Abstract
Background
The Dual Antiplatelet Therapy (DAPT) Study is large streamlined clinical trial designed to evaluate antiplatelet treatment strategies in a broadly inclusive population of subjects treated with coronary stents. Whether large streamlined trials can successfully include a representative group of study sites and patients has not been formally assessed.
Methods and Results
Within the NCDR CathPCI Registry, we compared characteristics and outcomes of DAPT-participating and non-participating hospitals. We also compared clinical and procedural characteristics of trial subjects undergoing PCI with drug-eluting stents (DES) to contemporaneous patients within the NCDR CathPCI Registry. Standardized differences between groups were estimated. Between September 2009 and July 2011, 1.1 million PCIs were performed among 1276 hospitals, of which 309 (24.2%) participated in the DAPT Study. Participating hospitals were larger (468 vs. 311 beds), more frequently located in urban settings (61.2% vs. 42.6%), and had higher annual PCI volumes (858 vs. 378) compared with non-participating hospitals, although hospital case mix and procedural outcomes were similar. Compared to CathPCI patients, trial patients undergoing PCI with DES were similar with respect to race, sex, and rates of diabetes, hypertension and smoking, although they had lower rates of prior cardiovascular disease.
Conclusions
Within the DAPT study, clinical trial sites had similar patient case mix and clinical outcomes as non-participating sites. While trial participants were representative of PCI patients with respect to race, sex and most comorbidities, they had a lower prevalence of chronic cardiovascular disease compared to registry patients. While a streamlined cardiovascular clinical trial may successfully involve a large number of hospitals and rapidly enroll a diverse population of patients, differences between eligible patients and those actually enrolled remained.
Clinical Trial Registration
URL: http://www.clinicaltrials.gov. Unique identifier: NCT00977938.
Keywords: clinical trial, stent, methodology, coronary disease
Randomized clinical trials (RCTs) have long been considered the gold standard for the comparison of treatments. However, limitations of RCTs have been well documented1-6 including the selection of patients and enrolling sites that may not accurately reflect the general clinical population and treatment environments, or protocol designs that compare treatments that may not be directly relevant to current clinical decision-making. There has been a growing interest, therefore, in the design and execution of more inclusive trials. Furthermore, the enrollment of sufficient sample sizes to compare treatment strategies with robust clinical endpoints may be challenging to achieve within a timeframe of clinical relevance. A proposed solution to these problems is greater use of large “streamlined” clinical trials, characterized as those that examine clinically relevant alternative treatments, include broad patient populations, and recruit patients from diverse practice settings with fewer protocol-defined specifications for data collection and site monitoring. 7, 8
In 2007, concerns arose regarding the occurrence of stent thrombosis in patients receiving drug-eluting stents (DES) for percutaneous coronary intervention. Existing randomized clinical trial data was largely from narrowly defined patient populations, often excluding patients with myocardial infarction or complex lesion anatomy, despite the reality that the large majority of such patients in routine clinical practice were being treated with drug-eluting stents. To address the public health concern regarding the prevention of stent thrombosis and its clinical sequelae such as death and myocardial infarction, the United States (US) Food and Drug Administration requested randomized trial data comparing different durations of dual antiplatelet therapy. The DAPT Study was designed as a large streamlined trial with few exclusion criteria to enroll a broad cohort of subjects undergoing percutaneous coronary intervention (PCI) with any FDA-approved DES. 9
Although large streamlined clinical trials would appear advantageous for generating broadly generalizable results to help inform clinical decision-making, few studies have examined whether such trials succeed in including hospitals and patients that are representative of routine practice environments. We therefore sought to determine whether the sites and subjects enrolled in the US DAPT Study were similar to typical PCI hospitals and patients in the United States, as reflected by the National Cardiovascular Data Registry (NCDR) CathPCI Registry.
Methods
We performed two main comparisons: 1) a comparison of characteristics of DAPT clinical trial sites compared with sites performing PCI in the NCDR CathPCI registry, a comprehensive national database of clinical practice locations, and 2) a comparison of DAPT-enrolled clinical trial subjects with patients reported to the CathPCI registry. The Figure shows the flow diagrams for the data used in hospital-level and patient-level comparisons.
Figure.
