Abstract
Much progress has been made in understanding autoimmune channelopathies, but the underlying pathogenic mechanisms are not always clear due to broad expression of some channel proteins. Recent studies show that autoimmune conditions that interfere with neurovascular coupling in the central nervous system (CNS) can lead to neurodegeneration. Cerebral blood flow that meets neuronal activity and metabolic demand is tightly regulated by local neural activity. This process of reciprocal regulation involves coordinated actions of a number of cell types, including neurons, glia, and vascular cells. In particular, astrocytic endfeet cover more than 90% of brain capillaries to assist blood-brain barrier (BBB) function, and wrap around synapses and nodes of Ranvier to communicate with neuronal activity. In this review, we highlight four types of channel proteins that are expressed in astrocytes, regarding their structures, biophysical properties, expression and distribution patterns, and related diseases including autoimmune disorders. Water channel aquaporin 4 (AQP4) and inwardly-rectifying potassium (Kir4.1) channels are concentrated in astrocytic endfeet, whereas some voltage-gated Ca2+ and two-pore-domain K+ channels are expressed throughout the cell body of reactive astrocytes. More channel proteins are found in astrocytes under normal and abnormal conditions. This research field will contribute to a better understanding of pathogenic mechanisms underlying autoimmune disorders.
Keywords: Autoimmunity, ion channel, water channel, neurovascular coupling, astrocyte, blood-brain barrier, neurodegenerative disease
1. Introduction
Autoimmune channelopathies involve autoantibodies to various types of channel proteins on cell membranes. In the nervous system, these channel proteins include ionotropic glutamate receptors, voltage-gated ion channels, other ion and water channels, G-protein coupled metabotropic receptors, etc (see [1–3] for a general overview of the field). Some of these channels are predominantly expressed in neurons and involved in synaptic transmission and intrinsic excitability. Their roles are largely reflected by autoimmune symptoms in neurophysiological dysfunctions. However, some channels have significant expression in other cell types in the nervous system, including glia. Autoimmunity to these channels can give rise to more complex symptoms and eventually lead to neurodegeneration. To understand the molecular mechanisms underlying autoimmune-mediated disorders will provide important clues for therapeutic interventions. In this review, we will focus on the channels that can regulate neurovascular coupling, whose dysfunction may lead to inflammation and neurodegeneration.
The neurovascular unit is a concept that describes neurons within their cellular context, including glia (astrocytes, microglia, and oligodendrocytes) and cells of the vasculature (endothelial cells, pericytes, and smooth muscle cells)[4]. The brain is protected by the BBB that is composed by endothelial cells and astrocytes. These BBB cells maintain the ionic and chemical environment of the neurons and mediate bidirectional communication between neural activity and blood flow. Increases in neural activity lead to greater metabolic demands, and require increased blood flow to supply sufficient nutrient support and allow for the removal of the end products of metabolism. As these elements remain in balance, the neurons are able to maintain normal functions, including synaptic transmission. In contrast, vascular abnormality can lead to defects in neural function and pathogeneses of neurodegenerative diseases such as Alzheimer's disease (AD), amyotrophic lateral sclerosis (ALS), and multiple sclerosis (MS) [4–6]. Imbalance between neural activity and blood supply can lead to neuronal dysfunction, and subsequently to altered neuronal regulation of vascular function, which further aggravates neuronal dysfunction [5, 7–10]. A better understanding of how astrocytes regulate neurovascular coupling will advance the prevention, diagnosis and therapy of these diseases.
Astrocytes are the most abundant non-neuronal cells of the CNS [11], and are involved in neurovascular coupling, myelination, and synaptic transmission [12–15]. Accumulating evidence indicates a critical role for astrocytes in MS, AD, ALS, epilepsy, stroke, spinal cord injury, and a variety of other neurological disorders [16]. Astrocytes are a diverse cell population distributed throughout the CNS and are divided into several major groups (Fig. 1). Protoplasmic astrocytes are found in gray matter and their processes contact synapses and blood vessel capillaries. Fibrous astrocytes are located in white matter and their endfeet contact nodes of Ranvier and capillaries [17, 18]. Radial glial cells are a more specialized population of astroglia that have a radial morphology and are usually vimentin-positive [19]. These cells play important roles in development, but some cell types in this category are also present in adulthood, such as Bergmann glia in the cerebellum and Muller cells in the retina [19]. In addition to serving as neuronal and glial precursor cells, they help to direct migration and synaptic development of newly generated neurons [19]. Future investigation in this field may identify new subpopulations of astrocytes with distinct properties and functions.
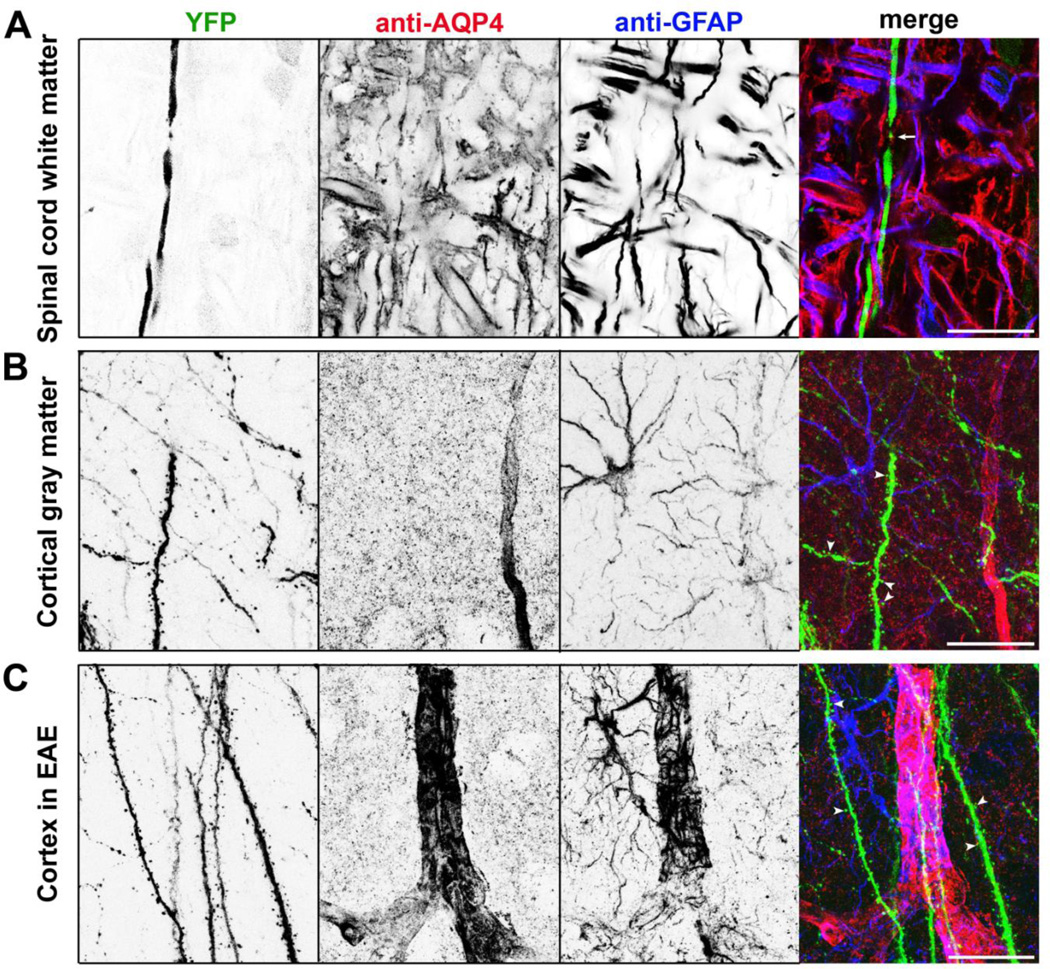
Figure 1. Astrocytes in neurovascular units in white and gray matter.
(A) Axons (YFP in green in merged), astrocytes (GFAP staining in blue in merged) and astrocytic endfeet (AQP4 staining in red in merged) in spinal cord white matter. The white arrow, a putative node of Ranvier. (B) Neurovascular units in cortical gray matter. Axons, dendrites and dendritic spines are in green. White arrowheads, dendritic spines. (C) Neurovascular units in cortical gray matter at the peak stage of EAE (Experimental Autoimmune Encephalomyelitis). These sections were from Thy1:YFP transgenic mice. Confocal image stacks (40 µm in thickness) were collapsed into 2D images. These images are modified from our recent paper [159].
Astrocytic functions are regulated by a number of channel proteins, which are embedded in the cell membrane and allow the passage of water or ions into or out of the cells. Ion and water channels are very important to the normal physiology of many cell types, and defects in channel functions can lead to various diseases. The water channel AQP4 regulates fluid homeostasis in the CNS and is the target of a disease-causing autoantibody in neuromyelitis optica (NMO) [20–22], a disease with some similarities to MS [23]. In the CNS, AQP4 is predominantly localized to astrocytic endfeet contacting blood capillaries. The inwardly rectifying K+ channel, Kir4.1, is also present in astrocytic endfeet and is involved in K+ buffering [24–26]. Kir4.1 is likely a target of the autoimmune response in a subgroup of MS patients [27]. Furthermore, other K+ channels, including two-pore and Ca2+-sensitive K+ channels, are also found in astrocytes [28–32]. Voltage-gated Kv1.4 channels are highly upregulated in activated astrocytes around experimental autoimmune encephalomyelitis (EAE; an animal model of MS) lesions [33], although their functional role in this context remains unclear. Astrocytes can participate in cell-to-cell communication by propagation of Ca2+ waves [34, 35], which require channel proteins in the plasma membrane and endoplasmic reticulum. Astrocytes can influence other cells through gap junctions and by releasing gliotransmitters [11, 36–39].
Here we provide an overview of channel proteins expressed in astrocytes and relevant autoimmune disorders. Dysregulated neurovascular coupling is a potential common pathogenic mechanism for these disorders. Four representative channels present in astrocytes are highlighted. This review starts with the molecular aspect of channel proteins, and discusses their structures, biophysical properties, expression and distribution patterns, and related diseases including autoimmune disorders. These channels may play critical roles in neurovascular coupling and are potential targets for treating autoimmune neurological disorders.
2. Astrocyte channel proteins
2.1. Water (AQP) channels
Aquaporins are channel proteins allowing water to be transported through the plasma membrane. They are divided into three subgroups: (1) classical aquaporins (AQP0, 1, 2, 4, 5 and 8) only transport water; (2) aquaglyceroporins (AQP3, 7, 9 and 10) transport both water and various small molecules; and (3) unorthodox aquaporins (AQP6, 11 and 12) are identified as aquaporins by sequence homology, but remain to be fully characterized [40]. AQP4 has two isoforms (M1 and M23), which differ only in the N-terminus by 22 residues (Fig. 2A) [41]. The aquaporin α-subunit contains 6 transmembrane (TM) domains, along with two additional membrane-embedded domains that do not span the entire bilayer (Fig. 2A) [42]. Although the monomeric α-subunit contains the water pore, aquaporins most often form stable tetramers [42]. AQP4 function is regulated by channel gating, subcellular distribution, phosphorylation, protein-protein interactions and orthogonal array formation (Fig. 2B) [43]. The orthogonal arrays are composed of aggregates of many AQP4 channels in one area of the cell surface to increase membrane permeability. Interestingly, the center of the array often contains the M23 isoform, while the outside edge contains the M1 isoform [44]. A recent study further showed that the AQP4 aggregation state determines its subcellular localization and functions. The M1 isoform is freely mobile in the plasma membrane and can diffuse into rapidly extending lamellipodial regions to support cell migration, whereas the M23 isoform forms large arrays that do not diffuse rapidly, but instead stably bind adhesion complexes and are polarized to astrocyte end-feet in vivo [45].
