Abstract
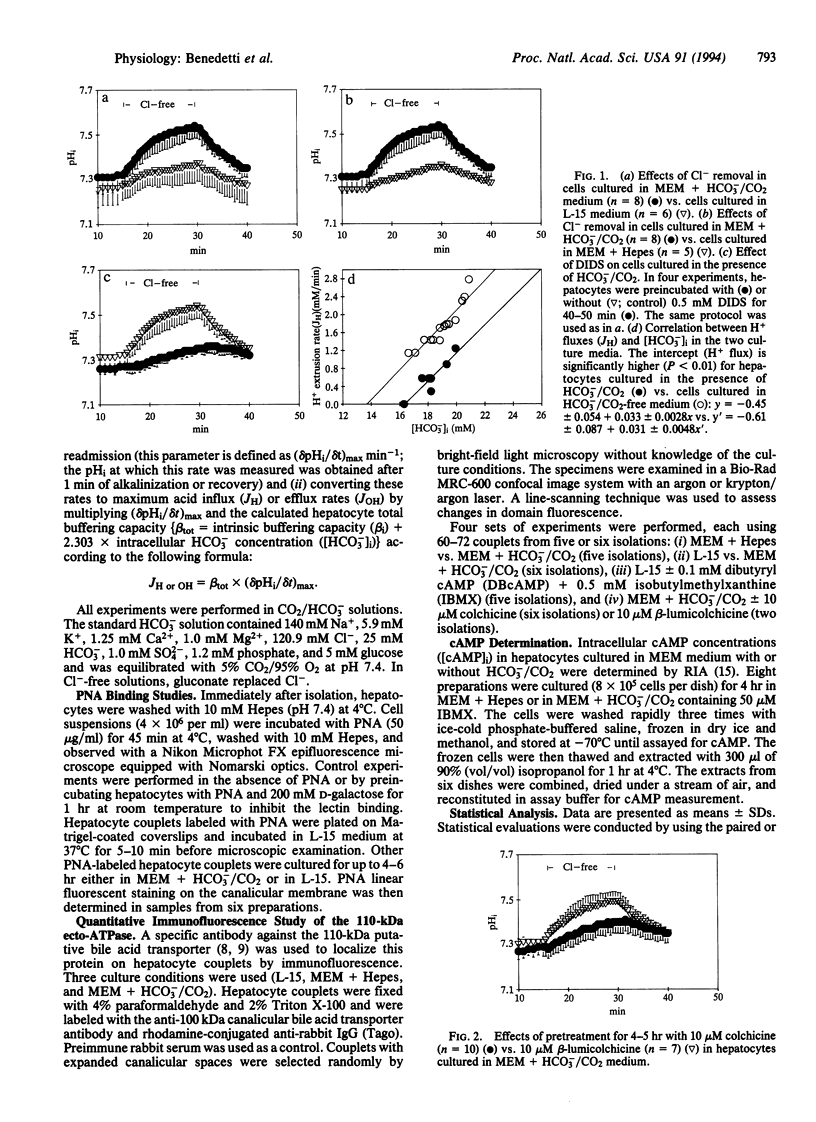
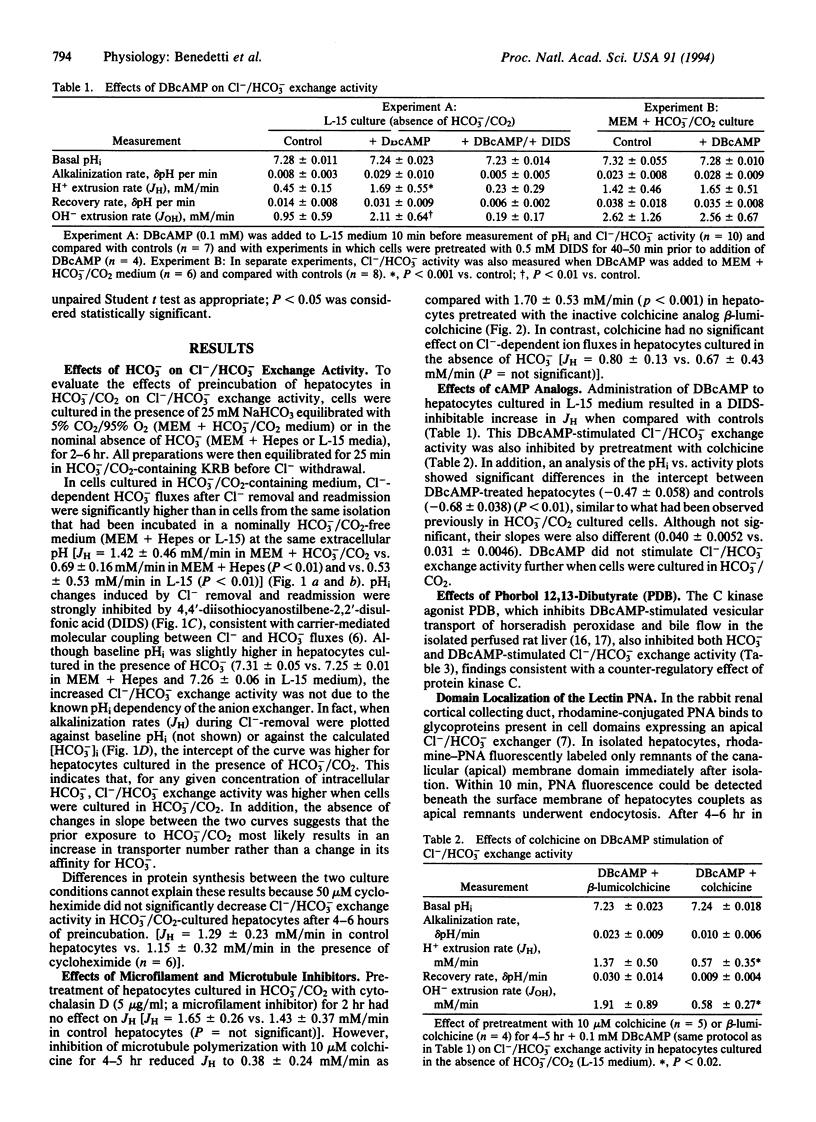
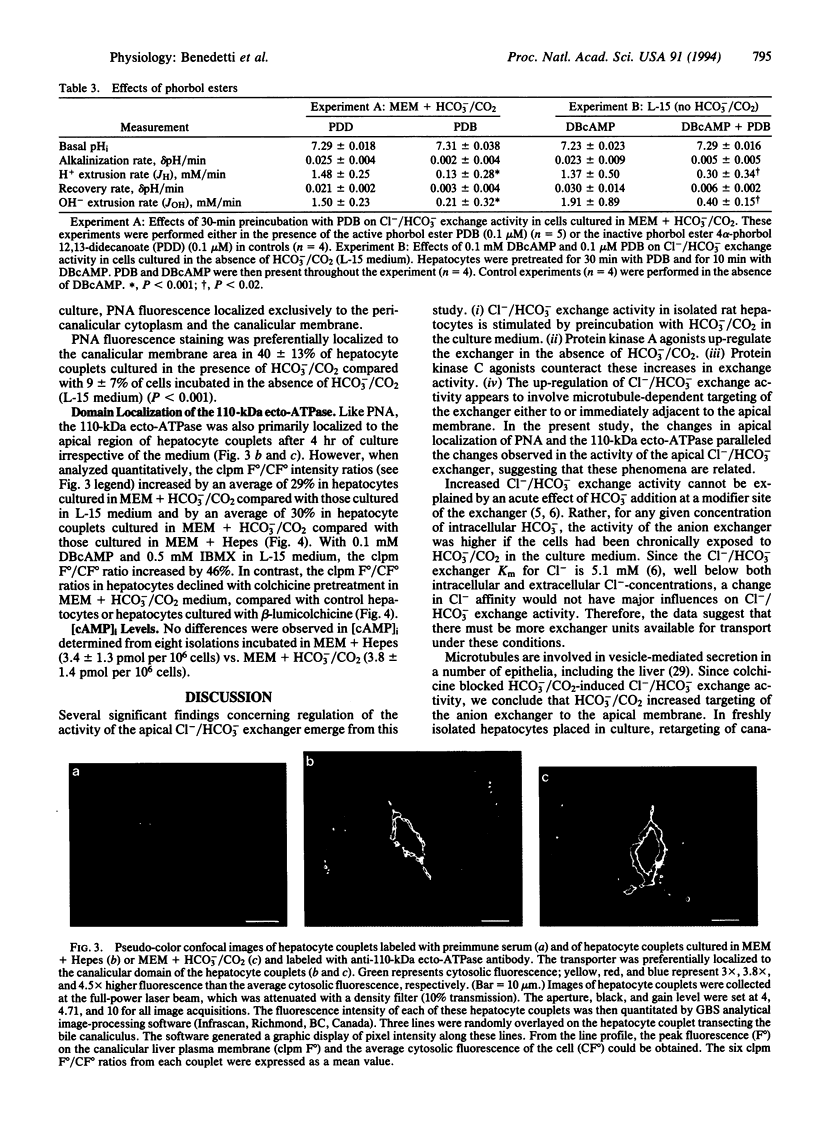
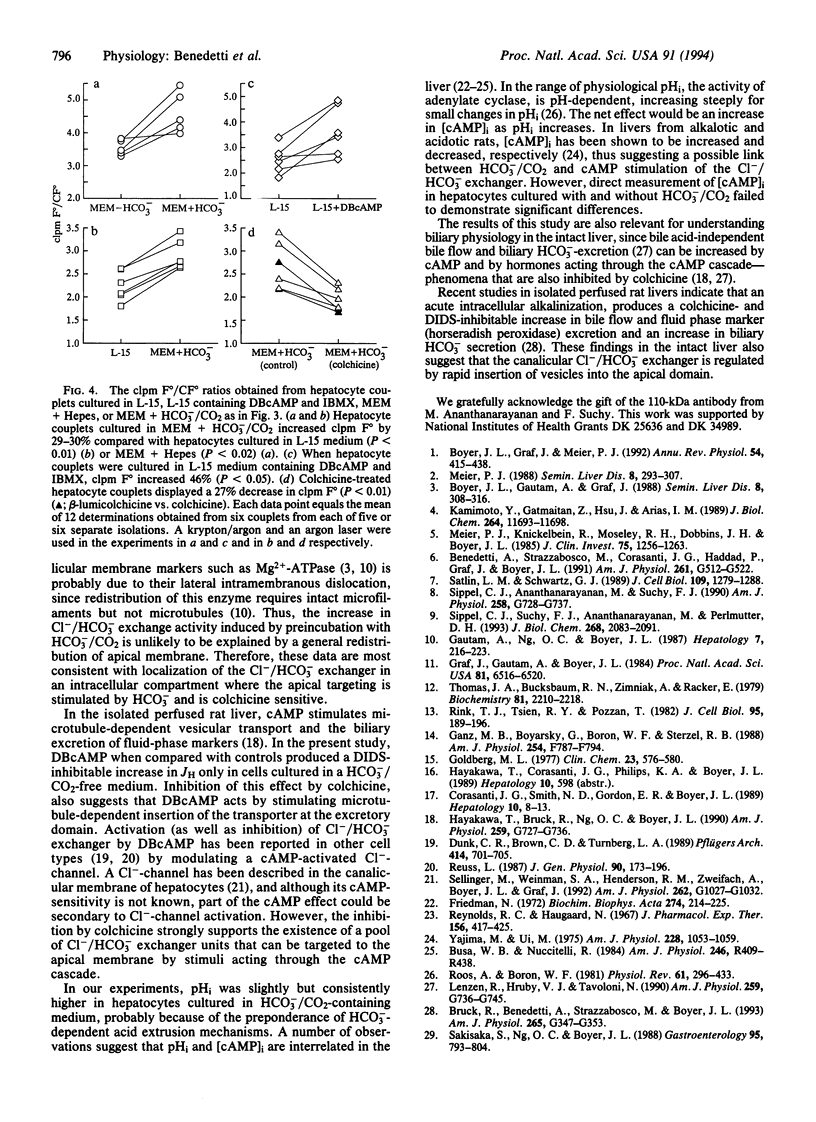
To test the hypothesis that rat hepatocyte canalicular Cl-/HCO3- exchange activity might be regulated by HCO3- or protein kinase-induced changes in the apical targeting of vesicles, isolated rat hepatocytes were cultured in the presence or absence of HCO3-/CO2.Cl-/HCO3- exchange activity increased in cells cultured in the presence of HCO3-/CO2 or when stimulated by dibutyryl cAMP. Both of these effects were blocked by either colchicine or the protein kinase C agonist phorbol 12,13-dibutyrate. Fluorescence and confocal microscopy, respectively, revealed increased pericanalicular-apical membrane localization of two canalicular markers, peanut agglutinin and a 110-kDa canalicular ecto-ATPase, when hepatocyte couplets were preincubated in HCO3-/CO2-containing medium, an effect that was again blocked by colchicine. Dibutyryl cAMP also stimulated canalicular localization of the 110-kDa protein. These findings suggest that hepatocyte Cl-/HCO3- exchange activity is regulated by HCO3-/CO2 and by protein kinase A and protein kinase C agonists through microtubule-dependent targeting of vesicles containing this exchanger to the canalicular domain.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benedetti A., Strazzabosco M., Corasanti J. G., Haddad P., Graf J., Boyer J. L. Cl(-)-HCO3- exchanger in isolated rat hepatocytes: role in regulation of intracellular pH. Am J Physiol. 1991 Sep;261(3 Pt 1):G512–G522. doi: 10.1152/ajpgi.1991.261.3.G512. [DOI] [PubMed] [Google Scholar]
