Abstract
Study Design Biomechanical study.
Objective Posterior instrumentation is used to stabilize the spine after a lumbar laminectomy. However, the effects on the adjacent segmental stability are unknown. Therefore, we studied the range of motion (ROM) and stiffness of treated lumbar spinal segments and cranial segments after a laminectomy and after posterior instrumentation in flexion and extension (FE), lateral bending (LB), and axial rotation (AR). These outcomes might help to better understand adjacent segment disease (ASD), which is reported cranial to the level on which posterior instrumentation is applied.
Methods We obtained 12 cadaveric human lumbar spines. Spines were axially loaded with 250 N for 1 hour. Thereafter, 10 consecutive load cycles (4 Nm) were applied in FE, LB, and AR. Subsequently, a laminectomy was performed either at L2 or at L4. Thereafter, load-deformation tests were repeated, after similar preloading. Finally, posterior instrumentation was added to the level treated with a laminectomy before testing was repeated. The ROM and stiffness of the treated, the cranial adjacent, and the control segments were calculated from the load-displacement data. Repeated-measures analyses of variance used the spinal level as the between-subject factor and a laminectomy or instrumentation as the within-subject factors.
Results After the laminectomy, the ROM increased (+19.4%) and the stiffness decreased (−18.0%) in AR. The ROM in AR of the adjacent segments also increased (+11.0%). The ROM of treated segments after instrumentation decreased in FE (−74.3%), LB (−71.6%), and AR (−59.8%). In the adjacent segments after instrumentation, only the ROM in LB was changed (−12.9%).
Conclusions The present findings do not substantiate a biomechanical pathway toward or explanation for ASD.
Keywords: human lumbar spine, single-level laminectomy, instrumentation, biomechanics, adjacent segment disease (ASD)
Introduction
When conservative treatment fails, symptomatic lumbar spinal stenosis is commonly treated with surgical decompression, often a laminectomy. Previously, it has been shown in vitro that a laminectomy reduces the threshold at which shear forces and torsion moments cause spondylolisthesis or spinal failure.1 2 Furthermore, it was also found that a laminectomy alters biomechanical behavior under submaximal loading.3 In clinical practice, a facet-sparing laminectomy can cause spondylolisthesis with a reported incidence of 8 to 31%.4 5 To prevent complications, surgeons often use posterior instrumentation to obtain arthrodesis (i.e., fusion of a segment by bone formation).6
However, rigid posterior instrumentation has several disadvantages associated with its use. The procedure of stabilization itself increases the probability of implant-related complications, including infection, pseudarthrosis, nerve injury, increased blood loss during surgery, extended surgery time, and instrumentation failure. Furthermore, the use of instrumentation significantly increases the costs of surgery,7 especially because early reoperations are more common after a laminectomy in combination with a spinal fusion.8 Finally, the use of posterior instrumentation has been associated with adjacent segment disease (ASD).7 9 ASD is a symptomatic deterioration of the intervertebral disk adjacent to a previous fusion and is seen predominantly at the adjacent cranial levels.10 11 The altered biomechanical behavior of the adjacent level, specifically an increased range of motion (ROM) and a decreased stiffness as evidence of instability, due to the use of posterior instrumentation at a lower level might explain the development of ASD.
Previously, we found that the adjacent biomechanical behavior was not substantially altered by a laminectomy in flexion-extension (FE), lateral bending (LB), and axial rotation (AR).3 However, to our best knowledge no literature is available on the effects of instrumented single-level facet-sparing laminectomy on the cranial adjacent segments. Therefore, the aim of this study was to investigate the effects of the instrumentation on the biomechanical behavior of the adjacent segments to biomechanically substantiate the possible etiology of ASD cranial to the level on which posterior spinal instrumentation is applied. Furthermore, we assessed to what extent the posterior instrumentation after a laminectomy actually restricts motion at the operated spine level.
Methods
Specimens and Specimen Preparation
We included 12 human cadavers (mean age: 82.5 years, range 66 to 91) in this study. The bodies were donated to the department of anatomy by last will in accordance with the Dutch legislation and were destined for medical education and research. Bodies were handled according to the guidelines of the anatomy department. None of the deceased subjects had any history of spinal injury, spinal surgery, or spinal metastatic disease. The freshly frozen (−20°C) cadavers were thawed before harvesting the lumbar spines (L1 to L5) and the subsequent testing. The excessive soft tissue and muscle tissue were carefully removed, keeping the anterior and posterior longitudinal ligaments as well as the facet joints intact. During the preparation, assessment, and biomechanical testing, specimens were kept hydrated using 0.9% saline-soaked gauzes. Anteroposterior, lateral, and oblique radiographs (Sedical, Digital Vet. DX–6, Arlington Heights, Illinois, United States) were made to determine whether the bridging osteophytes were present in the segments. The lumbar spines with bridging osteophytes were excluded from this study. The magnetic resonance imaging (Siemens Symphony 1.5 T, Syngo MR A30, software NUMARIS/4, Berlin, Germany), lateral and oblique radiographs, and visual inspection also confirmed that the facet joints were intact and no fractures of the pars interarticularis were present in the segments before the mechanical testing.