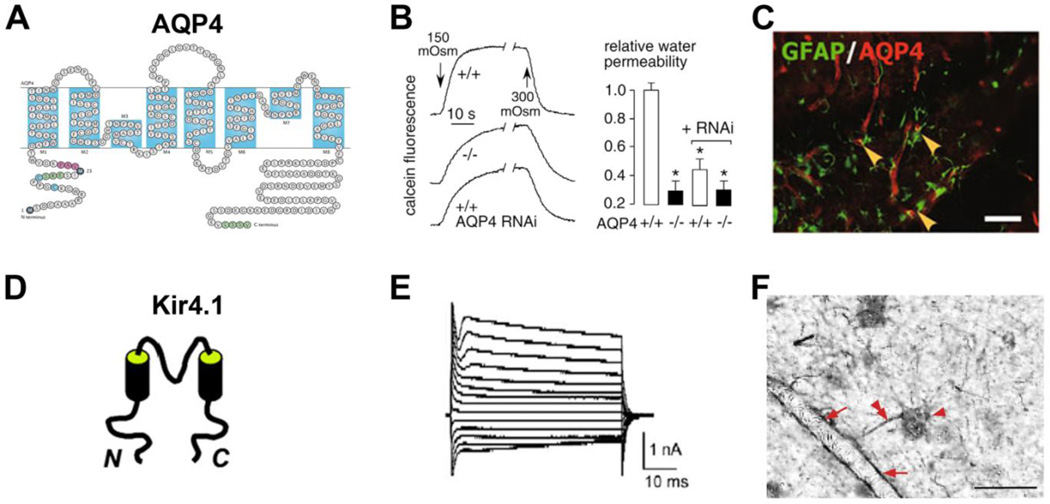
Figure 2. Structure and function of channel proteins highly concentrated in astrocytic endfeet.
(A) AQP4 structural diagram, showing eight membrane-embedded helical segments. Met1 and Met23 (black) are the translation initiation sites for the two AQP4 splice variants, M1 and M23. Residues highlighted in purple, green, and blue in the N-terminus are involved in regulating formation of orthogonal arrays of AQP4 tetramers. The AQP4 C-terminus contains a PDZ domain (in green) [reproduced from [42]]. (B) Osmotic water permeability of astroglial cells in AQP4 KO mice and in wildtype mice with and without AQP4 RNAi treatment. Changes in calcein fluorescence signal (left) in response to hypo-osmolar medium (150 mOsm) and return to iso-osmolarity (300 mOsm). The rate of water transport (right) in wildtype mice is three-fold higher than in AQP4 KO [reproduced from [160]]. (C) AQP4 (red) expression in GFAP (green) positive astrocytes of hippocampal dentate gyrus in a wildtype mouse. The colocalization of AQP4 and GFAP is revealed by yellow arrowheads. Scale bar, 40 µm. [reproduced from [52]]. (D) Structural diagram of the pore-forming subunit of Kir4.1 channel [reproduced from [69]]. (E) Representative whole-cell currents of wildtype astrocytes in CA1 stratum radiatum, which resulted from Kir4.1 channel activation [reproduced from [80]]. (F) Localization of Kir4.1 channels to astrocytic endfeet revealed by immunostaining in hippocampal tissue from a temporal lobe epilepsy patient. Red arrowhead, astrocyte soma; Red double arrowhead, astrocyte process; Red arrow, astrocyte endfeet. Scale Bar, 50 μm [modified from [84]].
Aquaporins are widely expressed throughout the body, likely in every type of tissue. Some channels seem restricted to certain tissues, such as AQP2 which is primarily expressed in kidney, while AQP3 has been reported in at least 20 tissues and confirmed to have functional roles in about half of these [40]. Some tissues with high fluid-handling express multiple aquaporins, such as the kidney and the eye with eight aquaporins each [46–48]. AQP1, 4, and 9 have known functions within the CNS [42]. AQP4 is highly expressed in astrocytic endfeet contacting both blood vessels and neuronal synapses (Fig. 2C), and is involved in regulation of the flow of water and solutes into and within the CNS. It is involved in bulk flow of cerebrospinal fluid into the brain parenchyma and helps to regulate the size of particles that can pass the BBB [49]. AQP4 is expressed at higher levels in more excitable brain regions than less excitable regions [50]. This is likely because more excitable areas have higher metabolic demand. In the CNS, AQP4 is confined to astrocytes and ependyma. It is enriched at glial-pial and glial-endothelial interfaces. Thus, AQP4 is a major player in neurovascular coupling with regulatory effects at both blood vessels and synapses.
Polarized expression of AQP4 in astrocytic endfeet allows for efficient regulation of water in the extracellular spaces adjacent to vascular and synaptic structures (Fig. 2B). AQP4 controlling extracellular water content helps to regulate ionic concentrations, influencing K+ buffering and calcium signaling. AQP4 KO mice may have improved function in cytotoxic models of edema, but perform worse in models of edema focused on blood vessel fluid leak [51]. AQP4 dysfunction leads to impaired water and solute handling abilities, and may contribute to the abnormal buildup of water (edema) or proteins that occurs in many neurological disorders [49]. Furthermore, AQP4 KO mice have impaired memory formation and reduced hippocampal synaptic plasticity [52–54]. AQP4 may also be a mediator of MS disease pathology, since EAE susceptibility is almost eliminated in AQP4 KO mice [55]. Interestingly, secretion of proinflammatory cytokines is also reduced from AQP4 KO astrocytes in vitro [55]. In contrast, AQP4 deficiency significantly increases the extent of neuron loss and demyelination, and leads to motor dysfunction in a spinal cord contusion injury model [56]. As might be expected from the changes in synaptic plasticity, AQP4 deficiency also affects seizure activity. AQP4 KO mice have a higher threshold for seizure activity induced by either pentylenetetrazole injection or by electrical stimulation [57, 58], but have a longer duration of generalized tonic-clonic seizures, likely due to altered K+ handling [58]. Perivascular loss of AQP4 in the hippocampus has been associated with temporal lobe epilepsy [59].
AQP4 was identified as the target of pathogenic autoantibodies in NMO, a spectrum of inflammatory CNS disorders of varying severity [60, 61]. NMO is a chronic relapsing condition mainly affecting the optic nerves and spinal cord, which can be easily misdiagnosed as MS. Autoantibodies to AQP4 are present in up to 80% of NMO patients. The symptoms include loss of vision, weakness or sensory disturbance associated with optic nerve inflammation or extensive spinal cord lesions, which can be identified on MRI. Different from MS, NMO is a primary astrocytopathy with secondary demyelination. NMO and MS also have the following two major differences. (1) AQP4 is reduced in NMO active lesions, whereas it is increased in active MS lesions. (2) NMO is caused by AQP4 autoantibodies and NMO lesions preferentially involve regions with high AQP4 expression.
AQP4-specific autoantibodies are produced concurrently with disease onset and have direct involvement in the disease pathology of NMO [62]. These antibodies target one of multiple binding sites located in the three extracellular loops. Disease-specific epitopes reside in extracellular loop C more than in loops A or E. IgG binding to intracellular epitopes lacks disease specificity. The binding can occur with AQP4 monomers, tetramers, and high order arrays [63]. This initiates an immune response that first damages astrocytes, and then results in BBB breakdown, myelin loss, and oligodendrocyte apoptosis [21, 64, 65]. The multiple autoantibodies with different binding features likely account for diverse pathological manifestations of NMO spectrum disorders in different CNS regions and at different developmental stages [66–68]. Despite some progress, the physiological and pathological functions of aquaporins remain to be fully understood.
2.2. Inwardly rectifying potassium (Kir) channels
The K+ channel superfamily consists of about 80 genes and can be categorized into three major families based on channel structure and function [69]. The voltage-gated K+ (Kv) channel family is composed of 12 different subfamilies denoted Kv1-12, encoded by about 40 separate genes [69–76]. Kv channel α-subunits have 6 TM segments, with one pore-forming loop located between segments 5 and 6. Four α-subunits tetramerize to form a functional potassium-selective channel. As a general rule, K+ channel α-subunits can form channel complexes with other members of the same subfamily. An additional specialized subfamily, the Slo family, can be grouped with the Kv channels, although these channels can be activated by intracellular signaling as well as the membrane potential [69]. The Slo family includes the Maxi-K (or BK) channel, or Slo1, which is activated by both voltage and Ca2+ [69]. The second family, inwardly rectifying K+ (Kir) channels, is composed of 7 subfamilies [69, 77]. The α- subunits of these channels have only two TM segments, which are connected by a pore-forming loop (Fig. 2D). [69]. Kir channels form tetramers with a single potassium-selective pore, and conduct current more efficiently in the inward direction [69, 77]. The third family, two-pore domain K+ (K2P) channels, has 15 members which can be divided into six subfamilies [69, 78]. The K2P α-subunit has four TM segments and two pore-forming loops, and two subunits dimerize to form a channel complex with dual potassium-selective pores [69, 78]. Most K2P channels are functionally characterized by currents having little voltage or time dependence, also known as “leak” K+ currents [69].
Kir channels are inwardly rectifying channels that preferentially allow K+ ions to flow into the cell (Fig. 2E). The Kir α-subunit contains two TM domains and one pore-forming loop (Fig. 2D), and four α-subunits homo-or hetero-tetramerize to form a functional channel. Fifteen Kir α-subunits have been identified in humans and rodents, and these are divided into seven subfamilies (Kir1-Kir7). Channels of the Kir2 subfamily are strong inward rectifiers and are considered the “classical” Kir channels [77]. Kir3 channels are also strong rectifiers, but are distinguished by their gating by G-protein-coupled receptors [77]. Channels in the Kir4, 5, and 7 families are intermediate inward rectifiers (Fig. 2E), and are known as “transport” channels along with Kir1.1, a weak inward rectifier [77]. Kir6 channels are also weak rectifiers and are sensitive to ATP [77]. Here we focus on the role of Kir4.1, an intermediate inward rectifier expressed in astrocytes.
