Abstract
Background
Gentamicin pharmacokinetics have not been described in patients undergoing short-daily hemodialysis (SDHD). The aim of this study is to describe gentamicin pharmacokinetics and dialytic clearance (Cldial) in SDHD patients and simulate gentamicin exposure after six dosing regimens to help guide future dosing.
Methods
Six anuric patients undergoing SDHD were enrolled. Patients received IV infusion of 2 mg/kg gentamicin on day 1 after first HD session followed by HD sessions on days 2, 3, and 4. Blood samples for determination of gentamicin concentrations were serially collected. Gentamicin pharmacokinetic parameters and Cldial and inter-individual variability terms (IIV) were estimated using NONMEM VII. Influence of patient weight on systemic clearance (Cls) and central volume of distribution (Vc) and influence of urea removal estimates on Cldial were assessed. The model was used to simulate gentamicin concentrations after six dosing regimens including pre- and post-dialysis as well as daily and every other day dosing.
Results
A two-compartment model with first-order elimination from central compartment described gentamicin pharmacokinetics. Population estimates for Cls and Cldial were 7.6 and 134 ml/min, respectively. Patient weight was statistically significantly associated with Cls and Vc
Pre-dialysis every other day regimens were as effective (Cmax ≥ 8 mg/L and AUC48 hrs ≥ 140 mg.hr/L) and less toxic (Cmin < 2 mg/L and AUC48 hrs < 240 mg.hr/L) than post-dialysis regimens.
Conclusions
Estimated gentamicin Cldial is higher than previous estimates with thrice-weekly regimens. Pre-dialysis every other day dosing may be recommended during SDHD.
Keywords: gentamicin, hemodialysis, pharmacokinetics, renal failure
Introduction
Bacterial infections remain the second leading cause of death in patients with chronic kidney disease, stage 5 (CKD-5) [1]. Aminoglycosides remain critical for treating multidrug-resistant gram negative and gram positive infections [2,3]. Optimization of aminoglycoside dosing to ensure maximal efficacy and minimal toxicity is essential. The best predictors of efficacy are the ratios of peak aminoglycoside plasma concentration (Cmax) or area under the curve (AUC) to the minimum inhibitory concentration (MIC). Hence, dosing regimens that maximize these are expected to maximize efficacy and prevent emergence of resistant strains [4].
Short daily hemodialysis (SDHD), usually 2 hours done 6 days per week, is an effective alternative to conventional thrice-weekly 4-hour hemodialysis. SDHD improves quality of life and reduces medical complications (i.e. blood pressure reduction, hyperkalemia, inflammation), associated with CKD-5[1,5,6].
The purpose of this study was to describe gentamicin pharmacokinetics in patients undergoing SDHD. Furthermore, the estimated gentamicin pharmacokinetic parameters were used to perform simulations to predict gentamicin exposure after six different dosing regimens.
Subjects and Methods
Six noninfected anuric subjects undergoing outpatient SDHD at the Indiana University Outpatient Dialysis Center (Indianapolis, IN) were enrolled in the study. Subjects were eligible for the study if they were 18 years of age or older, received regular SDHD six times weekly, suffered from no other acute illnesses, and had a postdialysis weight within 30% of ideal body weight [7]. Subjects were excluded from the study if they had a history of gentamicin allergy or if they received gentamicin within three weeks of enrollment. The study protocol was approved by the Indiana University-Purdue University-Indianapolis Institutional Review Board. All subjects provided written informed consent before participating in any study procedures. All study procedures were conducted in the Indiana Clinical Research Center (ICRC) as part of the Indiana Clinical and Translational Sciences Institute at Indiana University Hospital (Indianapolis, IN).