After the imaging, the top and bottom vertebrae (L1 and L5) were potted in a casting mold and partially buried in a low melting point (48°C) bismuth alloy (Cerrolow–147; 48.0% bismuth, 25.6% lead, 12.0% tin, 9.6% cadmium, and 4.0% indium; Fig. 1). The L1 and L5 vertebral bodies were firmly fixed into the alloy by adding screws into the vertebral body prior to submerging in the alloy. The disks were placed parallel based on the visual inspection. Because the muscle tissue was thoroughly and carefully removed, the intervertebral disk and corresponding end plates were clearly visible. All the articulating parts were kept free. Markers containing three LEDs were rigidly fixed with the screws to the casting mold containing L1 and the anterior surface of the vertebral bodies of L2, L3, and L4 and to the casting mold in which L5 was mounted.
Fig. 1.
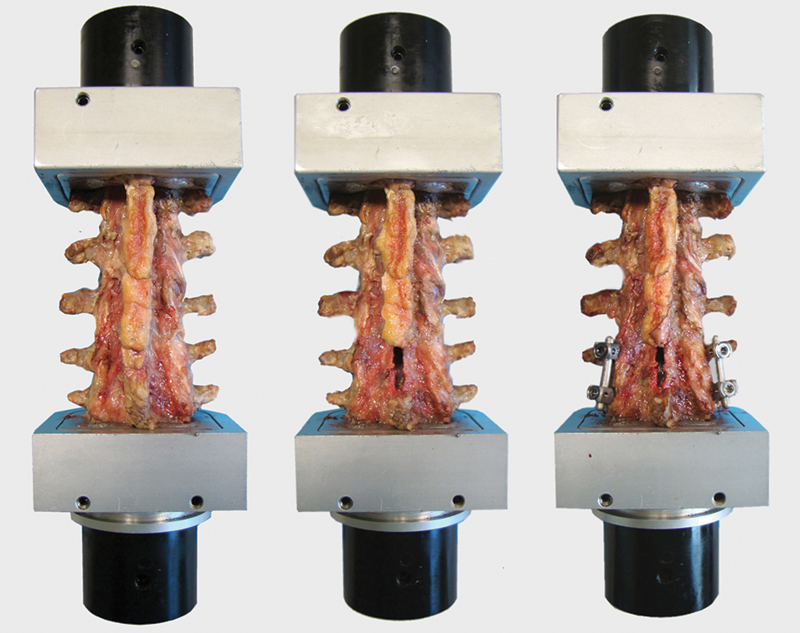
Biomechanical testing sequence with an untreated lumbar spine (left), a lumbar spine after laminectomy (middle), and a lumbar spine after laminectomy and posterior instrumentation (right). Note that laminectomy was performed on L4 (as shown in this figure) in six segments, and in the other six segments (instrumented), laminectomy was performed on level L2.
Biomechanical Testing
The test setup was similar to previous studies.12 13 14 15 The lumbar spines were placed horizontally in a custom-made four-point bending device in which FE, LB, and AR were applied using a hydraulic materials testing machine (Instron, model 8872; Instron and IST, Norwood, Massachusetts, United States). This setup guarantees equal moments at all levels of the lumbar spine.
Before testing, a compressive preload of 250 N was applied for 1 hour. A pure axial compressive force was applied using a pneumatic cylinder. The axial compression was calibrated using a load cell (Hottinger Baldwin Messtechnik, Force Transducer Type C2, Darmstadt, Germany). The amount of axial preload was chosen to allow for comparison with a previous work and to mimic physiologic conditions.12 13 14 15 During the testing, no compressive load was applied to prevent buckling of the multisegmented spine.16 The loads were applied up to a moment of 4 Nm at a constant angular velocity of 0.5 deg/s.17 When a moment of +4 Nm was measured, the Instron reversed its loading direction until −4 Nm was reached. Each movement direction was tested for 10 consecutive cycles.12 Force and displacement of the Instron were recorded and digitized at 100 Hz (Instron Fast Track 2). All the tests were performed at room temperature.
During testing, the motions of the LEDs of the casting mold containing L1; on the vertebral bodies of L2, L3, and L4; and of the casting mold in which L5 was mounted were recorded by an optoelectronic three-dimensional movement registration system with one array of three cameras (Optotrak 3020, Northern Digital Inc., Waterloo, Ontario, Canada). The three-dimensional resolution of this system at a distance of 2 m is 0.01 mm. Before testing, the axes of the Optotrak were aligned with the anatomic axes of the spines. Labview software was used for the data acquisition, synchronized with Instron data. The sampling rate was 100 samples per second.
After the first set of measurements (FE, LB, and AR), a laminectomy was performed at level L2 of six randomly chosen lumbar spines and at level L4 of the remaining six lumbar spines. The cranial adjacent levels (six times segment L1–L2 and six times segment L3–L4) were studied as well as the more distant untreated segments (six times segment L4–L5 and six times segment L2–L3), which were used as a control group. A laminectomy, analogous to standard clinical practice, was performed by removing the spinous process and part of the lamina, leaving the facet joints intact (Fig. 1).18 Again, a compressive preload of 250 N was applied for 1 hour. Thereafter, the lumbar spines were tested again in AR, LB, and FE for another set of 10 consecutive cycles. Before the last series of tests, a standard posterior lumbar fusion (PLF) was performed by placing the pedicle screws and rods (Medtronic, CD Horizon Legacy Spinal System, Minneapolis, Minnesota, United States) at the level of the laminectomy (Fig. 1). The screw placement was assessed on anteroposterior and lateral radiographs to confirm the correct position in the pedicle. No interbody devices were used.
