Abstract
Background
Modular and non-invasive expandable prostheses have been developed to provide a functional knee joint that allows future expansion as growth occurs in the contralateral extremity in children with bone sarcomas that require removal of the growth plate. This study aimed to evaluate the functional outcomes of paediatric patients who received either a non-invasive expandable or modular prosthesis for bone sarcomas arising around the knee.
Methods
We evaluated clinician-reported, patient-reported, and measured function in 42 paediatric patients at least one year (median age at assessment 19.1 years) after limb salvage surgery, and compared patients who received modular system prostheses (N=29, median age 15.5), who did not require lengthening procedures to those who received non-invasive expandable prostheses (N=13, median age 11.1) requiring lengthening procedures (median 5).
Results
The number of revisions and time to first revision did not differ between the two groups. There were no differences between the two groups in total scores on the Enneking Musculoskeletal Tumor Society Scale, the Toronto Extremity Salvage Scale, and the Functional Mobility Assessment. Children with non-invasive expandable prostheses climbed stairs (11.93±4.83 vs. 16.73±7.24 seconds, p=0.02) in less time than those with modular prostheses.
Conclusion
Our results suggest that the non-invasive expandable prosthesis produces similar functional results to the more traditional modular prosthesis.
Keywords: Functional Mobility Assessment, Expandable Prosthesis, Limb Sparing, Sarcoma
Introduction
Bone sarcomas frequently involve the metadiaphyseal junction and the growth plate of the long bones of children and adolescents. Surgical management of these tumors requires a wide resection with margins that extend into the normal tissue surrounding the tumor [1]. This may necessitate removal of the physis in the skeletally immature child when the tumor abuts or crosses the growth plate [2]. Reconstruction of the knee typically involves placement of a hinged knee type endoprosthesis [3]. In the skeletally immature patient, this creates a limb length inequality as the contralateral extremity continues to grow unabated [4] Custom prosthetic designs were initially unable to address this problem. Many children had to undergo amputation [5] or rotationplasty [6] to avoid future leg length discrepancy and to allow optimum mobility with an external prosthetic limb.
Technological advances to address this problem have resulted in the development of modular oncology prostheses which allow surgeons to periodically replace modular midsections with larger ones to compensate for differences in leg length [7] This design decreases the necessity for amputation and increases patient satisfaction by preserving the limb and cosmesis. However, the exchange of components requires multiple surgical procedures to be performed over time, and predisposes the patient to significant morbidity [5] Repeated bouts of tissue damage related to surgery, muscle atrophy related to post-surgical disuse, and a general decrease in mobility related to recovery-period immobility may impact optimal limb function and contribute to long-term physical disability and lower quality of life [8]
Among children with remaining growth potential, in an effort to avoid additional surgery, non-invasive expandable prostheses were designed to allow expansion of the prosthesis without an open procedure [9] The Repiphysis® non-invasive expandable prosthesis is an implant that allows expansion via external activation of a spring mechanism housed in the body of the implant. This device, like the modular systems appropriate for older children with little remaining growth potential, avoids amputation, allows the limb to be lengthened for optimal function, and eliminates the potential complications associated with an open surgical procedure. Although it was anticipated that the use of this device would optimize limb function and mobility in these children as they grew and reached final adult height, data about the functional outcomes after using this type of prosthesis versus (vs.) the modular system prosthesis was not available.
In this study, our aim was to compare range of motion and functional mobility outcomes among patients with bone sarcoma about the knee who underwent limb salvage surgery and insertion of modular system prostheses to those who underwent limb salvage surgery and insertion of non-invasive expandable prostheses.
Patients and Methods
Participants
Participants included children treated for lower extremity bone sarcoma at St. Jude Children’s Research Hospital. Forty-two children who underwent limb salvage surgery and received neoadjuvant chemotherapy and who returned for a follow-up visit during 18 consecutive months participated in this study. Inclusion criteria were: 1) diagnosis of lower-extremity bone sarcoma (Ewing sarcoma, osteosarcoma) after 1992; 2) limb-sparing surgical procedure at least one year prior to the scheduled visit; 3) completed chemotherapy and/or radiation; 4) at least 13 years of age or currently in the seventh year of school at time of the functional assessment; 5) no diagnosis of a neuromuscular disorder, developmental delay, or genetic disorder; 6) no local recurrence of disease; 7) no known cardiorespiratory abnormalities expected to affect physical function; and 8) no current injury to the lower-extremity such as a fracture. Patients who underwent amputation or rotationplasty were excluded. Study procedures and materials were approved by the Institutional Review Board. Consent/assent was obtained from all participants and guardians as appropriate prior to administration of study procedures.