- Boyer J. L., Gautam A., Graf J. Mechanisms of bile secretion: insights from the isolated rat hepatocyte couplet. Semin Liver Dis. 1988 Nov;8(4):308–316. doi: 10.1055/s-2008-1040552. [DOI] [PubMed] [Google Scholar]
- Boyer J. L., Graf J., Meier P. J. Hepatic transport systems regulating pHi, cell volume, and bile secretion. Annu Rev Physiol. 1992;54:415–438. doi: 10.1146/annurev.ph.54.030192.002215. [DOI] [PubMed] [Google Scholar]
- Bruck R., Benedetti A., Strazzabosco M., Boyer J. L. Intracellular alkalinization stimulates bile flow and vesicular-mediated exocytosis in IPRL. Am J Physiol. 1993 Aug;265(2 Pt 1):G347–G353. doi: 10.1152/ajpgi.1993.265.2.G347. [DOI] [PubMed] [Google Scholar]
- Busa W. B., Nuccitelli R. Metabolic regulation via intracellular pH. Am J Physiol. 1984 Apr;246(4 Pt 2):R409–R438. doi: 10.1152/ajpregu.1984.246.4.R409. [DOI] [PubMed] [Google Scholar]
- Corasanti J. G., Smith N. D., Gordon E. R., Boyer J. L. Protein kinase C agonists inhibit bile secretion independently of effects on the microcirculation in the isolated perfused rat liver. Hepatology. 1989 Jul;10(1):8–13. doi: 10.1002/hep.1840100103. [DOI] [PubMed] [Google Scholar]
