Abstract
Previous studies indicated that Environmentally Persistent Free Radicals (EPFRs) are formed in the post-flame, cool zone of combustion. They result from the chemisorption of gas-phase products of incomplete combustion (particularly hydroxyl- and chlorine-substituted aromatics) on Cu(II)O, Fe(III)2O3, and Ni(II)O domains of particulate matter (fly ash or soot particles). This study reports our detailed laboratory investigation on the lifetime of EPFRs on Zn(II)O/silica surface. Similarly, as in the case of other transition metals, chemisorption of the adsorbate on the Zn(II)O surface and subsequent transfer of electron from the adsorbate to the metal forms a surface-bound EPFR and a reduced metal ion center. The EPFRs are stabilized by their interaction with the metal oxide domain surface. The half-lives of EPFRs formed on Zn(II)O domains were the longest observed among the transition metal oxides studied and ranged from 3 to 73 days. These half-lives were an order of magnitude longer than those formed on nickel and iron oxides, and were 2 orders of magnitude longer compared to the EPFRs on copper oxide which have half-lives only on the order of hours. The longest-lived radicals on Zn(II)O correspond to the persistency in ambient air particles of almost a year. The half-life of EPFRs was found to correlate with the standard reduction potential of the associated metal.
Introduction
The incidence of chronic disease and exposure to airborne fine particulate matter (PM2.5) have been well-studied by a plethora of epidemiological studies; however, the precise mechanism of their toxicity has been elusive due to the complexity of the physical and chemical nature of PM2.5.1,2 Zinc, like iron is one of the most abundant metals in both fly ash and PM2.5.3,4 It is present in air, water, and soil, and is released from various geological and natural environmental processes. However, anthropogenic sources of zinc from industrial activities, metallurgical processing (galvanization), mining, and combustion of waste and fossil fuel for energy production account to the majority of its environmental input. The concentration of zinc in waste and fly ash from municipal waste incineration can reach ~2 mg g−1 and ~80 mg g−1, respectively.5 Zinc is second to iron as the dominant contributor of transition metals in PM2.5 with concentration as high as 157 ng m−3 in some urban areas.6,7 Particulate matter (PM) contains significant amount of transition metals that are known to produce reactive oxygen species (ROS). PM2.5 and combustion-generated PM such as cigarette smoke generates high amounts of ROS8,9 and have been implicated in the toxicity of PM.10–12
Previous studies of model particulate systems (Cu(II)O, Fe(III)2O3, and Ni(II)O on silica particles) demonstrated that various chlorine- and hydroxyl-substituted benzenes chemisorb on metal oxide surfaces and form EPFRs by the transfer of electron from the adsorbate to the metal ion which also results in the reduction of the metal.13,14 This process typically occurs at the post-combustion, cool-zone condition between 150 and 400 °C.
It has been amply demonstrated from biological studies of copper-bound EPFR that particle-radical systems are potent ROS generators resulting in pulmonary and cardiac dysfunction in animal models.15–17
Zinc, like iron and copper is not a hazardous metal by itself compared to nickel. However, if it is active in EPFR formation, the presence of Zn(II)O in airborne PM elevates its environmental and health risks as a potential source of these new pollutant systems. While previously identified atmospheric radicals have very short lifetimes of less than a second, EPFR radical-metal oxide systems are very persistent in the environment.13 This manuscript reports the results of a detailed laboratory study on the structure and persistency of EPFRs formed from various molecular adsorbates on particles consisting of 5% Zn(II)O on silica. We also compared the persistency of the EPFRs formed on Zn(II)O model particle surface with previously studied radical-metal oxide systems. Chlorinated adsorbates—1,2-dichlorobenzene, monochlorobenzene, and 2-mono-chlorophenol were chosen since they are abundant in incineration systems and the phenolic compounds—catechol, hydroquinone, and phenol are abundant components of biomass system.