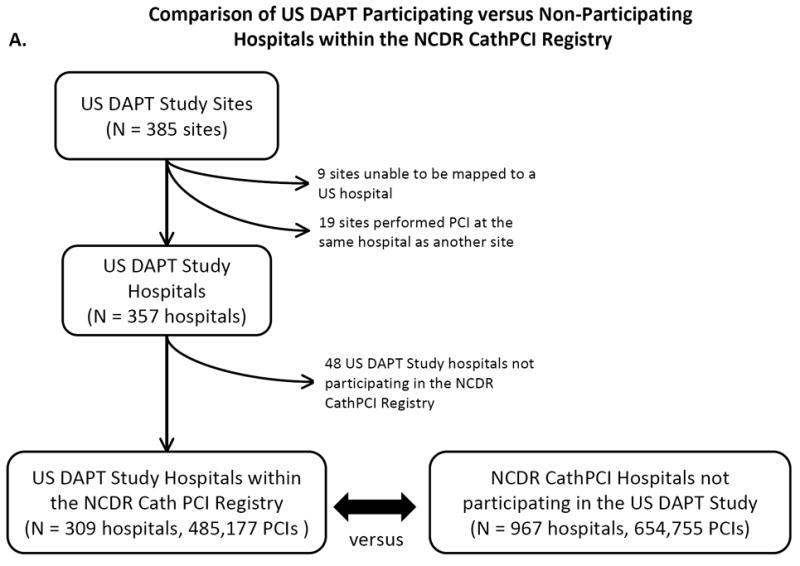
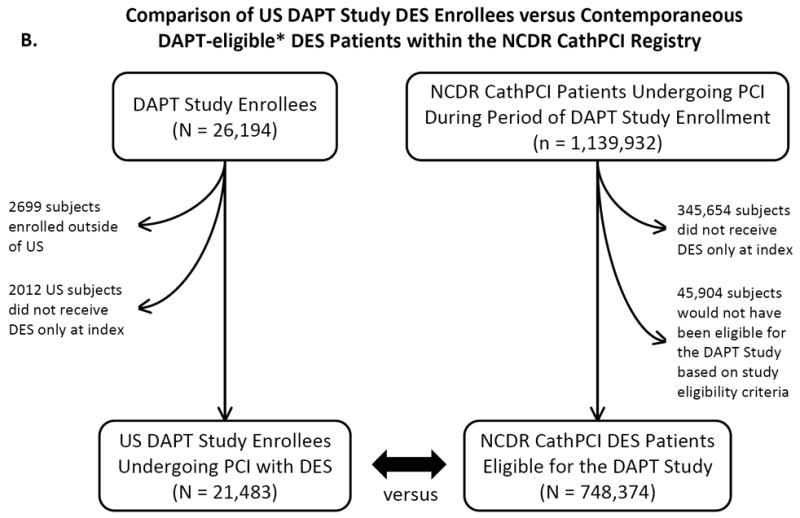
A. Study flow diagram for site-level comparison between US DAPT-participating and non-participating hospitals within the NCDR CathPCI Registry. B. Study flow diagram for patient-level comparison between subjects enrolled in the US DAPT Study treated with DES versus subjects eligible to be enrolled in the DAPT Study receiving DES within the NCDR CathPCI Registry.
The Dual Antiplatelet Therapy (DAPT) Study
The DAPT Study is an ongoing international, multicenter, randomized clinical trial that compares 30 months versus 12 months of dual antiplatelet therapy after PCI with coronary stents. The rationale and design of the DAPT Study have previously been described 9. Inclusion criteria for the trial were purposefully broad in order to evaluate DES-treated subjects representative of patients seen in routine clinical practice. The study included subjects >18 years of age undergoing PCI with an FDA-approved stent. The main exclusion criteria were: planned surgery requiring discontinuation of antiplatelet therapy within 30 months after enrollment, pregnancy, life expectancy <3 years, concomitant use of warfarin or another anticoagulant, and hypersensitivity or allergy to any component of dual antiplatelet therapy. For this analysis, all DES-treated subjects enrolled in the DAPT Study from sites within the US were included (herein referred to as the “DAPT-enrolled” population). Study enrollment commenced on September 1, 2009 and completed on July 1, 2011.
National Cardiovascular Data Registry – CathPCI Registry
The CathPCI is registry co-sponsored by the American College of Cardiology and the Society for Cardiovascular Angiography and Interventions, and includes more than 1200 hospitals in all 50 US states, contributing data on more than 600,000 PCI procedures per year. 10 Data submitted to the registry are filtered for completeness and consistency, and a random sample of records are audited annually. 11 CathPCI hospitals represent more than three-quarters of all PCI-performing hospitals in the US, as identified by American Hospital Association. 12 We identified hospitals participating in the registry and registry patients who underwent PCI with DES from September 1, 2009 through July 1, 2011, contemporaneous with the enrollment period of the DAPT Study. Because unique identifiers are not submitted to the NCDR CathPCI registries, individuals undergoing multiple procedures during the study period may be represented more than once in the dataset.
Statistical Analysis
Participating versus Non-Participating Hospital Comparison
We compared hospital and patient characteristics between hospitals that participated in the DAPT Study versus hospitals that did not participate, among those hospitals contributing data to the NCDR CathPCI registry. All PCI procedures were included in this comparison independent of stent type or procedure indication. To identify DAPT sites within the NCDR CathPCI registry, we first linked each DAPT site with the corresponding hospital where cardiac catheterizations were performed. Sites that performed PCI at more than three locations without a clearly dominant location, as well as those that could not be linked to a hospital, were excluded from this study. The hospital list of DAPT Study sites was then cross-matched with a list of CathPCI-participating hospitals during the period of DAPT Study enrollment. Information was gathered through a mix of web searches, communications with site principal investigators and research coordinators, and direct telephone communications with hospital catheterization laboratories.
A wide range of characteristics were compared among patients at DAPT-participating hospitals versus non-participating hospitals, including demographic characteristics, medical history, presenting symptoms, procedural characteristics and in-hospital outcomes. The characteristics and outcomes were drawn from data collected by the NCDR CathPCI Registry, and definitions of the data elements are available at https://www.ncdr.com/webncdr/cathpci/home/datacollection. Hospital characteristics were compared between these groups, and included number of beds, location and community type, profit type, association with a fellowship, internship, or residency program, geographical region, annual PCI volume, and the number of physicians performing PCI procedures.
