Abstract
Autism spectrum disorder (ASD) has been associated with various sensory atypicalities across multiple domains. Interoception, the ability to detect and attend to internal bodily sensations, has been found to moderate the experience of body ownership, a known difference in ASD that may affect social function. However, interoception has not been empirically examined in ASD. In the current study, 45 children (21 with ASD and 24 controls) ages 8 to 17 years completed a heartbeat perception paradigm as a measure of interoceptive ability. A subset of these children also completed the rubber hand illusion task, a multisensory paradigm probing the malleability of perceived body ownership. Although the heartbeat perception paradigm yielded comparable interoceptive awareness (IA) overall across both groups, children with ASD were superior at mentally tracking their heartbeats over longer intervals, suggesting increased sustained attention to internal cues in ASD. In addition, IA was negatively correlated with rubber hand illusion susceptibility in both groups, supporting a previously demonstrated inverse relationship between internal awareness and one's ability to incorporate external stimuli into one's perception of self. We propose a tradeoff between attention to internal cues and attention to external cues, whereby attentional resources are disproportionately allocated to internal, rather than external, sensory cues in ASD.
Keywords: Interoception, Autism spectrum disorder, Sensory processing
Introduction
Autism spectrum disorder (ASD) is associated with atypical sensory processing across multiple sensory modalities. Much of this literature focuses on sensory processing as it is traditionally defined: the perception of, integration of, and response to physical energy from the external environment. Sensory stimuli can be categorized on a continuum of distance from the perceiver, whereby distal cues are emitted from some distance (e.g., light) and more proximal cues are perceived only through close or direct physical contact. (e.g., heat). Proprioception and interoception lie on the extreme proximal end of the continuum because the physical source of stimulus energy is within the individual's body. Although there are some studies investigating proprioceptive ability in ASD (Blanche, Reinoso, Chang, & Bodison, 2012; Fuentes, Mostofsky, & Bastian, 2011; Izawa et al., 2012), there are no published studies of interoception in individuals with ASD.
Interoceptive awareness (IA) can be broadly defined as the conscious perception of internal bodily cues such as heartbeat and breathing (Craig, 2003) and is related to empathic abilities (Fukushima, Terasawa, & Umeda, 2011) and emotional experiences (Barrett, 2004; Wiens, 2005), both of which are affected in individuals with ASD (American Psychiatric Association, 2013). Interestingly, heightened IA has been identified in two disorders that often co-occur with ASD: depression and anxiety (Paulus & Stein, 2010), suggesting that IA may become maladaptive if excessive attention to interoceptive input is associated with negative affect (Arch & Craske, 2006; Cioffi, 1991; Flink, Nicholas, Boersma, & Linton, 2009; Spek, van Ham, & Nyklicek, 2013). To determine whether attention to inter-oceptive cues is “excessive,” it is important to deconstruct the broad concept of IA into components of detection and attention.
Neurobiological evidence suggests that detection and attention are neurally separable (Sarter, Givens, & Bruno, 2001), with intervals of repeated sensory stimulation longer than 60 s representing sustained attention (Grahn & Manly, 2012). The ability to detect and accurately count one's heartbeats is one common measure of interoceptive ability (Schandry, 1981). By measuring accuracy over four temporal intervals of increasing duration and attentional demand, Schandry's (1981) heartbeat detection task allows separate measurement of interoceptive detection and attention in addition to IA as a whole, although prior studies have mainly addressed the holistic construct of IA.
Whereas IA is a measure of internal body awareness, the rubber hand illusion (RHI) paradigm (Botvinick & Cohen, 1998) has been used as a measure of external body awareness; the illusion reflects an ability to perceive a rubber hand as one's own by way of integrating visual, tactile, and proprioceptive information from the external environment and incorporating it into the sense of self. In adults, individuals with greater IA are more resistant to the RHI (Tsakiris, Tajadura-Jimenez, & Costantini, 2011). The authors suggest that IA modulates the experience of body ownership and that the RHI may result from a trade-off of attentional resources between internal and external cues. If this relationship between IA and RHI susceptibility holds true for children, it would be expected that those with ASD might have heightened interoceptive ability given the reduced susceptibility to the RHI in both children (Cascio, Foss-Feig, Burnette, Heacock, & Cosby, 2012) and adults (Paton, Hohwy, & Enticott, 2012) with ASD.