Results and discussion
EPFR formation
Adsorption of Zn(II)O particles supported on silica with various molecular aromatic adsorbates yielded an electron paramagnetic resonance (EPR) signal centered at ~3400 Gauss with a narrow line width (ΔHp–p) of ~7 to 11 Gauss depending on experimental conditions. These paramagnetic signals are characteristics of aromatic radical species. Table 1 presents the spectral parameters and radical concentration for each adsorbate molecule. The radical signal originates from the electronic interaction between the chemisorbed species and metal ion center. This interaction is a result of a one-electron transfer from the adsorbed molecule towards the metal center and the formation of radical species. Based on our previous research, it is known that the overall position and g-value of the EPFR spectra are superposition of the contributing components: F-centers (trapped electrons in the matrix) and combination of phenoxyl and semiquinone-type radicals associated with metal center.13,14 Depending on the relative concentration of these two types of radical, an overall shift of the EPR signal is observed. In the current study, the initial g-value of the EPFR species was essentially similar for most of the adsorbates (~2.003 to 2.004) with the exception of phenol whose g-value was 2.0042. A narrower signal was detected for phenol (ΔHp–p = 6.8 Gauss) while other species produced wider signals (7.2 Gauss for 2-monochlorophenol and ~10 Gauss for other adsorbates). A narrower EPR signal for adsorbed phenol is consistent with our studies on other metal oxides, where phenol was found to produce predominantly one type of radical—a surface-associated phenoxyl species.13,14
Table 1.
Spectral characteristics of EPFRs formed on Zn(II)O/silica from different adsorbates at 230 °C
| Adsorbate | ΔHp–p | g-value | Intensitya |
|---|---|---|---|
| PH | 6.8 | 2.0042 | 4.3 |
| MCBz | 10.0 | 2.0037 | 10.4 |
| 2-MCP | 7.3 | 2.0038 | 13.4 |
| 1,2-DCBz | 9.1 | 2.0038 | 17.5 |
| HQ | 10.2 | 2.0030 | 32.7 |
| CT | 10.7 | 2.0036 | 66.4 |
×1018 (spins per g of Zn).
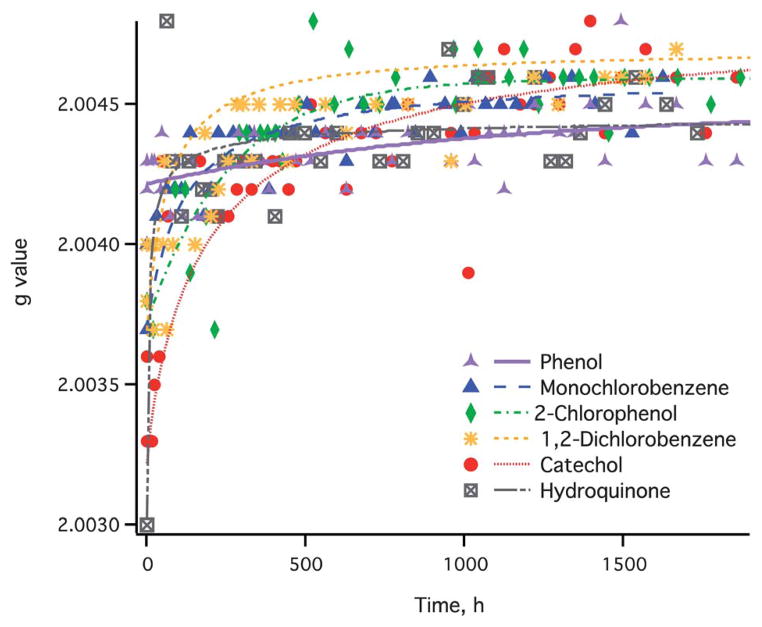
Exposure of the EPFR radicals to ambient air affects both the signal width and the position. Two behaviors can be distinguished. The shape and the location of the EPR signal for EPFR formed from phenol adsorbates were least affected by air exposure. In this case, only a slight increase of the overall g-value was registered (cf. Fig. 1), while at the same time, the EPR signal width had dropped considerably from 6.8 to 3.9 Gauss. A small shift in g-value with simultaneous shrinking of the peak width indicates the small contribution from other radicals present on the surface other than phenoxyl radical species. On the other hand, other adsorbates had shown a transient increase of the overall g-value for the first few hours of air exposure, and eventually stabilized at ~2.0045—a g-value similar to that observed for radicals formed from phenoxyl adsorbates after prolonged air exposure (cf. Fig. 1). At the same time, the peak width had also decreased significantly from ~10 Gauss to below 5 Gauss. The initial change of both peak width and position of the paramagnetic signal for different adsorbates and their eventual convergence after prolonged air exposure indicate transformation of the surface radicals in ambient conditions that eventually lead to a common species remaining on the surface. As a result, one can anticipate that once the EPFR-containing particulate exits the combustor exhaust into the ambient atmosphere, combustion-derived PM will finally contain the same type of radical regardless of the adsorbates.