Kir channels are broadly expressed. Kir2 channels are present in skeletal and cardiac muscle cells and smooth muscle cells of the vasculature, and in neurons throughout the brain at low levels. Kir3 channels are also expressed throughout the brain. Kir6 channels are expressed in the pancreas, heart, vascular smooth muscle, and certain areas of the brain such as hypothalamus. Kir1.1 is found in kidney and Kir7.1 is expressed in the retina and choroid plexus [77]. Kir4.1 channels are expressed in the CNS and localized in astrocytic endfeet, and so are well-situated to remove K+ ions from extracellular spaces adjacent to blood vessels and synapses, in what is often referred to as a “K+ buffering” role (Fig. 2F). A loss of Kir4.1 function leads to neuronal dysfunction and seizure activity, as shown by several different studies. In animal models, mice with a Kir4.1 genetic polymorphism causing increased seizure susceptibility have impaired astrocytic K+ and glutamate uptake [79]. A conditional KO under a GFAP promoter confirms that this effect is mediated by astrocytic Kir4.1, also showing impaired K+ and glutamate uptake, ataxia, seizures, and premature death [80]. Synaptic function is also affected in the Kir4.1 conditional KO, causing enhanced short-term potentiation [80]. Thus, the function of astrocytic Kir4.1 is clearly coupled to neuronal activity. Although Kir4.1 and AQP4 are both expressed in astrocytic endfeet, they appear to have no direct functional interaction. Kir4.1 channel expression and function are no different in AQP4 KO mice [81, 82], and inhibition or knockdown of Kir4.1 causes no change in the water permeability of AQP4 [82]. Also, no change in AQP4 expression has been observed in Kir4.1 KO mice [83]. However, AQP4 may support K+ buffering by altering the concentration in the extracellular space.
Several diseases are known to be related to Kir4.1. Hippocampal sclerosis in temporal lobe epilepsy has been shown to be associated with loss of Kir4.1 in astrocytic endfeet [84]. In animal models of epilepsy, disruption of the BBB leads to serum albumin leakage into the CNS and uptake in astrocytes, mediated by the TGFβ receptor. This has led to downregulation of Kir4.1 and the development of neuronal hyperexcitability and epileptiform activity [85, 86]. Reduced Kir4.1 activity likely leads to increased extracellular K+ and glutamate, contributing to brain excitability and epileptogenesis [87]. Furthermore, deficits of astrocytic Kir4.1 channels may contribute to neuronal dysfunction in Huntington’s disease model mice [88]. Symptom onset in mouse models is associated with the reduction of Kir4.1 functional expression, but not classic astrogliosis. As a result, elevated extracellular K+ levels (by ~2 mM) depolarize striatal medium spiny neurons and increase their excitability. This can be rescued by overexpressing functional Kir4.1, ameliorating several deficits associated with the mouse models [88]. Kir4.1 has also been shown to have effects in demyelinating conditions. A spinal cord injury model shows up to 80% reduction in Kir4.1 expression in the lesion area, and demonstrates that a previously discovered neuroprotective agent, 17-β-oestradiol, can partially restore Kir4.1 expression and function [89].
A recent study of serum IgG has identified Kir4.1 as the target of autoantibodies present in about half of the MS patients studied [27]. These autoantibodies are essentially absent in people with other neurological diseases and in healthy donors. Injection of serum IgG from MS patients causes a loss of Kir4.1 expression, alters GFAP expression in astrocytes, and leads to activation of the complement cascade at the Kir4.1 expressing site in the cerebellum [27]. These results show that those autoantibodies are indeed pathogenic. They bind to the first extracellular loop of Kir4.1. Therefore, Kir4.1 is likely a target of the autoantibody response in a subgroup of MS patients. Serum antibodies to Kir4.1 are found in the majority of children with acquired CNS demyelinating diseases but not in children with other diseases or in healthy controls [90]. This appears consistent with the results that Kir4.1 KO mice have impaired myelination and oligodendrocyte maturation in development, leading to neuronal degeneration and a poor survivability to only about 2 – 3 weeks of age [91]. However, the prevalence of Kir4.1 autoantibodies in MS patients has been questioned in a couple of recent studies [92, 93]. What causes the discrepancy currently remains unknown. It may be partially due to technical differences between the studies. For instance, Kir4.1 antibodies may differ in recognizing monomers, tetramers or high-order polymers of channel proteins [94]. Nonetheless, this is still a highly significant research field with many unknowns.
2.3. Voltage-gated calcium (Cav) channels
Cav channels are synthesized as a single α-subunit protein containing four homologous domains that contain six TM segments each (Fig. 3A). There are ten known α-subunit genes in the Cav family, which are divided into three subfamilies, Cav1-3. They also have classical labels based on properties and originally discovered location. The Cav1 family contains four L-type (“Long-lasting” conductance) channels that are important for gene transcription involving calcium-mediated signal pathways [95, 96]. The Cav2 family contains P-type (“Purkinje cell”) channels (Cav2.1) and N-type (“Neuronal”) channels (Cav2.2) that are important for neurotransmitter release from synaptic terminals, as well as R-type (“toxin Resistant”) channels (Cav2.3) [95, 96]. The Cav3 family contains three T-type (“Transient” current) channels that are important for modulation of neuronal excitability and firing patterns [95, 96]. L-, P- and N-type channels are all activated by high voltages, while R-type channels are activated by intermediate voltages and T-type channels by low voltage [95]. In addition, most Cav channel α-subunits exist in complex with other subunits that regulate their activity: α2δ, β, and γ subunits [97]. The four α2δ subunit genes encode two subunits each, α2 and δ [97]. There are four different β subunit genes and eight γ-subunit genes [97]. In addition to voltage, Cav channels can be regulated by G-protein coupled receptors (GPCRs), either by direct interaction or through cAMP-mediated signaling [96].
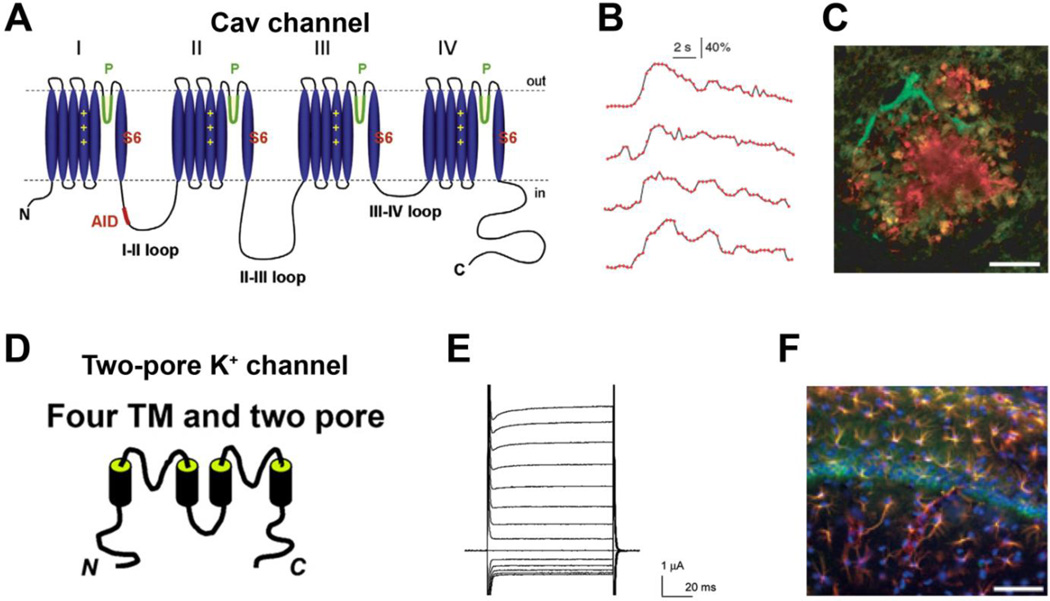
Figure 3. Structure, function and localization of astrocytic voltage-gated Ca2+ channels and two-pore K+ channels.
(A) Structural diagram of Cav channels. The α-interacting domain (AID; shown in red on the I–II linker) binds the Cavβ subunit with high affinity. The pore-forming loops between TM segments S5 and S6 are marked with ‘P’, and the voltage-sensor domain in TM segment S4 is indicated by ‘+’ signs [reproduced from [161]]. (B) Traces of Ca2+ activity measured by fluorescence of the Ca2+ indicator Fluo-4 along an astrocyte process imaged by two-photon microscopy of a brain slice [modified from [100]]. (C) Expression of Cav channels in reactive astrocytes near an amyloid-β plaque in a transgenic mouse model of Alzheimer’s disease, revealed by immunostaining and confocal microscopy. Green, Cav1.2 antibody; red, amyloid-β antibody. Scale bar, 20 µm [modified from [105]]. (D) Structural diagram of two-pore domain K+ channels [reproduced from [69]]. (E) Whole-cell current from a Xenopus laevis oocyte expressing TASK-3 channels [modified from [121]]. (F) Hippocampal astrocytes co-immunostained with TASK-2 (green) and GFAP (red). Nuclei are indicated by DAPI staining in blue [modified from [124]].
Cav channels are primarily expressed in neurons in the CNS. The Cav1 and Cav3 families are mainly targeted somatodendritically, while Cav2 family members are targeted to synaptic terminals. Cav channels have no or low-level expression in astrocytes under normal conditions, but they may be highly induced or upregulated in reactive astrocytes.
Calcium signaling is the major form of excitability or activity for astrocytes, and can directly affect neurotransmission by release of glutamate from astrocyte endfeet contacting synapses (Fig. 3B,C). Besides the influx of calcium through Cav channels at the cell membrane, calcium signaling in astrocytes also involves intracellular mechanisms of calcium release, termed store-operated calcium release. This mechanism is calcium-dependent, so that a small current passing through the plasma membrane can be amplified by intracellular calcium release from the endoplasmic reticulum mediated by inositol triphosphate receptors (IP3R) [98]. Furthermore, this calcium release can self-propagate by further stimulation of adjacent IP3Rs both within the cell and intercellularly through gap junctions in a travelling “calcium wave” that can modulate activity at endfoot processes over a wider area [98]. Interestingly, calcium signaling can be differentially regulated at the level of the astrocyte process [99]; Calcium signaling can therefore be matched to needs of surrounding neurovascular unit at the process level. As shown by two-photon imaging, this local Ca2+ signaling in astrocytic processes modulates neurotransmission in nearby synapses [100], and is dependent upon IP3R2-mediated intracellular calcium release. Astrocytic Ca2+ signaling is required for cholinergic-induced synaptic plasticity (LTP), also mediated by IP3R signaling, and results in glutamate release to act upon synaptic glutamate receptors [101]. Calcium signaling can also be affected by AQP4-mediated changes in extracellular osmolarity [102].
Neuronal Cav channels are involved in many neurological diseases, and disease-causing mutations have been mapped throughout the α1-subunit in various channels [103]. Cav1 (L-type) channel mutations have been linked to retinal dysfunction and to disorders of cardiac and skeletal muscle [103]. Cav3 (T-type) channel mutations have been linked to autism and epilepsy, while Cav2.1 (P-type) channel mutations are associated with ataxia and migraine [103]. Although these are likely mediated by Cav channel dysfunction in neurons, astrocytes may also play a role in disease processes. Cav1.3 (L-type) and Cav2.1 (P-type) channel expression is induced in hippocampal reactive astrocytes following pilocarpine-induced status epilepticus [104]. In APP transgenic mice, Cav1.2 channels are induced in reactive astrocytes surrounding amyloid plaques, and their expression increases with the age of mice and the number of plaques [105]. The potential beneficial or detrimental aspects of upregulation of Cav channels in astrocytes have not yet been fully explored, but could alter neuronal responses over a wide area through intracellular and intercellular Ca2+ wave activity.