Enrolled versus Eligible Patient Analysis
We compared characteristics between the enrolled DAPT Study subjects versus the population of NCDR CathPCI registry subjects undergoing PCI with DES who may have been eligible for enrollment based on the trial’s inclusion/exclusion criteria (“DAPT-eligible” patients). To exclude NCDR CathPCI registry patients who would not have been eligible for the DES arms of the DAPT Study, we excluded patients not prescribed a thienopyridine at discharge. Because data on pregnancy, life expectancy, and concurrent warfarin therapy were not available within the CathPCI registry, these DAPT Study exclusion criteria were not applied to the CathPCI registry population.
Enrolled DAPT subjects were compared with DAPT-eligible registry patients for selected characteristics that were similarly defined and recorded in the DAPT Study and the NCDR CathPCI registry. These data elements included sociodemographic information (age, sex, race, and ethnicity); medical history (body mass index, diabetes, and smoking); cardiovascular history (hypertension, prior myocardial infarction [MI], prior congestive heart failure, peripheral vascular disease, prior PCI, and prior coronary artery bypass grafting [CABG]); presentation (non-ST elevation MI or ST-elevation MI); and procedural characteristics (treated vessel and thienopyridine therapy at discharge). Because of the lack of patient identifiers, we could not identify individual subjects enrolled in the DAPT Study within NCDR CathPCI data to exclude them from the registry group. However, because of the very large number of registry patients compared to trial enrollees, this is unlikely to influence the comparison. We also repeated this comparison limiting the population to those undergoing PCI within DAPT-participating hospitals.
All comparisons were made using standardized differences due to the large sample sizes which may render comparisons via p-values less useful.13 By convention, standardized differences of >10% were considered significant. All analyses were conducted using SAS version 9.2 (SAS Institute Inc., Cary, NC). Analyses for the DAPT Study were performed at the Harvard Clinical Research Institute, and approved by the Institutional Review Board of Partners Healthcare. Analyses by the NCDR are approved by Chesapeake Research Review, Inc.
Results
Between September 1, 2009 and July 1, 2011, the DAPT Study enrolled 26,194 patients, of whom 23,495 were enrolled in the US and 2699 were enrolled outside the US. Among US-enrolled patients, 21,481 underwent PCI with DES at 385 unique study sites.
Contemporaneously, 1,139,932 PCI procedures were performed at 1276 hospitals in the NCDR CathPCI registry from September 1, 2009 through July 1, 2011. Of these, 748,374 procedures at 1270 hospitals were performed using DES and no BMS, among subjects who would have met eligibility criteria for enrollment in the DAPT Study. A total of 385 US sites participated in the DAPT Study; 48 of these sites (12.5%) did not participate in the NCDR CathPCI registry, 19 (5.0%) performed PCI at the same hospital as another DAPT site, and 9 (2.3%) could not be mapped to a unique hospital. Therefore, 309 DAPT-participating hospitals also participated in the NCDR CathPCI registry, representing 24.2% of all NCDR CathPCI participating hospitals. These 309 hospitals performed 485,177 PCIs during the study period, representing 42.6% of all PCIs performed within the registry.
DAPT-Participating vs. Non-Participating Hospitals
Patient characteristics for PCIs at DAPT-participating hospitals were no different from those at non-participating hospitals. There were no significant differences in demographics, comorbidities, admission symptoms, indications for PCI, or procedural characteristics between the two groups (Supplemental Appendix, Tables 1 and 2).
However, there were marked differences with regard to hospital characteristics between DAPT-participating and non-participating hospitals (Table 1). Hospitals participating in DAPT were more frequently located in an urban setting (61.2% vs. 42.6%, standardized difference (SD) 37.8%), located in the Northeast (19.1% vs. 11.4%, SD 21.6%), and affiliated with an internship, residency, or fellowship program (53.7% vs. 34.1%, SD 40.3%). Furthermore, DAPT-participating hospitals had more beds (468 ± 242 vs. 311 ± 182, SD 73.3%), had a higher average annual PCI volume (858 ± 533 procedures vs. 378 ± 328, SD 108.6%), and had more physicians performing PCI procedures (14.6 ± 9.5 vs. 10.3 ± 7.0, SD 51.3%). Participating hospitals were less frequently private or community hospitals compared with non-participating hospitals (80.6% vs. 93.2%, SD -38.0%).
Table 1.