Fig. 1.
EPR signal peak position with increasing particle exposure time to air.
Persistence and lifetime of EPFRs
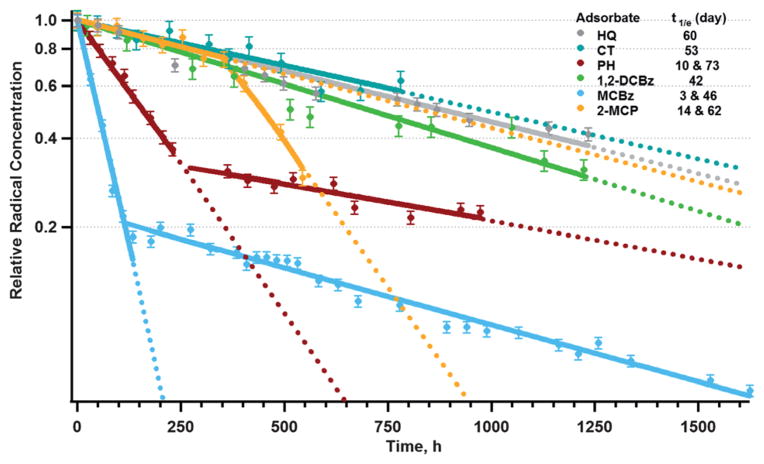
Changes in the EPR signal position and width are indicative of either the transformation of one radical to another radical or the variation in their rates of decay. Studies of the decay profiles of EPFRs on Zn(II)O/silica formed from different adsorbates in ambient air showed that for some adsorbate molecules, two different decay rates were observed—a ‘short’ one with a half-life of 3–15 days, and a ‘long’ one with a half-life of 46–73 days (phenol, monochlorobenzene, and 2-monochlorophenol) (cf. Fig. 2). For the other 3 adsorbates (hydroquinone, catechol and 1,2-dichlorobenzene) only the long decay rate of 42–60 days was observed. The differences in the half-life of the radicals from different adsorbates provide a valuable information on the types of radical present on Zn(II)O/silica surface. Hydroquinone, catechol, and 1,2-dichlorobenzene molecules upon reaction with the surface lead to a very similar species: dihydroxybenzenes14 and corresponding radicals (p-semiquinone from hydroquinone and o-semiquinone from catechol and 1,2-dichlorobenzene). At the same time, surface decomposition of the species leads to the formation of surface phenoxyl radicals. This is not the case with phenol, monochlorobenzene, and 2-monochlorophenol. The first two molecules have been shown to form phenoxyl species, and the reaction of phenoxyl radicals with the surface can lead to the formation of o-dihydroxybenzene radicals (o-semiquinone radical).13,14 2-Monochlorophenol forms mixture of phenoxyl and o-semiquinone radicals. The decays were driven by the most dominant EPFRs as evidenced by the good exponential fit. Considering the mechanistic differences of radical formation from different adsorbates and observed decay rates, we conclude that in the case of Zn(II)O surface, the long-lived species are semiquinone-type radicals, while the shorter-lived species are phenoxyl (or chlorophenoxyl) EPFRs.
Fig. 2.
First-order decay profile of EPFRs on Zn(II)O/silica surface dosed with different adsorbates at 230 °C on exposure to air at ambient condition.
