence ADHD are not well-understood. The current study explored one approach to elucidating psychological mechanisms of genetic risk for ADHD: personality traits. The personality traits of conscientiousness and neuroticism partially mediated the relationship between an additive catecholamine genetic risk composite score (i.e., risk genotypes of DRD4, DAT1, and ADRA2A) and inattentive and hyperactive-impulsive ADHD symptoms, whether rated by parents or by teachers. However, when controlling for the covariance between inattention, hyperactivity, and oppositional-defiance, the mediation effect involving conscientiousness appeared most specific to ADHD inattention, while the mediation effect involving neuroticism appeared more generally related to disruptive behavior problems, particularly inattention and oppositional-defiance.
Given prior work demonstrating low to moderate correlations across informant for ADHD symptoms in addition to prior false positives in genetic research, the consistency of the results of the current study across informant was striking. Even though teacher and parent symptom scores are only moderately correlated, both showed an association with the catecholamine composite score and both showed mediation in relation to conscientiousness and neuroticism. This indicates that effects were not attributable to shared rater variance (i.e., parent ratings of both ADHD and personality). It also suggests there is a common liability for ADHD detected in both environments that is related to this genetic risk measure via these personality mechanisms.
Since personality traits are associated with some of the same genetic risks that have been found to underlie ADHD, including the 7-repeat form of the DRD4 III exon VNTR polymorphism (Ekelund and Lichtermann 1999; Keltikangas-Jarvinen et al. 2003; Lakatos et al. 2003) and have shown well-established associations with psychopathology (Watson et al. 2006), including ADHD, they provide a potential phenotype by which to better understand and grapple with the nature of the association between genetic risk and ADHD. To this end, study results suggested that personality traits partially mediate associations between genetic risk and inattentive and hyperactive-impulsive ADHD symptoms. Thus, personality traits may be one means by which to disentangle developmental trajectories to ADHD.
These results are consistent with prior work suggesting that while there is substantial genetic overlap between the inattentive and hyperactive-impulsive ADHD symptom domains, there are also significant specific genetic and environmental influences on inattention versus hyperactivity-impulsivity (Levy et al. 2001; McLoughlin et al. 2007). Importantly, there was some evidence of personality mediation effects generally for ADHD and externalizing symptoms, as well as evidence of specificity of mediation effects. While mediation effects involving conscientiousness appeared to be specific to ADHD inattention, mediation effects involving neuroticism appeared to generalize to several forms of behavior problems, including inattention and oppositional-defiance. These findings are in line with previous work finding specificity of relations between personality and temperament traits and ADHD symptom domains (Martel and Nigg 2006; Nigg et al. 2002). Current study findings are also in line with work that extends this idea to the broader domain of disruptive behavior disorders. ADHD and common comorbid disorders like ODD, CD, and Reading Disorder appear to share genetic and environmental influences, as well as being subject to specific genetic and environmental influences (Coolidge et al. 2000; Martin et al. 2006; Waldman et al. 2001).
Of course, the relations between ADHD and personality traits remain controversial and cannot be disentangled in this cross-sectional report. It is possible that personality traits may predispose individuals to psychopathology, be influenced or changed by psychopathology, share a common cause with psychopathology, and/or lie on the same continuum as psychopathology (Van Leeuwen et al. 2007; Watson et al. 2006). Results of the current study could be consistent with any of these ideas. Longitudinal research would also be helpful in order to determine whether there is in fact a causal pathway between genetic risk, conscientiousness, and ADHD symptoms, although work to date suggests that this is the direction of effects (Eisenberg et al. 2001). It is further acknowledged that three-occasion data would be a more powerful way to test the meditational hypotheses of the current study due to the information it would provide about temporal ordering of effects (Kraemer et al. 2001). Since personality traits did not fully mediate genetic risk associations with ADHD symptoms, exploration of other relevant psychological mechanisms (e.g., executive function; Doyle et al. 2005) that might mediate the relationship between genetic risk and ADHD should be explored. Finally, as additional genetic markers associated with ADHD are identified with new technologies and methods (i.e., genome-wide association studies, regional sequencing), it will be important to try to elucidate specificity and diversity in equifinality of pathways to ADHD involving individual, additive, and dominant genetic risk.