Each subject received a 2 mg/kg gentamicin intravenous (IV) infusion over 30 min (n=5) or 60 min (n=1) on day 1 after the first SDHD session. Blood samples were collected at the end of the dialysis session immediately before drug administration and then at 0.5, 1, 1.5, 2, 3, and 5 hours after the end of dialysis/start of the gentamicin infusion. On day 2, blood samples were collected just before the start, at the middle (1 hr), and at the end of the second SDHD session and then at 0.5, 1, 2, and 4 hours after the end of the dialysis session. On days 3 and 4, blood samples were collected before the start, at the middle (1 hr), and at the end of the dialysis sessions. All samples were collected in nonheparinized evacuated blood collection tubes and allowed to clot. Samples were centrifuged and serum was harvested and stored at −70°C until analyzed in batch. Dialysis procedures were performed using a new unused cellulose triacetate high-flux dialyzer (Exeltra 150 Baxter Healthcare, Deerfield, IL). The dialyzer had an ultrafiltration coefficient of 31.5 ml/hr per mmHg and a surface area of 1.5 m2. Serum gentamicin concentrations were determined using an Enzyme Multiplied Immunoassay Technique (EMIT; Syva Co., Dade Behring Inc., Cupertino, CA) [8]. The lower limit of quantification for the assay was 0.5 μg/ml with calibration curves constructed for concentrations up to 10 μg/ml. Intraday and interday coefficients of variation were less than 12% at 1 μg/ml and 8 μg/ml. Urea nitrogen and creatinine concentrations were determined by colorimetric methods [8,9]. This assay had intra-and inter-assay coefficients of variation of <5% for both urea nitrogen and creatinine.
Gentamicin serum concentrations from all subjects were used simultaneously to perform population compartmental pharmacokinetic modeling using NONMEM (Version VII; Globomax LLC, MD, USA). Initially, gentamicin concentrations on day 1 only (after the first SDHD session and before the second session) were used to describe gentamicin disposition without the effect of dialysis. A two compartment structural pharmacokinetic model [8] was used to describe gentamicin pharmacokinetics during inter-dialysis periods (equations 1 and 2):
| (1) |
| (2) |
Where X(1) and X(2) are the amounts of drug in the central and peripheral compartments, dX(1)/dt and dX(2)/dt are the rates of change in drug amount over time for central and peripheral compartments, Vc and Vp are the apparent volumes of distribution in the central and peripheral compartments, respectively, R0 is the zero-order infusion rate, Cls and Cld are the systemic and distribution clearances, respectively.
The above model was used to obtain population parameter estimates during inter-dialysis periods. Subsequently, gentamicin concentrations from days 1-4 were used in the overall model that describes the additional effect of SDHD on gentamicin pharmacokinetics. Effect of dialysis on gentamicin elimination was modeled using a third compartment representing the dialyzer. Gentamicin dialysis clearance (Cldial) into the third compartment was turned on and off according to the schedule of dialysis sessions. Equation 1 was modified to express Cldial as shown in equation 3, whereas equation 5 was used to describe change in gentamicin amounts in the dialysis compartment and equation 4 described change in gentamicin amounts in peripheral compartment.
| (3) |
| (4) |
| (5) |
An indicator variable “DIAL” with values of 1 or 0 was used to turn the dialysis compartment on and off, respectively, depending on the timing of daily dialysis sessions [8]. Transfer of drug between the three compartments was assumed to be following first order processes.
Inter-individual variability (IIV) on Cls, Vc, and Cldial was modeled using an exponential IIV model assuming log-normal distribution of the between-subject variability in population parameter estimates. Each subject's estimated Cls, Vc, and Cldial were therefore related to the corresponding population estimate. ^Residual unexplained variability (RUV), including intra-individual variability, was modeled using an additive error term.The best structural model to describe observed data was chosen based on goodness-of-fit plots, minimum value of objective function (OFV), as well as individual plots of observed and model-predicted concentrations versus time.
The final structural model was used to test the effects of subject and dialysis covariates on the model parameters. The effects of subject weight on population parameter and IIV estimates for Cls and Vc were examined. Similarly, to determine if there was a significant impact of urea removal on gentamicin removal , the effects of single-pool Kt/Vurea (spKt/V), equilibrated Kt/Vurea (eKt/V), and weekly standard Kt/V (as dialyzer-specific covariates) on Cldial were evaluated. All urea removal estimates were calculated using standard methods previously described by Leypoldt et al [9]. Covariates were kept in the model if their addition resulted in a statistically significant decrease in OFV (a decrease of 3.84 units is considered statistically significant at α=0.05 using a chi-square test). Relationship between subject weight and each of Cls and Vc was described using a power model after correcting each subject's weight for the median weight value according to equation 6:
| (6) |
Where Ɵ1 is the typical value (population estimate) of the parameter (Cls or Vc) in a subject weighing 91 kg (median weight for the six subjects), WT is the subject weight, and Ɵ2 is the power term describing the effect of subject weight on typical value of the parameter. A similar approach was used to test the effects of dialyzer-specific covariates on Cldial but with no correction of subject values to the median of the population.