Comparative lifetimes of EPFRs
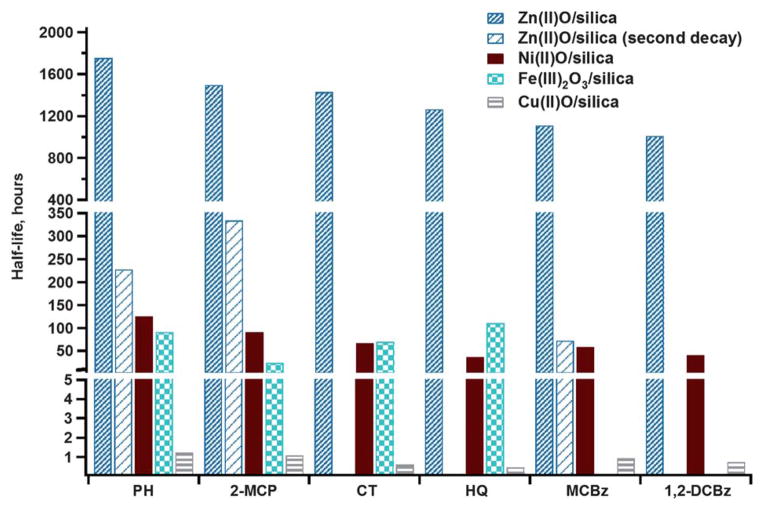
The persistence of the EPFRs formed on Zn(II)O surface was far longer compared to those previously formed from similar adsorbates on Fe(III)2O3, Ni(II)O, and Cu(II)O13,14 (cf. Fig. 3). While the half-lives measured for Cu(II)O were on the order of hours and on the order of days for both Fe(III)2O3 and Ni(II)O, the overall half-lives on Zn(II)O were on the order of months. Such long half-lives of EPFRs on Zn(II)O translate to almost a year-long presence of radicals on particles in ambient air and comprise a potentially hazardous environmental pollutants.
Fig. 3.
Comparative half-lives of EPFRs for different adsorbates on Ni(II)O, Cu(II)O, Fe(III)2O3, and Zn(II)O dosed with different adsorbates at 230 °C.
The significant differences in the half-life of EPFR on Zn(II)O were driven by the second decay rate (longer-lived semiquinone radical). In fact, the half-lives of shorter lived radicals (i.e. phenoxyl or chlorophenoxyl) were comparable with those observed on Fe(III)2O3 and Ni(II)O. The half-lives of EPFRs of chlorinated compounds, 1,2-dichlorobenzene and monochlorobenzene were so short and virtually non-measurable on Fe(III)2O3 but were not structurally different from the other EPFRs for Cu(II)O. This is attributed to the greater propensity of Fe(III)2O3 to form surface hypochlorites compared to Cu(II)O, Ni(II)O, and Zn(II)O.13,14
The study suggests that metals and transition metals with similar properties can catalyze the formation of EPFRs. It is evident from the results that their stability and persistency are most importantly determined by the metals they are bound to, as in the case of Zn(II)O which have much longer half-lives.
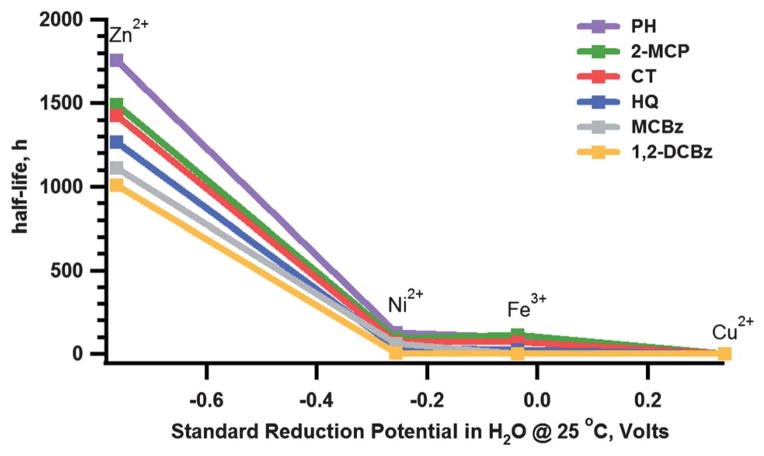
We see two possible contributing effects that might explain the observed persistency. First, it is possibly the consequence of all the electrons in the 3d sublevels being paired. It is known that complexes formed with zinc are highly stable due to their small ionic radius which enhance their interaction and also to the absence of a crystal-field splitting energy (CFSE). Since Zn2+ is a closed-shell transition metal ion, when the one-electron reduction occurs, it is likely that the electron from the 2p orbital of oxygen transfers to the 4s orbital of zinc and not to the 3d orbital. This process does not alter the CFSE of zinc resulting to a very stable radical. In contrast with the other transition metals used in the previous studies, the electron is accommodated to the 3d orbital leading to the distortion of CFSE as evidenced by the faster decay rate of EPFRs bound on Cu(II)O, Ni(II)O, and Fe(III)2O3. Second, the correlation between the half-life of the EPFRs and the reduction potential of the metal (cf. Fig. 4) indicates that the Gibb’s Free Energy of the reaction between the EPFR–Zn complex and the interacting species (whether molecular oxygen or any other species) is less spontaneous for metal that has more negative reduction potential. This correlation indicates that the stability of the radical is related with the reducibility of the metal or their ability to accept electron and suggests that the persistency of the EPFRs is rather dependent on their internal reaction with the metal oxide surface and not their reactivity toward molecular oxygen. However, to fully elucidate and established the governing mechanism behind the observed stability and persistency of EPFRs on Zn(II)O, additional studies are needed which is beyond the scope of this paper.