- Dunk C. R., Brown C. D., Turnberg L. A. Stimulation of Cl/HCO3 exchange in rat duodenal brush border membrane vesicles by cAMP. Pflugers Arch. 1989 Sep;414(6):701–705. doi: 10.1007/BF00582138. [DOI] [PubMed] [Google Scholar]
- Friedmann N. Effects of glucagon and cyclic AMP on ion fluxes in the perfused liver. Biochim Biophys Acta. 1972 Jul 3;274(1):214–225. doi: 10.1016/0005-2736(72)90295-7. [DOI] [PubMed] [Google Scholar]
- Ganz M. B., Boyarsky G., Boron W. F., Sterzel R. B. Effects of angiotensin II and vasopressin on intracellular pH of glomerular mesangial cells. Am J Physiol. 1988 Jun;254(6 Pt 2):F787–F794. doi: 10.1152/ajprenal.1988.254.6.F787. [DOI] [PubMed] [Google Scholar]
- Gautam A., Ng O. C., Boyer J. L. Isolated rat hepatocyte couplets in short-term culture: structural characteristics and plasma membrane reorganization. Hepatology. 1987 Mar-Apr;7(2):216–223. doi: 10.1002/hep.1840070203. [DOI] [PubMed] [Google Scholar]
- Goldberg M. L. Radioimmunoassay for adenosine 3', 5'-cyclic monophosphate and guanosine 3',5'-cyclic monophosphate in human blood, urine, and cerebrospinal fluid. Clin Chem. 1977 Mar;23(3):576–580. [PubMed] [Google Scholar]
- Graf J., Gautam A., Boyer J. L. Isolated rat hepatocyte couplets: a primary secretory unit for electrophysiologic studies of bile secretory function. Proc Natl Acad Sci U S A. 1984 Oct;81(20):6516–6520. doi: 10.1073/pnas.81.20.6516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa T., Bruck R., Ng O. C., Boyer J. L. DBcAMP stimulates vesicle transport and HRP excretion in isolated perfused rat liver. Am J Physiol. 1990 Nov;259(5 Pt 1):G727–G735. doi: 10.1152/ajpgi.1990.259.5.G727. [DOI] [PubMed] [Google Scholar]