The goal of the current study was to (a) characterize interoceptive ability (detection, attention, and overall awareness) and (b) examine the relationship between IA and susceptibility to the RHI in children with and without ASD. This will help to clarify the relationship between perception of and attention to internal and external cues that affect body perception and how that relationship may differ in children with ASD. We hypothesized a group difference in IA given the well-established literature confirming sensory-perceptual abnormalities in ASD; however, the previous literature did not suggest a strong directional prediction. Importantly, because perception and attention are both psychologically and neurally distinguishable, and because the degree of attention seems to influence whether IA is adaptive versus maladaptive, we predicted potential group differences at the shortest and longest durations measured, with the longest interval requiring increased sustained attention (Grahn & Manly, 2012). Based on the established relationship between IA and susceptibility to the RHI in typical adults (Tsakiris et al., 2011), we expected to replicate this inverse relationship in children, at least among the typically developing group.
Method
Participants
In total, 30 children with ASD and 27 children with typical development (TD) ages 8 to 17 years participated in the study. Diagnosis of ASD was confirmed with research-reliable administration of the Autism Diagnostic Observation Schedule (ADOS; Lord et al., 2000) and the Autism Diagnostic Interview-Revised (ADI-R; Lord, Rutter, & Le Couteur, 1994) as well as judgment of a licensed clinical psychologist based on DSM-IV-TR (Diagnostic and statistical manual of mental disorders, 4th edition, text revision) criteria. Participants in the TD group were excluded for having diagnosed psychiatric or learning disorders, having a first-degree relative with an ASD, or having elevated scores on two screening measures: the Social Communication Questionnaire (SCQ; Rutter, Bailey, & Lord, 2003) and the Child Behavior Checklist (CBCL). No participants had known genetic or neurological problems, nor had they experienced head injuries. Of the original sample, 9 participants with ASD and 3 with TD were excluded after study completion based on experimenter's judgment of comprehension of or compliance with task instructions. The final sample consisted of 21 children with ASD and 24 children with TD. Of this sample, 2 participants in each group did not complete the RHI task and 1 participant with ASD was determined to be an outlier in this task (see Results). Therefore, the sample for the second set of analyses consisted of 18 children with ASD and 22 children with TD. Groups were matched on mean age and IQ measured by the Wechsler Abbreviated Scales of Intelligence (WASI; Wechsler, 1999) in both analysis samples. See Table 1 for participant characteristics. Parents gave informed consent, and participants gave informed assent. Procedures were approved by the Vanderbilt institutional review board.
Table 1.
Participant characteristics by group for each set of analyses.
| Interoceptive ability analysis |
Rubber hand and interoceptive ability analysis |
|||
|---|---|---|---|---|
| ASD (n = 21) | TD (n = 24) | ASD (n = 18) | TD (n = 22) | |
| Age | 12.28 (2.8) | 11.52 (2.5) | 12.75 (2.7) | 11.63 (2.6) |
| IQ | 109.4 (17.9) | 113.2 (14.3) | 106.8 (18.0) | 114.1 (12.7) |
| % Male | 90.5 | 83.3 | 88.8 | 81.8 |
Note: Group means are shown with standard deviations in parentheses. There were no statistically significant between-group differences on age, IQ, or gender in either analysis sample.
Interoception task
Participants monitored their heartbeat according to the mental tracking method (Schandry, 1981) over four temporal intervals: 25, 35, 45, and 100 s. Actual heart rate was monitored using a pulse oximeter (Biopac OXY100C) with a finger transducer (Biopac TSD123A) and recorded using AcqKnowledge software (sampling rate of 50 Hz). During each of these intervals, participants were instructed to sit quietly, focus internally, and count their own heartbeats without taking their pulse or putting their hand on their chest. The order of intervals was randomized across participants. Each participant was given verbal instructions and completed a 30-s practice session to verify task comprehension. If participants reported zero heartbeats, instructions were repeated and an additional practice session was administered. If they were unable to detect their heartbeat after two practice sessions, testing was terminated. Thus, only participants who could detect their heartbeat to some degree were included in this study. At the end of each interval, the participant verbally reported the number of heartbeats counted, and accuracy was calculated for each interval as the absolute value of the difference between the number of recorded and reported heartbeats divided by the number of recorded heartbeats, giving a percentage. An overall measure of IA was calculated by averaging across all four intervals.