Table 1 shows the characteristics of the patients. Twenty-nine (69%) patients had modular prostheses. The remaining 13 patients had non-invasive expandable prostheses (31%). The median age of the patients at the time of limb salvage surgery was 13.7 (range, 6.1 – 21.7) years, and the median age at functional mobility assessment was 19.1 (range, 10.5 – 26.8) years. Most patients were white (n=36; 86%) and the majority were male (n=23; 55%). Ninety percent of patients had osteosarcoma (n=38).
Table I.
Patient characteristics for all patients and according to type of prosthesis
| Prosthesis Type
|
||||
|---|---|---|---|---|
| All Patients | Modular system prosthesis | Non-invasive expandable prosthesis | P-value | |
|
|
||||
| (n=42) | (n=29) | (n=13) | ||
| Gender | ||||
| Male | 23 (55%) | 13 (45%) | 10 (77%) | 0.09 |
| Female | 19 (45%) | 16 (55%) | 3 (23%) | |
| Race | ||||
| White | 36 (86%) | 26 (90%) | 10 (77%) | |
| Black | 5 (12%) | 3 (10%) | 2 (15%) | 0.62a |
| Other | 1 (2%) | 0 (0%) | 1 (8%) | |
| Diagnosis | ||||
| Osteosarcoma | 38 (90%) | 26 (90%) | 12 (92%) | 0.71 |
| Ewing Sarcoma | 4 (10%) | 3 (10%) | 1 (8%) | |
| Limb Salvage Site | ||||
| Femur | 32 (76%) | 23 (79%) | 9 (69%) | 0.70 |
| Tibia | 10 (24%) | 6 (21%) | 4 (31%) | |
| Leg Length Discrepancy > 2 Centimeters | 3 (7%) | 3 (10%) | 0 (0%) | <0.01 |
| Number of Open Surgical Revisions | ||||
| At least one [N (%)] | 20 (48%) | 14 (48%) | 6 (46%) | |
| Mean (SD) | 0.93 (1.42) | 0.93 (1.41) | 0.92 (1.49) | 0.76 |
| Median | 0 | 0 | 0 | |
| Min – Max | 0 – 6 | 0 – 6 | 0 – 5 | |
| Age at Initial Surgery (years) | ||||
| Mean (SD) | 14.0 (3.8) | 15.6 (3.5) | 10.5 (1.4) | |
| Median | 13.7 | 15.5 | 11.1 | <0.001 |
| Min – Max | 6.1 – 21.7 | b6.1 – 21.7 | 8.2 – 12.2 | |
| Time to First Revision (years)c | ||||
| Mean (SD) | 2.7 (2.2) | 2.8 (2.5) | 2.6 (1.3) | |
| Median | 2.0 | 1.98 | 2.80 | 0.78 |
| Min – Max | 0.1 – 9.3 | 0.1 – 9.3 | 0.7 – 4.2 | |
| Age at Functional Assessment (years) | ||||
| Mean (SD) | 18.6 (4.0) | 20.5 (3.1) | 14.3 (2.1) | <0.001 |
| Median | 19.1 | 21.1 | 14.4 | |
| Min – Max | 10.5 – 26.8 | 13.0 – 26.8 | 10.5 – 18.6 | |
| Time from Initial Surgery to Functional Assessment (years) | ||||
| Mean (SD) | 4.6 (3.0) | 4.9 (3.3) | 3.8 (2.0) | 0.47 |
| Median | 4.2 | 4.8 | 3.2 | |
| Min – Max | 1.0 – 12.7 | 1.0 – 12.7 | 1.0 – 7.5 | |
Abbreviations: SD: standard deviation; Min: minimum value; Max: maximum value
P-value compares white vs. black (patients of other races were excluded from the comparison)
Patient who was 6.1 years of age at the time of surgery was treated in 1996 prior to availability of the non-invasive expandable prosthesis
For patients with revisions
Surgical Management
Those patients felt to have a potential for developing limb length discrepancy greater than 4–6 centimeters (cm) at final adult height had surgical reconstruction with an expandable prosthesis (Repiphysis®, Microport Orthopedics, Arlington, TN) designed for non-invasive lengthening (Figures 1 and 2). The device is custom designed for each child, based on pre-operative imaging that takes into account the estimated length of the resection, the child’s growth potential and the estimated limb length discrepancy. Expansion capacity (range 3.5 to 11 centimeters) is related to the length of the resected bone [10]. Patients who were skeletally mature or who had potential for minimal leg length difference (<4 cm) had a modular endoprosthesis placed. None of the patients had their patella removed; none had extra-articular resections. The surgical procedures in either case were performed by the same surgeon (M.D.N.), and consisted of wide local resection and placement of either cemented or press fit stems. Patients with resection of the proximal tibia had reconstruction utilizing a gastrocnemius muscle flap to secure the patellar tendon. This group of patients had their surgical limb casted or splinted in full extension for six weeks to allow healing of the extensor mechanism. Patients with non-invasive expandable prostheses were considered for external limb lengthening when leg lengths differed by 2 centimeters by radiography. Lengthening was done as an outpatient procedure; fluoroscopy was used to monitor the lengthening and localize the expansion mechanism of the prosthesis, a compressed spring housed inside a titanium tube and covered by a polymer tube. During the expansion a transmitter ring was placed over the limb, creating an electromagnetic field to heat the titanium, unlocking it from the polymer and allowing the spring to decompress. Twenty seconds of exposure was typically required to achieve desired expansion.