Fig. 4.
Relation of standard reduction potential (Mx+ to M0) of the metal with EPFR half-life.
Experimental
Particle surrogate samples (5% Zn(II)O supported on silica) were prepared by impregnation of silica gel with zinc(II) nitrate hexahydrate using the method of incipient wetness followed by calcination. Silica gel powder (Sigma-Aldrich, grade 923, 100–200 mesh size) was introduced into a sufficient amount of 0.308 M aqueous solution of zinc(II) nitrate hexahydrate (Sigma-Aldrich, zinc(II) nitrate hexahydrate, 99+%) for incipient wetness to occur. The resulting paste was equilibrated for 24 h at room temperature, dried at 120 °C for 12 h, and calcined in air at 450 °C for 5 h.
The adsorbate chemicals, catechol—CT (99+%), hydroquinone—HQ (99+%), phenol—PH (99+%), 2-mono-chlorophenol—2-MCP (99+%), monochlorobenzene—MCBz (99.8% anhydrous), and 1,2-dichlorobenzene—1,2-DCBz (99% HPLC grade) were used as received without further purification. All chemicals were purchased from Sigma-Aldrich unless otherwise noted.
The particulate samples were exposed to the vapors of the adsorbates using a custom-made vacuum exposure system.13,14 The system consisted of a vacuum gauge, dosing vial port, equilibration chamber, and 2 reactors. Prior to adsorption, each sample was oxidized in air in situ at 450 °C. Vapors of the molecular adsorbates were introduced into the equilibration chamber at the desired pressure (~1.33 KPa), and the particles were exposed to the adsorbate vapors at 230 °C for 5 min. Once the exposure was completed, the port and dosing tube were evacuated for 1 h at the dosing temperature and pressure of 1.33 Pa. The reactor was then sealed under vacuum, and the sample was cooled to room temperature prior to EPR measurements.
To determine the persistency and stability of the EPFRs, kinetic studies were performed. EPFR/Zn(II)O/silica samples were continuously exposed to room air at ambient temperature and the EPR signal was measured periodically to determine the radical concentration as a function of time.
All EPR measurements were performed using a Bruker EMX-2.0/2.7 EPR spectrometer with dual cavities, X-band, 100 kHz, and microwave frequency, 9.53 GHz. The spectra were obtained at room temperature. The typical operating parameters were: microwave power 1 mW, modulation amplitude 4 G, sweep width 200 G, time constant 40.960 ms, and sweep time 167.7 s.
Conclusions
The adsorption of various aromatic adsorbates on Zn(II)O supported on silica yielded EPFR–Zn complexes with the longest lifetimes observed compared to previously studied metals: Cu, Ni, and Fe. The lifetime of EPFR on zinc ranged from 3–73 days, which translates to an almost year-long presence of the EPFRs for some of the adsorbates. The most stable species with very long lifetimes (over 50 days in ambient air) are attributed to surface-bound semiquinone species. Decay of the radicals is probably a result of their internal oxidation with the metal oxide. Significant differences in the EPFR half-life correlate with the standard reduction potential of the associated metal.
Environmental impact.
The half-lives of EPFRs formed on zinc oxide domains were the longest observed among transition metal oxides studied. The half-lives of EPFR on zinc oxide range from 3.0 to 73 days and are an order of magnitude longer than those formed on nickel and iron oxides and by 2 orders of magnitude longer compared to copper oxides which have half-lives only on the order of hours. The longest-lived radicals on ZnO correspond to the persistency in ambient air particles for a year. Such long lifetimes comprise a potentially high impact on the environment, as exposure to the EPFRs has been shown to result in adverse health effects.