The current study has several limitations. First, a sample enriched for ADHD-related problems was used in the present study; the present findings should be replicated in other populations (e.g., epidemiological samples). Because family data was not available for the entire sample, population stratification, while unlikely based on analytic checks involving ethnicity, cannot be ruled out. In addition, the current study relied on single markers of genetic risk formed into an additive genetic composite to elucidate a way of thinking about the etiological process of ADHD from gene to disorder; other complementary genetic approaches (e.g., genome-wide analysis, dense marker array) should also be explored in relation to these types of questions because, for example, this type of candidate gene study may be examining a polymorphism that is in linkage disequilibrium with “true” susceptibility variant(s). While a catecholaminergic genetic risk composite showed relationship with ADHD symptoms in the current study, seemingly as a more robust indicator of the additive genetic risk of dopaminergic and adrenergic alleles, further exploration of the nature of the association between single and composite genetic risk alleles and psychopathology is needed. Although findings held using both maternal and teacher report on ADHD symptoms, suggesting that results were not due entirely to shared source variance, the chance for falsepositives in genetic association studies always remains. Personality traits were measured via maternal report only. Observational measures of personality will be useful for replication attempts as well as for exploring mediation relationships further (Goldsmith and Rothbart 1999).
The current study makes an important contribution by elucidating the process by which genetic risk may lead to ADHD symptoms. A better understanding of pathways between genetic risk and ADHD may pave the way for earlier prevention, assessment, and intervention efforts. Results of the current study suggest that low conscientiousness and high neuroticism partially mediate the relationship between catecholamine risk and inattentive and hyperactive-impulsive ADHD symptoms with some specificity to symptom domains, suggesting that these traits may be able to identify children at risk for ADHD earlier in their development.
Acknowledgments
This research was supported by NIH National Institute of Mental Health Grant R01-MH63146, MH59105, and MH70542 to Karen Friderici and Joel Nigg. We are indebted to the families and staff who made this study possible.
Contributor Information
Michelle M. Martel, Email: mmartel@uno.edu, Psychology Department, University of New Orleans, 2000 Lakeshore Drive; 2005 Geology & Psychology Building, New Orleans, LA 70148, USA.
Molly Nikolas, Psychology Department, Michigan State University, East Lansing, MI, USA.
Katherine Jernigan, Microbiology and Molecular Genetics, Michigan State University, East Lansing, MI, USA.
Karen Friderici, Microbiology and Molecular Genetics, Michigan State University, East Lansing, MI, USA.
Joel T. Nigg, Psychiatry Department, Oregon Health and Sciences University, Portland, OR, USA
References
- Achenbach TM. Manual for the Child Behavior Checklist/4–18 and 1991 Profile. Burlington: University of Vermont Department of Psychiatry; 1991. [Google Scholar]
- Arnold LE, Abikoff HB, Cantwell DP, Conners CK, Elliott G, Greenhill LL, et al. National institute of mental health collaborative multimodal treatment study of children with ADHD (the MTA). Design challenges and choices. Archives of General Psychiatry. 1997;54:865–870. doi: 10.1001/archpsyc.1997.01830210113015. [DOI] [PubMed] [Google Scholar]
- Auerbach JG, Benjamin J, Faroy M, Geller V, Ebstein R. DRD4 related to infant attention and information processing: a developmental link to ADHD? Psychiatric Genetics. 2001;11:31–35. doi: 10.1097/00041444-200103000-00006. [DOI] [PubMed] [Google Scholar]