Figure 1.
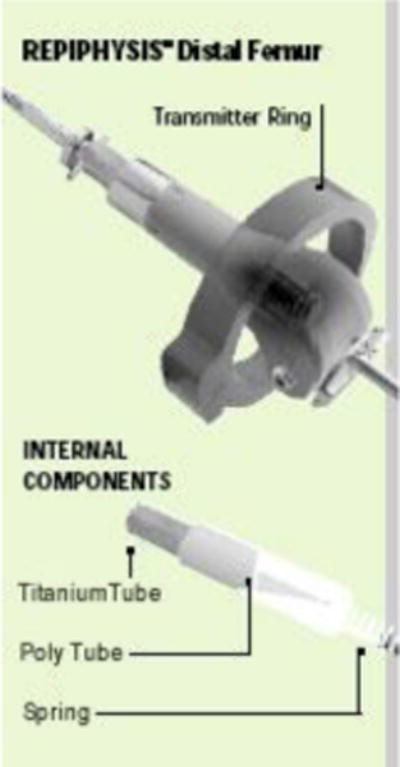
Drawing of Repiphysis® expandable prosthesis
Figure 2.
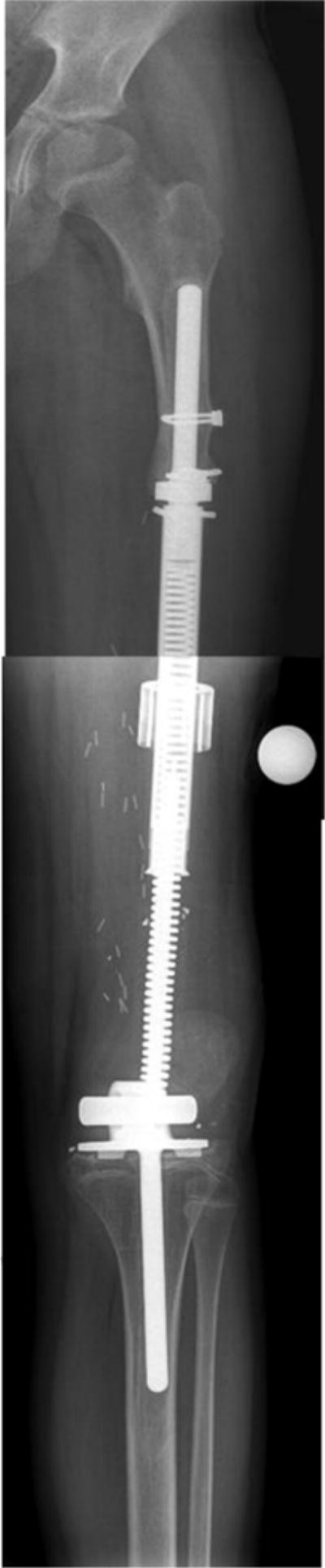
Radiograph of 14 year old male with osteosarcoma status post limb sparing surgery with implantation of Repiphysis® expandable prosthesis
Rehabilitation
Patients in both groups were allowed early weight bearing; active and passive motion in the immediate post-operative period was allowed unless the patient’s surgical procedure required a gastrocnemius muscle flap (N=10). Physical therapy was initiated immediately post initial surgery for both groups of patients. Rehabilitation continued 3 to 5 times per week until the patient was able to ambulate independently without an assistive device, on level and uneven surfaces and up and down stairs, or until progress with range of motion, strength and ambulation remained static for four weeks. Physical therapy intervention was provided after each internal or external limb lengthening using the same criteria.
Range of motion
Hip and knee flexion and extension were measured in the affected limb using standard goniometric technique [11,12] Active hip flexion was measured with the patient in the supine position with the goniometer pivot point on the greater trochanter, the stationary arm parallel to the long access of the trunk and spine, and the moveable arm parallel to the long axis of the femur. Active hip extension was measured with the patient in the side-lying position with the same reference points. Knee flexion and extension were measured with the patient in the supine position with the goniometer pivot point at the femoral condyle, the stationary arm parallel to the long axis of the femur and the moveable arm parallel to the long axis of the fibula. The contralateral leg was stabilized with the hip and knee in comfortable flexion for both hip and knee measures.