Characteristics of DAPT-participating vs. non-participating hospitals
| Characteristic | DAPT-Participating Hospital (n = 309) | Non-Participating Hospital (n = 967) | Standardized Difference (%) |
|---|---|---|---|
| Number of CMS-certified beds | 468 ± 242 | 311 ±182 | 73.3 |
| Community type | |||
| Rural | 9.4 | 19.3 | -28.7 |
| Suburban | 29.5 | 38.1 | -18.3 |
| Urban | 61.2 | 42.6 | 37.8 |
| Profit type | |||
| Government | 1.9 | 1.5 | 3.8 |
| Private/community | 80.6 | 93.2 | -38.0 |
| University | 17.5 | 5.4 | 38.7 |
| Internship, residency, or fellowship program | 53.7 | 34.1 | 40.3 |
| Hospital region | |||
| Midwest | 27.8 | 30.0 | -4.8 |
| Northeast | 19.1 | 11.4 | 21.6 |
| South | 36.9 | 37.5 | -1.3 |
| West | 16.2 | 20.8 | -11.9 |
| Average annual PCI volume | 858 ± 533 | 378 ± 328 | 108.6 |
| Number of unique physicians doing PCI procedures | 14.6 ± 9.5 | 10.3 ± 7.0 | 51.3 |
Values are percent or mean ± standard deviation.
Abbreviations: CMS, Centers for Medicare and Medicaid Services; PCI, percutaneous coronary intervention.
Crude in-hospital mortality rates after PCI were not different at DAPT-participating hospitals compared to non-participating hospitals (1.30% vs. 1.45%, SD 1.3%). Unadjusted bleeding rates within 72 hours after PCI were also not different (Table 2).
Table 2.
In-hospital outcomes of PCIs at DAPT-participating vs. non-participating hospitals
| Outcome | DAPT-Participating Hospital (n = 485177 PCIs) | Non-Participating Hospital (n = 654755 PCIs) | Standardized Difference (%) |
|---|---|---|---|
| All-Cause Death (%) | 1.30 | 1.45 | -1.3 |
| Bleeding Complication (%) | 5.48 | 5.96 | -2.1 |
| Tranfusion (%) | 2.63 | 2.68 | -0.3 |
| Renal Failure | 0.25 | 0.24 | 0.11 |
Results are for all PCIs occurring in the study period, independent of stent type used.
DAPT-Enrolled Drug-Eluting Stent Patients vs. US Drug-Eluting Stent Patients
Patients undergoing PCI with DES who were enrolled in the DAPT Study were not different from patients contemporaneously undergoing PCI with DES within the NCDR CathPCI registry with respect to race and sex, as well as clinical characteristics such as diabetes and hypertension. (Table 3) Patients enrolled in the trial were younger than registry patients (62.2 ± 10.6 years vs. 64.3 ± 11.6, SD -19.2%), and prior cardiovascular history, including history of peripheral vascular disease, congestive heart failure, myocardial infarction, and prior PCI and CABG, was less frequent in DAPT-enrolled patients compared to NCDR CathPCI patients.
Table 3.
Baseline clinical characteristics of patients receiving DES enrolled in the DAPT trial and those eligible to be enrolled in the CathPCI registry
| Characteristic | DAPT US DES Enrolled (n = 21481) | CathPCI DAPT-Eligible DES Patients (n = 748374) | Standardized Difference (%) |
|---|---|---|---|
| Age | 62.2 ± 10.6 | 64.3 ± 11.6 | -19.2 |
| Female | 28.8 | 32.5 | -8.2 |
| Race | |||
| American Indian or Alaska Native | 0.4 | 0.5 | -1.2 |
| Asian | 1.1 | 2.3 | -9.4 |
| Black | 7.1 | 7.3 | -0.8 |
| Hispanic ethnicity | 4.4 | 4.8 | -1.5 |
| Native Hawaiian | 0.2 | 0.2 | 0.9 |
| White | 89.4 | 88.9 | 1.4 |
| BMI (kg/m2) | 30.6 ± 5.9 | 30.2 ± 6.3 | 7.2 |
| Co-morbidities | |||
| Smoker | 25.5 | 25.7 | -0.5 |
| Diabetes | 33.8 | 36.5 | -5.6 |
| Not on insulin | 22.3 | 23.4 | -2.6 |
| On insulin | 11.5 | 13.1 | -4.9 |
| Hypertension | 79.1 | 82.3 | -8.2 |
| Peripheral arterial disease | 7.3 | 11.8 | -15.7 |
| Prior CHF | 6.8 | 10.5 | -13.4 |
| Prior MI | 23.8 | 29.6 | -13.2 |
| Prior procedures | |||
| PCI | 35.6 | 42.5 | -14.1 |
| CABG | 14.5 | 18.5 | -11.0 |
| Presentation | |||
| Non-STEMI | 14.6 | 16.5 | -5.1 |
| STEMI | 8.6 | 11.4 | -9.2 |
Values are percent or mean ± standard deviation.
Abbreviations: BMI, body mass index; CABG, coronary artery bypass grafting; CHF, congestive heart failure; MI, myocardial infarction; PCI, percutaneous coronary intervention; STEMI, ST elevation myocardial infarction.
With regard to procedural characteristics, patients enrolled in the DAPT Study were less likely to have multi-vessel PCI (11.1% vs. 15.0%, SD -11.8%), but rates of left main coronary artery stenting and vein graft procedures were not different (Table 4). Length of stay was significantly shorter in enrolled patients compared to those eligible. Patients enrolled in DAPT more often received paclitaxel-eluting stents and were more frequently discharged on prasugrel compared to eligible patients within the NCDR, as one of the clinical studies contributing to DAPT mandated use of these two in combination.14 Similar results were seen when the NCDR population was limited to those hospitals that participated in the DAPT study (Supplemental Tables 3 and 4).