The final model (including covariates) was used to simulate gentamicin plasma concentrations using NONMEM VII after six different dosing regimens, A1, A2, B1, B2, C1, and C2, as shown in Table 1 in order to determine the best dosing strategy in patients undergoing SDHD. All doses were simulated as 30 min infusions and plasma concentrations were simulated for a 1-week interval. Two of those regimens (B1 and C1) have been tested in a previous study [10]. Previous studies have proposed the use of AUC ≥ 140 mg.hr/L/48 hr and Cmax ≥ 8 mg/L as pharmacodynamic targets indicating achievement of concentrations high enough to cause bacterial killing[8,11]. Similarly, AUC ≤ 240 mg.hr/L/48 hr and Cmin < 2 mg/L were proposed as targets indicating achievement of concentrations low enough between doses to avoid toxicity (i.e. non-toxic). We used these same targets to evaluate the performance of six different dosing regimens.
Table 1.
Dosing regimens used to simulate gentamicin exposure.
| Regimen | Initial loading dose (mg/kg) | Follow-up doses (mg/kg) | Timing of follow-up doses |
|---|---|---|---|
| A1 | 2 | 1 | Immediately after every other SDHD |
| A2 | 2 | 0.5 | Immediately after each SDHD |
| B1 | 3.1 | 2.75 | 1 hr before every other SDHD |
| B2 | 3.1 | 1.375 | 1 hr before each SDHD |
| C1 | 3 | 3 | 1 hr before every other SDHD |
| C2 | 3 | 1.5 | 1 hr before each SDHD |
Areas under the simulated plasma concentration versus time curves (AUC) were calculated using the linear trapezoidal method for the time intervals 0-48 (AUC0-48), 48-96 (AUC48-96), and 96-144 (AUC96-144) based on the simulations. Percentages of simulated subjects achieving a 48 hr interval AUC ≥140 mg.hr/L/48 hr, a 48 hr interval AUC ≤240 mg.hr/L/48 hr, a peak plasma concentration ≥ 8 mg/L (30 min after the end of infusion), and a trough (pre-dialysis for regimen A and pre-dose for regimens B and C) plasma concentration < 2 mg/L were calculated.
Results
Six subjects (1 female and 5 male subjects) enrolled in and completed the study. Subjects had a median age of 54 years (28-59) and a median weight of 91 kg (59-110). No adverse effects related to gentamicin were reported. The dialysis blood flow rate in all subjects was 500 mL/min. The median (range) dialysis duration was 2.5 (2.0-2.5) hours. Observed gentamicin plasma concentration-time profiles were described by a two-compartment model with first order elimination from the central compartment. The effect of dialysis was described by a third compartment with Cldial controlling the one-way transfer of drug from the central to the dialysis compartment during intra-dialysis periods. Table 2 shows the model-estimated population PK parameters with the associated IIV where applicable. Addition of IIV terms on only Cls, Vc, and Cldial led to significant improvement in the model evident by a significant decrease in the OFV.
Table 2.
Gentamicin population PK parameter estimates for the structural and the covariate models.
| Structural Model | Final Covariate Model | |||||
|---|---|---|---|---|---|---|
| Parameter | Population Estimate | Relative standard error % | Inter-individual Variability | Population Estimate | Relative standard error % | Inter-individual Variability |
| Cls1 (ml/min) | 6 | 20.8 | 55.7 | 7.6 | 5.5 | 0.3 |
| Vc2 (L) | 9.5 | 38.1 | 90.7 | 12.1 | 23.2 | 50.7 |
| Vp3 (L) | 10.6 | 2.2 | NA6 | 10.5 | 5.1 | NA |
| Cld4 (ml/min) | 116.2 | 12.3 | NA | 128.7 | 8.8 | NA |
| Cldial5 (ml/min) | 139.5 | 15.2 | 34.5 | 134.2 | 15.9 | 29.3 |
Cls, systemic clearance
Vc, apparent volume of distribution for central compartment
Vp, apparent volume of distribution for peripheral compartment
Cld, distribution clearance between central and peripheral compartments
Cldial, dialysis clearance
NA, no inter-individual variability was estimated for that parameter
The structural PK model was used to examine the effects of different subject and dialysis covariates on the estimated PK model parameters and the associated IIV parameters. Subject weight was significantly associated with Cls and Vc as evident from drop in OFV of 15 and 5 points upon addition of subject weight as a covariate on Cls and Vc, respectively. The addition of subject weight as a covariate on Cls led to a marked decrease in IIV estimate from 55.7% to 0.3%. Similarly, addition of subject weight as a covariate on Vc led to a decrease in IIV from 90.7% to 50.7%. The relationship between subject weight and each of Cls and Vc was explained by equations 7 and 8, respectively.