Physical function
The Enneking Musculoskeletal Tumor Society Scale (MSTS) [13], the Toronto Extremity Salvage Scale (TESS) [14], and the Functional Mobility Assessment (FMA) [15] were used to evaluate clinician-reported, self-reported, and measured physical function in the two groups of patients. The MSTS was completed by a clinician to quantify activity limitations. The six items (lower extremity version) were scored on a 0 (worst) to 5 (best) scale and included pain, function, emotional acceptance, supports (brace, cane, and crutches), walking ability, and gait. A maximum raw score of 30 was possible, indicating normal function. Results are presented as a sum and as a proportion of the total score [13] The TESS is a questionnaire that was self-administered. On the TESS, patients were asked to indicate the degree of difficulty performing everyday activities such as dressing, grooming, mobility, work, sports, and leisure [14] The items were rated on a scale ranging from 1 to 5, with the total score calculated as a percentage. The FMA is a performance measure and, like the MSTS, includes six specific items: 1) pain, 2) function with two specific measures: Timed Up and Down Stairs (TUDS) time and Timed Up and Go (TUG) time, 3) supports, 4) satisfaction with walking quality, 5) participation in work, school, sports, and 6) endurance as measured by the 9-minute run-walk test. The raw scores in each subcategory in the FMA are converted to a table score (Table II) from 0 (worst) to 5 (best), with a maximum score of 70 points [15]
Table II.
Functional Mobility Assessment
| Function
| ||||||||
|---|---|---|---|---|---|---|---|---|
| Pain 0–10 scale | TUDS | TUG | ||||||
|
| ||||||||
| Score | (0 = none, 10 = worst) | Time (seconds) | HR (beats per minute) | RPE (6–20 scale) | Time (seconds) | HR (beats per minute) | RPE (6–20 scale) | |
|
| ||||||||
| 5 | 0 | ≤ 8 | ≤ 127 | ≤ 7 | ≤ 4 | ≤ 127 | ≤ 7 | |
| 4 | 1–2 | 9 to 12 | 128 to 137 | 8 to 9 | 5 | 128 to 137 | 8 to 9 | |
| 3 | 3–4 | 13 to 16 | 138 to 147 | 10 to 11 | 6 | 138 to 147 | 10 to 11 | |
| 2 | 5–6 | 17 to 20 | 148 to 157 | 12 to 13 | 7 | 148 to 157 | 12 to 13 | |
| 1 | 7–8 | 21 to 24 | 158 to 167 | 14 to 15 | 8 | 1 58 to 167 | 14 to 15 | |
| 0 | 9–10 | > 24 | >167 | > 15 | > 8 | > 167 | > 15 | |
|
| ||||||||
| Subtotal Score | ||||||||
| Score | Supports | Satisfaction with my walking quality | Participation | Endurance (9-minute walk run)
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Distance (feet) | PCI | HR | RPE | |||||||
|
| ||||||||||
| 5 | None | Very satisfied | Participate in work or school and sports. | ≥ 4000 | ≤ 0.20 | <115 | ≤ 7 | |||
| 4 | 1 crutch or cane < 5 hours a day | Happy with my walking quality. | Participate in all activities including work/school, but limited sports. | 3000 to 3999 | 0.21 to 0.41 | 115 to 134 | 8 to 9 | |||
| 3 | 1 crutch or cane all day | Happy but hope it improves. | Limited in work or school and sports. | 2250 to 2999 | 0.42 to 0.62 | 135 to 154 | 10 to 11 | |||
| 2 | 2 crutches or canes < 5 hours a day | Disappointed and want to improve. | Limited in work or school and do not participate in sports. | 1500 to 2249 | 0.63 to 0.83 | 155 to 174 | 12 to 13 | |||
| 1 | 2 crutches or canes > 5 hours a day | Disappointed and afraid it will not improve. | Limited in work or school and activities of daily living. | 1000 to 1499 | 0.84 to 1.09 | 175 to 194 | 14 to 15 | |||
| 0 | Wheelchair | Very unhappy and rarely leave the house. | Unable to go to work or school. | <1000 | ≥ 1.10 | ≥195 | > 15 | |||
|
| ||||||||||
| Subtotal | ||||||||||
|
| ||||||||||
| Total (add all 14 boxes (range 0–70) | ||||||||||
Timed up and down stairs (TUDS), timed up and go (TUG), heart rate (HR), rating of perceived exertion (RPE), physiological cost index (PCI)PCI=(HR end of walk-HR beginning of walk)/meters walked/9 minutes
(Copyright [Pediatric Blood and Cancer 2007;49:183–189]) This material is reproduced with permission of John Wiley & Sons, Inc.
Statistical analyses
Means, standard deviations, medians, ranges, frequencies and percentages were calculated to describe the demographic and treatment characteristics of the study participants. The exact Wilcoxon rank sum test and Fisher’s exact test were used to compare diagnosis and demographic variables between the two groups. Descriptive statistics for range of motion and measures on the MSTS, TESS, and FMA were calculated. Associations between prosthesis group and physical function outcomes and range of motion were examined using exact Wilcoxon rank sum tests. All tests were two-sided. SAS version 9.2 (SAS Institute Inc., Cary NC) was used for all analyses.