Autoantibodies to Cav channels are involved in neurological disorders. The Lambert-Eaton myasthenic syndrome (LEMS) is caused by autoimmunity to Cav channels, interfering with presynaptic neuromuscular transmission. This disorder is characterized by proximal limb weakness and dysfunction of the autonomic nervous system. Approximately two-thirds of LEMS patients have an underlying small cell lung carcinoma (SCLC) [106]. Multiple autoantibodies against different Cav channel isoforms have been identified. These channel proteins are present both at neuromuscular junctions and the surface of SCLC cells. Autoantibodies from LEMS patients directly bind to multiple α1 subunits, as well as the cytoplasmic β3 subunit, over-expressed in HEK293 cells [107]. Paraneoplastic cerebellar degeneration (PCD) has a pathological hallmark, an extensive loss of Purkinje cells. PCD is also often associated with SCLC. In a subset of PCD patients, autoantibodies to Cav channels are found [108, 109]. The expression levels of P/Q and N-type Cav channels are high in the cerebellum, especially in the Purkinje and granule cells. These channels are also found at the neuromuscular junction and on the surface of SCLC. Knockout mice of P/Q-type Cav channel develop ataxia and dystonia [110], consistent with the notion that Cav autoantibodies inhibit channel activity, thereby leading to neurophysiological dysfunction. Interestingly, LEMS, but not PCD, patients respond favorably to immunotherapy, although Cav autoantibodies are pathogenic in both cases. The autoantibodies likely induce irreversible neuronal degeneration in PCD patients. Is it possible that astrocytic Cav channels are involved in PCD? So far, neurons are the predominant targets for Cav atuoantibodies. Although some Cav channels are expressed in activated astrocytes, their role in autoimmune disorders is still not known.
2.4. Two-pore-domain K+ (K2P) channels
K2P channel α-subunits have four TM segments and two pore-forming loops (Fig. 3D). They dimerize to form two K+ permeable pores. They contribute to “leak currents” and help to set the resting membrane potential of the cell (Fig. 3E). The 15 known members of this family are named for their defining characteristic. TWIK (Tandem-pore-domain Weak Inwardly-rectifying K+ channel), THIK (Halothane- Inhibited), TREK (TWIK-RElated), TRAAK (Arachidonic Acid-stimulated), TASK (Acid-Sensitive), TALK (ALkaline pH activated), TRESK (TWIK-RElated Spinal) [78]. As the names imply, two-pore domain K+ channels can be modulated by a number of different factors, including pH, neurotransmitters, anesthetics, osmolarity, and oxygen tension [69, 78].
K2P channels are expressed in both neurons and glia in the CNS. They are normally present throughout astrocytes and are not polarized to endfeet (Fig. 3F). TASK-1, -2, and -3 are expressed in cerebellar astrocytes [111]. TWIK-1 and TREK-1 are highly expressed in hippocampal astrocytes, while TASK-1 is more weakly expressed [112]. These channels, especially TREK-1, mediate a large portion of the passive membrane K+ conductance that maintains the resting membrane potential of mature hippocampal astrocytes [112]. TREK-1 channels can be modulated by GPCRs, which apparently render them permeable to glutamate and thus promote glutamate release from astrocytes [113]. In neurons, TWIK-1 can also be activated by the 5-HT1A receptor through the Gαi/PKA-mediated signaling pathway, allowing serotonin to reduce neuronal excitability [114]. Members of the TREK family may be involved in neuroprotection, and modulated by neuroprotective factors including riluzole, polyunsaturated fatty acids and lysophospholipids [115].
K2P channels are involved in autoimmunity of several diseases, including MS. TASK-1 deficiency reduces the severity of EAE mainly through inhibiting T cell proliferation and cytokine production [116, 117]. Blocking TASK-1 with the aromatic carbamide A293 significantly reduces the EAE severity, the extent of cellular infiltration, and the degree of demyelination [117, 118]. TASK-1 is expressed in T cells and regulates the synthesis of proinflammatory cytokines and T cell proliferation. Moreover, another study showed that expression of TASK-2 in CD4+ T cells correlates with disease activity in rheumatoid arthritis patients [119]. Therefore, TASK-1 is a very promising drug target to treat inflammatory demyelinating disorders. However, the role of K2P channels in astrocytes in autoimmunity remains unclear. Their autoantibodies have not yet been described. Nonetheless, their important physiological functions are confirmed in knockout mice and disease models. TASK-1 KO mice perform worse in ischemia, resulting in larger lesion volumes, but it is unclear whether this effect is mediated within the brain or by altered blood pressure due to loss of TASK-1 in the heart, adrenal gland, or vasculature [120]. A TASK-3 mutation has been identified as the cause of a mental retardation dysmorphism syndrome and completely abolishes channel currents both in the homodimeric form and when heterodimerized with TASK-1 [121]. A sheep model of fetal alcohol syndrome causes significant cerebellar Purkinje cell loss due to ethanol induced changes in pH, and this effect can be abolished when TASK-1 and TASK-3 are blocked concurrently with ethanol administration [122]. In the pilocarpine model of epilepsy, both TASK-1 and TASK-2 are upregulated in hippocampal astrocytes [123, 124]. Interestingly, TASK-2 expression increases in the astrocytic endfeet as well as the soma, which may indicate dysregulation of the BBB [124]. Future investigations will continue to illuminate significant roles of other K2P channels in health and disease pathology.
2.5. Other astrocytic channels and autoimmune disorders
In this review, we have discussed four different channels that are present in astrocytes and known to have important effects in neurovascular coupling. There are actually many other channels found in astrocytes that could also play important roles in neurovascular coupling (Table 1). There are a number of K+ channels implicated in astrocytes. We recently showed that Kv1.4 is highly upregulated in activated astrocytes within and surrounding lesions in the spinal cord of mice induced with EAE [33]. Kv1.6 expression has been reported in astrocyte culture [125] but does not appear to be altered in reactive astrocytes [126]. The BK channel, mainly present in neurons, is expressed in some astrocytes. Its β subunits BKβ4 (strong expression) and BKβ1 (weak expression) subunits are expressed in astrocytes in some brain regions [127]. A recent study suggests that spontaneous Ca2+ oscillations keep BK channels activated and increase basal extracellular (perivascular) levels of K+ [128]. Another study found that BK channels are reduced along with AQP4 and Kir4.1 levels in astrocytic endfeet both in AD patient tissues and in amyloid precursor protein transgenic mice [126]. Astrocytic BK channels do appear to play a significant role in neurovascular coupling, likely through regulation of the extracellular K+ level in conjunction with Kir4.1 to control vasodilation or vasoconstriction. Ca2+ concentration in astrocyte endfeet is known to affect vasodilation and vasoconstriction [32], and this is mediated at least in part by BK channel activation by Ca2+ [32, 128]. Interestingly, in a model of subarachnoid hemorrhage, BK channel activation instead produced vasoconstriction, suggesting a biphasic vascular response to K+ levels [128].
Table 1.
Channel proteins expressed in astrocytes
| Resting Astrocytes | Activated Astrocytes | |
|---|---|---|
| Water channel | ||
| AQP4[42, 49, 50, 52] | AQP4 [18] | |
| Inward Rectifier K+ channels | ||
| Kir4.1[80, 162] | ||
| Kir2.3[163, 164] | ||
| Kv channels | ||
| BK channel [126]. | ||
| Kv1.6[125] | Kv1.4[33] | |
| Two-pore domain K+ channels | ||
| TWIK-1[112] | ||
| TREK-1[112] | ||
| TASK-1[111] | TASK-1[111] | |
| TASK-2[111] | TASK-2[111] | |
| TASK-3[111] | ||
| Cav channels | ||
| Cav1.2[105] | ||
| Cav1.3[104] | ||
| Cav2.1[104] | ||
| Nav channels | ||
| Nav1.1[129] | Nav1.2[129] | |
| Nav1.2[129] | Nav1.5[129] | |
| Nav1.3[129] | ||
| Nav1.6[129] | ||
| TRP channels | ||
| TRPV1[130] | TRPV4[131, 132] | |
| TRPV4[132] | ||
| TRPC1[133] | ||
| Gap junctions | ||
| Connexin 30[165] | ||
| Connexin 43[166] | ||
Other cation channels are expressed in some astrocytes at low levels. These include voltage-gated sodium (Nav) channels Nav1.1, 1.2, 1.3, and 1.6 [129]. Of these, only Nav1.2 is slightly upregulated in reactive astrocytes in MS lesions. In contrast, Nav 1.5 is induced and highly upregulated in reactive astrocytes in MS lesions, cerebrovascular accidents, and brain tumors [129]. Transient receptor potential (TRP) channels are permeable to more than one cation, and TRPV1 [130], TRPV4 [131, 132], and TRPC1 [133] channels have recently been reported to be expressed in astrocytes. TRPV4 is involved in astrocytic calcium signaling [131, 132] and appears to be upregulated in reactive astrocytes in ischemic conditions [131]. TRPV4 may also interact with AQP4 function [134]. Finally, we discuss gap junctions expressed in astrocytes. Connexins are proteins which hexamerize to form a “hemichannel” in the cell membrane which allows the passage of ions and molecules smaller than about 1 kD into or out of the cell [135, 136]. When coupled to a hemichannel on an adjacent cell, a gap junction is formed, which allows molecules to pass between the cytoplasm of the two cells. The major connexins expressed in astrocytes are Cx43 and Cx30, and these are thought to have a role in several diseases including MS and AD [137, 138] (Table 1). As these and other channels are more fully studied, they may be found to play important roles in neurovascular coupling. Since this is a research field that is still being intensively investigated, the presence and function of certain types of channel in astrocytes sometimes may remain to be confirmed.
3. Future Perspectives
In the past, reactive astrocytes have been viewed in a negative light, as proinflammatory cells that form glial scars which hinder remyelination and axon growth. We now know from both in vivo and in vitro studies that activated astrocytes also mediate CNS myelination by promoting the migration, proliferation, and differentiation of oligodendrocyte progenitor cells [13, 14, 139, 140]. Astrocytes promote myelination in response to electrical activity by releasing the cytokine leukemia inhibitory factor (LIF) [139]. Various factors produced from astrocytes can impact myelination [141], such as platelet-derived growth factor (PDGF), fibroblast growth factor (FGF), ciliary neurotrophic factor (CNTF), and insulin-like growth factor (IGF), as well as gliotransmitters including glutamate and ATP. Because ion channels are clearly involved in myelination-promoting functions of astrocytes, especially gliotransmission and the modulation of electrical activity, the role of astrocytic ion channels in myelination will remain an important topic for future research.