In the first six lumbar spines, a laminectomy was performed three times on L2 and three times on L4, and then the tests were performed in order A: FE, LB, AR, laminectomy, AR, LB, FE, instrumentation, FE, LB, AR. In the second six lumbar spines, a laminectomy was performed three times on L2 and three times on L4, and the tests were performed in order B: AR, FE, LB, laminectomy, LB, FE, AR, instrumentation, AR, FE, LB. The testing order was changed to correct for possible effects of test sequence.
Data Analysis
Using Instron forces, the dimension of the four-point bending device, and Optotrak LED displacements, a Matlab (Mathworks, Natick, Massachusetts, United States) computer program calculated the load-displacement curves in the loaded direction for L2 relative to L1, for L3 relative to L2, for L4 relative to L3, and for L5 relative to L4. For each individual test (FE, LB, and AR), the ROM (degrees) and stiffness (Nm/degree) of the untreated spines and the spines after a single-level laminectomy and after subsequent instrumentation were calculated per motion segment (L1–L2, L2–L3, L3–L4, and L4–L5) from the load-displacement data. The ROM was calculated between an applied load of −4 Nm and +4 Nm. The tenth cycle was used for analysis.12 The stiffness was estimated by means of a least squares fit of a straight line through a fitted curve of the load-displacement data with the slope of the fitted line representing the stiffness. The stiffness was calculated between −1.0 and +1.0 Nm.
Statistical Methods
The effects of the laminectomy and instrumentation on the ROM and stiffness were tested using repeated-measures analysis of variance with the spinal level as the between-subject factor and the laminectomy or instrumentation as the within-subject factor. Levels L2–L3 and L4–L5 were considered as an intervention group with respect to a laminectomy at L2 and L4, respectively. Levels L1–L2 and L3–L4 were considered as cranial adjacent for a laminectomy at L2 and L4, respectively. The control groups consisted of L2–L3 and L4–L5 with respect to a laminectomy at L4 and L2, respectively.
First, the ROM and stiffness in FE, LB, and AR of the treated, adjacent, and control segments before and after a laminectomy were compared. Second, the ROM and stiffness in FE, LB, and AR of the treated, adjacent, and control segments before and after instrumented laminectomy were compared. A significance level of 5% was used. The statistical analyses were performed using SPSS for Mac version 20.0 (SPSS Inc., Chicago, Illinois, United States).
Results
An overview of specimen characteristics is presented in Table 1. Tables 2 and 3 provide an overview of the effects of a laminectomy and instrumentation on the ROM and stiffness, respectively. The effects of segment level and interaction effects with treatment are presented in Table 4.
Table 1. Overview of specimens.
| Specimen | Sex | Age (y) | Laminectomy |
|---|---|---|---|
| 1 | Female | 83 | L2 |
| 2 | Male | 76 | L2 |
| 3 | Female | 91 | L4 |
| 4 | Female | 86 | L4 |
| 5 | Male | 82 | L2 |
| 6 | Male | 82 | L4 |
| 7 | Male | 66 | L4 |
| 8 | Male | 72 | L2 |
| 9 | Male | 90 | L2 |
| 10 | Male | 86 | L4 |
| 11 | Female | 86 | L4 |
| 12 | Male | 90 | L2 |
Table 2. The effect of laminectomy and instrumentation on the ROM of spinal segments in FE, LB, and AR.
| Untreated versus laminectomy | Laminectomy versus instrumentation | |||||||
|---|---|---|---|---|---|---|---|---|
| ROM | Segments | Untreated (degrees) | After laminectomy (degrees) | After instrumentation (degrees) | % | p value | % | p value |
| FE | ||||||||
| Laminectomy | 5 × L2–L3 and 6 × L4–L5 | 5.28 (± 2.28) (E = 11) | 5.81 (± 2.76) (E = 11) | 1.41 (± 0.90) (E = 10) | +10.1 | 0.106 (E = 11) | –74.3 | 0.001 (E = 10) |
| Adjacent | 6 × L1–L2 and 6 × L3–L4 | 4.42 (± 1.70) (E = 12) | 4.46 (± 1.74) (E = 12) | 4.85 (± 1.70) (E = 11) | +0.9 | 0.863 (E = 12) | +8.2 | 0.978 (E = 11) |
| Control | 6 × L2–L3 and 6 × L4–L5 | 4.51 (± 1.94) (E = 12) | 4.90 (± 2.10) (E = 12) | 4.25 (± 1.82) (E = 12) | +8.6 | 0.043 (E = 12) | −13.2 | 0.130 (E = 12) |
| LB | ||||||||
| Laminectomy | 5 × L2–L3 and 6 × L4–L5 | 4.87 (± 2.31) (E = 11) | 4.96 (± 2.28) (E = 11) | 1.33 (± 0.97) (E = 10) | +1.9 | 0.416 (E = 11) | −71.6 | 0.001 (E = 10) |
| Adjacent | 6 × L1–L2 and 6 × L3–L4 | 4.00 (± 2.22) (E = 12) | 4.32 (± 2.41) (E = 12) | 3.86 (± 1.82) (E = 11) | +8.1 | 0.076 (E = 12) | −12.9 | 0.025 (E = 11) |
| Control | 6 × L2–L3 and 6 × L4–L5 | 3.86 (± 2.40) (E = 12) | 4.37 (± 2.65) (E = 12) | 4.10 (± 2.58) (E = 12) | +13.4 | 0.073 (E = 12) | −6.2 | 0.265 (E = 12) |
| AR | ||||||||
| Laminectomy | 5 × L2–L3 and 6 × L4–L5 | 2.65 (± 1.09) (E = 11) | 3.17 (± 1.19) (E = 11) | 1.32 (± 0.57) (E = 10) | +19.4 | 0.001 (E = 11) | −59.8 | <0.001 (E = 10) |
| Adjacent | 6 × L1–L2 and 6 × L3–L4 | 2.13 (± 1.43) (E = 12) | 2.37 (± 1.48) (E = 12) | 2.18 (± 1.49) (E = 11) | +11.0 | 0.043 (E = 12) | −8.9 | 0.097 (E = 11) |
| Control | 6 × L2–L3 and 6 × L4–L5 | 2.58 (± 1.61) (E = 12) | 2.69 (± 1.78) (E = 12) | 2.65 (± 1.64) (E = 12) | +4.3 | 0.096 (E = 12) | −1.3 | 0.597 (E = 12) |
Abbreviations: ANOVA, analysis of variance; AR, axial rotation; E, number of excluded measurements (see Note below and text); FE, flexion and extension; LB, lateral bending; ROM, range of motion.