- Bush G, Valera EM, Seidman LJ. Functional neuroimaging of Attention-Deficit/Hyperactivity Disorder: a review and suggested future directions. Biological Psychiatry. 2005;57:1273–1284. doi: 10.1016/j.biopsych.2005.01.034. [DOI] [PubMed] [Google Scholar]
- Caspi A, Block J, Block JH, Klopp B, Lynam D, Moffitt TE, et al. A “common-language” version of the California Child Q-Set for personality assessment. Psychological Assessment. 1992;4:512–523. [Google Scholar]
- Conners CK. Conners rating scales-revised. Toronto: Multi-Health Systems; 1997. [Google Scholar]
- Coolidge FL, Thede LL, Young SE. Heritability and the comorbidity of attention deficit hyperactivity disorder with behavior disorders and executive function deficits: a preliminary investigation. Developmental Neuropsychology. 2000;17:273–287. doi: 10.1207/S15326942DN1703_1. [DOI] [PubMed] [Google Scholar]
- Curran SG, West SG, Finch JF. The robustness of test statistics to nonnormality and specification error in confirmatory factor analysis. Psychological Methods. 1996;1:16–29. [Google Scholar]
- Depue RA, Lenzenweger MF. Toward a developmental psychopathology of personality disturbance: A neurobehavioral dimensional model. In: Cicchetti D, Cohen D, editors. Developmental psychopathology (Vol. 2): Developmental neuroscience. Hoboken: Wiley; 2006. pp. 762–796. [Google Scholar]
- Derryberry D, Tucker DM. Motivation, self-regulation, and self-organization. In: Cicchetti D, Cohen D, editors. Developmental psychopathology (vol. 2): Developmental neuroscience. Hoboken: Wiley; 2006. pp. 502–532. [Google Scholar]
- Doyle AE, Faraone SV, Seidman LJ, Willcutt EG, Nigg JT, Waldman ID, et al. Are endophenotypes based on measures of executive functions useful for molecular genetic studies of ADHD? Journal of Child Psychology and Psychiatry. 2005;46:778–803. doi: 10.1111/j.1469-7610.2005.01476.x. [DOI] [PubMed] [Google Scholar]
- Dragan WL, Oniszczenko W. An association between dopamine D4 receptor and transporter gene polymorphisms and personality traits, assessed using NEO-FFI in a Polish female population. Personality and Individual Differences. 2007;43:531–540. [Google Scholar]
- DuPaul GJ, Power TJ, Anastopolous AD, Reid R. ADHD rating scale—IV: Checklists, norms, & clinical interpretation. New York: Guilford press; 1998. [Google Scholar]
- Durston S. A review of the biological bases of ADHD: what have we learned from imaging studies? Mental Retardation and Developmental Disabilities Research Reviews. 2003;9:184–195. doi: 10.1002/mrdd.10079. [DOI] [PubMed] [Google Scholar]
- Eisenberg N, Cumberland A, Spinrad TL, Fabes RA, Shepard SA, Reiser M, et al. The relations of regulation and emotionality to children’s externalizing and internalizing problem behavior. Child Development. 2001;72:1112–1134. doi: 10.1111/1467-8624.00337. [DOI] [PubMed] [Google Scholar]
- Ekelund J, Lichtermann D. Association between novelty-seeking and the type 4 dopamine receptor gene in a large Finnish cohort sample. American Journal of Psychiatry. 1999;156:1453–1455. doi: 10.1176/ajp.156.9.1453. [DOI] [PubMed] [Google Scholar]
- Faraone SV, Perlis RH, Doyle AE, Smoller JW, Goralnick JJ, Holmgren MA, et al. Molecular genetics of attention-deficit/hyperactivity disorder. Biological Psychiatry. 2005;57:1313–1323. doi: 10.1016/j.biopsych.2004.11.024. [DOI] [PubMed] [Google Scholar]
- Gardini S, Cloninger CR, Venneri A. Individual differences in personality traits reflect structural variance in specific brain regions. Brain Research Bulletin. 2009;79:265–270. doi: 10.1016/j.brainresbull.2009.03.005. [DOI] [PubMed] [Google Scholar]
- Gizer IR, Waldman ID, Barr CL, Feng Y, Wigg KG, Misener VL, et al. Relations between multi-informant assessments of ADHD symptoms, DAT1, and DRD4. Journal of Abnormal Psychology. 2008;117:869–880. doi: 10.1037/a0013297. [DOI] [PubMed] [Google Scholar]
- Goldsmith HH, Rothbart MK. The laboratory temperament assessment battery: Locomotor version 3.1. 1999 [Google Scholar]