Astrocytes are now recognized as an important player in MS, ALS, AD, epilepsy, stroke, spinal cord injury, and other neurological disorders [16]. Astrocytes modify their morphology and function in response to neuroinflammation or injury, by altering expression of many genes, including ion channel genes. These changes are regulated by specific signaling pathways that produce a measured, context-dependent response to the insult [16]. Although they normally do not fire action potentials, astrocytes express Na+ channels and K+ channels [80, 112, 142–147]. Many ion channels are also upregulated in activated astrocytes (Table 1). Future studies of the mechanisms and functional importance of ion channel alterations in reactive astrocytosis will be vital for the understanding and treatment of many neurological diseases.
Relatively little is known about the factors that regulate the targeting of astrocytic channels and the induction of channel expression in reactive astrocytes. In vitro studies of protein kinase C epsilon (PKCε) show that this kinase can upregulate Cav channels in astrocytes [148], and can alter cytoskeletal protein expression to induce a stellate morphology which may be analogous to reactive gliosis [149, 150]. It will be interesting to determine what other transcription factors and signal pathways are activated in reactive astrocytes, which might in turn induce or upregulate channel expression or trafficking. The localization of channel proteins to specific subcellular areas such as the endfeet may have important roles in normal astrocyte function and in reactive astrocytosis. Some of the details of trafficking and anchoring of AQP4 and Kir4.1 have been shown [151–153], but the targeting mechanisms of other channels are unexplored in astrocytes and may be an important topic for future research. It would also be interesting to determine whether astrocytic channel activity itself can induce or regulate signaling pathways, perhaps through promoting the upregulation of other channels during reactive astrocytosis.
Astrocytic channels play important roles in autoimmune diseases. Autoantibodies can be generated against different epitopes in the same channel protein or in multiple isoforms of the channel family. Moreover, autoantibodies can be generated against different proteins of the same signaling complex, leading to similar disease symptoms. For instance, autoantibodies against Kv channel complexes can lead to limbic encephalitis (acute onset of memory loss, confusion and seizure), Morvan’s syndrome, neuromyotonia (muscle fasciculations and cramps). Kv-associated proteins, including contactin-associated protein 2 (CASPR2) and leucine-rich glioma inactivated protein 1 (LGI1), are also targets for the autoantibodies [154–158]. Therefore, it will be interesting to determine whether autoimmunity to astrocytes alters neurovascular coupling and hence gives rise to inflammation and neurodegeneration in general.
4. Conclusion
The function or dysfunction of astrocytes within the neurovascular unit may have broad implications for health and disease pathology. The neurovascular unit is known to be perturbed in many devastating diseases, and many questions remain about the role of reactive astrocytes in the progression of, or the response to, disease processes. Astrocytic ion and water channels are situated at key interfaces between astrocytes and either neurons or blood vessels. Ion channels are the second largest target class for approved drugs after GPCRs. Understanding the mechanisms of astrocytic channel activity will promote the research and discovery of potential targets for drug therapy in diseases involving neurovascular abnormalities, and will inform the strategies necessary to restore health within the neurovascular unit. This research field should pave the road not only to understand the pathogenic mechanisms underlying autoimmunity, but also to develop novel antibody-based therapies to treat neurological disorders.
Take-home message.
Autoimmunity to channel proteins on cell membranes leads to imbalance of water and ion metabolism, and hence different diseases.
Some water and ion channels are expressed in astrocytes controlling neurovascular coupling.
Neurovascular coupling dysfunction may cause neurodegeneration in autoimmune disorders.
Acknowledgement
This work was supported by a grant from the US National Institute of Neurological Disorders and Stroke/National Institutes of Health (R01NS062720) to C.G. We apologize to authors whose work is not included in this review due to space constrains.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lang B, Vincent A. Autoantibodies to ion channels at the neuromuscular junction. Autoimmunity Reviews. 2003;2(2):94–100. doi: 10.1016/s1568-9972(02)00146-5. [DOI] [PubMed] [Google Scholar]
- 2.Vincent A. Developments in autoimmune channelopathies. Autoimmunity Reviews. 12(6):678–681. doi: 10.1016/j.autrev.2012.10.016. [DOI] [PubMed] [Google Scholar]
- 3.van Coevorden-Hameete, et al. Molecular and cellular mechanisms underlying antineuronal antibody mediated disorders of the central nervous system. Autoimmunity Reviews. 2014;13:299–312. doi: 10.1016/j.autrev.2013.10.016. [DOI] [PubMed] [Google Scholar]
- 4.Zlokovic BV. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron. 2008;57(2):178–201. doi: 10.1016/j.neuron.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 5.Quaegebeur A, Lange C, Carmeliet P. The neurovascular link in health and disease: molecular mechanisms and therapeutic implications. Neuron. 2011;71(3):406–424. doi: 10.1016/j.neuron.2011.07.013. [DOI] [PubMed] [Google Scholar]
- 6.Ransohoff RM, Brown MA. Innate immunity in the central nervous system. Journal of Clinical Investigation. 2012;122(4):1164–1171. doi: 10.1172/JCI58644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jacobs AH, Tavitian B. Noninvasive molecular imaging of neuroinflammation. J Cereb Blood Flow Metab. 2012;32(7):1393–1415. doi: 10.1038/jcbfm.2012.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kovacs R, Heinemann U, Steinhauser C. Mechanisms underlying blood-brain barrier dysfunction in brain pathology and epileptogenesis: role of astroglia. Epilepsia. 2012;53(Suppl 6):53–59. doi: 10.1111/j.1528-1167.2012.03703.x. [DOI] [PubMed] [Google Scholar]
- 9.Guo S, Lo EH. Dysfunctional cell-cell signaling in the neurovascular unit as a paradigm for central nervous system disease. Stroke. 2009;40(3 Suppl):S4–S7. doi: 10.1161/STROKEAHA.108.534388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Evans MC, et al. Magnetic resonance imaging of pathological processes in rodent models of amyotrophic lateral sclerosis. Amyotrophic Lateral Sclerosis. 2012;13(3):288–301. doi: 10.3109/17482968.2011.623300. [DOI] [PubMed] [Google Scholar]
- 11.Nedergaard M, Ransom B, Goldman SA. New roles for astrocytes: redefining the functional architecture of the brain. Trends Neurosci. 2003;26(10):523–530. doi: 10.1016/j.tins.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 12.Petzold GC, Murthy VN. Role of astrocytes in neurovascular coupling. Neuron. 2011;71(5):782–797. doi: 10.1016/j.neuron.2011.08.009. [DOI] [PubMed] [Google Scholar]
- 13.Nash B, Ioannidou K, Barnett SC. Astrocyte phenotypes and their relationship to myelination. J Anat. 2011;219(1):44–52. doi: 10.1111/j.1469-7580.2010.01330.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sorensen A, et al. Astrocytes, but not olfactory ensheathing cells or Schwann cells, promote myelination of CNS axons in vitro. Glia. 2008;56(7):750–763. doi: 10.1002/glia.20650. [DOI] [PubMed] [Google Scholar]
- 15.Eroglu C, Barres BA. Regulation of synaptic connectivity by glia. Nature. 2010;468(7321):223–231. doi: 10.1038/nature09612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sofroniew MV, Vinters HV. Astrocytes: biology and pathology. Acta Neuropathol. 2010;119(1):7–35. doi: 10.1007/s00401-009-0619-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barres BA. The mystery and magic of glia: a perspective on their roles in health and disease. Neuron. 2008;60(3):430–440. doi: 10.1016/j.neuron.2008.10.013. [DOI] [PubMed] [Google Scholar]
- 18.Jukkola P, Gray V, Guerrero T, Gu C. Astrocytes differentially respond to inflammatory autoimmune insults and imbalances of neural activity. Acta Neuropathologica Communications. 2013;1:70. doi: 10.1186/2051-5960-1-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sild M, Ruthazer ES. Radial glia: progenitor, pathway, and partner. Neuroscientist. 2011;17(3):288–302. doi: 10.1177/1073858410385870. [DOI] [PubMed] [Google Scholar]
- 20.Lennon VA, et al. A serum autoantibody marker of neuromyelitis optica: distinction from multiple sclerosis. Lancet. 2004;364(9451):2106–2112. doi: 10.1016/S0140-6736(04)17551-X. [DOI] [PubMed] [Google Scholar]
- 21.Hinson SR, et al. Molecular outcomes of neuromyelitis optica (NMO)-IgG binding to aquaporin-4 in astrocytes. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(4):1245–1250. doi: 10.1073/pnas.1109980108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Waters P, Vincent A. Detection of anti-aquaporin-4 antibodies in neuromyelitis optica: current status of the assays. International Ms Journal. 2008;15(3):99–105. [PubMed] [Google Scholar]
- 23.Barnett MH, Sutton I. Neuromyelitis optica: not a multiple sclerosis variant. Current Opinion in Neurology. 2012;25(3):215–220. doi: 10.1097/WCO.0b013e3283533a3f. [DOI] [PubMed] [Google Scholar]
- 24.Higashimori H, Sontheimer H. Role of Kir4.1 channels in growth control of glia. GLIA. 2007;55(16):1668–1679. doi: 10.1002/glia.20574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stephan J, et al. Kir4.1 channels mediate a depolarization of hippocampal astrocytes under hyperammonemic conditions in situ. GLIA. 2012;60(6):965–978. doi: 10.1002/glia.22328. [DOI] [PubMed] [Google Scholar]
- 26.Bay V, Butt AM. Relationship between glial potassium regulation and axon excitability: a role for glial Kir4.1 channels. GLIA. 2012;60(4):651–660. doi: 10.1002/glia.22299. [DOI] [PubMed] [Google Scholar]
- 27.Srivastava R, et al. Potassium channel KIR4.1 as an immune target in multiple sclerosis. N Engl J Med. 2012;367(2):115–123. doi: 10.1056/NEJMoa1110740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Armstrong WE, et al. Immunocytochemical localization of small-conductance, calciumdependent potassium channels in astrocytes of the rat supraoptic nucleus. Journal of Comparative Neurology. 2005;491(3):175–185. doi: 10.1002/cne.20679. [DOI] [PubMed] [Google Scholar]