Note: Effects of laminectomy and instrumentation on treated, adjacent, and control segments were analyzed with ANOVA. If a measurement was excluded, the ANOVA also excludes its corresponding value, noted with an E.
Table 3. The effect of laminectomy and instrumentation on the stiffness of spinal segments in FE, LB, and AR.
| Untreated versus laminectomy | Laminectomy versus instrumentation | |||||||
|---|---|---|---|---|---|---|---|---|
| Stiffness | Segments | Untreated (degrees) | After laminectomy(degrees) | After instrumentation (degrees) | % | p value | % | p value |
| FE | ||||||||
| Laminectomy | 5 × L2–L3 and 6 × L4–L5 | 0.81 (± 0.44) (E = 9) | 0.70 (± 0.35) (E = 9) | X | −14.4 | 0.129 (E = 9) | X | X |
| Adjacent | 6 × L1–L2 and 6 × L3–L4 | 1.56 (± 1.20) (E = 12) | 1.33 (± 1.10) (E = 12) | 1.24 (± 0.68) (E = 11) | −14.7 | 0.080 (E = 12) | −9.7 | 0.320 (E = 11) |
| Control | 6 × L2–L3 and 6 × L4–L5 | 1.14 (± 0.58) (E = 12) | 1.10 (± 0.56) (E = 12) | 1.14 (± 0.56) (E = 11) | −4.3 | 0.417 (E = 12) | −0.5 | 0.358 (E = 11) |
| LB | ||||||||
| Laminectomy | 5 × L2–L3 and 6 × L4–L5 | 1.49 (± 1.91) (E = 9) | 1.29 (± 1.24) (E = 9) | X | −13.4 | 0.604 (E = 9) | X | X |
| Adjacent | 6 × L1–L2 and 6 × L3–L4 | 1.73 (± 1.32) (E = 12) | 1.50 (± 0.91) (E = 12) | 1.87 (± 1.52) (E = 11) | −13.6 | 0.231 (E = 12) | +22.5 | 0.369 (E = 11) |
| Control | 6 × L2–L3 and 6 × L4–L5 | 1.90 (± 1.57) (E = 12) | 1.67 (± 1.43) (E = 12) | 1.63 (± 1.00) (E = 11) | −12.2 | 0.006 (E = 12) | +20.5 | 0.730 (E = 11) |
| AR | ||||||||
| Laminectomy | 5 × L2–L3 and 6 × L4–L5 | 2.63 (± 1.58) (E = 11) | 2.16 (± 1.33) (E = 11) | X | −18.0 | 0.005 (E = 11) | X | X |
| Adjacent | 6 × L1–L2 and 6 × L3–L4 | 2.43 (± 1.34) (E = 9) | 2.16 (± 1.21) (E = 9) | 2.35 (± 1.44) (E = 8) | −10.8 | 0.533 (E = 9) | +19.3 | 0.386 (E = 8) |
| Control | 6 × L2–L3 and 6 × L4–L5 | 2.42 (± 1.13) (E = 11) | 2.25 (± 1.16) (E = 11) | 2.40 (± 1.08) (E = 11) | −6.8 | 0.177 (E = 11) | +6.4 | 0.302 (E = 11) |
Abbreviations: ANOVA, analysis of variance; AR, axial rotation; E, number of excluded measurements (see Note below and text); FE, flexion and extension; LB, lateral bending; X, not analyzed.
Note: Effects of laminectomy and instrumentation on treated, adjacent, and control segments were analyzed with ANOVA. If a measurement was excluded, the ANOVA also excludes its corresponding value, noted with an E.