Results
Participants
Table I shows the characteristics of all the patients according to their type of prosthesis. There were no differences in the distributions of gender, race, tumor type, site of surgery (femur vs. tibia), or time until first revision with respect to type of prosthesis. There was evidence of a significant difference in patient age at the time of surgery (p<0.001). As expected, patients who received the modular system prosthesis were older than those who received the non-invasive expandable prosthesis (median ages, 15.5 years versus 11.1 years). Consequently, ages at the time of evaluation differed between the two groups (p<0.001); the median age at evaluation for patients who received the modular system prosthesis was 21.1 years, compared to 14.4 years for patients who received the expandable prosthesis. The number of open surgical revisions did not differ between groups. Fourteen participants with modular system prostheses required 26 repairs or revisions, five for fractures and 21 for prosthetic loosening or hardware failure. Six participants with non-invasive expandable prostheses had 13 repairs or revisions, one for fracture and 12 for prosthetic loosening. Participants with modular system prostheses did not have lengthening procedures. Those with non-invasive expandable prostheses had 1 to 14 (median 5) external lengthening procedures between the original surgery and the functional assessment.
Function
The means and standard deviations, medians and ranges for participants’ scores on the MSTS, TESS and FMA (including the individual FMA items) are shown in Table III according to type of endoprosthesis. No significant differences were observed between the non-invasive expandable prosthesis or modular system prosthesis groups in total scores on the MSTS, TESS or FMA. There were also no differences between the two groups on most individual FMA items. However, participants with non-invasive expandable prostheses on average completed the timed up and down stairs (11.93±4.83 vs. 16.73±7.24 seconds, p=0.02) in less time than participants with modular prostheses. Range of motion values in the hips and knees were on average, adequate for daily activities for both participant groups. There was some evidence that hip flexion differed between the groups (p=0.05) (Table IV). The median value for hip flexion was 115 (range, 48 – 160) degrees for patients who received the modular system prosthesis, compared to 108 (range, 30 – 142) degrees for patients who received the non-invasive expandable prosthesis. There were no difference in functional or range of motion outcomes when participants with tibial versus participants with femoral tumors were compared.
Table III.
Functional Mobility according to Type of Prosthesis
| Variable | Prosthesis Type | Mean | SD | Median | Min | Max | P-value |
|---|---|---|---|---|---|---|---|
| Total Scores | |||||||
| MSTS Score (%) | MP | 0.69 | 0.15 | 0.70 | 0.27 | 0.93 | 0.29 |
| NIEP | 0.73 | 0.15 | 0.73 | 0.30 | 0.90 | ||
| MSTS Score | MP | 20.55 | 4.59 | 21.00 | 8.00 | 28.00 | 0.29 |
| NIEP | 21.92 | 4.39 | 22.00 | 9.00 | 27.00 | ||
| TESS Score | MP | 85.24 | 10.67 | 87.86 | 60.61 | 99.29 | 0.58 |
| NIEP | 87.00 | 10.90 | 92.19 | 61.67 | 98.57 | ||
| FMA Total Score | MP | 46.90 | 7.98 | 47.00 | 31.00 | 61.00 | 0.37 |
| NIEP | 49.15 | 4.71 | 49.00 | 40.00 | 58.00 | ||
| Pain (raw score) | MP | 1.28 | 2.17 | 0.00 | 0.00 | 6.00 | 0.71 |
| NIEP | 1.38 | 3.01 | 0.00 | 0.00 | 10.00 | ||
| TUDS Time (raw) | MP | 16.73 | 7.24 | 14.88 | 7.34 | 35.69 | 0.02 |
| NIEP | 11.93 | 4.83 | 10.43 | 6.88 | 21.41 | ||
| TUDS HR (raw) | MP | 118.48 | 17.20 | 122.00 | 74.00 | 150.00 | 0.43 |
| NIEP | 123.00 | 16.94 | 125.00 | 94.00 | 145.00 | ||
| TUDS RPE (raw) | MP | 10.76 | 3.07 | 11.00 | 6.00 | 17.00 | 0.13 |
