Abstract
Viral single strands (SS) are converted to the duplex from (RF) by a soluble enzyme fraction uninfected Escherichia coli [Schekman et al. (1975) J. Biol. Chem. 250, 5859-5865]. When reactions were supplemented with a soluble enzyme fraction from phi X174-infected cells, replication of phi X174 superhelical RF I DNA was observed. The activity supplied by infected cells was absent in cells treated with chloramphenicol or in cells infected with a phi X174 phage mutant in cistron A (cis A). A host function coded by the rep gene, essential in vivo for RF replication (but not for SS leads to RF), was supplied by enzyme fractions from either infected or uninfected cells. Based on complementation assays, the cisA-dependent and the rep-dependent proteins have each been purified about 1000-fold. The synthetic products of the enzymatic reaction were identified as RF I and RF II in which viral (+) and complementary (-) strands were newly synthesized.
Full text
PDF
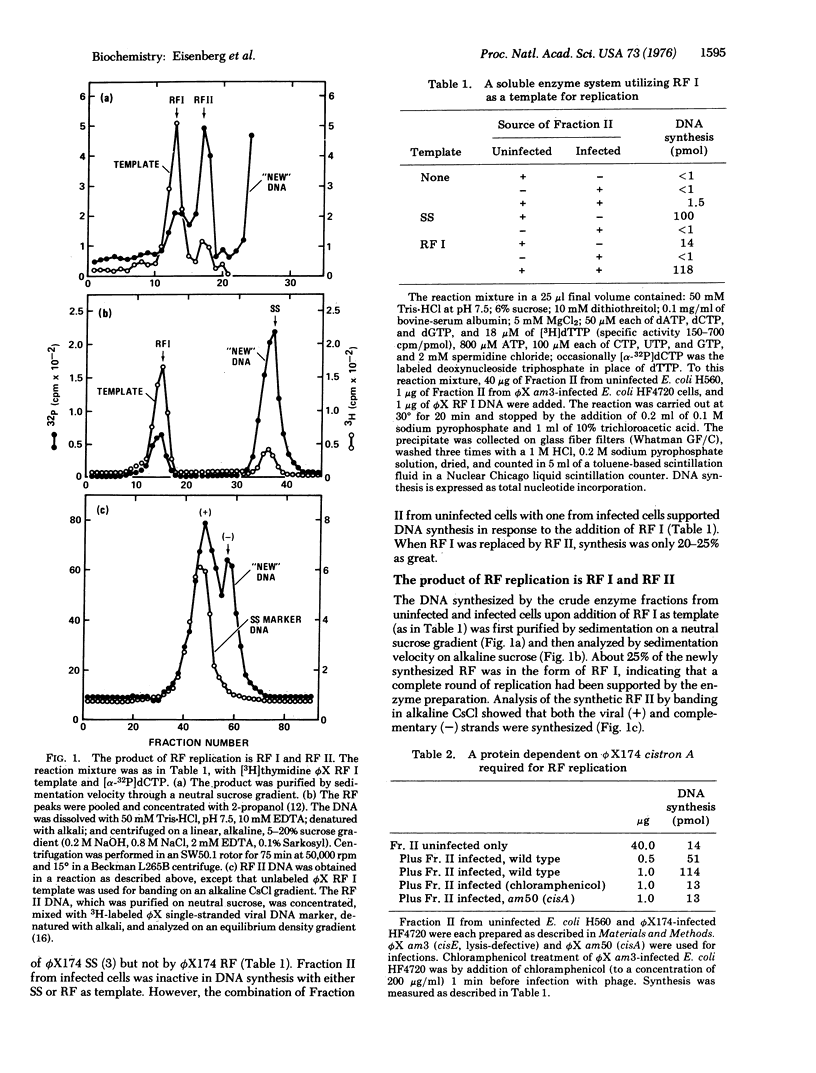
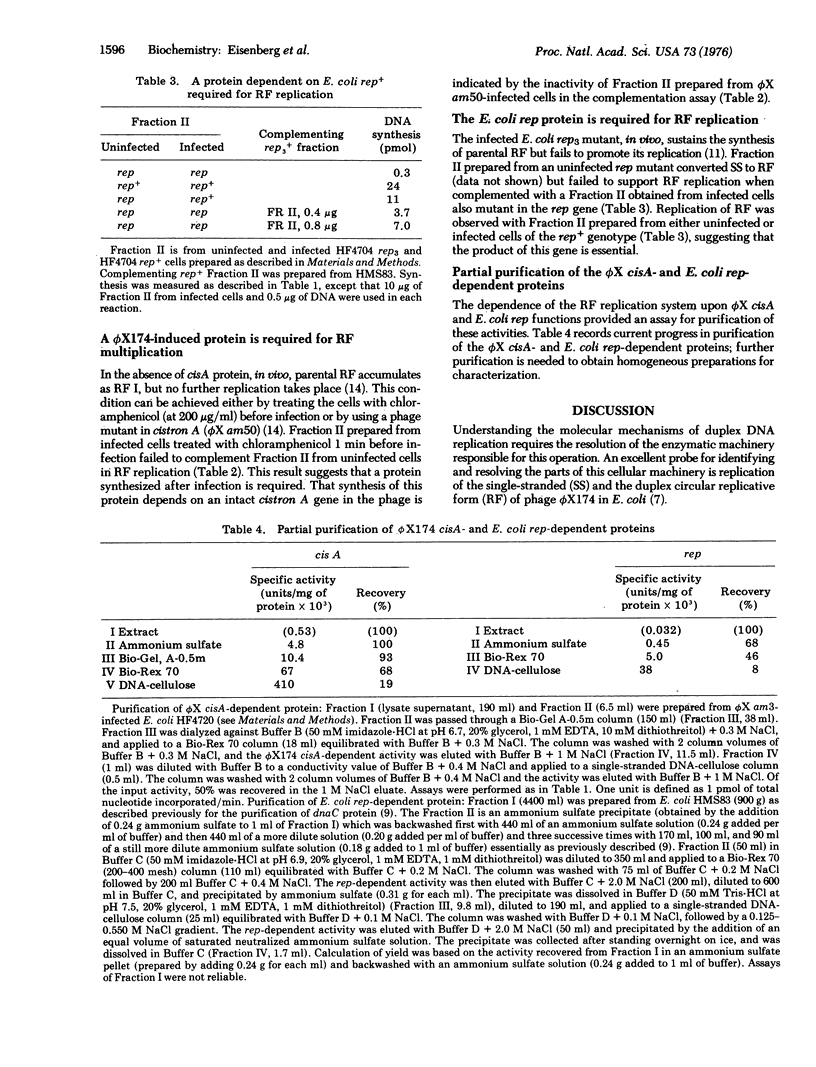

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brunk C. F., Leick V. Rapid equilibrium isopycnic CsC1 gradients. Biochim Biophys Acta. 1969 Mar 18;179(1):136–144. doi: 10.1016/0005-2787(69)90129-4. [DOI] [PubMed] [Google Scholar]
- Denhardt D. T., Iwaya M., Larison L. L. The rep mutation. II. Its effect on Escherichia coli and on the replication of bacteriophage phi X174. Virology. 1972 Aug;49(2):486–496. doi: 10.1016/0042-6822(72)90500-4. [DOI] [PubMed] [Google Scholar]
- Eisenberg S., Harbers B., Hours C., Denhardt D. T. The mechanism of replication of phiX174 DNA. XII. Non-random location of gaps in nascent phiX174 RF II DNA. J Mol Biol. 1975 Nov 25;99(1):107–123. doi: 10.1016/s0022-2836(75)80162-8. [DOI] [PubMed] [Google Scholar]
- Francke B., Ray D. S. Formation of the parental replicative form DNA of bacteriophage phi-X174 and initial events in its replication. J Mol Biol. 1971 Nov 14;61(3):565–586. doi: 10.1016/0022-2836(71)90065-9. [DOI] [PubMed] [Google Scholar]
- Geider K., Kornberg A. Conversion of the M13 viral single strand to the double-stranded replicative forms by purified proteins. J Biol Chem. 1974 Jul 10;249(13):3999–4005. [PubMed] [Google Scholar]