| (7) |
| (8) |
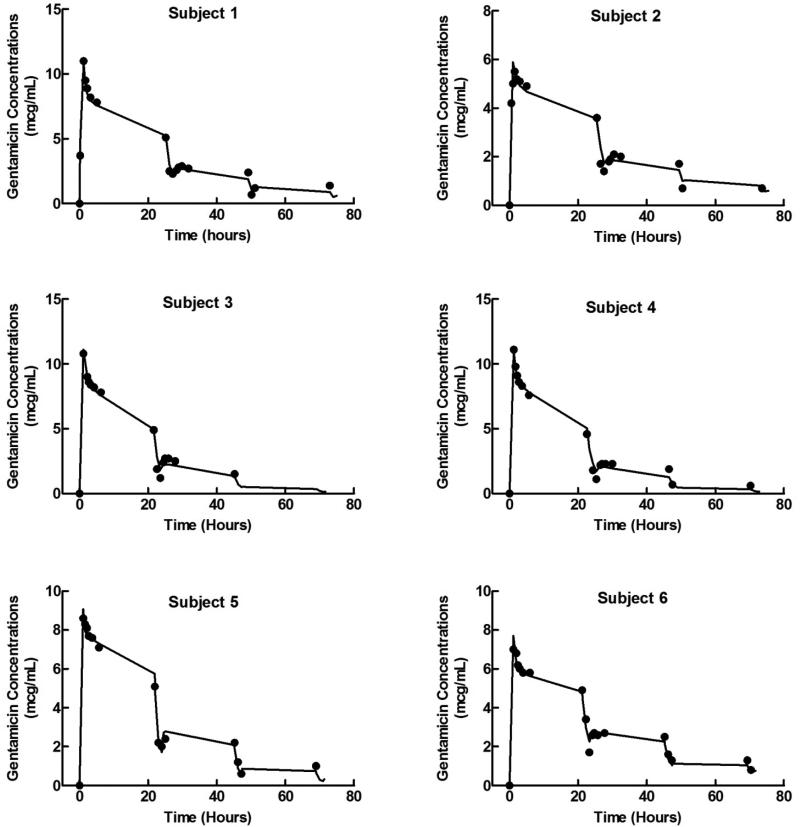
where Cls,TV and Vc,TV are the population estimates of systemic clearance and apparent volume of distribution in central compartment and WT is the subject's weight. The power terms describing the effects of subject weight on the Cls and Vc were estimated to be 1.97 with a relative standard error of 24.8% and 2.27 with a relative standard error of 54.6%, respectively. Table 2 shows the final parameter estimates from the final covariate model. The final covariate model was able to accurately predict the observed gentamicin plasma concentration-time profiles for the six subjects as shown in Figure 1. There was no statistically significant impact of spKt/V, eKt/V, and weekly standard Kt/V (as dialyzer-specific covariates) on Cldial (data not shown).
Figure 1.
Observed and model predicted gentamicin plasma concentration-time profiles for the six subjects. Solid lines represent the model individual predicted concentrations and the closed circles represent the observed concentrations.