Table 4.
Procedural characteristics of patients receiving DES enrolled in the DAPT trial versus DAPT-eligible patients within the CathPCI registry
| Procedural Characteristic | DAPT US DES Enrolled (n = 21481) | CathPCI DAPT-Eligible (n = 748374) | Standardized Difference (%) |
|---|---|---|---|
| High Risk Feature | |||
| Left Main | 1.5 | 1.8 | -2.3 |
| Proximal LAD | 19.0 | 15.8 | 8.5 |
| Arterial graft | 0.7 | 0.5 | 2.2 |
| Vein graft | 3.6 | 5.4 | -8.7 |
| Multi-vessel | 11.1 | 15.0 | -11.8 |
| Stent Type | |||
| Everolimus | 47.7 | 62.9 | -31.1 |
| Zotarolimus | 14.7 | 10.4 | 13.0 |
| Paclitaxel | 21.9 | 13.7 | 21.6 |
| Sirolimus | 13.1 | 8.6 | 14.3 |
| Multiple Types | 2.7 | 4.3 | -9.0 |
| Discharge medications | |||
| Aspirin | 99.4 | 98.1 | 11.0 |
| Clopidogrel | 69.2 | 86.9 | -43.8 |
| Prasugrel* | 30.7 | 13.7 | 40.2 |
| Outcome | |||
| Length of stay (days, mean ± SD) | 1.3 ± 1.2 | 2.54 ± 7.01 | -25.3 |
| Length of stay (days, median, interquartile range) | 1.00 (1.00, 1.00) | 1.00 (1.00, 3.00) | - |
Values are percent unless otherwise noted.
Abbreviations: LAD, left anterior descending; SD, standard deviation. Missing data were < 4% for all elements.
One of the 4 manufacturer-sponsored studies contributing to the DAPT Study mandated treatment with prasugrel after PCI.
Discussion
There has been an increased awareness of the limitations of traditional randomized clinical trials to inform clinical-relevant decisions faced by physicians. Although observational studies using registry or claims data may often overcome some of these limitations through the inclusion of large populations of unselected patients 15, they introduce the potential for confounding. More recently, there have been calls for trials occupying a middle ground, ranging from streamlined trials that may employ risk-based rather than comprehensive site monitoring strategies and limited central event adjudication 8, to “pragmatic” or “practical” trials that may forgo these elements. 16 Common to all such trials, however, are the goals to “specifically to answer the questions faced by decision makers,”7 the incorporation of standard clinical alternatives to the intervention of interest, the use of purposefully broad inclusion criteria with minimal exclusions, and the preservation of randomization to minimize bias. The DAPT Study, the largest randomized trial of PCI patients to date, was designed as one such trial 9. The goal of this study was to assess whether the study design and execution resulted in the inclusion of hospitals and patients representative of those seen in clinical practice.
We found that the DAPT Study involved a large percentage of US hospitals and that DAPT-participating hospitals had very similar outcomes to non-participating hospitals. Participating hospitals tended to be larger, academic hospitals with higher procedural volumes, and were more often located in urban settings compared to non-participating hospitals. While most hospitals were located in the South among both groups, there was a higher prevalence of hospitals in the Northeast among participating versus non-participating centers. The observed differences in hospital characteristics between participating and non-participating sites may represent targeting sites that serve a larger and more geographically concentrated population of patients to enable more rapid enrollment and ensure timely study completion and dissemination of results. Further examination into the characteristics of smaller rural hospitals that nevertheless participated in the trial may improve our ability to broaden the number centers that participate in clinical research, and further enhance the generalizability of research findings.
The inability to enroll sufficient numbers of women and minority patients has been a significant critique of prior randomized clinical trials. 3-6, 17-19 We found that the DAPT Study was able to enroll a population of patients that was similar with respect to race and sex as those patients undergoing PCI with DES in the community. Patients enrolled in the study also underwent similar rates of complex procedures such as vein graft and left main interventions. However, DAPT-enrolled patients tended to be younger and have lower cardiovascular disease burden than US PCI patients, as well as shorter lengths of stay. There are multiple possible mechanisms for this difference, including subjects’ views on informed consent for a randomized trial or the investigators perception regarding a subject’s ability to comply with the trial protocol. The presence of screening and consent procedures has been found to bias trials toward inclusion of healthier subjects than observed in general practice even in studies intended to be broadly inclusive. 20, 21 While we observed that the adjusted outcomes for hospitals did not differ between participating and non-participating hospitals, determining how clinical trial results might be formally “adjusted” to reflect the practice in the general population is an area warranting further investigation.
A major strength of this study is the ability to link study sites within a large comprehensive national registry with a large clinical trial. As a result, we were able to critically examine challenges to the generalizability of a large clinical trial in a manner not otherwise possible. We believe that this study is the first to formally examine whether a representative group of practice settings and patients can be represented in a large trial of this type. Furthermore, clinical trials embedded in registries have been promoted as a potential solution to improving the generalizability and feasibility of conducting randomized trials. 22-24 For example, the Thrombus Aspiration in ST-Elevation Myocardial Infarction in Scandinavia (TASTE) trial evaluated the role of aspiration thrombectomy among patient with ST-elevation myocardial infarction using routinely collected baseline and outcome data from a comprehensive national registry. 23 Similarly, the Study of Access site for Enhancement of PCI for Women (SAFE-PCI) utilized the NCDR CathPCI registry in order to identify sites with sufficient radial artery catheterization experience and leveraged existing data collection forms to increase the efficiency of trial enrollment and execution. 24, 25 We believe our study further highlights the potential uses of registry data to inform clinical trial external validity and address a key critique of RCTs.