- 29.Benesova J, et al. Quantification of astrocyte volume changes during ischemia in situ reveals two populations of astrocytes in the cortex of GFAP/EGFP mice. J Neurosci Res. 2009;87(1):96–111. doi: 10.1002/jnr.21828. [DOI] [PubMed] [Google Scholar]
- 30.Bouhy D, et al. Inhibition of the Ca2-dependent K channel, KCNN4/KCa3.1, improves tissue protection and locomotor recovery after spinal cord injury. Journal of Neuroscience. 2011;31(45):16298–16308. doi: 10.1523/JNEUROSCI.0047-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Filosa JA, et al. Local potassium signaling couples neuronal activity to vasodilation in the brain. Nat Neurosci. 2006;9(11):1397–1403. doi: 10.1038/nn1779. [DOI] [PubMed] [Google Scholar]
- 32.Girouard H, et al. Astrocytic endfoot Ca2+ and BK channels determine both arteriolar dilation and constriction. Proc Natl Acad Sci U S A. 2010;107(8):3811–3816. doi: 10.1073/pnas.0914722107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jukkola PI, et al. K+ channel alterations in the progression of experimental autoimmune encephalomyelitis. Neurobiol Dis. 2012;47(2):280–293. doi: 10.1016/j.nbd.2012.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Charles AC, et al. Intercellular signaling in glial cells: calcium waves and oscillations in response to mechanical stimulation and glutamate. Neuron. 1991;6(6):983–992. doi: 10.1016/0896-6273(91)90238-u. [DOI] [PubMed] [Google Scholar]
- 35.Cornell-Bell AH, et al. Glutamate induces calcium waves in cultured astrocytes: longrange glial signaling. Science. 1990;247(4941):470–473. doi: 10.1126/science.1967852. [DOI] [PubMed] [Google Scholar]
- 36.Shigetomi E, et al. Two forms of astrocyte calcium excitability have distinct effects on NMDA receptor-mediated slow inward currents in pyramidal neurons. J Neurosci. 2008;28(26):6659–6663. doi: 10.1523/JNEUROSCI.1717-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Halassa MM, Fellin T, Haydon PG. The tripartite synapse: roles for gliotransmission in health and disease. Trends Mol Med. 2007;13(2):54–63. doi: 10.1016/j.molmed.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 38.Perea G, Navarrete M, Araque A. Tripartite synapses: astrocytes process and control synaptic information. Trends Neurosci. 2009;32(8):421–431. doi: 10.1016/j.tins.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 39.Volterra A, Meldolesi J. Astrocytes, from brain glue to communication elements: the revolution continues. Nat Rev Neurosci. 2005;6(8):626–640. doi: 10.1038/nrn1722. [DOI] [PubMed] [Google Scholar]
- 40.Rojek A, et al. A current view of the mammalian aquaglyceroporins. Annu Rev Physiol. 2008;70:301–327. doi: 10.1146/annurev.physiol.70.113006.100452. [DOI] [PubMed] [Google Scholar]
- 41.Umenishi F, Verkman AS. Isolation and functional analysis of alternative promoters in the human aquaporin-4 water channel gene. Genomics. 1998;50(3):373–377. doi: 10.1006/geno.1998.5337. [DOI] [PubMed] [Google Scholar]
- 42.Papadopoulos MC, Verkman AS. Aquaporin water channels in the nervous system. Nat Rev Neurosci. 2013;14(4):265–277. doi: 10.1038/nrn3468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yukutake Y, Yasui M. Regulation of water permeability through aquaporin-4. Neuroscience. 2010;168(4):885–891. doi: 10.1016/j.neuroscience.2009.10.029. [DOI] [PubMed] [Google Scholar]
- 44.Rossi A, et al. Super-resolution imaging of aquaporin-4 orthogonal arrays of particles in cell membranes. J Cell Sci. 2012;125(Pt 18):4405–4412. doi: 10.1242/jcs.109603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smith AJ, et al. Aggregation state determines the localization and function of M1- and M23-aquaporin-4 in astrocytes. Journal of Cell Biology. 204(4):559–573. doi: 10.1083/jcb.201308118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hamann S, et al. Aquaporins in complex tissues: distribution of aquaporins 1 – 5 in human and rat eye. Am J Physiol. 1998;274(5 Pt 1):C1332–C1345. doi: 10.1152/ajpcell.1998.274.5.C1332. [DOI] [PubMed] [Google Scholar]
- 47.Radin MJ, et al. Aquaporin-2 regulation in health and disease. Vet Clin Pathol. 2012;41(4):455–470. doi: 10.1111/j.1939-165x.2012.00488.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tran TL, et al. Aquaporins 6 – 12 in the human eye. Acta Ophthalmol. 2013;91(6):557–563. doi: 10.1111/j.1755-3768.2012.02547.x. [DOI] [PubMed] [Google Scholar]
- 49.Iliff JJ, et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid beta. Sci Transl Med. 2012;4(147):147ra111. doi: 10.1126/scitranslmed.3003748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Aoyama M, et al. Region-specific expression of a water channel protein, aquaporin 4, on brain astrocytes. J Neurosci Res. 2012;90(12):2272–2280. doi: 10.1002/jnr.23117. [DOI] [PubMed] [Google Scholar]
- 51.Verkman AS, et al. Three distinct roles of aquaporin-4 in brain function revealed by knockout mice. Biochim Biophys Acta. 2006;1758(8):1085–1093. doi: 10.1016/j.bbamem.2006.02.018. [DOI] [PubMed] [Google Scholar]
- 52.Fan Y, et al. Aquaporin-4 promotes memory consolidation in Morris water maze. Brain Struct Funct. 2013;218(1):39–50. doi: 10.1007/s00429-011-0373-2. [DOI] [PubMed] [Google Scholar]
- 53.Scharfman HE, Binder DK. Aquaporin-4 water channels and synaptic plasticity in the hippocampus. Neurochem Int. 2013 doi: 10.1016/j.neuint.2013.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Skucas VA, et al. Impairment of select forms of spatial memory and neurotrophin-dependent synaptic plasticity by deletion of glial aquaporin-4. J Neurosci. 2011;31(17):6392–6397. doi: 10.1523/JNEUROSCI.6249-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li L, et al. Proinflammatory role of aquaporin-4 in autoimmune neuroinflammation. Faseb J. 2011;25(5):1556–1566. doi: 10.1096/fj.10-177279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kimura A, et al. Protective role of aquaporin-4 water channels after contusion spinal cord injury. Ann Neurol. 2010;67(6):794–801. doi: 10.1002/ana.22023. [DOI] [PubMed] [Google Scholar]
- 57.Binder DK, et al. Increased seizure threshold in mice lacking aquaporin-4 water channels. Neuroreport. 2004;15(2):259–262. doi: 10.1097/00001756-200402090-00009. [DOI] [PubMed] [Google Scholar]
- 58.Binder DK, et al. Increased seizure duration and slowed potassium kinetics in mice lacking aquaporin-4 water channels. Glia. 2006;53(6):631–636. doi: 10.1002/glia.20318. [DOI] [PubMed] [Google Scholar]
- 59.Eid T, et al. Loss of perivascular aquaporin 4 may underlie deficient water and K+ homeostasis in the human epileptogenic hippocampus. Proc Natl Acad Sci U S A. 2005;102(4):1193–1198. doi: 10.1073/pnas.0409308102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lennon VA, et al. IgG marker of optic-spinal multiple sclerosis binds to the aquaporin-4 water channel. Journal of Experimental Medicine. 2005;202(4):473–477. doi: 10.1084/jem.20050304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hinson SR, McKeon A, Lennon VA. Neurological autoimmunity targeting aquaporin-4. Neuroscience. 168(4):1009–1018. doi: 10.1016/j.neuroscience.2009.08.032. [DOI] [PubMed] [Google Scholar]
- 62.Bennett JL, et al. Intrathecal pathogenic anti-aquaporin-4 antibodies in early neuromyelitis optica. Ann Neurol. 2009;66(5):617–629. doi: 10.1002/ana.21802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Iorio R, et al. Astrocytic autoantibody of neuromyelitis optica (NMO-IgG) binds to aquaporin-4 extracellular loops, monomers, tetramers and high order arrays. J Autoimmun. 2013;40:21–27. doi: 10.1016/j.jaut.2012.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Brosnan CRCS. The Astrocyte in Multiple Sclerosis Revisited. Glia. 2013;61:453–465. doi: 10.1002/glia.22443. [DOI] [PubMed] [Google Scholar]
- 65.Jarius S, Wildemann B. AQP4 antibodies in neuromyelitis optica: diagnostic and pathogenetic relevance. Nature Reviews Neurology. 2010;6(7):383–392. doi: 10.1038/nrneurol.2010.72. [DOI] [PubMed] [Google Scholar]
- 66.Roemer SF, et al. Pattern-specific loss of aquaporin-4 immunoreactivity distinguishes neuromyelitis optica from multiple sclerosis. Brain. 2007;130(Pt 5):1194–1205. doi: 10.1093/brain/awl371. [DOI] [PubMed] [Google Scholar]
- 67.Popescu BF, et al. Neuromyelitis optica unique area postrema lesions: nausea, vomiting, and pathogenic implications. Neurology. 76(14):1229–1237. doi: 10.1212/WNL.0b013e318214332c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.McKeon A, et al. CNS aquaporin-4 autoimmunity in children. Neurology. 2008;71(2):93–100. doi: 10.1212/01.wnl.0000314832.24682.c6. [DOI] [PubMed] [Google Scholar]
- 69.Gu C, Barry J. Function and mechanism of axonal targeting of voltage-sensitive potassium channels. Prog Neurobiol. 2011;94(2):115–132. doi: 10.1016/j.pneurobio.2011.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gu C, Gu Y. Clustering and activity tuning of Kv1 channels in myelinated hippocampal axons. Journal of Biological Chemistry. 2011;286(29):25835–25847. doi: 10.1074/jbc.M111.219113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gu Y, et al. Alternative splicing regulates kv3.1 polarized targeting to adjust maximal spiking frequency. Journal of Biological Chemistry. 2012;287(3):1755–1769. doi: 10.1074/jbc.M111.299305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gu Y, Gu C. Dynamics of Kv1 channel transport in axons. PLoS ONE [Electronic Resource] 2010;5(8):e11931. doi: 10.1371/journal.pone.0011931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Barry J, et al. Activation of conventional kinesin motors in clusters by Shaw voltagegated K+ channels. J Cell Sci. 2013;126(Pt 9):2027–2041. doi: 10.1242/jcs.122234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gu C, et al. The microtubule plus-end tracking protein EB1 is required for Kv1 voltagegated K+ channel axonal targeting. Neuron. 2006;52(5):803–816. doi: 10.1016/j.neuron.2006.10.022. [DOI] [PubMed] [Google Scholar]
- 75.Xu M, et al. The axon-dendrite targeting of Kv3 (Shaw) channels is determined by a targeting motif that associates with the T1 domain and ankyrin G. Journal of Neuroscience. 2007;27(51):14158–14170. doi: 10.1523/JNEUROSCI.3675-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Xu M, et al. Kinesin I transports tetramerized Kv3 channels through the axon initial segment via direct binding. Journal of Neuroscience. 2010;30(47):15987–16001. doi: 10.1523/JNEUROSCI.3565-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hibino H, et al. Inwardly rectifying potassium channels: their structure, function, and physiological roles. Physiol Rev. 2010;90(1):291–366. doi: 10.1152/physrev.00021.2009. [DOI] [PubMed] [Google Scholar]