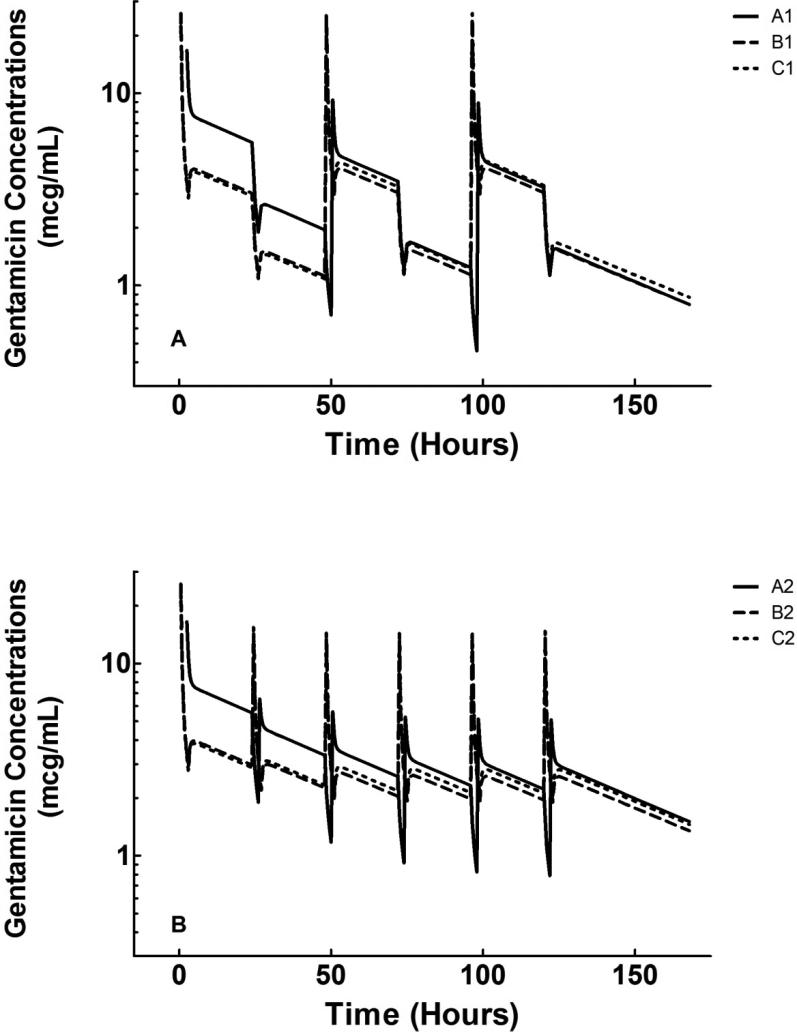
Gentamicin exposure was simulated after six dosing regimens. Figure 2 shows the simulated plasma concentration-time profiles (mean of 1000 simulations) for both every other day and daily regimens. Post-dialysis dosing regimens (A1 and A2) led to about 90% of simulated subjects achieving Cmax ≥ 8 mg/L after the first dose but almost none of the subjects achieving this target after the second and third doses. With pre-dialysis dosing, about 70-80% of simulated subjects achieved a Cmax ≥ 8 mg/L after all doses of the every other day regimens (B1 and C1) and after the first dose of the daily dosing regimens (B2 and C2). (Tables 3 and 4)
Figure 2.
Simulated concentration-time profiles (mean of 1000 simulations) for every other day (A) and daily (B) regimens. Regimen A1, initial dose of 2 mg/kg followed by follow-up doses of 1 mg/kg after every other SDHD session; Regimen A2, initial dose of 2 mg/kg followed by follow-up doses of 0.5 mg/kg after each SDHD session; Regimen B1, initial dose of 3.1 mg/kg followed by follow-up doses of 2.75 mg/kg after every other SDHD session; Regimen B2, initial dose of 3.1 mg/kg followed by follow-up doses of 1.375 mg/kg after each SDHD session; Regimen C1, initial dose of 3 mg/kg followed by follow-up doses of 3 mg/kg after every other SDHD session; Regimen C2, initial dose of 3 mg/kg followed by follow-up doses of 1.5 mg/kg after each SDHD session.
Table 3.