However, there are several limitations to our study. First, we were not able to apply all of the DAPT Study exclusion criteria to the NCDR CathPCI registry population as certain information (pregnancy, life expectancy <3 years, and concurrent warfarin or other anticoagulant therapy) was not available from the registry. The lack of information on oral anticoagulation in the NCDR is notable, as its prevalence may differ substantially between trial patients and NCDR patients. However, by limiting the cohort to DES subjects for the comparison of DAPT-enrolled vs. eligible patients, we believe we have likely excluded a large number of patients on chronic anticoagulation for whom some guidelines recommend BMS for PCI. 26, 27 Nevertheless, the inability to exclude such patients from the NCDR CathPCI sample may explain some of the observed imbalance in baseline comorbidities and demographic characteristics or contribute to unobserved differences between groups. Next, while the DAPT study is the largest randomized post-marketing study of coronary stents conducted to date, it may not be representative of clinical trials in other disciplines or other cardiovascular trials conducted in a different manner. Additionally, although the NCDR CathPCI registry includes the large majority of PCI-performing hospitals in the US, systematic differences may exist between included and excluded hospitals, such as those that are part of the Veteran’s Administration system. Data from the NCDR registries are site generated and not comprehensively subject to querying or adjudication for accuracy. Furthermore, the DAPT Study includes randomization to prolonged dual antiplatelet therapy or placebo 12 months after enrollment. However, because we did not have data on NCDR CathPCI patients 12 months after their procedures, we were not able to compare the DAPT Study randomized population to similar patients who might have been eligible for randomization had they participated in the study. Next, because the NCDR does not include unique patient identifiers, patients may be represented more than once in the dataset. Finally, due to very large sample size within the NCDR population, we used standardized differences to examine differences between groups, with a cutoff of 10% for significance. Other methods and cutoffs for statistical significance could have been selected.
In conclusion, we demonstrate that a large streamlined clinical trial can be conducted in a manner that involves a very large number of hospitals, enrolls rapidly, and includes a diverse population of patients, while also preserving key design features to minimize bias: randomization and central adjudication. While sites participating in such a trial had similar adjusted clinical outcomes, they still differ from non-participating sites in many ways, a factor which should be considered when generalizing results to clinical practice. In addition, patient enrolled in the DAPT Study were, on average, a lower risk population compared to potentially eligible patients, despite limited exclusion criteria. These findings have important implications for the evaluation and interpretation large inclusive randomized trials.
Supplementary Material
Acknowledgments
Sources of Funding
Funding for the DAPT Study is provided by four U.S. manufacturers of drug-eluting stents (Abbott Vascular, Boston Scientific, Cordis, and Medtronic Cardiovascular) and the current manufacturers of thienopyridine medications (Eli Lilly/Daiichii Sankyo and Bristol-Myers Squibb/Sanofi-Aventis) with supplementary funding from the Health and Human Services 1R01FD003870-01 (PI Laura Mauri, M.D). The NCDR CathPCI Registry is funded by the American College of Cardiology Foundation. Dr. Yeh is supported by a Career Development Award (1K23HL118138) from the National Heart, Lung, and Blood Institute.
Footnotes
Disclosures
Dr. Yeh receives funding from the Harvard Clinical Research Institute
Dr. Czarny has no disclosures or conflicts of interest to report.
Dr. Normand is the Director of the Massachusetts Data Analysis Center, contracted by the Massachusetts Department of Public Health, and is funded to collect, validate, analyze, and disseminate evidence of quality of cardiovascular care at acute care non-federal hospitals in Massachusetts.
Dr. Kereiakes has the following disclosures: Consulting fees: Modest: Harvard Clinical Research Institute; Significant: Boston Scientific, Abbott Vascular
Dr. Holmes has no disclosures or conflicts of interest to report.
Dr. Brindis is Senior Medical Officer, External Affairs of the National Cardiovascular Data Registry
Dr. Weaver has the following disclosures: Consulting for Data Safety and Monitoring Committees for Boston Scientific Corporation and for Biotronik Corporation.
Dr. Rumsfeld is Chief Scientific Officer for the National Cardiovascular Data Registry.
Dr. Roe has the following disclosures: Research funding: Eli Lilly, Revalesio, Sanofi-Aventis, American College of Cardiology, American Heart Association, Familial Hypercholesterolemia Foundation; consulting or honoraria: Astra Zeneca, Sanofi-Aventis, Janssen Pharmaceuticals, Merck, Regeneron, and Daiichi-Sankyo. All conflicts of interest are listed at www.dcri.org.
Ms. Lee has no disclosures.
Ms. Driscoll-Shempp is an employee of the Harvard Clinical Research Institute.