Visual counting control task
To control for potential confounds related to attention, working memory, and general counting ability, a visual counting control task was added partially through data collection, resulting in 8 children in the TD group and 12 in the ASD group (33% and 57% of the respective samples) completing this task. A dim visual stimulus briefly flashed on a screen over the same four intervals as the interoception task at a rate equivalent to the average heart rate for the age range of the sample (Fleming et al., 2011) based on normative data.
Rubber hand task
The methods and results from a partially overlapping (15 of the 40 participants) sample completing this task have been described in a previous report (Cascio, Foss-Feig, Burnette, et al., 2012). Briefly, participants placed their left hand into one chamber of a two-chambered box. This chamber was opaque, obscuring the view of the hand. Inside a second transparent chamber was a realistic rubber left hand. The experimenter instructed children to attend to the rubber hand visually throughout the experiment. Participants made a series of baseline judgments as to the position of their left index finger. Next, the experimenter manually delivered brush strokes to both the visible rubber hand and the hidden actual hand at a rate of approximately 0.5 to 1 Hz for 3 min. After the 3-min block of brushing, participants repeated the proprioceptive judgments. For each participant, this was done under two conditions: synchronous (experimental), where the real and rubber hands were stroked simultaneously, and asynchronous (control), where the strokes were offset by approximately 500 ms. Proprioceptive drift was calculated for the synchronous condition as the difference in centimeters between the perceived location of the left index finger before and after brushing.
Analysis approach
We applied a linear mixed model (LMM) to our interoception data, which allowed us to examine interoceptive ability as it relates to all time intervals tested. In addition, to directly test for hypothesized differences in attention as they relate to interoceptive awareness, we repeated this analysis with only the shortest (25 s) and longest (100 s) intervals. The 25-s interval was chosen because it required the least attentional demand, and the 100-s interval was chosen based on previous literature suggesting that attention to repeated sensory stimulation over intervals longer than 60 s represents sustained attention and is fundamentally different from shorter durations (Grahn & Manly, 2012). Finally, to determine the relation between proprioceptive drift in the RHI and IA in this sample, and its relation to group, we performed a regression analysis.
Results
Interoceptive ability
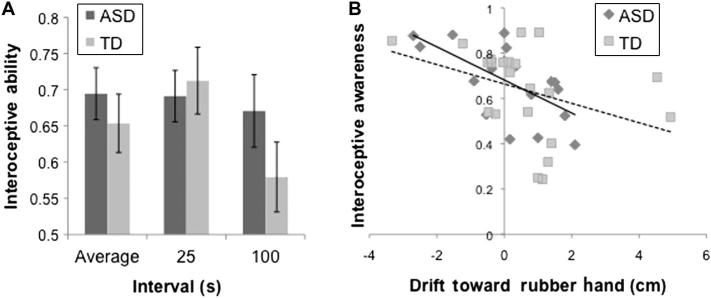
The LMM including all four time intervals (25, 35, 45, and 100 s) revealed a significant main effect of interval, Wald χ2(3) = 11.56, p = .009, in which performance decreased as interval length increased. There was no main effect of group or Group × Interval interaction; however, visual inspection of the estimated marginal means indicated that performance in the ASD group was more stable across the intervals than performance in the TD group. The additional analysis examining only the 25- and 100-s intervals also demonstrated a significant main effect of interval, Wald χ2(1) = 8.75, p = .003; however, this was qualified by a significant Group Interval interaction, Wald χ2(1) = 4.56, p = .03. Performance for the 100-s interval significantly decreased relative to the 25-s interval in the TD group (mean IA: .71 vs. .58), paired t(23) = 3.63, p = .001, Cohen's d = 1.50), whereas in the ASD group performance was constant across these two intervals (mean IA: .69 vs. .67), paired t(20) = 0.525, p = .605. These data are summarized in Fig. 1A.
Fig. 1.