AUC, peak and trough gentamicin plasma concentrations on days 1, 3, and 5 following each of the six simulated dosing regimens
| Regimen | First Day |
Third Day |
Fifth Day |
||||||
|---|---|---|---|---|---|---|---|---|---|
| AUC (mg.hr/L) | Cmax (mg/L) | Cmin (mg/L) | AUC (mg.hr/L) | Cmax (mg/L) | Cmin (mg/L) | AUC (mg.hr/L) | Cmax (mg/L) | Cmin (mg/L) | |
| A1 | 208±29 | 10.7±2.1 | 1.9±0.3 | 132±16 | 6.3±1 | 1.2±0.2 | 123±15 | 5.9±0.9 | 1.2±0.2 |
| A2 | 225±33 | 10.7±1.9 | 3.3±0.3 | 137±17 | 4.3±0.6 | 2.3±0.2 | 123±15 | 3.8±0.5 | 2.2±0.2 |
| B1 | 132±32 | 9.2±1.7 | 1.1±0.4 | 132±35 | 8.9±1.8 | 1.1±0.5 | 132±36 | 8.9±1.8 | 1.1±0.5 |
| B2 | 169±38 | 9.1±1.6 | 2.2±0.6 | 128±35 | 5.5±1.2 | 1.9±0.6 | 125±34 | 5.3±1.2 | 1.9±0.6 |
| C1 | 128±30 | 8.9±1.7 | 1.1±0.4 | 142±37 | 9.7±1.9 | 1.2±0.5 | 144±39 | 9.7±1.9 | 1.2±0.5 |
| C2 | 170±38 | 8.8±1.6 | 2.3±0.6 | 138±36 | 5.9±1.4 | 2.1±0.6 | 135±36 | 5.8±1.3 | 2.1±0.6 |
Data presented as mean ± SD. AUC values are for 48 hr intervals.
Table 4.
Percent of simulated subjects achieving AUC and peak and trough concentration targets following each of the six simulated dosing regimens
| Regimen | First Day |
Third Day |
Fifth Day |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AUC (mg.hr/L) |
AUC (mg.hr/L) |
AUC (mg.hr/L) |
|||||||||||||
| ≤240 | ≥140 | 140-240 | Cmax ≥ 8 mg/L | Cmin < 2 mg/L | ≤240 | ≥140 | 140-240 | Cmax ≥ 8 mg/L | Cmin < 2 mg/L | ≤240 | ≥140 | 140-240 | Cmax ≥ 8 mg/L | Cmin < 2 mg/L | |
| A1 | 86.9 | 98.6 | 85.5 | 87.1 | 58.5 | 100 | 32.7 | 32.7 | 0.2 | 99.5 | 100 | 11.8 | 11.8 | 0 | 99.9 |
| A2 | 42.3 | 99.7 | 42 | 89.4 | 0 | 100 | 43.5 | 43.5 | 0 | 9.3 | 100 | 11.2 | 11.2 | 0 | 18.9 |
| B1 | 100 | 37.2 | 37.2 | 77.8 | 97.2 | 99.9 | 37.2 | 37.1 | 70.8 | 93.9 | 99.8 | 37.7 | 37.5 | 70.9 | 93.5 |
| B2 | 96.4 | 76.5 | 72.9 | 77.9 | 39.1 | 99.7 | 32.1 | 31.8 | 2.1 | 57.8 | 99.8 | 29.1 | 28.9 | 1.3 | 60.5 |
| C1 | 100 | 32.2 | 32.2 | 72.7 | 98 | 99.3 | 48.6 | 47.9 | 81.5 | 91.5 | 99.1 | 50.1 | 49.2 | 82.3 | 89.8 |
| C2 | 96.1 | 75.9 | 72 | 71.8 | 32.8 | 99.1 | 44.1 | 43.2 | 6.9 | 46.5 | 99.5 | 41.7 | 41.2 | 5.2 | 47.5 |
Data presented as percent of 1000 simulated subjects achieving AUC between 140 and 240 mg.hr/L/48 hrs, below 240 mg.hr/L/48 hrs, or above 140 mg.hr/L/48 hrs and percent of 1000 simulated subjects achieving Cmax ≥ 8 mg/L and Cmin < 2 mg/L. AUC on fifth day excludes the last 24 hours of the week period.
The calculated 48 hr AUC values based on simulations showed that, although all subjects achieved AUC ≥ 140 mg.hr/L/48 after the first dose in regimens “A1” and “A2”, only 33-44% and 12% achieved it after the second and third doses, respectively. Pre-dialysis every other day regimens resulted in 37-50% of simulated subjects achieving an AUC ≥ 140 mg.hr/L/48 hr. Similarly, less than 50% of simulated subjects achieved this target after the pre-dialysis daily regimens (except for following first dose).