Table 4. Effects of level and interaction effects level × laminectomy and level × instrumentation on ROM and stiffness.
| Segment level (p value) | Segment level × laminectomy (p value) | Segment level (p value) | Segment level × instrumentation (p value) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Segments | ROM | Stiffness | ROM | Stiffness | ROM | Stiffness | ROM | Stiffness | |
| FE | |||||||||
| Laminectomy | 5 × L2–L3 and 6 × L4–L5 | 0.414 | 0.665 | 0.255 | 0.229 | 0.626 | X | 0.545 | X |
| Adjacent | 6 × L1–L2 and 6 × L3–L4 | 0.721 | 0.448 | 0.516 | 0.325 | 0.864 | 0.226 | 0.987 | 0.959 |
| Control | 6 × L2–L3 and 6 × L4–L5 | 0.707 | 0.626 | 0.535 | 0.279 | 0.716 | 0.701 | 0.883 | 0.123 |
| LB | |||||||||
| Laminectomy | 5 × L2–L3 and 6 × L4–L5 | 0.441 | 0.780 | 0.151 | 0.209 | 0.594 | X | 0.467 | X |
| Adjacent | 6 × L1–L2 and 6 × L3–L4 | 0.576 | 0.291 | 0.178 | 0.786 | 0.668 | 0.174 | 0.165 | 0.195 |
| Control | 6 × L2–L3 and 6 × L4–L5 | 0.917 | 0.337 | 0.368 | 0.951 | 0.935 | 0.548 | 0.376 | 0.304 |
| AR | |||||||||
| Laminectomy | 5 × L2–L3 and 6 × L4–L5 | 0.563 | 0.987 | 0.249 | 0.979 | 0.928 | X | 0.161 | X |
| Adjacent | 6 × L1–L2 and 6 × L3–L4 | 0.108 | 0.826 | 0.384 | 0.294 | 0.189 | 0.850 | 0.647 | 0.396 |
| Control | 6 × L2–L3 and 6 × L4–L5 | 0.416 | 0.550 | 0.039 | 0.387 | 0.390 | 0.414 | 0.179 | 0.621 |
Abbreviations: AR, axial rotation; FE, flexion and extension; LB, lateral bending; ROM, range of motion; X, not analyzed.
The ROM and stiffness in all motion directions of specimen number 2 (L2–L3 and L3–L4) and specimen number 12 (L3–L4) were excluded from analysis due to severely irregular load-displacement curves, leaving 45 segments to be analyzed. The data of segments L3–L4 and L4–L5 of specimen number 3 after instrumentation were excluded due to a loosened marker attached to the vertebral body of L4. Both the ROM and stiffness data of these measurements (FE, LB, and AR) were excluded. Due to a limited ROM of treated segments after instrumentation, the resultant load-displacement curves were too irregular to accurately define stiffness. Therefore, we did not calculate stiffness at the instrumented level (as shown with an X in Tables 3 and 4). Furthermore, the resultant range of ROM measurements was too small (<1 degree) in 22 measurements to allow for reliable assessment of stiffness, which were therefore excluded from the final analysis. In a few cases, not all 10 cycles were used due to irregular curves or other errors related to calculation.
Spinal Segments after Laminectomy and after Instrumented Laminectomy (L2–L3 and L4–L5)
The ROM increased significantly for AR (+19.4%; p = 0.001) in the segments treated with a laminectomy, although no significant effects were observed for FE and LB. As for the ROM, the stiffness after a laminectomy was only affected in AR (−18.0%; p = 0.005). Again, no significant effects were found for FE and LB. After instrumentation, the ROM decreased significantly in FE (−74.3%; p = 0.001), LB (−71.6%; p = 0.001), and AR (−59.8%; p < 0.001) compared with after a laminectomy. The stiffness of instrumented segments was not calculated, as stated previously.
Adjacent Segments (L1–L2 and L3–L4)
The ROM of the adjacent segments in AR increased significantly (+11.0%; p = 0.043) after a laminectomy. No significant effects on the ROM in FE and LB of the adjacent segments were observed. After a laminectomy, no significant effects on the spinal stiffness of the adjacent segments in any of the three directions were found. Instrumentation caused a significant decrease of the ROM of the adjacent segments in LB (−12.9%; p = 0.025) compared with after a laminectomy. No significant effects on the ROM in FE and AR were found. The stiffness of the segments adjacent to the instrumented segments was not affected in FE, LB, and AR.
Control Segments (L2–L3 and L4–L5)
After a laminectomy, the ROM in FE of the control segments increased significantly (p = 0.043) by 8.6%. In addition, the stiffness of the control segments in LB was significantly decreased (−12.2%; p = 0.006). After instrumentation, the ROM and stiffness of control segments were not different from after a laminectomy.
Effects of Segment Level
Finally, Table 4 shows that other than the interaction between the segment level and a laminectomy in the control group of AR (p = 0.039), no significant effects of the segment level or interactions between the treatment (both laminectomy and instrumentation) and segment level were found.
Discussion
In the present study, we quantified the effects of a single-level facet-sparing laminectomy and posterior instrumentation on the ROM and stiffness of the adjacent spinal segments in 12 fresh frozen human cadaveric lumbar spines. We found that a laminectomy increases the ROM of both treated and adjacent segments in AR, although AR stiffness was only decreased at the treated level. As expected, posterior instrumentation significantly and substantially decreased the ROM in FE, LB, and AR, but in adjacent segments only a decrease in LB ROM was found. The spinal stiffness of the adjacent segments was unaffected by instrumentation.