- Goldsmith HH, Lemery S, Essex MJ. Temperament as a liability factor for childhood behavioral disorders: The concept of liability. In: DiLalla LF, editor. Behavior genetics principles: Perspectives in development, personality, and psychopathology. Washington: American Psychological Association; 2004. pp. 19–39. [Google Scholar]
- Hutchinson AD, Mathias JL, Banich MT. Corpus callosum morphology in children and adolescents with attention deficit hyperactivity disorder: a meta-analytic review. Neuropsychology. 2008;22:341–349. doi: 10.1037/0894-4105.22.3.341. [DOI] [PubMed] [Google Scholar]
- Hutchison KE, Stallings M, McGeary J, Bryan A. Population stratification in the candidate gene study: fatal flaw or red herring? Psychological Bulletin. 2004;130:66–79. doi: 10.1037/0033-2909.130.1.66. [DOI] [PubMed] [Google Scholar]
- Jensen PS, Martin D, Cantwell DP. Comorbidity in ADHD: implications for research, practice, and DSM-V. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36:1065–1079. doi: 10.1097/00004583-199708000-00014. [DOI] [PubMed] [Google Scholar]
- John OP, Caspi A, Robins RW, Moffitt TE, Stouthamer-Loeber M. The little-five: exploring the nomological network of the five-factor model of personality in adolescent boys. Child Development. 1994;65:160–178. [PubMed] [Google Scholar]
- Keltikangas-Jarvinen L, Elovainio M, Kivimaki M, Lichtermann D, Ekelund J, Peltonen L. Association between the type 4 dopamine receptor gene polymorphism and novelty seeking. Psychosomatic Medicine. 2003;65:471–476. doi: 10.1097/01.psy.0000041547.31072.25. [DOI] [PubMed] [Google Scholar]
- Kraemer HC, Stice E, Kazdin A, Offord D, Kupfer D. How do risk factors work together? Mediators, moderators, and independent, overlapping, and proxy risk factors. American Journal of Psychiatry. 2001;158:848–856. doi: 10.1176/appi.ajp.158.6.848. [DOI] [PubMed] [Google Scholar]
- Kustanovich V, Ishi J, Crawford L, Yang M, McGough JJ, McCracken JT, et al. Transmission disequilibrium testing of dopamine-related candidate gene polymorphisms in ADHD: confirmation of association of ADHD with DRD4 and DRD5. Molecular Psychiatry. 2004;9:711–717. doi: 10.1038/sj.mp.4001466. [DOI] [PubMed] [Google Scholar]
- Lahey BB, Applegate B, McBurnett K, Biederman J, et al. DSM-IV field trials for attention deficit hyperactivity disorder in children and adolescents. American Journal of Psychiatry. 1994;151:1673–1685. doi: 10.1176/ajp.151.11.1673. [DOI] [PubMed] [Google Scholar]
- Lakatos K, Nemoda Z, Birkas E, Ronai Z, Kovacs E, Ney K, et al. Association of D4 dopamine receptor gene and serotonin transporter polymorphisms with infants’ response to novelty. Molecular Psychiatry. 2003;8:90–97. doi: 10.1038/sj.mp.4001212. [DOI] [PubMed] [Google Scholar]
- Levy F, Hay DA, McStephen M, Wood CH, Waldman I. Attention-deficit/hyperactivity disorder: a category or a continuum? Genetic analysis of a large-scale twin study. American Academy of Child & Adolescent Psychiatry. 1997;36:737–744. doi: 10.1097/00004583-199706000-00009. [DOI] [PubMed] [Google Scholar]
- Levy F, McStephen M, Hay DA. The diagnostic genetics of ADHD symptoms and subtypes. In: Levy F, Hay DA, editors. Attention, Genes and ADHD. Brunner-Routledge: East Sussex; 2001. pp. 35–57. [Google Scholar]
- Martel MM, Nigg JT. Child ADHD and personality/ temperament traits of reactive and effortful control, resiliency, and emotionality. Journal of Child Psychology & Psychiatry. 2006;47:1175–1183. doi: 10.1111/j.1469-7610.2006.01629.x. [DOI] [PubMed] [Google Scholar]
- Martin NC, Levy F, Pieka J, Hay DA. A genetic study of attention deficit hyperactivity disorder, conduct disorder, oppositional defiant disorder and reading disability: aetiological overlaps and implications. International Journal of Disability, Development, and Education. 2006;53:21–34. [Google Scholar]
- McCartney K, Burchinal MR, Bub KL. Best practices in quantitative methods for developmentalists. Monographs of the Society for Research in Child Development. 2006;71:105–126. doi: 10.1111/j.1540-5834.2006.07103001.x. [DOI] [PubMed] [Google Scholar]