(A) Mean IA did not differ between groups; however, there was a Group × Interval interaction, whereby individuals with TD showed decreased abilities from shortest to longest interval and individuals with ASD showed similar abilities across intervals. Error bars represent ±1 standard error. (B) Higher IA was associated with less drift toward the rubber hand.
Performance on the visual counting task was between 90% and 100% for all four intervals in both groups and did not differ between groups, supporting the assumption that children in both groups were able to attend to and count a repeating stimulus over the relevant time intervals.
Relationship between IA and RHI susceptibility
Inspection of the rubber hand drift data revealed a single extreme outlier in the ASD group whose data point was greater than 3 standard deviations below the group mean and, thus, was removed from analyses involving the rubber hand task. Group, IA, and their interaction together accounted for 18% of the variance in rubber hand drift, F(3,36) = 2.617, p = .066. IA uniquely accounted for 16% of the variance in rubber hand drift, F(1,36) = 7.11, p = .011, with higher IA predicting reduced susceptibility to the RHI. Group and the interaction of group and IA did not uniquely account for a significant amount of variance in rubber hand drift (ps > .05). These results are summarized in Fig. 1B.
Discussion
There was no group difference in IA overall, as measured by heartbeat perception using the mental tracking method. Unlike other clinical groups (Ehlers & Breuer, 1996; Eley, Stirling, Ehlers, Gregory, & Clark, 2004; Paulus & Stein, 2010; Pollatos et al., 2008), ASD does not seem to be characterized by either atypically enhanced or diminished IA. However, when considering differences between the shortest (25 s) and longest (100 s) tracking intervals, children with ASD did not show the same decrease in accuracy for the longest time interval as their TD counterparts. This suggests that individuals with ASD demonstrate a heightened ability to sustain attention to internal cues over longer durations. Thus, in contrast to various psychiatric populations that show a general deficit or advantage in heartbeat detection accuracy that could be attributed to either perceptual or attentional differences, ASD seems to be characterized by a specific increased ability to sustain attention to these internal bodily cues.
Previous work suggests that deficits in sustained attention in ASD beyond that accounted for by developmental delay are attributable to motivation (Garretson, Fein, & Waterhouse, 1990), with salient or motivating stimuli presenting little to no difficulty. A recent neuroimaging study investigating sustained visual attention in ASD reported aberrant neural response patterns, including impaired default mode suppression and enhanced cerebellar recruitment, but in the absence of behavioral differences (Christakou et al., 2013). The default mode is important for internally directed processing; its persistence along with intact sustained attention for motivating stimuli in ASD suggests that internal sensory stimuli may have a particular salience for individuals with ASD. Future studies should examine the neural substrates of attention to internal stimuli in ASD because interoceptive attention recruits a limbic and paralimbic network (Farb, Segal, & Anderson, 2013) that is distinct from the frontoparietal attention network. In addition, our study highlights a need for introducing a distinction between perceptual and attentional components of body awareness (Mehling et al., 2012).
How might enhanced attention to internal cues relate to the core symptoms of ASD? In anxiety and depression, sustained attention to internal cues is thought to be maladaptive, whereby too much internal focus leads to perseverative negative thoughts about the self (Cioffi, 1991). Perhaps the sustained attention we see in ASD is similarly maladaptive, but instead of leading to negative cognitive patterns, heightened attention to internal cues may lead to decreased attention to external stimuli, which provides a putative link between decreased social interaction and repetitive patterns of behavior that directs the focus of attention inward. In addition, individuals with ASD are often under-responsive to environmental sensory stimuli (Baranek et al., 2013; Rogers & Ozonoff, 2005; Watson et al., 2011).
The current study extends the negative relationship between IA and susceptibility to the RHI reported in adults (Tsakiris et al., 2011) to children, further supporting an inverse relationship between internal and external sensory awareness for bodily perception. Individuals with ASD are less susceptible to the RHI (Cascio, Foss-Feig, Burnette, et al., 2012; Paton et al., 2012) and have heightened sustained attention to interoceptive cues, a pattern opposite that seen in individuals with negative body image perceptions and eating disorders (Eshkevari, Rieger, Longo, Haggard, & Treasure, 2012; Mussap & Salton, 2006; Pollatos et al., 2008). Nevertheless, the consistent inverse relationship between these two tasks across multiple groups is indicative of a competition between internal and external sensory input.