Previously, we studied the effects of an uninstrumented laminectomy on the adjacent biomechanics.3 In that study, we found a significant increase in the ROM at the treated level after a laminectomy in FE, LB, and AR, ranging from 7 to 12%. Although our current results only statistically confirmed this effect of a laminectomy for AR (+19.4%; p = 0.001), the results in FE were comparable but not significant (+10.1%; p = 0.106), although LB results did not show this increase (+1.9%; p = 0.416). The differences between these studies can possibly be explained by the differences in the state of degeneration between the studies. Interestingly, in both the present and previous study, the effects of a laminectomy were most substantial for AR.3 A possible explanation is that a laminectomy disrupts the integrity of the posterior arch and AR moments are largely resisted by the arch, whereas the intervertebral disk contributes substantially to resistance against FE and LB. Similar effects of a laminectomy on AR were previously described.19 The posterior arches of segments L1–L2 and L3–L4 level are supported by those of the treated segments (L2–L3 and L4–L5), which could cause a laminectomy at level L2 or L4 to also increase the ROM and decrease the stiffness at the cranial adjacent segment, albeit to a lesser extent. Although changes in the average values in the present data do suggest such effects to some extent, these effects were only found to be statistically significant for the ROM in AR.
In previous work, we elaborated on the question why significant alterations in the ROM do not always coincide with significant alterations in the corresponding stiffness.3 We measured the stiffness between −1 Nm and +1 Nm, which basically represents the neutral zone. We believe the spinous process, part of the lamina, and the posterior attached ligaments are most likely either not strained or strained only within the toe region of their stress-strain curves. At 4 Nm, these structures would be strained more substantially and would contribute more to the resistance against movement.
New in this study was the investigation of the effects of a single-level lumbar laminectomy and additional instrumentation on adjacent segments. To our best knowledge, only Cardoso et al and Delank et al investigated the effects of a laminectomy and instrumentation on adjacent levels.20 21 However, these authors used different types of (multilevel) decompressive techniques, such as facetectomy, and performed other (multilevel) constructs, making it difficult to compare results.
We found that, as expected, instrumentation substantially reduces the ROM by as much as 60 to 74% at the level at which instrumentation is used. In addition, instrumentation slightly but significantly decreased the ROM of the adjacent segment in LB by 13%, but FE and AR ROM and stiffness in all directions was unaffected. This result does not seem to offer a biomechanical explanation for ASD. However, in this study we did not investigate the effect of a posterior lumbar interbody fusion (PLIF) on the adjacent levels. It is progressively becoming clear that in particular PLIF and PLF carry a risk of ASD,11 which is receiving increasing interest among spine surgeons. ASD is one reason reoperations are necessary and have a higher incidence in elderly patients.22 The probability of undergoing a revision surgery for ASD was 5.8% at 5 years and 10.4% and 10 years postoperatively.22 The prevalence of ASD requiring reoperation in patients older than 60 years of age was 21.9% at 10 years postoperatively.22 PLIF procedures showed significantly lower survival rates than PLF procedures. ASD is often found at the level cranial to the spinal fusion.10 The exact pathogenesis is not well understood. First, it has been argued that ASD merely reflects the progression of the physiologic degeneration to other than the primarily affected segment.23 Second, it has been suggested that the application of instrumentation could negatively affect the spinal stability of the adjacent level, which could speed up the process of degeneration.23 The first hypothesis does not explain why mainly the cranial adjacent segment progresses into a state of degeneration. The second hypothesis is not supported by the biomechanical results presented here. If subjects would, in daily life, impose the same amount of total lumbar motion as before surgery, it is clear that after instrumentation, the ROM of adjacent segments would increase. However, this still does not explain the predominance of ASD in the segments cranial to the treated segment.
In clinical practice, topping-off procedures are used with the aim of preventing or slowing down ASD.24 Topping-off procedures combine a rigid fusion with a flexible pedicle screw system at the cranial adjacent level to prevent ASD.25 In our opinion, these procedures would only be beneficial when the stability is negatively affected at the adjacent level. However, in contrast, we found that the spinal ROM at the adjacent level after instrumentation was decreased, and the instability coincided with an increase in the ROM. Apparently, instrumentation not only stabilizes the treated segment but also supports the posterior arch of the lower level of the adjacent segment. Therefore, our results do not support the use of topping-off procedures to restabilize the adjacent levels after posterior instrumentation.
One limitation of the present study is that we were not able to evaluate the distal adjacent levels. The segment distal to L4–L5 was not included as L5 was our bottom vertebra. Although data of the segments distal to L2–L3 were available, these included only six segments, two of which had to be excluded due to irregular load-deformation curves. Nevertheless, in clinical practice the biomechanical behavior of the cranial adjacent segments has more relevance.
During the analysis, we found three unexpected significant results. The ROM in FE of the control segments increased significantly (p = 0.043) by 8.6%. The stiffness in LB of the control segments decreased significantly (p = 0.006) after a laminectomy. These effects may indicate tissue creep due to the repeated testing or dehydration, which would suggest that we slightly overestimated the ROM and underestimated the stiffness after a laminectomy and even more so after instrumentation. In addition, a significant (p = 0.039) interaction between the segment level and a laminectomy in the control group of AR was found, for which we can offer no explanation.
The stiffness was calculated in the neutral zone as this been hypothesized to be a clinically relevant indicator of lumbar spine instability.26 27 Other studies determined the neutral zone as the zone between the points of the largest changes in flexibility in the load-displacement curve.28 Unfortunately, these points could not reliably be detected in too many curves, as there were often small irregularities in load-displacement curves, possibly caused by degenerative deformities as a consequence of our aged sample. Consequently, we decided to measure the stiffness between −1 Nm and +1 Nm in this study.