- McCracken JT, Smalley SL, McGough JJ, Crawford L, Del’Homme M, Cantor RM, et al. Evidence for linkage of a tandem duplication polymorphism upstream of the dopamine D4 receptor gene (DRD4) with attention deficit hyperactivity disorder (ADHD) Molecular Psychiatry. 2000;5:531–536. doi: 10.1038/sj.mp.4000770. [DOI] [PubMed] [Google Scholar]
- McCrae RR, Costa PT. Validation of the five-factor model of personality across instruments and observers. Journal of Personality and Social Psychology. 1987;52:81–90. doi: 10.1037//0022-3514.52.1.81. [DOI] [PubMed] [Google Scholar]
- McLoughlin G, Ronald A, Kuntsi J, Asherton P, Plomin R. Genetic support for the dual nature of attention deficit hyperactivity disorder: substantial genetic overlap between the inattentive and hyperactive-impulsive components. Journal of Abnormal Child Psychology. 2007;35:999–1008. doi: 10.1007/s10802-007-9149-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meulenbelt I, Droog S, Trommelen GJ, Boomsa DI, Slagboom P. High-yield non-invasive human genomic DNA isolation method for genetic studies in geographically dispersed families and populations. American Journal of Human Genetics. 1995;57:1252–1254. [PMC free article] [PubMed] [Google Scholar]
- Miller CJ, Miller SR, Newcorn JH, Halperin JM. Personality characteristics associated with persistent ADHD in late adolescence. Journal of Abnormal Child Psychology. 2008;36:165–173. doi: 10.1007/s10802-007-9167-7. [DOI] [PubMed] [Google Scholar]
- Muthen LK, Muthen BO. Mplus user’s guide. 5th ed. Los Angeles: Muthen & Muthen; 1998–2008. [Google Scholar]
- Nigg JT. On inhibition/disinhibition in developmental psychopathology: views from cognitive and personality psychology and a working inhibition taxonomy. Psychological Bulletin. 2000;126:220–246. doi: 10.1037/0033-2909.126.2.220. [DOI] [PubMed] [Google Scholar]
- Nigg JT. Temperament and developmental psychopathology. Journal of Child Psychology and Psychiatry. 2006;47:395–422. doi: 10.1111/j.1469-7610.2006.01612.x. [DOI] [PubMed] [Google Scholar]
- Nigg JT, John OP, Blaskey LG, Huang-Pollock CL, Willcutt EG, Hinshaw SP, et al. Big five dimensions and ADHD symptoms: links between personality traits and clinical symptoms. Journal of Personality and Social Psychology. 2002;83:451–469. doi: 10.1037/0022-3514.83.2.451. [DOI] [PubMed] [Google Scholar]
- Nigg J, Nikolas M, Friderici K, Park L, Zucker RA. Genotype and neuropsychological response inhibition as resilience promoters for attention-deficit/hyperactivity disorder, oppositional defiant disorder, and conduct disorder under conditions of psychosocial adversity. Development and Psychopathology. 2007;19:767–786. doi: 10.1017/S0954579407000387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyman ES, Loukola A, Ekelund J, Veijola J, Jourkamaa M, Taanila A, et al. Impact of the dopamine receptor gene family on temperament traits in a population-based birth cohort. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2009;150B:854–865. doi: 10.1002/ajmg.b.30908. [DOI] [PubMed] [Google Scholar]
- Park L, Nigg JT, Waldman ID, Nummy KA, Huang-Pollock C, Rappley M, et al. Association and linkage of α-2A adrenergic receptor gene polymorphism with childhood ADHD. Molecular Psychiatry. 2005;10:572–580. doi: 10.1038/sj.mp.4001605. [DOI] [PubMed] [Google Scholar]
- Parker JDA, Majeski SA, Collin VT. ADHD symptoms and personality: relationships with the five-factor model. Personality and Individual Differences. 2004;36:977–987. [Google Scholar]
- Puig-Antich J, Ryan N. Kiddie schedule for affective disorders and schizophrenia. Pittsburgh: Western Psychiatric Institute; 1986. [Google Scholar]
- Rothbart MK, Posner MI. Temperament, attention, and developmental psychopathology. In: Cicchetti D, Cohen D, editors. Developmental psychopathology (vol. 2): Developmental neuroscience. Hoboken: Wiley; 2006. pp. 465–501. [Google Scholar]
- Rubia K, Halari R, Smith AB, Mohammad M, Scott S, Brammer MJ. Shared and disorder-specific prefrontal abnormalities in boys with pure attention-deficit/hyperactivity disorder compared to boys with pure CD during interference inhibition and attention allocation. Journal of Child Psychology and Psychiatry. 2009;50:669–678. doi: 10.1111/j.1469-7610.2008.02022.x. [DOI] [PubMed] [Google Scholar]