| NIEP | 9.23 | 2.49 | 9.00 | 6.00 | 13.00 | ||
| TUG Time (raw) | MP | 7.14 | 2.28 | 6.54 | 3.91 | 13.06 | 0.16 |
| NIEP | 6.04 | 1.00 | 5.75 | 4.50 | 7.72 | ||
| TUG HR (raw) | MP | 94.83 | 16.85 | 96.00 | 65.00 | 129.00 | 0.34 |
| NIEP | 100.31 | 14.18 | 100.00 | 78.00 | 129.00 | ||
| TUG RPE (raw) | MP | 8.55 | 2.23 | 8.00 | 6.00 | 15.00 | 0.85 |
| NIEP | 8.69 | 2.93 | 9.00 | 6.00 | 16.00 | ||
| Supports | MP | 4.59 | 1.09 | 5.00 | 1.00 | 5.00 | 0.83 |
| NIEP | 4.69 | 1.11 | 5.00 | 1.00 | 5.00 | ||
| Satisfaction | MP | 3.59 | 1.18 | 3.00 | 1.00 | 5.00 | 0.47 |
| NIEP | 3.85 | 1.34 | 4.00 | 1.00 | 5.00 | ||
| Participation | MP | 3.52 | 1.21 | 4.00 | 0.00 | 5.00 | 0.18 |
| NIEP | 4.08 | 0.64 | 4.00 | 3.00 | 5.00 | ||
| Endurance Distance (feet) | MP | 2077.90 | 533.55 | 2145.00 | 1230.00 | 3380.00 | 0.45 |
| NIEP | 2222.73 | 591.14 | 1950.00 | 1310.00 | 3161.00 | ||
| Endurance PCI (raw) | MP | 0.85 | 0.35 | 0.80 | 0.43 | 1.92 | 0.46 |
| NIEP | 0.87 | 0.33 | 0.93 | 0.27 | 1.46 | ||
| Endurance HR (raw) | MP | 137.93 | 21.21 | 141.00 | 96.00 | 173.00 | 0.17 |
| NIEP | 151.85 | 32.46 | 150.00 | 94.00 | 196.00 | ||
| Endurance RPE (raw) | MP | 12.55 | 2.91 | 13.00 | 6.00 | 18.00 | 0.71 |
| NIEP | 12.31 | 2.95 | 12.00 | 8.00 | 19.00 | ||
Abbreviations: MP: Modular prosthesis; NIEP: non invasive expandable prosthesis; SD: standard deviation; Min: minimum value; Max: maximum value; MSTS: Musculoskeletal Tumor Society; TESS: Toronto extremity salvage scale; FMA: functional mobility assessment; TUDS: timed up and down stairs; TUG: timed up and go; RPE: Rate of perceived exertion; HR: heart rate; PCI: physiologic cost index
Table IV.
Active range of motion in degrees according to type of prosthesis
| Variable | Prosthesis Type | Mean | SD | Median | Min | Max | P-value |
|---|---|---|---|---|---|---|---|
| Hip Extension | MP | 14.45 | 7.74 | 14.00 | 0.00 | 32.00 | 0.45 |
| NIEP | 12.54 | 7.16 | 12.00 | 0.00 | 25.00 | ||
| Hip Flexion | MP | 110.66 | 21.87 | 115.00 | 48.00 | 160.00 | 0.05 |
| NIEP | 93.15 | 32.90 | 108.00 | 30.00 | 142.00 | ||
| Knee Extension | MP | −1.79 | 3.21 | 0.00 | −12.00 | 0.00 | 0.44 |
| NIEP | −1.85 | 4.58 | 0.00 | −16.00 | 0.00 | ||
| Knee Flexion | MP | 102.41 | 26.74 | 112.00 | 22.00 | 138.00 | 0.70 |
| NIEP | 98.85 | 28.68 | 109.00 | 50.00 | 132.00 |
Abbreviations: MP: Modular prosthesis; NIEP: non-invasive expandable prosthesis; SD: standard deviation; Min: minimum value; Max: maximum value
Discussion
Progress in the oncologic management of children and adolescents with lower limb malignancies has improved their long-term survival. This progress has been accompanied by rapid developments to improve surgical techniques and to design prosthetic implants that not only improve functional outcomes, but also that limit the need for multiple subsequent surgical interventions. Because malignant bone tumors of the lower extremity frequently require resection of the involved physis, which in the skeletally immature child can result in significant limb length discrepancy at maturity, an implantable prosthesis that has the ability to “grow” with the child has a unique advantage. Although the expandable prosthesis requires replacement with a permanent prosthesis once adult height is achieved, it reduces the number of times the child has to be hospitalized for a surgical procedure while growing, minimizing the potential for infection and other surgical complications [7,9]. In this study, we demonstrate that such a prosthesis results in good functional outcomes among patients evaluated one year or longer after the reconstruction. Younger patients who received the non-invasive expandable prosthesis had similar function to older patients whose potential for a limb length difference (less than 4–6 centimeters) allowed for placement of a modular prosthesis.