- Kamimoto Y., Gatmaitan Z., Hsu J., Arias I. M. The function of Gp170, the multidrug resistance gene product, in rat liver canalicular membrane vesicles. J Biol Chem. 1989 Jul 15;264(20):11693–11698. [PubMed] [Google Scholar]
- Meier P. J., Knickelbein R., Moseley R. H., Dobbins J. W., Boyer J. L. Evidence for carrier-mediated chloride/bicarbonate exchange in canalicular rat liver plasma membrane vesicles. J Clin Invest. 1985 Apr;75(4):1256–1263. doi: 10.1172/JCI111824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier P. J. Transport polarity of hepatocytes. Semin Liver Dis. 1988 Nov;8(4):293–307. doi: 10.1055/s-2008-1040551. [DOI] [PubMed] [Google Scholar]
- Reuss L. Cyclic AMP inhibits Cl-/HCO3- exchange at the apical membrane of Necturus gallbladder epithelium. J Gen Physiol. 1987 Aug;90(2):173–196. doi: 10.1085/jgp.90.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos A., Boron W. F. Intracellular pH. Physiol Rev. 1981 Apr;61(2):296–434. doi: 10.1152/physrev.1981.61.2.296. [DOI] [PubMed] [Google Scholar]
- Sakisaka S., Ng O. C., Boyer J. L. Tubulovesicular transcytotic pathway in isolated rat hepatocyte couplets in culture. Effect of colchicine and taurocholate. Gastroenterology. 1988 Sep;95(3):793–804. doi: 10.1016/s0016-5085(88)80030-1. [DOI] [PubMed] [Google Scholar]