The 1-hour, 250-N axial preload applied can only partly simulate effects of loading due to gravity and muscle forces. We did not apply axial loading during our test as the application of compression to a multisegmented spine combined with bending causes buckling.16 Possibly, the short preload period did not correspond with a daily loading pattern. Due to losses of fluids in the disk in daily life, the effects of a laminectomy might be enhanced. We repeated each load cycle 10 times, thereby allowing for some visco- and poroelastic behavior to mimic in vivo loading.12 29 30 Furthermore, during daily in vivo loading, the lumbar spine is often subjected to a combination of different loading directions. Combined loading of the lumbar spine was not investigated in this study.
In conclusion, we found that although posterior instrumentation stabilizes the segments treated by a laminectomy, it does not negatively alter adjacent spinal biomechanics (i.e., increase the ROM or decrease the stiffness). Based on our results, the spinal segments cranial to posterior fusion techniques do not become less stable after instrumentation. Therefore, we postulate that altered biomechanical behavior due a laminectomy with or without stabilization does not lead substantial alterations at the adjacent level and therefore cannot explain ASD.
Acknowledgments
The authors thank Medtronic, The Netherlands, for providing the instrumentation necessary to perform this study.
Footnotes
Disclosures Arno Bisschop, none Roderick M. Holewijn, none Idsart Kingma, none Agnita Stadhouder, none Pieter-Paul A. Vergroesen, none Albert J. van der Veen, none Jaap H. van Dieën, none Barend J. van Royen, none
References
- 1.Bisschop A, Mullender M G, Kingma I. et al. The impact of bone mineral density and disc degeneration on shear strength and stiffness of the lumbar spine following laminectomy. Eur Spine J. 2012;21(3):530–536. doi: 10.1007/s00586-011-1968-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bisschop A, van Dieën J H, Kingma I. et al. Torsion biomechanics of the spine following lumbar laminectomy: a human cadaver study. Eur Spine J. 2013;22(8):1785–1793. doi: 10.1007/s00586-013-2699-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bisschop A van Engelen S J Kingma I et al. Single level lumbar laminectomy alters segmental biomechanical behavior without affecting adjacent segments Clin Biomech (Bristol, Avon) 2014; July 3 (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 4.Fox M W, Onofrio B M, Onofrio B M, Hanssen A D. Clinical outcomes and radiological instability following decompressive lumbar laminectomy for degenerative spinal stenosis: a comparison of patients undergoing concomitant arthrodesis versus decompression alone. J Neurosurg. 1996;85(5):793–802. doi: 10.3171/jns.1996.85.5.0793. [DOI] [PubMed] [Google Scholar]
- 5.Fu Y S, Zeng B F, Xu J G. Long-term outcomes of two different decompressive techniques for lumbar spinal stenosis. Spine (Phila Pa 1976) 2008;33(5):514–518. doi: 10.1097/BRS.0b013e3181657dde. [DOI] [PubMed] [Google Scholar]
- 6.Benz R J, Garfin S R. Current techniques of decompression of the lumbar spine. Clin Orthop Relat Res. 2001;(384):75–81. doi: 10.1097/00003086-200103000-00010. [DOI] [PubMed] [Google Scholar]
- 7.Deyo R A, Nachemson A, Mirza S K. Spinal-fusion surgery—the case for restraint. N Engl J Med. 2004;350(7):722–726. doi: 10.1056/NEJMsb031771. [DOI] [PubMed] [Google Scholar]
- 8.Brodke D S, Annis P, Lawrence B D, Woodbury A M, Daubs M D. Reoperation and revision rates of 3 surgical treatment methods for lumbar stenosis associated with degenerative scoliosis and spondylolisthesis. Spine (Phila Pa 1976) 2013;38(26):2287–2294. doi: 10.1097/BRS.0000000000000068. [DOI] [PubMed] [Google Scholar]
- 9.Ekman P, Möller H, Shalabi A, Yu Y X, Hedlund R. A prospective randomised study on the long-term effect of lumbar fusion on adjacent disc degeneration. Eur Spine J. 2009;18(8):1175–1186. doi: 10.1007/s00586-009-0947-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Celestre P C, Montgomery S R, Kupperman A I, Aghdasi B, Inoue H, Wang J C. Lumbar clinical adjacent segment pathology: predilection for proximal levels. Spine (Phila Pa 1976) 2014;39(2):172–176. doi: 10.1097/BRS.0000000000000094. [DOI] [PubMed] [Google Scholar]
- 11.Radcliff K E, Kepler C K, Jakoi A. et al. Adjacent segment disease in the lumbar spine following different treatment interventions. Spine J. 2013;13(10):1339–1349. doi: 10.1016/j.spinee.2013.03.020. [DOI] [PubMed] [Google Scholar]
- 12.Bisschop A, Kingma I, Bleys R L. et al. Effects of repetitive movement on range of motion and stiffness around the neutral orientation of the human lumbar spine. J Biomech. 2013;46(1):187–191. doi: 10.1016/j.jbiomech.2012.10.014. [DOI] [PubMed] [Google Scholar]