Other limb sparing procedures have been developed in attempts to decrease the number of additional surgeries and ultimately to preserve function of the reconstructed limb. These procedures have had varying degrees of success. Avedian et al. [16] recently reported excellent functional outcomes (MSTS scores from 28–30 out of 30) among six patients who had limb sparing surgeries using multiplanar osteotomy resection with limited wide margins and intercalary allograft reconstruction. However, all six patients had surgical complications; three required additional procedures to remedy joint or soft tissue integrity problems. These patients were similar in age to our cohort at initial resection (11–19 years) and were 25–66 months post surgery when functional outcome was assessed. This study did not report range of motion, timed mobility or self-reported function. Agarwal et al. [17] reviewed the records of 25 children who had diaphyseal resections with small remaining epiphyseal or metaphyseal segments to generate guidelines for selecting a reconstruction method and implant type. Eight of these children (ages 2–14 years) had physis-sparing procedures. Four had excellent outcomes with no surgical revisions; one had an angular deformity, two required plate revision and one required amputation because of infection. At one year after surgery, MSTS scores ranged from 28–30 for the six patients who were evaluable. MSTS scores were not obtained on the patient who had amputation or the patient who had local recurrence and pulmonary metastases one year after surgery. Range of motion values, timed mobility and self-reported function were not reported for these eight patients. These patients were younger than our cohort at both initial surgery and at evaluation of function. Finally, Zhang et al. [18] evaluated functional outcome an average of 47 months following custom prosthetic replacement among eleven patients (mean age 17; range 14–23 years) with osteosarcoma of the proximal tibia and proximal tibiofibular joint. Six patients remained free of disease at the time of evaluation, three died of pulmonary metastases, and two had recurrent disease. One patient with local recurrence underwent amputation. The mean MSTS score for the six patients was 70% (corresponds to 21/30), mean knee flexion was 85 degrees and mean knee extension was −20 degrees. These patients were similar in age to our cohort. Range of motion outcomes appeared to be somewhat worse than those measured in our cohort.
There are certain limitations that should be considered when interpreting the results of our study. First, this was not a randomized study. We compared functional outcomes among patients selected for non-invasive expandable prosthesis because of their young age and potential limb growth to those of older patients who were approaching skeletal maturity and received an endoprosthesis. More optimal comparison groups would have been children in whom skeletal growth was expected but who received a more traditional modular device that required subsequent surgical intervention to achieve limb growth or children who receive amputation or rotationplasty. It is possible that our approach actually underestimates the positive impact of the non-invasive expandable prosthesis on functional outcomes. Our study also suffers from the usual problem encountered in assessment of new surgical intervention in that we have a relatively small sample size. This makes it difficult to evaluate the potential contributions of additional patient disease, treatment, and personal characteristics on functional outcomes.
In summary, we found that functional outcomes among children who received a non-invasive expandable prosthesis are similar to older children whose expected skeletal growth does not require an expandable endoprosthetic implant. Although longer follow-up will be needed to evaluate potential technical or biomechanical problems with the non-invasive expandable prosthesis, this new device shows promise to avoid further surgery in younger children while achieving acceptable limb function in children and adolescents with lower extremity bone malignancies.
Acknowledgments
Support: Authors at St. Jude Children’s Research Hospital received support from a Cancer Center Support Grant, CA 21765, from the National Institutes of Health and the American Lebanese Syrian Associated Charities (ALSAC).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Role of the Funding Source
The funding source did not play a role in the study design, data collection, data analysis, data interpretation or writing of this manuscript.
Conflict of Interest Statement
Each author certifies that he or she has no commercial associations (e.g. consultancies, stock ownership, equity interest, patent/licensing arrangement, etc.) that might pose a conflict of interest in connection with the submitted article.
Contributor Information
Kirsten K. Ness, Email: kiri.ness@stjude.org, St. Jude Children’s Research Hospital, Department of Epidemiology and Cancer Control, Mail Stop 735, 262 Danny Thomas Place, Memphis, TN 38105 USA.
Michael D. Neel, Email: mneel@orthomemphis.com, St. Jude Children’s Research Hospital, Department of Surgery, Mail Stop 721, 262 Danny Thomas Place, Memphis, TN 38105 USA.
Sue C. Kaste, Email: sue.kaste@stjude.org, St. Jude Children’s Research Hospital, Department of Radiological Sciences, Mail Stop 220, 262 Danny Thomas Place, Memphis, TN 38105 USA.
Catherine A. Billups, Email: catherine.billups@stjude.org, St. Jude Children’s Research Hospital, Department of Biostatistics, Mail Stop 723, 262 Danny Thomas Place, Memphis, TN 38105 USA.
Victoria G. Marchese, Email: vmarche1@jhu.edu, Johns Hopkins University, Center for Technology in Education, 6740 Alexander Bell Drive, Suite 302, Columbia, MD 21046 USA.
Bhaskar N. Rao, Email: bhaskar.rao@stjude.org, St. Jude Children’s Research Hospital, Department of Surgery, Mail Stop 721, 262 Danny Thomas Place, Memphis, TN 38105 USA.
Najat C. Daw, Email: ndaw@mdanderson.org, Division of Pediatrics, Unit 87, MD Anderson Cancer Center, 1515 Holcombe Blvd, Houston TX 77030 USA.