- Shaffer D, Fisher P, Lucas C, Dulcan MK, Schwab-Stone M. NIMH diagnostic interview schedule for children, version IV (NIMH DISC-IV): description, differences from previous versions and reliability of some common diagnoses. Journal of American Academy of Child and Adolescent Psychiatry. 2000;39:28–38. doi: 10.1097/00004583-200001000-00014. [DOI] [PubMed] [Google Scholar]
- Shaw P, Gornick M, Lerch J, Addington A, Seal J, Greenstein D, et al. Polymorphisms of the dopamine D4 receptor, clinical outcome, and cortical structure in attention-deficit/hyperactivity disorder. Archives of General Psychiatry. 2007;64:921–931. doi: 10.1001/archpsyc.64.8.921. [DOI] [PubMed] [Google Scholar]
- Sheese BE, Voelker PM, Rothbart MK, Posner MI. Parenting quality interacts with genetic variation in dopamine receptor D4 to influence temperament in early childhood. Development and Psychopathology. 2007;19:1039–1046. doi: 10.1017/S0954579407000521. [DOI] [PubMed] [Google Scholar]
- Shiner R, Caspi A. Personality differences in childhood and adolescence: measurement, development, and consequences. Journal of Child Psychology and Psychiatry. 2003;44:2–32. doi: 10.1111/1469-7610.00101. [DOI] [PubMed] [Google Scholar]
- Simon JJ, Walther S, Fiebach CJ, Friederich H, Stippich C, Weisbrod M, Kaiser S. Neural reward processing is modulated by approach- and avoidance-related personality traits. NeuroImage. 2009 doi: 10.1016/j.neuroimage.2009.09.016. in press). [DOI] [PubMed] [Google Scholar]
- Valera EM, Faraone SV, Murray KE, Seidman LJ. Meta-analysis of structural imaging findings in attention-deficit/ hyperactivity disorder. Biological Psychiatry. 2007;61:1361–1369. doi: 10.1016/j.biopsych.2006.06.011. [DOI] [PubMed] [Google Scholar]
- Van Leeuwen K, Mervielde I, De Clercq BJ, De Fruyt F. Extending the spectrum idea: child personality, parenting and psychopathology. European Journal of Personality. 2007;21:63–89. [Google Scholar]
- Vandenbergh DJ, Persico AM, Hawkins AL, Griffin CA, Li X, Jabs EW, et al. Human dopamine transporter gene (DAT1) maps to chromosome 5p15.3 and displays a VNTR. Genomics. 1992;14:1104–1106. doi: 10.1016/s0888-7543(05)80138-7. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang G, Fowler JS, Ding Y. Imaging the effects of methylphndiate on brain dopamine: new model on its therapeutic actions for attention-deficit/hyperactivity disorder. Biological Psychiatry. 2005;57:1410–1415. doi: 10.1016/j.biopsych.2004.11.006. [DOI] [PubMed] [Google Scholar]
- Waldman ID, Gizer IR. The genetics of attention deficit hyperactivity disorder. Clinical Psychology Review. 2006;26:396–432. doi: 10.1016/j.cpr.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Waldman ID, Rhee SH, Levy F, Hay DA. Causes of the overlap among symptoms of attention deficit hyperactivity disorder, oppositional defiant disorder, and conduct disorder. In: Levy F, Hay DA, editors. Attention, genes and ADHD. Brunner-Routledge: East Sussex; 2001. pp. 115–138. [Google Scholar]
- Watson D, Kotov R, Gamez W. Basic dimensions of temperament in relation to personality and psychopathology. In: Krueger RF, Tackett JL, editors. Personality and psychopathology. New York: Guildford; 2006. pp. 7–38. [Google Scholar]
- Wechsler D. Wechsler intelligence scale for children: administration and scoring manual. 3rd ed. New York: Psychological Corporation; 1991. [Google Scholar]
- Whittle S, Allen NB, Lubman DI, Yucel M. The neurobiological basis of temperament: towards a better understanding of psychopathology. Neuroscience and Biobehavioral Reviews. 2006;30:511–525. doi: 10.1016/j.neubiorev.2005.09.003. [DOI] [PubMed] [Google Scholar]
- Yamagata S, Takahashi Y, Kijima N, Maekawa H, Ono Y, Ando J. Genetic and environmental etiology of effortful control. Twin Research and Human Genetics. 2005;8:300–306. doi: 10.1375/1832427054936790. [DOI] [PubMed] [Google Scholar]