Acknowledgments
This research was supported by the National Institute of Environmental Health Sciences, Superfund Research Program under Center Grant P42ES013648-3
Notes and references
- 1.Dockery DW, Pope CA, Xu X, Spengler JD, Ware JH, Fay ME, Ferris BG, Speizer FE. N Engl J Med. 1993;329:1753–1759. doi: 10.1056/NEJM199312093292401. [DOI] [PubMed] [Google Scholar]
- 2.Pope CA, III, Burnett RT, Thun MJ, Calle EE, Krewski D, Ito K, Thurston GD. JAMA, J Am Med Assoc. 2002;287:1132–1141. doi: 10.1001/jama.287.9.1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Struis RPWJ, Ludwig C, Lutz H, Scheidegger AM. Environ Sci Technol. 2004;38:3760–3767. doi: 10.1021/es0346126. [DOI] [PubMed] [Google Scholar]
- 4.Gao Y, Nelson ED, Field MP, Ding Q, Li H, Sherrell RM, Gigliotti CL, Van Ry DA, Glenn TR, Eisenreich SJ. Atmos Environ. 2002;36:1077–1086. [Google Scholar]
- 5.Chandler AJ, Eighmy TT, Hartlen J, Hjelmar O, Kosson DS, Sawell SE, Van der Sloot HA, Vehlow J. The International Ash Working Group (IAWG) Elsevier Science B.V; Amsterdam: 1997. [Google Scholar]
- 6.Kothai P, Prathibha P, Saradhi IV, Pandit GG, Puranik VD. Int J Civ Environ Eng. 2009;1:27–30. doi: 10.1007/s10661-009-1090-7. [DOI] [PubMed] [Google Scholar]
- 7.Martuzevicius D, Grinshpun SA, Reponen T, Garny RL, Shukla R, Lockey J, Hu S, McDonald R, Biswas P, Kliucininkas L, LeMasters G. Atmos Environ. 2004;38:1091–1105. [Google Scholar]
- 8.Miljevic B, Fairfull-Smith KE, Bottle SE, Ristovski ZD. Atmos Environ. 2010;44:2224–2230. [Google Scholar]
- 9.Dellinger B, Pryor WA, Cueto B, Squadrito GL, Deutsch WA. Proc Combust Inst. 2000;28:2675–2681. [Google Scholar]
- 10.Peters DC, Epstein FH, McVeigh ER. Magn Reson Med. 2001;45:562–567. doi: 10.1002/mrm.1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Valavanidis A, Fiotakis K, Bakeas E, Vlahogianni T. Redox Rep. 2005;10:37–51. doi: 10.1179/135100005X21606. [DOI] [PubMed] [Google Scholar]
- 12.Gurgueira SA, Lawrence J, Coull B, Murthy GGK, Gonzalez-Flecha B. Environ Health Perspect. 2002;110:749–755. doi: 10.1289/ehp.02110749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vejerano E, Lomnicki S, Dellinger B. Environ Sci Technol. 2011;45:589–594. doi: 10.1021/es102841s. [DOI] [PubMed] [Google Scholar]
- 14.Lomnicki S, Truong H, Vejerano E, Dellinger B. Environ Sci Technol. 2008;42:4982–4988. doi: 10.1021/es071708h. [DOI] [PubMed] [Google Scholar]
- 15.Fahmy B, Ding L, You D, Lomnicki S, Dellinger B, Cormier SA. Environ Toxicol Pharmacol. 2010;29:173–182. doi: 10.1016/j.etap.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lord K, Moll D, Lindsey JK, Mahne S, Raman G, Dugas T, Cormier S, Troxlair D, Lomnicki S, Dellinger B, Varner K. J Recept Signal Transduction. 2011;31:157–167. doi: 10.3109/10799893.2011.555767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Balakrishna S, Lomnicki S, McAvey KM, Cole RB, Dellinger B, Cormier SA. Part Fibre Toxicol. 2009;6:11. doi: 10.1186/1743-8977-6-11. [DOI] [PMC free article] [PubMed] [Google Scholar]