Discussion
Estimated gentamicin systemic clearance was 7.6 ml/min with a mean calculated elimination half-life of approximately 35 hrs. This estimate of half-life during inter-dialysis periods is similar to what was estimated previously [8]. Similar estimates of gentamicin systemic clearance during interdialytic periods have also been reported [11,12]. Dialytic clearance was estimated to be 134 ml/min in our study. Previous studies have reported dialysis clearance values between 78-116 ml/min [8,11-14].
Results of the simulations showed that with post-dialysis dosing, although 80-90% of subjects may achieve a Cmax ≥ 8 mg/L following the first dose, none of the simulated subjects achieved that target after subsequent doses. On the other hand, pre-dialysis dosing regimens performed similarly and better than post-dialysis regimens in terms of percent of simulated subjects achieving Cmax ≥ 8 mg/L. More than 70% of simulated subjects achieved a Cmax ≥ 8 mg/L after all doses of every other day dosing regimens (B1 and C1). These results indicate that pre-dialysis dosing of gentamicin may perform better than post-dialysis regimens in achieving effective concentrations due to the presence of remaining gentamicin concentrations from previous dose. The above results also show that every other day pre-dialysis dosing performs better than daily dosing in terms of achieving efficacy targets. This might be due to the smaller dose used with daily regimens and the inability to increase such dose due to the associated risk of toxicity.
In terms of the toxicity target, every other day regimens performed generally better than daily regimens. Less than 20% of the subjects achieved a Cmin < 2 mg/L after the daily dosing regimen (A2) and only 33-61% achieved the same target after the daily regimens (B2 and C2). These results indicate that daily dosing in patients on SDHD may lead to sustained high trough concentrations potentially predisposing to aminoglycoside toxicity. Hence, every other day dosing is more suitable to allow for adequate removal of gentamicin between doses. The above results also show that the pre-dialysis and post-dialysis dosing may perform the same when it comes to achieving non-toxic concentrations. This is expected given the fact that dosing before or after the dialysis would have a greater effect on the peak rather than the trough concentrations.
Achievement of pre-determined exposure targets was also assessed using 48 hr interval AUC values. All six dosing regimens performed similarly in terms of achieving a toxicity target of AUC ≤ 240 mg.hr/L/48 hr with all subjects achieving this target (except for first dose of regimen A2). Unlike the results of the peak concentrations, the pre-dialysis regimens performed worse than the post-dialysis regimens in terms of achieving an AUC ≥ 140 mg.hr/L/48 hr after the first dose. After subsequent doses regimen “C” performed consistently better than regimen “B” with almost 50% of the subjects achieving the AUC target in regimens “C1” and “C2” after all subsequent doses assessed. Although worse after the first dose, regimen “C” still performed better than regimen “A” after subsequent doses. Taking these results together with the results of peak concentrations, we can conclude that pre-dialysis dosing performs generally better than post-dialysis dosing in terms of achieving effective gentamicin concentrations.
The results were compared to those obtained previously from simulations of every other day dosing in subjects undergoing thrice-weekly hemodialysis [8]. Percentages of simulated subjects achieving Cmax ≥ 8 mg/L were similar between the two studies for all doses. On the other hand, all three regimens performed better in our study in terms of achieving the toxicity target of Cmin < 2 mg/L. This can be due to differences in the effectiveness of the dialyzer used in our study or due to the more effective removal of gentamicin with more frequent dialysis sessions. Such results indicate that regimens B1 and C1 may be as effective and less toxic when given to subjects undergoing SDHD compared to subjects on thrice-weekly hemodialysis. The more frequent dialysis with SDHD is expected to enhance gentamicin removal leading to lower trough concentrations while achieving peak concentrations similar to those achieved with thrice-weekly hemodialysis.
This study has some limitations. Data used to develop the pharmacokinetic model were obtained from a relatively small sample of dialysis patients. Also, our data did not include pharmacodynamics measurements of drug efficacy or institutional MIC values. This study, hence, represents a pharmacodynamics simulation study that predicts drug efficacy based on simulated exposure. This is a relatively common approach that has been used before to help guide dosing regimens in special patient populations.
Conclusions
Gentamicin is effectively removed during SDHD using the Exeltra 150 dialyzer with an estimated Cldial higher than reported in previous studies. Results of the simulations indicate that pre-dialysis every other day regimens may be as effective and less toxic than post-dialysis regimens and that the same every other day regimens can be used for subjects undergoing thrice-weekly or SDHD.