- 78.Enyedi P, Czirjak G. Molecular background of leak K+ currents: two-pore domain potassium channels. Physiol Rev. 2010;90(2):559–605. doi: 10.1152/physrev.00029.2009. [DOI] [PubMed] [Google Scholar]
- 79.Inyushin M, et al. Potassium channel activity and glutamate uptake are impaired in astrocytes of seizure-susceptible DBA/2 mice. Epilepsia. 2010;51(9):1707–1713. doi: 10.1111/j.1528-1167.2010.02592.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Djukic B, et al. Conditional knock-out of Kir4.1 leads to glial membrane depolarization, inhibition of potassium and glutamate uptake, and enhanced short-term synaptic potentiation. J Neurosci. 2007;27(42):11354–11365. doi: 10.1523/JNEUROSCI.0723-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ruiz-Ederra J, Zhang H, Verkman AS. Evidence against functional interaction between aquaporin-4 water channels and Kir4.1 potassium channels in retinal Muller cells. J Biol Chem. 2007;282(30):21866–21872. doi: 10.1074/jbc.M703236200. [DOI] [PubMed] [Google Scholar]
- 82.Zhang H, Verkman AS. Aquaporin-4 independent Kir4.1 K+ channel function in brain glial cells. Mol Cell Neurosci. 2008;37(1):1–10. doi: 10.1016/j.mcn.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hsu MS, et al. Laminar-specific and developmental expression of aquaporin-4 in the mouse hippocampus. Neuroscience. 2011;178:21–32. doi: 10.1016/j.neuroscience.2011.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Heuser K, et al. Loss of perivascular Kir4.1 potassium channels in the sclerotic hippocampus of patients with mesial temporal lobe epilepsy. J Neuropathol Exp Neurol. 2012;71(9):814–825. doi: 10.1097/NEN.0b013e318267b5af. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cacheaux LP, et al. Transcriptome profiling reveals TGF-beta signaling involvement in epileptogenesis. J Neurosci. 2009;29(28):8927–8935. doi: 10.1523/JNEUROSCI.0430-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ivens S, et al. TGF-beta receptor-mediated albumin uptake into astrocytes is involved in neocortical epileptogenesis. Brain. 2007;130(Pt 2):535–547. doi: 10.1093/brain/awl317. [DOI] [PubMed] [Google Scholar]
- 87.de Lanerolle NC, Lee TS, Spencer DD. In: Histopathology of Human Epilepsy, in Jasper's Basic Mechanisms of the Epilepsies [Internet] 4th edition. JL AM Noebels, Rogawski MA, Olsen RW, Delgado-Escueta AV., editors. National Center for Biotechnology Information (US); Bethesda (MD): 2012. [PubMed] [Google Scholar]
- 88.Tong, et al. Astrocytic Kir4.1 ion channel deficits contribute to neuronal dysfunction in Hungtington's disease model mice. Nature Neuroscience. 2014;17:694–703. doi: 10.1038/nn.3691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Olsen ML, et al. Spinal cord injury causes a wide-spread, persistent loss of Kir4.1 and glutamate transporter 1: benefit of 17 beta-oestradiol treatment. Brain. 2010;133(Pt 4):1013–1025. doi: 10.1093/brain/awq049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Verena, et al. Potassium channel KIR4.1-specific antibodies in children with acquired demyelinating CNS disease. Neurology. 2014;82:470–473. doi: 10.1212/WNL.0000000000000097. [DOI] [PubMed] [Google Scholar]
- 91.Neusch C, et al. Kir4.1 potassium channel subunit is crucial for oligodendrocyte development and in vivo myelination. J Neurosci. 2001;21(15):5429–5438. doi: 10.1523/JNEUROSCI.21-15-05429.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nerrant, et al. Lack of confirmation of anti-inward rectifying potassium channel 4.1 antibodies as reliable markers of multiple sclerosis. Multiple Sclerosis Journal. 2014 doi: 10.1177/1352458514531086. [DOI] [PubMed] [Google Scholar]
- 93.Brickshawana A, et al. Investigation of the KIR4.1 potassium channel as a putative antigen in patients with multiple sclerosis: a comparative study. Lancet Neurology. 13(8):795–806. doi: 10.1016/S1474-4422(14)70141-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Schirmer L, et al. Differential loss of KIR4.1 immunoreactivity in multiple sclerosis lesions. Annals of Neurology. 75(6):810–828. doi: 10.1002/ana.24168. [DOI] [PubMed] [Google Scholar]
- 95.Lehmann-Horn F, Jurkat-Rott K. Voltage-gated ion channels and hereditary disease. Physiol Rev. 1999;79(4):1317–1372. doi: 10.1152/physrev.1999.79.4.1317. [DOI] [PubMed] [Google Scholar]
- 96.Zamponi GW, Currie KP. Regulation of Ca(V)2 calcium channels by G protein coupled receptors. Biochim Biophys Acta. 2013;1828(7):1629–1643. doi: 10.1016/j.bbamem.2012.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Buraei Z, Yang J. The β subunit of voltage-gated Ca2+ channels. Physiol Rev. 2010;90(4):1461–1506. doi: 10.1152/physrev.00057.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Leybaert L, Sanderson MJ. Intercellular Ca(2+) waves: mechanisms and function. Physiol Rev. 2012;92(3):1359–1392. doi: 10.1152/physrev.00029.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Arizono M, et al. Receptor-selective diffusion barrier enhances sensitivity of astrocytic processes to metabotropic glutamate receptor stimulation. Sci Signal. 2012;5(218):ra27. doi: 10.1126/scisignal.2002498. [DOI] [PubMed] [Google Scholar]
- 100.Di Castro MA, et al. Local Ca2+ detection and modulation of synaptic release by astrocytes. Nat Neurosci. 2011;14(10):1276–1284. doi: 10.1038/nn.2929. [DOI] [PubMed] [Google Scholar]
- 101.Navarrete M, et al. Astrocytes mediate in vivo cholinergic-induced synaptic plasticity. PLoS Biol. 2012;10(2):e1001259. doi: 10.1371/journal.pbio.1001259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Thrane AS, et al. Critical role of aquaporin-4 (AQP4) in astrocytic Ca2+ signaling events elicited by cerebral edema. Proc Natl Acad Sci U S A. 2011;108(2):846–851. doi: 10.1073/pnas.1015217108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Cain SM, Snutch TP. Voltage-gated calcium channels and disease. Biofactors. 2011;37(3):197–205. doi: 10.1002/biof.158. [DOI] [PubMed] [Google Scholar]
- 104.Xu JH, et al. Ca(v)1.2, Ca(v)1.3, and Ca(v)2.1 in the mouse hippocampus during and after pilocarpine-induced status epilepticus. Hippocampus. 2007;17(3):235–251. doi: 10.1002/hipo.20263. [DOI] [PubMed] [Google Scholar]
- 105.Willis M, et al. L-type calcium channel CaV 1.2 in transgenic mice overexpressing human AbetaPP751 with the London (V717I) and Swedish (K670M/N671L) mutations. J Alzheimers Dis. 2010;20(4):1167–1180. doi: 10.3233/JAD-2010-091117. [DOI] [PubMed] [Google Scholar]
- 106.Titulaer MJ, Lang B, Verschuuren JJ. Lambert-Eaton myasthenic syndrome: from clinical characteristics to therapeutic strategies. Lancet Neurology. 10(12):1098–1107. doi: 10.1016/S1474-4422(11)70245-9. [DOI] [PubMed] [Google Scholar]
- 107.Hajela R, et al. Lambert-eaton syndrome antibodies target multiple subunits of voltagegated Ca2+ channels. Muscle & Nerve. 2014 doi: 10.1002/mus.24295. In press. [DOI] [PubMed] [Google Scholar]
- 108.Graus F, et al. P/Q type calcium-channel antibodies in paraneoplastic cerebellar degeneration with lung cancer. Neurology. 2002;59(5):764–766. doi: 10.1212/wnl.59.5.764. [DOI] [PubMed] [Google Scholar]
- 109.Mason WP, et al. Small-cell lung cancer, paraneoplastic cerebellar degeneration and the Lambert-Eaton myasthenic syndrome. Brain. 1997;120(Pt 8):1279–1300. doi: 10.1093/brain/120.8.1279. [DOI] [PubMed] [Google Scholar]
- 110.Jun K, et al. Ablation of P/Q-type Ca(2+) channel currents, altered synaptic transmission, and progressive ataxia in mice lacking the alpha(1A)-subunit. Proceedings of the National Academy of Sciences of the United States of America. 1999;96(26):15245–15250. doi: 10.1073/pnas.96.26.15245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Rusznak Z, et al. Differential distribution of TASK-1, TASK-2 and TASK-3 immunoreactivities in the rat and human cerebellum. Cell Mol Life Sci. 2004;61(12):1532–1542. doi: 10.1007/s00018-004-4082-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zhou M, et al. TWIK-1 and TREK-1 are potassium channels contributing significantly to astrocyte passive conductance in rat hippocampal slices. J Neurosci. 2009;29(26):8551–864. doi: 10.1523/JNEUROSCI.5784-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Woo DH, et al. TREK-1 and Best1 channels mediate fast and slow glutamate release in astrocytes upon GPCR activation. Cell. 2012;151(1):25–40. doi: 10.1016/j.cell.2012.09.005. [DOI] [PubMed] [Google Scholar]
- 114.Deng PY, et al. Serotonin inhibits neuronal excitability by activating two-pore domain k+ channels in the entorhinal cortex. Mol Pharmacol. 2007;72(1):208–218. doi: 10.1124/mol.107.034389. [DOI] [PubMed] [Google Scholar]
- 115.Mathie A, Veale EL. Therapeutic potential of neuronal two-pore domain potassium-channel modulators. Curr Opin Investig Drugs. 2007;8(7):555–562. [PubMed] [Google Scholar]
- 116.Bittner S, et al. The TASK1 channel inhibitor A293 shows efficacy in a mouse model of multiple sclerosis. Exp Neurol. 2012;238(2):149–155. doi: 10.1016/j.expneurol.2012.08.021. [DOI] [PubMed] [Google Scholar]
- 117.Bittner S, et al. TASK1 modulates inflammation and neurodegeneration in autoimmune inflammation of the central nervous system. Brain. 2009;132(Pt 9):2501–2516. doi: 10.1093/brain/awp163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bittner S, et al. The TASK1 channel inhibitor A293 shows efficacy in a mouse model of multiple sclerosis. Exp Neurol. 238(2):149–155. doi: 10.1016/j.expneurol.2012.08.021. [DOI] [PubMed] [Google Scholar]
- 119.Bittner S, et al. Expression of K2P5.1 potassium channels on CD4+ T lymphocytes correlates with disease activity in rheumatoid arthritis patients. Arthritis Research & Therapy. 13(1):R21. doi: 10.1186/ar3245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Muhammad S, et al. Expression of the kcnk3 potassium channel gene lessens the injury from cerebral ischemia, most likely by a general influence on blood pressure. Neuroscience. 2010;167(3):758–764. doi: 10.1016/j.neuroscience.2010.02.024. [DOI] [PubMed] [Google Scholar]