Neurobiological evidence suggests that the insula plays a key role in evaluating the motivational significance of interoceptive cues, which has been proposed as an underlying mechanism for human awareness (Craig, 2002, 2009). The insula contains von Economo neurons, large-diameter spindle cells that are relatively rare throughout the rest of the brain and are thought to play a role in intuition (Allman, Watson, Tetreault, & Hakeem, 2005) via the ability to rapidly convey information about physiological state and emotion (Craig, 2009). When Santos and colleagues (2011) discovered higher numbers of von Economo neurons in insular cortex in ASD, they speculated that this might lead to enhanced interoception, a conjecture that is supported by the results of the current study. In addition, increased insula response in ASD while viewing pictures of food under fasting conditions (Cascio, Foss-Feig, Heacock, et al., 2012) has been interpreted as increased attention to the internal perception of hunger. Together, these studies provide neural evidence that the processing of interoceptive cues may be atypical in this population and could contribute to the core deficits of the disorder.
This study was the first to show behavioral evidence for increased sustained attention to interoceptive cues in ASD and to explore the relationship between IA and body awareness. Methodological differences limited the ability to directly replicate previous findings of reduced susceptibility to the RHI in ASD (Cascio, Foss-Feig, Burnette, et al., 2012), but they revealed an inverse relation between RHI susceptibility and interoceptive awareness, confirming previous findings in healthy adults (Tsakiris et al., 2011). In addition, future studies should directly test the proposed relationship of increased attention to internal cues prohibiting sufficient attentional resources to process external environmental stimuli and how this altered focus may contribute to core symptoms of ASD.
Acknowledgments
This work was supported by the Slifka/Ritvo Innovation in Autism Research Award and the National Institute of Mental Health (K01 MH090232 awarded to C.J.C.). The National Center for Advancing Translational Sciences (UL1 TR000445) provided database support. The authors thank Whitney Loring for assistance with diagnostic testing of research participants and thank Kali Hobson, Michael Hogue, and Reagan Allen for assistance with data collection.
Footnotes
Uncited reference
References
- Allman JM, Watson KK, Tetreault NA, Hakeem AY. Intuition and autism: A possible role for von Economo neurons. Trends in Cognitive Science. 2005;9:367–373. doi: 10.1016/j.tics.2005.06.008. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association . Diagnostic and statistical manual of mental disorders. 5th ed. American Psychiatric Association; Arlington, VA: 2013. [Google Scholar]
- Arch JJ, Craske MG. Mechanisms of mindfulness: Emotion regulation following a focused breathing induction. Behaviour Research and Therapy. 2006;44:1849–1858. doi: 10.1016/j.brat.2005.12.007. [DOI] [PubMed] [Google Scholar]
- Baranek GT, Watson LR, Boyd BA, Poe MD, David FJ, McGuire L. Hyporesponsiveness to social and nonsocial sensory stimuli in children with autism, children with developmental delays, and typically developing children. Development and Psychopathology. 2013;25:307–320. doi: 10.1017/S0954579412001071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett LF. Feelings or words? Understanding the content in self-report ratings of experienced emotion. Journal of Personality and Social Psychology. 2004;87:266–281. doi: 10.1037/0022-3514.87.2.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanche EI, Reinoso G, Chang MC, Bodison S. Proprioceptive processing difficulties among children with autism spectrum disorders and developmental disabilities. American Journal of Occupational Therapy. 2012;66:621–624. doi: 10.5014/ajot.2012.004234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botvinick M, Cohen J. Rubber hands “feel” touch that eyes see. Nature. 1998;391:756. doi: 10.1038/35784. [DOI] [PubMed] [Google Scholar]