- Henry T. J., Knippers R. Isolation and function of the gene A initiator of bacteriophage phi-chi 174, a highly specific DNA endonuclease. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1549–1553. doi: 10.1073/pnas.71.4.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane H. E., Denhardt D. T. The rep mutation. IV. Slower movement of replication forks in Escherichia coli rep strains. J Mol Biol. 1975 Sep 5;97(1):99–112. doi: 10.1016/s0022-2836(75)80025-8. [DOI] [PubMed] [Google Scholar]
- Schekman R., Weiner A., Kornberg A. Multienzyme systems of DNA replication. Science. 1974 Dec 13;186(4168):987–993. doi: 10.1126/science.186.4168.987. [DOI] [PubMed] [Google Scholar]
- Schekman R., Weiner J. H., Weiner A., Kornberg A. Ten proteins required for conversion of phiX174 single-stranded DNA to duplex form in vitro. Resolution and reconstitution. J Biol Chem. 1975 Aug 10;250(15):5859–5865. [PubMed] [Google Scholar]
- Schekman R., Wickner W., Westergaard O., Brutlag D., Geider K., Bertsch L. L., Kornberg A. Initiation of DNA synthesis: synthesis of phiX174 replicative form requires RNA synthesis resistant to rifampicin. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2691–2695. doi: 10.1073/pnas.69.9.2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinsheimer R. L. Bacteriophage phi-X174 and related viruses. Prog Nucleic Acid Res Mol Biol. 1968;8:115–169. [PubMed] [Google Scholar]
- Wickner S., Hurwitz J. Conversion of phiX174 viral DNA to double-stranded form by purified Escherichia coli proteins. Proc Natl Acad Sci U S A. 1974 Oct;71(10):4120–4124. doi: 10.1073/pnas.71.10.4120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner W., Brutlag D., Schekman R., Kornberg A. RNA synthesis initiates in vitro conversion of M13 DNA to its replicative form. Proc Natl Acad Sci U S A. 1972 Apr;69(4):965–969. doi: 10.1073/pnas.69.4.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner W., Schekman R., Geider K., Kornberg A. A new form of DNA polymerase 3 and a copolymerase replicate a long, single-stranded primer-template. Proc Natl Acad Sci U S A. 1973 Jun;70(6):1764–1767. doi: 10.1073/pnas.70.6.1764. [DOI] [PMC free article] [PubMed] [Google Scholar]