Dr. Mauri receives institutional research support from Abbott, Boston Scientific, Cordis, Medtronic, Eli Lilly, Daiichi Sankyo, Bristol Myers Squibb, Sanofi-Aventis; and has consulted for Cordis, Medtronic, Boston Scientific and Biotronik.
References
- 1.Lauer MS. Commentary: How the debate about comparative effectiveness research should impact the future of clinical trials. Stat Med. 2012;31:3051–3. doi: 10.1002/sim.5400. [DOI] [PubMed] [Google Scholar]
- 2.Luce BR, Kramer JM, Goodman SN, Connor JT, Tunis S, Whicher D, Schwartz JS. Rethinking randomized clinical trials for comparative effectiveness research: the need for transformational change. Ann Intern Med. 2009;151:206–209. doi: 10.7326/0003-4819-151-3-200908040-00126. [DOI] [PubMed] [Google Scholar]
- 3.Gurwitz JH, Col NF, Avorn J. The exclusion of the elderly and women from clinical trials in acute myocardial infarction. JAMA. 1992;268:1417–22. [PubMed] [Google Scholar]
- 4.Harris DJ, Douglas PS. Enrollment of women in cardiovascular clinical trials funded by the National Heart, Lung, and Blood Institute. N Engl J Med. 2000;343:475–80. doi: 10.1056/NEJM200008173430706. [DOI] [PubMed] [Google Scholar]
- 5.Lee PY, Alexander KP, Hammill BG, Pasquali SK, Peterson ED. Representation of elderly persons and women in published randomized trials of acute coronary syndromes. JAMA. 2001;286:708–13. doi: 10.1001/jama.286.6.708. [DOI] [PubMed] [Google Scholar]
- 6.Rothwell PM. External validity of randomised controlled trials: “to whom do the results of this trial apply?”. Lancet. 2005;365:82–93. doi: 10.1016/S0140-6736(04)17670-8. [DOI] [PubMed] [Google Scholar]
- 7.Tunis SR, Stryer DB, Clancy CM. Practical clinical trials: increasing the value of clinical research for decision making in clinical and health policy. JAMA. 2003;290:1624–1632. doi: 10.1001/jama.290.12.1624. [DOI] [PubMed] [Google Scholar]
- 8.Calvo G, McMurray JJ, Granger CB, Alonso-Garcia A, Armstrong P, Flather M, Gomez-Outes A, Pocock S, Stockbridge N, Svensson A, Van de Werf F. Large streamlined trials in cardiovascular disease. Eur Heart J. 2014;35:544–8. doi: 10.1093/eurheartj/eht535. [DOI] [PubMed] [Google Scholar]
- 9.Mauri L, Kereiakes DJ, Normand SL, Wiviott SD, Cohen DJ, Holmes DR, Bangalore S, Cutlip DE, Pencina M, Massaro JM. Rationale and design of the dual antiplatelet therapy study, a prospective, multicenter, randomized, double-blind trial to assess the effectiveness and safety of 12 versus 30 months of dual antiplatelet therapy in subjects undergoing percutaneous coronary intervention with either drug-eluting stent or bare metal stent placement for the treatment of coronary artery lesions. Am Heart J. 2010;160:1035–41. 1041 e1. doi: 10.1016/j.ahj.2010.07.038. [DOI] [PubMed] [Google Scholar]
- 10.Masoudi FA, Ponirakis A, Yeh RW, Maddox TM, Beachy J, Casale PN, Curtis JP, De Lemos J, Fonarow G, Heidenreich P, Koutras C, Kremers M, Messenger J, Moussa I, Oetgen WJ, Roe MT, Rosenfield K, Shields TP, Jr, Spertus JA, Wei J, White C, Young CH, Rumsfeld JS. Cardiovascular care facts: a report from the national cardiovascular data registry: 2011. J Am Coll Cardiol. 2013;62:1931–47. doi: 10.1016/j.jacc.2013.05.099. [DOI] [PubMed] [Google Scholar]
- 11.Messenger JC, Ho KK, Young CH, Slattery LE, Draoui JC, Curtis JP, Dehmer GJ, Grover FL, Mirro MJ, Reynolds MR, Rokos IC, Spertus JA, Wang TY, Winston SA, Rumsfeld JS, Masoudi FA Science N and Quality Oversight Committee Data Quality W. The National Cardiovascular Data Registry (NCDR) Data Quality Brief: the NCDR Data Quality Program in 2012. J Am Coll Cardiol. 2012;60:1484–8. doi: 10.1016/j.jacc.2012.07.020. [DOI] [PubMed] [Google Scholar]
- 12.Concannon TW, Nelson J, Kent DM, Griffith JL. Evidence of systematic duplication by new percutaneous coronary intervention programs. Circ Cardiovasc Qual Outcomes. 2013;6:400–8. doi: 10.1161/CIRCOUTCOMES.111.000019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Austin PC. Using the standardized difference to compare the prevalence of a binary variable between two groups in observational research. Communications in Statistics - Simulation and Computation. 2009;38:1228–1234. [Google Scholar]