- Satlin L. M., Schwartz G. J. Cellular remodeling of HCO3(-)-secreting cells in rabbit renal collecting duct in response to an acidic environment. J Cell Biol. 1989 Sep;109(3):1279–1288. doi: 10.1083/jcb.109.3.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellinger M., Weinman S. A., Henderson R. M., Zweifach A., Boyer J. L., Graf J. Anion channels in rat liver canalicular plasma membranes reconstituted into planar lipid bilayers. Am J Physiol. 1992 Jun;262(6 Pt 1):G1027–G1032. doi: 10.1152/ajpgi.1992.262.6.G1027. [DOI] [PubMed] [Google Scholar]
- Sippel C. J., Ananthanarayanan M., Suchy F. J. Isolation and characterization of the canalicular membrane bile acid transport protein of rat liver. Am J Physiol. 1990 May;258(5 Pt 1):G728–G737. doi: 10.1152/ajpgi.1990.258.5.G728. [DOI] [PubMed] [Google Scholar]
- Sippel C. J., Suchy F. J., Ananthanarayanan M., Perlmutter D. H. The rat liver ecto-ATPase is also a canalicular bile acid transport protein. J Biol Chem. 1993 Jan 25;268(3):2083–2091. [PubMed] [Google Scholar]
- Thomas J. A., Buchsbaum R. N., Zimniak A., Racker E. Intracellular pH measurements in Ehrlich ascites tumor cells utilizing spectroscopic probes generated in situ. Biochemistry. 1979 May 29;18(11):2210–2218. doi: 10.1021/bi00578a012. [DOI] [PubMed] [Google Scholar]
- Uajima M., Ui M. Hydrocortisone restoration of the pH-dependent metabolic responses to catecholamines. Am J Physiol. 1975 Apr;228(4):1053–1059. doi: 10.1152/ajplegacy.1975.228.4.1053. [DOI] [PubMed] [Google Scholar]