References
- 1.Mercuri M, Capanna R, Manfrini M, et al. The management of malignant bone tumors in children and adolescents. Clin Orthop Relat Res. 1991;264:156–168. [PubMed] [Google Scholar]
- 2.Finn HA, Simon MA. Limb-salvage surgery in the treatment of osteosarcoma in skeletally immature individuals. Clin Orthop Relat Res. 1991:108–118. [PubMed] [Google Scholar]
- 3.Kneisl JS, Finn HA, Simon MA. Mobile knee reconstructions after resection of malignant tumors of the distal femur. Orthop Clin North Am. 1991;22:105–119. [PubMed] [Google Scholar]
- 4.Arndt CA, Crist WM. Common musculoskeletal tumors of childhood and adolescence. N Engl J Med. 1999;341:342–352. doi: 10.1056/NEJM199907293410507. [DOI] [PubMed] [Google Scholar]
- 5.Weisstein JS, Goldsby RE, O’Donnell RJ. Oncologic approaches to pediatric limb preservation. J Am Acad Orthop Surg. 2005;13:544–554. doi: 10.5435/00124635-200512000-00007. [DOI] [PubMed] [Google Scholar]
- 6.Cammisa FP, Jr, Glasser DB, Otis JC, Kroll MA, Lane JM, Healey JH. The Van Nes tibial rotationplasty. A functionally viable reconstructive procedure in children who have a tumor of the distal end of the femur. J Bone Joint Surg Am. 1990;72:1541–1547. [PubMed] [Google Scholar]
- 7.Neel MD, Letson GD. Modular endoprostheses for children with malignant bone tumors. Cancer Control. 2001;8:344–348. doi: 10.1177/107327480100800406. [DOI] [PubMed] [Google Scholar]
- 8.Nagarajan R, Clohisy DR, Neglia JP, et al. Function and quality-of-life of survivors of pelvic and lower extremity osteosarcoma and Ewing’s sarcoma: the Childhood Cancer Survivor Study. Br J Cancer. 2004;91:1858–1865. doi: 10.1038/sj.bjc.6602220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Neel MD, Wilkins RM, Rao BN, Kelly CM. Early multicenter experience with a noninvasive expandable prosthesis. Clin Orthop Relat Res. 2003;415:72–81. doi: 10.1097/01.blo.0000093899.12372.25. [DOI] [PubMed] [Google Scholar]
- 10.Saghieh S, Abboud MR, Muwakkit SA, Saab R, Rao B, Haidar R. Seven-year experience of using Repiphysis expandable prosthesis in children with bone tumors. Pediatr Blood Cancer. 2010;55:457–463. doi: 10.1002/pbc.22598. [DOI] [PubMed] [Google Scholar]
- 11.Piriyaprasarth P, Morris ME. Psychometric properties of measurement tools for quantifying knee joint position and movement: a systematic review. Knee. 2007;14:2–8. doi: 10.1016/j.knee.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 12.Pua YH, Wrigley TV, Cowan SM, Bennell KL. Intrarater test-retest reliability of hip range of motion and hip muscle strength measurements in persons with hip osteoarthritis. Arch Phys Med Rehabil. 2008;89:1146–1154. doi: 10.1016/j.apmr.2007.10.028. [DOI] [PubMed] [Google Scholar]
- 13.Enneking WF, Dunham W, Gebhardt MC, Malawar M, Pritchard DJ. A system for the functional evaluation of reconstructive procedures after surgical treatment of tumors of the musculoskeletal system. Clin Orthop Relat Res. 1993;286:241–246. [PubMed] [Google Scholar]
- 14.Davis AM, Wright JG, Williams JI, Bombardier C, Griffin A, Bell RS. Development of a measure of physical function for patients with bone and soft tissue sarcoma. Qual Life Res. 1996;5:508–516. doi: 10.1007/BF00540024. [DOI] [PubMed] [Google Scholar]
- 15.Marchese VG, Rai SN, Carlson CA, et al. Assessing functional mobility in survivors of lower-extremity sarcoma: reliability and validity of a new assessment tool. Pediatr Blood Cancer. 2007;49:183–189. doi: 10.1002/pbc.20932. [DOI] [PubMed] [Google Scholar]
- 16.Avedian RS, Haydon RC, Peabody TD. Multiplanar osteotomy with limited wide margins: a tissue preserving surgical technique for high-grade bone sarcomas. Clin Orthop Relat Res. 2010;468:2754–2764. doi: 10.1007/s11999-010-1362-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Agarwal M, Puri A, Gulia A, Reddy K. Joint-sparing or physeal-sparing diaphyseal resections: the challenge of holding small fragments. Clin Orthop Relat Res. 2010;468:2924–2932. doi: 10.1007/s11999-010-1458-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang Y, Yang Z, Li X, et al. Custom prosthetic reconstruction for proximal tibial osteosarcoma with proximal tibiofibular joint involved. Surg Oncol. 2008;17:87–95. doi: 10.1016/j.suronc.2007.11.003. [DOI] [PubMed] [Google Scholar]