- 121.Barel O, et al. Maternally inherited Birk Barel mental retardation dysmorphism syndrome caused by a mutation in the genomically imprinted potassium channel KCNK9. Am J Hum Genet. 2008;83(2):193–199. doi: 10.1016/j.ajhg.2008.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ramadoss J, et al. Acid-sensitive channel inhibition prevents fetal alcohol spectrum disorders cerebellar Purkinje cell loss. Am J Physiol Regul Integr Comp Physiol. 2008;295(2):R596–R603. doi: 10.1152/ajpregu.90321.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kim JE, et al. Region-specific alterations in astroglial TWIK-related acid-sensitive K+-1 channel immunoreactivity in the rat hippocampal complex following pilocarpine-induced status epilepticus. J Comp Neurol. 2008;510(5):463–474. doi: 10.1002/cne.21767. [DOI] [PubMed] [Google Scholar]
- 124.Kim JE, Kwak SE, Kang TC. Upregulated TWIK-related acid-sensitive K+ channel-2 in neurons and perivascular astrocytes in the hippocampus of experimental temporal lobe epilepsy. Epilepsia. 2009;50(4):654–663. doi: 10.1111/j.1528-1167.2008.01957.x. [DOI] [PubMed] [Google Scholar]
- 125.Smart SL, Bosma MM, Tempel BL. Identification of the delayed rectifier potassium channel, Kv1.6, in cultured astrocytes. Glia. 1997;20(2):127–134. doi: 10.1002/(sici)1098-1136(199706)20:2<127::aid-glia4>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 126.Wilcock DM, Vitek MP, Colton CA. Vascular amyloid alters astrocytic water and potassium channels in mouse models and humans with Alzheimer's disease. Neuroscience. 2009;159(3):1055–1069. doi: 10.1016/j.neuroscience.2009.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Seidel KN, et al. Expression of the voltage- and Ca2+-dependent BK potassium channel subunits BKbeta1 and BKbeta4 in rodent astrocytes. Glia. 2011;59(6):893–902. doi: 10.1002/glia.21160. [DOI] [PubMed] [Google Scholar]
- 128.Koide M, et al. Inversion of neurovascular coupling by subarachnoid blood depends on large-conductance Ca2+-activated K+ (BK) channels. Proc Natl Acad Sci U S A. 2012;109(21):E1387–E195. doi: 10.1073/pnas.1121359109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Black JA, Newcombe J, Waxman SG. Astrocytes within multiple sclerosis lesions upregulate sodium channel Nav1.5. Brain. 2010;133(Pt 3):835–846. doi: 10.1093/brain/awq003. [DOI] [PubMed] [Google Scholar]
- 130.Mannari T, et al. Astrocytic TRPV1 ion channels detect blood-borne signals in the sensory circumventricular organs of adult mouse brains. Glia. 2013;61(6):957–971. doi: 10.1002/glia.22488. [DOI] [PubMed] [Google Scholar]
- 131.Butenko O, et al. The increased activity of TRPV4 channel in the astrocytes of the adult rat hippocampus after cerebral hypoxia/ischemia. PLoS One. 2012;7(6):e39959. doi: 10.1371/journal.pone.0039959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Dunn KM, et al. TRPV4 channels stimulate Ca2+-induced Ca2+ release in astrocytic endfeet and amplify neurovascular coupling responses. Proc Natl Acad Sci U S A. 2013;110(15):6157–6162. doi: 10.1073/pnas.1216514110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Reyes RC, Verkhratsky A, Parpura V. TRPC1-mediated Ca(2+) and Na(+) signalling in astroglia: Differential filtering of extracellular cations. Cell Calcium. 2013;54(2):120–125. doi: 10.1016/j.ceca.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Benfenati V, et al. An aquaporin-4/transient receptor potential vanilloid 4 (AQP4/TRPV4) complex is essential for cell-volume control in astrocytes. Proc Natl Acad Sci U S A. 2011;108(6):2563–2568. doi: 10.1073/pnas.1012867108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Laird DW. Life cycle of connexins in health and disease. Biochem J. 2006;394(Pt 3):527–543. doi: 10.1042/BJ20051922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Giaume C, et al. Connexin and pannexin hemichannels in brain glial cells: properties, pharmacology, and roles. Front Pharmacol. 2013;4:88. doi: 10.3389/fphar.2013.00088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Koulakoff A, et al. Glial connexin expression and function in the context of Alzheimer's disease. Biochim Biophys Acta. 2012;1818(8):2048–2057. doi: 10.1016/j.bbamem.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 138.Masaki K, et al. Connexin 43 astrocytopathy linked to rapidly progressive multiple sclerosis and neuromyelitis optica. PLoS One. 2013;8(8):e72919. doi: 10.1371/journal.pone.0072919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ishibashi T, et al. Astrocytes promote myelination in response to electrical impulses. Neuron. 2006;49(6):823–832. doi: 10.1016/j.neuron.2006.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Watkins TA, et al. Distinct stages of myelination regulated by gamma-secretase and astrocytes in a rapidly myelinating CNS coculture system. Neuron. 2008;60(4):555–569. doi: 10.1016/j.neuron.2008.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Moore CS, et al. How factors secreted from astrocytes impact myelin repair. J Neurosci Res. 2011;89(1):13–21. doi: 10.1002/jnr.22482. [DOI] [PubMed] [Google Scholar]
- 142.Black JA, et al. Glial cells have heart: rH1 Na+ channel mRNA and protein in spinal cord astrocytes. Glia. 1998;23(3):200–208. [PubMed] [Google Scholar]
- 143.Black JA, et al. Type II sodium channels in spinal cord astrocytes in situ: immunocytochemical observations. Glia. 1994;12(3):219–227. doi: 10.1002/glia.440120307. [DOI] [PubMed] [Google Scholar]
- 144.Black JA, et al. Sodium channel mRNAs I, II and III in the CNS: cell-specific expression. Brain Res Mol Brain Res. 1994;22(1 – 4):275–289. doi: 10.1016/0169-328x(94)90056-6. [DOI] [PubMed] [Google Scholar]
- 145.Neusch C, et al. Lack of the Kir4.1 channel subunit abolishes K+ buffering properties of astrocytes in the ventral respiratory group: impact on extracellular K+ regulation. J Neurophysiol. 2006;95(3):1843–1852. doi: 10.1152/jn.00996.2005. [DOI] [PubMed] [Google Scholar]
- 146.Olsen ML, et al. Functional expression of Kir4.1 channels in spinal cord astrocytes. Glia. 2006;53(5):516–528. doi: 10.1002/glia.20312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Schaller KL, et al. A novel, abundant sodium channel expressed in neurons and glia. J Neurosci. 1995;15(5 Pt 1):3231–3242. doi: 10.1523/JNEUROSCI.15-05-03231.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Burgos M, et al. PKCepsilon upregulates voltage-dependent calcium channels in cultured astrocytes. Glia. 2007;55(14):1437–1448. doi: 10.1002/glia.20555. [DOI] [PubMed] [Google Scholar]
- 149.Burgos M, et al. PKCepsilon induces astrocyte stellation by modulating multiple cytoskeletal proteins and interacting with Rho A signalling pathways: implications for neuroinflammation. Eur J Neurosci. 2007;25(4):1069–1078. doi: 10.1111/j.1460-9568.2007.05364.x. [DOI] [PubMed] [Google Scholar]
- 150.Burgos M, et al. A proteomic analysis of PKCepsilon targets in astrocytes: implications for astrogliosis. Amino Acids. 2011;40(2):641–651. doi: 10.1007/s00726-010-0691-3. [DOI] [PubMed] [Google Scholar]
- 151.Noell S, et al. Evidence for a role of dystroglycan regulating the membrane architecture of astroglial endfeet. Eur J Neurosci. 2011;33(12):2179–2186. doi: 10.1111/j.1460-9568.2011.07688.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Potokar M, et al. Regulation of AQP4 surface expression via vesicle mobility in astrocytes. Glia. 2013;61(6):917–928. doi: 10.1002/glia.22485. [DOI] [PubMed] [Google Scholar]
- 153.Steiner E, et al. Loss of astrocyte polarization upon transient focal brain ischemia as a possible mechanism to counteract early edema formation. Glia. 2012;60(11):1646–1659. doi: 10.1002/glia.22383. [DOI] [PubMed] [Google Scholar]
- 154.Tan KM, et al. Clinical spectrum of voltage-gated potassium channel autoimmunity. Neurology. 2008;70(20):1883–1890. doi: 10.1212/01.wnl.0000339405.94708.8d. [DOI] [PubMed] [Google Scholar]
- 155.Kleopa KA, et al. Neuromyotonia and limbic encephalitis sera target mature Shakertype K+ channels: subunit specificity correlates with clinical manifestations. Brain. 2006;129(6):1570–1584. doi: 10.1093/brain/awl084. [DOI] [PubMed] [Google Scholar]
- 156.Irani SR, et al. Antibodies to Kv1 potassium channel-complex proteins leucine-rich, glioma inactivated 1 protein and contactin-associated protein-2 in limbic encephalitis, Morvan's syndrome and acquired neuromyotonia. Brain. 133(9):2734–2748. doi: 10.1093/brain/awq213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Lancaster E, et al. Investigations of caspr2, an autoantigen of encephalitis and neuromyotonia. Annals of Neurology. 69(2):303–311. doi: 10.1002/ana.22297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Lai M, et al. Investigation of LGI1 as the antigen in limbic encephalitis previously attributed to potassium channels: a case series. Lancet Neurology. 9(8):776–785. doi: 10.1016/S1474-4422(10)70137-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Jukkola P, et al. Astrocytes differentially respond to inflammatory autoimmune insults and imbalances of neural activity. Acta Neuropathol Commun. 2013;1(1):70. doi: 10.1186/2051-5960-1-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Saadoun S, et al. Involvement of aquaporin-4 in astroglial cell migration and glial scar formation. J Cell Sci. 2005;118(Pt 24):5691–5698. doi: 10.1242/jcs.02680. [DOI] [PubMed] [Google Scholar]
- 161.Buraei Z, Yang J. Structure and function of the beta subunit of voltage-gated Ca(2)(+) channels. Biochim Biophys Acta. 2013;1828(7):1530–1540. doi: 10.1016/j.bbamem.2012.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Heuser K, et al. Loss of perivascular Kir4.1 potassium channels in the sclerotic hippocampus of patients with mesial temporal lobe epilepsy. Journal of Neuropathology & Experimental Neurology. 71(9):814–825. doi: 10.1097/NEN.0b013e318267b5af. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Perillan PR, et al. Transforming growth factor-beta 1 regulates Kir2.3 inward rectifier K+ channels via phospholipase C and protein kinase C-delta in reactive astrocytes from adult rat brain. J Biol Chem. 2002;277(3):1974–1980. doi: 10.1074/jbc.M107984200. [DOI] [PubMed] [Google Scholar]
- 164.Seifert G, et al. Analysis of astroglial K+ channel expression in the developing hippocampus reveals a predominant role of the Kir4.1 subunit. J Neurosci. 2009;29(23):7474–7488. doi: 10.1523/JNEUROSCI.3790-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Koulakoff A, et al. Glial connexin expression and function in the context of Alzheimer's disease. Biochimica et Biophysica Acta. 1818(8):2048–2057. doi: 10.1016/j.bbamem.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 166.Masaki K, et al. Connexin 43 astrocytopathy linked to rapidly progressive multiple sclerosis and neuromyelitis optica. PLoS ONE [Electronic Resource] 8(8):e72919. doi: 10.1371/journal.pone.0072919. [DOI] [PMC free article] [PubMed] [Google Scholar]