- Cascio CJ, Foss-Feig JH, Burnette CP, Heacock JL, Cosby AA. The rubber hand illusion in children with autism spectrum disorders: Delayed influence of combined tactile and visual input on proprioception. Autism. 2012;16:406–419. doi: 10.1177/1362361311430404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cascio CJ, Foss-Feig JH, Heacock JL, Newsom CR, Cowan RL, Benningfield MM, et al. Response of neural reward regions to food cues in autism spectrum disorders. Journal of Neurodevelopmental Disorders. 2012;4:9. doi: 10.1186/1866-1955-4-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christakou A, Murphy CM, Chantiluke K, Cubillo AI, Smith AB, Giampietro V, et al. Disorder-specific functional abnormalities during sustained attention in youth with attention deficit hyperactivity disorder (ADHD) and with autism. Molecular Psychiatry. 2013;18:236–244. doi: 10.1038/mp.2011.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cioffi D. Beyond attentional strategies: Cognitive-perceptual model of somatic interpretation. Psychological Bulletin. 1991;109:25–41. doi: 10.1037/0033-2909.109.1.25. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel? Interoception: The sense of the physiological condition of the body. Nature Reviews Neuroscience. 2002;3:655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- Craig AD. Interoception: The sense of the physiological condition of the body. Current Opinion in Neurobiology. 2003;13:500–505. doi: 10.1016/s0959-4388(03)00090-4. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel – now? The anterior insula and human awareness. Nature Reviews Neuroscience. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- Ehlers A, Breuer P. How good are patients with panic disorder at perceiving their heartbeats? Biological Psychology. 1996;42:165–182. doi: 10.1016/0301-0511(95)05153-8. [DOI] [PubMed] [Google Scholar]
- Eley TC, Stirling L, Ehlers A, Gregory AM, Clark DM. Heart-beat perception, panic/somatic symptoms, and anxiety sensitivity in children. Behaviour Research and Therapy. 2004;42:439–448. doi: 10.1016/S0005-7967(03)00152-9. [DOI] [PubMed] [Google Scholar]
- Eshkevari E, Rieger E, Longo MR, Haggard P, Treasure J. Increased plasticity of the bodily self in eating disorders. Psychological Medicine. 2012;42:819–828. doi: 10.1017/S0033291711002091. [DOI] [PubMed] [Google Scholar]
- Farb NAS, Segal ZV, Anderson AK. Attentional modulation of primary interoceptive and exteroceptive cortices. Cerebral Cortex. 2013;23:114–126. doi: 10.1093/cercor/bhr385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming S, Thompson M, Stevens R, Heneghan C, Pluddemann A, Maconochie I, et al. Normal ranges of heart rate and respiratory rate in children from birth to 18 years of age: A systematic review of observational studies. The Lancet. 2011;377:1011–1018. doi: 10.1016/S0140-6736(10)62226-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flink IK, Nicholas MK, Boersma K, Linton SJ. Reducing the threat value of chronic pain: A preliminary replicated single-case study of interoceptive exposure versus distraction in six individuals with chronic back pain. Behaviour Research and Therapy. 2009;47:721–728. doi: 10.1016/j.brat.2009.05.003. [DOI] [PubMed] [Google Scholar]
- Fuentes CT, Mostofsky SH, Bastian AJ. No proprioceptive deficits in autism despite movement-related sensory and execution impairments. Journal of Autism and Developmental Disorders. 2011;41:1352–1361. doi: 10.1007/s10803-010-1161-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukushima H, Terasawa Y, Umeda S. Association between interoception and empathy: Evidence from heartbeat-evoked brain potential. International Journal of Psychophysiology. 2011;79:259–265. doi: 10.1016/j.ijpsycho.2010.10.015. [DOI] [PubMed] [Google Scholar]
- Garretson HB, Fein D, Waterhouse L. Sustained attention in children with autism. Journal of Autism and Developmental Disorders. 1990;20:101–114. doi: 10.1007/BF02206860. [DOI] [PubMed] [Google Scholar]
- Grahn J, Manly T. Common neural recruitment across diverse sustained attention tasks. PLoS ONE. 2012;7:e49556. doi: 10.1371/journal.pone.0049556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izawa J, Pekny SE, Marko MK, Haswell CC, Shadmehr R, Mostofsky SH. Motor learning relies on integrated sensory inputs in ADHD, but over-selectively on proprioception in autism spectrum conditions. Autism Research. 2012;5:124–136. doi: 10.1002/aur.1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH, Jr., Leventhal BL, DiLavore PC, et al. The Autism Diagnostic Observation Schedule-Generic: A standard measure of social and communication deficits associated with the spectrum of autism. Journal of Autism and Developmental Disorders. 2000;30:205–223. [PubMed] [Google Scholar]