- 13.Busscher I, van der Veen A J, van Dieën J H, Kingma I, Verkerke G J, Veldhuizen A G. In vitro biomechanical characteristics of the spine: a comparison between human and porcine spinal segments. Spine (Phila Pa 1976) 2010;35(2):E35–E42. doi: 10.1097/BRS.0b013e3181b21885. [DOI] [PubMed] [Google Scholar]
- 14.Busscher I, van Dieën J H, Kingma I, van der Veen A J, Verkerke G J, Veldhuizen A G. Biomechanical characteristics of different regions of the human spine: an in vitro study on multilevel spinal segments. Spine (Phila Pa 1976) 2009;34(26):2858–2864. doi: 10.1097/BRS.0b013e3181b4c75d. [DOI] [PubMed] [Google Scholar]
- 15.van Engelen S J, Ellenbroek M H, van Royen B J, de Boer A, van Dieën J H. Validation of vibration testing for the assessment of the mechanical properties of human lumbar motion segments. J Biomech. 2012;45(10):1753–1758. doi: 10.1016/j.jbiomech.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 16.Patwardhan A G, Havey R M, Meade K P, Lee B, Dunlap B. A follower load increases the load-carrying capacity of the lumbar spine in compression. Spine (Phila Pa 1976) 1999;24(10):1003–1009. doi: 10.1097/00007632-199905150-00014. [DOI] [PubMed] [Google Scholar]
- 17.Wilke H J, Wenger K, Claes L. Testing criteria for spinal implants: recommendations for the standardization of in vitro stability testing of spinal implants. Eur Spine J. 1998;7(2):148–154. doi: 10.1007/s005860050045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Detwiler P W Spetzler C B Taylor S B Crawford N R Porter R W Sonntag V K Biomechanical comparison of facet-sparing laminectomy and Christmas tree laminectomy J Neurosurg 200399(2, Suppl):214–220. [DOI] [PubMed] [Google Scholar]
- 19.Schmoelz W, Huber J F, Nydegger T, Dipl-Ing I, Claes L, Wilke H J. Dynamic stabilization of the lumbar spine and its effects on adjacent segments: an in vitro experiment. J Spinal Disord Tech. 2003;16(4):418–423. doi: 10.1097/00024720-200308000-00015. [DOI] [PubMed] [Google Scholar]
- 20.Cardoso M J, Dmitriev A E, Helgeson M, Lehman R A, Kuklo T R, Rosner M K. Does superior-segment facet violation or laminectomy destabilize the adjacent level in lumbar transpedicular fixation? An in vitro human cadaveric assessment. Spine (Phila Pa 1976) 2008;33(26):2868–2873. doi: 10.1097/BRS.0b013e31818c63d3. [DOI] [PubMed] [Google Scholar]
- 21.Delank K S, Gercek E, Kuhn S. et al. How does spinal canal decompression and dorsal stabilization affect segmental mobility? A biomechanical study. Arch Orthop Trauma Surg. 2010;130(2):285–292. doi: 10.1007/s00402-009-1002-x. [DOI] [PubMed] [Google Scholar]
- 22.Lee J C, Kim Y, Soh J W, Shin B J. Risk factors of adjacent segment disease requiring surgery after lumbar spinal fusion: comparison of posterior lumbar interbody fusion and posterolateral fusion. Spine (Phila Pa 1976) 2014;39(5):E339–E345. doi: 10.1097/BRS.0000000000000164. [DOI] [PubMed] [Google Scholar]
- 23.Helgeson M D, Bevevino A J, Hilibrand A S. Update on the evidence for adjacent segment degeneration and disease. Spine J. 2013;13(3):342–351. doi: 10.1016/j.spinee.2012.12.009. [DOI] [PubMed] [Google Scholar]
- 24.Liu H Y, Zhou J, Wang B. et al. Comparison of topping-off and posterior lumbar interbody fusion surgery in lumbar degenerative disease: a retrospective study. Chin Med J (Engl) 2012;125(22):3942–3946. [PubMed] [Google Scholar]
- 25.Siewe J, Otto C, Knoell P. et al. Comparison of standard fusion with a “topping off” system in lumbar spine surgery: a protocol for a randomized controlled trial. BMC Musculoskelet Disord. 2011;12:239. doi: 10.1186/1471-2474-12-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Panjabi M M The stabilizing system of the spine. Part I. Function, dysfunction, adaptation, and enhancement J Spinal Disord 199254383–389., discussion 397 [DOI] [PubMed] [Google Scholar]
- 27.Panjabi M M The stabilizing system of the spine. Part II. Neutral zone and instability hypothesis J Spinal Disord 199254390–396., discussion 397 [DOI] [PubMed] [Google Scholar]
- 28.Smit T H, van Tunen M S, van der Veen A J, Kingma I, van Dieën J H. Quantifying intervertebral disc mechanics: a new definition of the neutral zone. BMC Musculoskelet Disord. 2011;12:38. doi: 10.1186/1471-2474-12-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Koeller W, Meier W, Hartmann F. Biomechanical properties of human intervertebral discs subjected to axial dynamic compression. A comparison of lumbar and thoracic discs. Spine (Phila Pa 1976) 1984;9(7):725–733. doi: 10.1097/00007632-198410000-00013. [DOI] [PubMed] [Google Scholar]
- 30.Zilch H, Rohlmann A, Bergmann G, Kölbel R. Material properties of femoral cancellous bone in axial loading. Part II: Time dependent properties. Arch Orthop Trauma Surg. 1980;97(4):257–262. doi: 10.1007/BF00380706. [DOI] [PubMed] [Google Scholar]