Acknowledgments
Supported by grants from The National Kidney Foundation of Indiana, Inc., Indianapolis, IN and the Indiana Clinical and Translational Sciences Institute funded, in part by Grant Number Grant # RR025761 from the National Institutes of Health, National Center for Research Resources, Clinical and Translational Sciences Award.
Footnotes
Conflict of Interest Statement: The above authors have no conflicts of interested related to this paper.
References
- 1.Usrds 2010 annual data report: Atlas of end-stage renal disease in the united states.; Us renal data system. National Institutes of Health, National Institutes of Diabetes amd Digestive and Kidney Diseases; Bethesda, MD: 2010. [Google Scholar]
- 2.Drusano GL, Louie A. Optimization of aminoglycoside therapy. Antimicrob Agents Chemother. 2011;55:2528–2531. doi: 10.1128/AAC.01314-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Le T, Bayer AS. Combination antibiotic therapy for infective endocarditis. Clin Infect Dis. 2003;36:615–621. doi: 10.1086/367661. [DOI] [PubMed] [Google Scholar]
- 4.Craig WA. Pharmacokinetic/pharmacodynamic parameters: Rationale for antibacterial dosing of mice and men. Clin Infect Dis. 1998;26:1–10. doi: 10.1086/516284. quiz 11-12. [DOI] [PubMed] [Google Scholar]
- 5.Decker BS, Kays MB, Chambers M, Kraus MA, Moe SM, Sowinski KM. Vancomycin pharmacokinetics and pharmacodynamics during short daily hemodialysis. Clin J Am Soc Nephrol. 2010;5:1981–1987. doi: 10.2215/CJN.03450410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vos PF, Zilch O, Kooistra MP. Clinical outcome of daily dialysis. Am J Kidney Dis. 2001;37:S99–S102. doi: 10.1053/ajkd.2001.20761. [DOI] [PubMed] [Google Scholar]
- 7.Green B, Duffull SB. Development of a dosing strategy for enoxaparin in obese patients. Br J Clin Pharmacol. 2003;56:96–103. doi: 10.1046/j.1365-2125.2003.01849.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sowinski KM, Magner SJ, Lucksiri A, Scott MK, Hamburger RJ, Mueller BA. Influence of hemodialysis on gentamicin pharmacokinetics, removal during hemodialysis, and recommended dosing. Clin J Am Soc Nephrol. 2008;3:355–361. doi: 10.2215/CJN.02920707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leypoldt JK, Jaber BL, Zimmerman DL. Predicting treatment dose for novel therapies using urea standard kt/v. Semin Dial. 2004;17:142–145. doi: 10.1111/j.0894-0959.2004.17212.x. [DOI] [PubMed] [Google Scholar]
- 10.Dang L, Duffull S. Development of a semimechanistic model to describe the pharmacokinetics of gentamicin in patients receiving hemodialysis. J Clin Pharmacol. 2006;46:662–673. doi: 10.1177/0091270006286902. [DOI] [PubMed] [Google Scholar]
- 11.Teigen MM, Duffull S, Dang L, Johnson DW. Dosing of gentamicin in patients with end-stage renal disease receiving hemodialysis. J Clin Pharmacol. 2006;46:1259–1267. doi: 10.1177/0091270006292987. [DOI] [PubMed] [Google Scholar]
- 12.Vercaigne LM, Ariano RE, Zacharias JM. Bayesian pharmacokinetics of gentamicin in a haemodialysis population. Clin Pharmacokinet. 2004;43:205–210. doi: 10.2165/00003088-200443030-00004. [DOI] [PubMed] [Google Scholar]
- 13.Amin NB, Padhi ID, Touchette MA, Patel RV, Dunfee TP, Anandan JV. Characterization of gentamicin pharmacokinetics in patients hemodialyzed with high-flux polysulfone membranes. Am J Kidney Dis. 1999;34:222–227. doi: 10.1016/s0272-6386(99)70347-1. [DOI] [PubMed] [Google Scholar]
- 14.Dager WE, King JH. Aminoglycosides in intermittent hemodialysis: Pharmacokinetics with individual dosing. Ann Pharmacother. 2006;40:9–14. doi: 10.1345/aph.1G064. [DOI] [PubMed] [Google Scholar]