- 14.Garratt KN, Lee DP, Rose EM, Windle KJ, Liao H, Nwachuku CE, Winters KJ, Bowman TS, Dawkins KD. Rationale and design of the TAXUS Liberte Post-Approval Study: examination of patients receiving the TAXUS Liberte stent with concomitant prasugrel therapy in routine interventional cardiology practice. Am Heart J. 2012;163:142–8 e6. doi: 10.1016/j.ahj.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 15.Kunz LM, Yeh RW, Normand SL. Comparative effectiveness research: does one size fit all? Statistics in medicine. 2012;31:3062–5. doi: 10.1002/sim.5482. discussion 3066-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tunis SR, Stryer DB, Clancy CM. Practical clinical trials: increasing the value of clinical research for decision making in clinical and health policy. JAMA. 2003;290:1624–32. doi: 10.1001/jama.290.12.1624. [DOI] [PubMed] [Google Scholar]
- 17.Dhruva SS, Redberg RF. Variations between clinical trial participants and Medicare beneficiaries in evidence used for Medicare national coverage decisions. Arch Intern Med. 2008;168:136–40. doi: 10.1001/archinternmed.2007.56. [DOI] [PubMed] [Google Scholar]
- 18.Heiat A, Gross CP, Krumholz HM. Representation of the elderly, women, and minorities in heart failure clinical trials. Arch Intern Med. 2002;162:1682–8. doi: 10.1001/archinte.162.15.1682. [DOI] [PubMed] [Google Scholar]
- 19.Steg PG, Lopez-Sendon J, Lopez de Sa E, Goodman SG, Gore JM, Anderson FA, Jr, Himbert D, Allegrone J, Van de Werf F. External validity of clinical trials in acute myocardial infarction. Arch Intern Med. 2007;167:68–73. doi: 10.1001/archinte.167.1.68. [DOI] [PubMed] [Google Scholar]
- 20.Tu JV, Willison DJ, Silver FL, Fang J, Richards JA, Laupacis A, Kapral MK. Impracticability of informed consent in the Registry of the Canadian Stroke Network. N Engl J Med. 2004;350:1414–21. doi: 10.1056/NEJMsa031697. [DOI] [PubMed] [Google Scholar]
- 21.de Boer SP, Lenzen MJ, Oemrawsingh RM, Simsek C, Duckers HJ, van der Giessen WJ, Serruys PW, Boersma E. Evaluating the ‘all-comers’ design: a comparison of participants in two ‘all-comers’ PCI trials with non-participants. Eur Heart J. 2011;32:2161–7. doi: 10.1093/eurheartj/ehr126. [DOI] [PubMed] [Google Scholar]
- 22.Lauer MS, D’Agostino RB., Sr The randomized registry trial--the next disruptive technology in clinical research? N Engl J Med. 2013;369:1579–81. doi: 10.1056/NEJMp1310102. [DOI] [PubMed] [Google Scholar]
- 23.Frobert O, Lagerqvist B, Olivecrona GK, Omerovic E, Gudnason T, Maeng M, Aasa M, Angeras O, Calais F, Danielewicz M, Erlinge D, Hellsten L, Jensen U, Johansson AC, Karegren A, Nilsson J, Robertson L, Sandhall L, Sjogren I, Ostlund O, Harnek J, James SK. Thrombus aspiration during ST-segment elevation myocardial infarction. N Engl J Med. 2013;369:1587–97. doi: 10.1056/NEJMoa1308789. [DOI] [PubMed] [Google Scholar]
- 24.Hess CN, Rao SV, Kong DF, Aberle LH, Anstrom KJ, Gibson CM, Gilchrist IC, Jacobs AK, Jolly SS, Mehran R, Messenger JC, Newby LK, Waksman R, Krucoff MW. Embedding a randomized clinical trial into an ongoing registry infrastructure: unique opportunities for efficiency in design of the Study of Access site For Enhancement of Percutaneous Coronary Intervention for Women (SAFE-PCI for Women) Am Heart J. 2013;166:421–8. doi: 10.1016/j.ahj.2013.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rao SV, Hess CN, Barham B, Aberle LH, Anstrom KJ, Patel TB, Jorgensen JP, Mazzaferri EL, Jr, Jolly SS, Jacobs A, Newby LK, Gibson CM, Kong DF, Mehran R, Waksman R, Gilchrist IC, McCourt BJ, Messenger JC, Peterson ED, Harrington RA, Krucoff MW. A Registry-Based Randomized Trial Comparing Radial and Femoral Approaches in Women Undergoing Percutaneous Coronary Intervention: The SAFE-PCI for Women (Study of Access Site for Enhancement of PCI for Women) Trial. JACC Cardiovascular interventions. 2014;7:857–67. doi: 10.1016/j.jcin.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 26.Sourgounis A, Lipiecki J, Lo TS, Hamon M. Coronary stents and chronic anticoagulation. Circulation. 2009;119:1682–8. doi: 10.1161/CIRCULATIONAHA.108.834861. [DOI] [PubMed] [Google Scholar]
- 27.Becker RC, Meade TW, Berger PB, Ezekowitz M, Connor CM, Vorchheimer DA, Guyatt GH, Mark DB, Harrington RA and American College of Chest P. The primary and secondary prevention of coronary artery disease: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition) Chest. 2008;133:776S–814S. doi: 10.1378/chest.08-0685. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