- Lord C, Rutter M, Le Couteur A. Autism Diagnostic Interview-Revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders. 1994;24:659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- Masterton BA, Biederman GB. Proprioceptive versus visual control in autistic children. Journal of Autism and Developmental Disorders. 1983;13:141–152. doi: 10.1007/BF01531815. [DOI] [PubMed] [Google Scholar]
- Mehling WE, Price C, Daubenmier JJ, Acree M, Bartmess E, Stewart A. The Multidimensional Assessment of Interoceptive Awareness (MAIA). PLoS ONE. 2012;7:e48230. doi: 10.1371/journal.pone.0048230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mussap AJ, Salton N. A “rubber-hand” illusion reveals a relationship between perceptual body image and unhealthy body change. Journal of Health Psychology. 2006;11:627–639. doi: 10.1177/1359105306065022. [DOI] [PubMed] [Google Scholar]
- Paton B, Hohwy J, Enticott PG. The rubber hand illusion reveals proprioceptive and sensorimotor differences in autism spectrum disorders. Journal of Autism and Developmental Disorders. 2012;42:1870–1883. doi: 10.1007/s10803-011-1430-7. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Stein MB. Interoception in anxiety and depression. Brain Structure and Function. 2010;214:451–463. doi: 10.1007/s00429-010-0258-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollatos O, Kurz AL, Albrecht J, Schreder T, Kleemann AM, Schopf V, et al. Reduced perception of bodily signals in anorexia nervosa. Eating Behaviors. 2008;9:381–388. doi: 10.1016/j.eatbeh.2008.02.001. [DOI] [PubMed] [Google Scholar]
- Rogers SJ, Ozonoff S. Annotation: What do we know about sensory dysfunction in autism? A critical review of the empirical evidence. Journal of Child Psychology and Psychiatry. 2005;46:1255–1268. doi: 10.1111/j.1469-7610.2005.01431.x. [DOI] [PubMed] [Google Scholar]
- Rutter M, Bailey A, Lord C. The Social Communication Questionnaire. Western Psychological Services; Los Angeles: 2003. [Google Scholar]
- Santos M, Uppal N, Butti C, Wicinski B, Schmeidler J, Giannakopoulos P, et al. Von Economo neurons in autism: A stereologic study of the frontoinsular cortex in children. Brain Research. 2011;1380:206–217. doi: 10.1016/j.brainres.2010.08.067. [DOI] [PubMed] [Google Scholar]
- Sarter M, Givens B, Bruno J. The cognitive neuroscience of sustained attention: Where top-down meetings bottom-up. Brain Research Reviews. 2001;35:146–160. doi: 10.1016/s0165-0173(01)00044-3. [DOI] [PubMed] [Google Scholar]
- Schandry R. Heart beat perception and emotional experience. Psychophysiology. 1981;18:483–488. doi: 10.1111/j.1469-8986.1981.tb02486.x. [DOI] [PubMed] [Google Scholar]
- Spek AA, van Ham NC, Nyklicek I. Mindfulness-based therapy in adults with an autism spectrum disorder: A randomized controlled trial. Research in Developmental Disabilities. 2013;34:246–253. doi: 10.1016/j.ridd.2012.08.009. [DOI] [PubMed] [Google Scholar]
- Tsakiris M, Tajadura-Jimenez A, Costantini M. Just a heartbeat away from one’s body: Interoceptive sensitivity predicts malleability of body-representations. Proceedings of the Royal Society B: Biological Sciences. 2011;278:2470–2476. doi: 10.1098/rspb.2010.2547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson LR, Patten E, Baranek GT, Poe M, Boyd BA, Freuler A, et al. Differential associations between sensory response patterns and language, social, and communication measures in children with autism or other developmental disabilities. Journal of Speech, Language, and Hearing Research. 2011;54:1562–1576. doi: 10.1044/1092-4388(2011/10-0029). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. WASI: Wechsler Abbreviated Scale of Intelligence. Harcourt Assessment; San Antonio, TX: 1999. [Google Scholar]
- Wiens S. Interoception in emotional experience. Current Opinion in Neurology. 2005;18:442–447. doi: 10.1097/01.wco.0000168079.92106.99. [DOI] [PubMed] [Google Scholar]