Abstract
Background
Vitamin D supplementation may be an inexpensive intervention to reduce heart failure (HF) incidence. However, there are insufficient data to support this hypothesis. This study evaluates whether vitamin D plus calcium (CaD) supplementation is associated with lower rates of HF in post-menopausal women and whether the effects differ between those at high versus low risk for HF.
Methods and Results
Analyses were restricted to 35,983 (of original 36,282) women aged 50 to 79 years old in the Women’s Health Initiative randomized trial of CaD supplementation who were randomized 1:1 in a double-blinded fashion to receive 1,000 mg/day of calcium plus 400 IU/day of vitamin D3 or placebo. Overall, 744 adjudicated incident HF cases (intervention, 363; control, 381) occurred during a median follow up of 7.1 (interquartile range, 1.6) years. CaD supplementation, compared to placebo, was not associated with reduced HF risk in the overall population, hazard ratio (HR), 0.95; P=0.46. However, CaD supplementation had differential effects (P-interaction=0.005) in subgroups stratified by baseline risk status of HF defined by the presence (high-risk=17,449) or absence (low-risk=18,534) of preexisting HF precursors including coronary heart diseases, diabetes, or hypertension: 37% (HR, 0.63 [95% CI, 0.46 to 0.87]) lower risk of HF in the low-risk versus HR, 1.06; P=0.51, in the high-risk subgroups.
Conclusions
CaD supplementation did not significantly reduce HF incidence in the overall cohort, however, it was beneficial among postmenopausal women without major HF precursors while of little value in high-risk subgroups. Additional studies are warranted to confirm these findings and investigate the underlying mechanism.
Heart failure (HF) is a major public health problem with an enormous burden of morbidity, disability, and associated health care costs1. HF incidence rates are likely to remain high due to a rapidly aging population and a high prevalence of major precursors of HF, including hypertension, diabetes, and coronary heart disease (CHD). It is therefore imperative to identify effective interventions for prevention of HF.
The role of vitamin D in HF pathogenesis in humans is not well established, partly owing to the lack of randomized controlled trial data on this relationship. However, it has been proposed that vitamin D can directly affect cardiac functioning through interaction with cardiomyocyte vitamin D receptors (VDR)2, 3 or indirectly by modifying the incidence of hypertension, diabetes, CHD, or other major precursors of HF4. Evidence for this relationship derives from trials in HF and chronic kidney disease (CKD) patients5–10. Among CKD patients, it has been suggested that insufficient vitamin D may lead to cardiac malfunction in the presence of excess parathyroid hormone (PTH) levels precipitated by low vitamin D levels5, 7–10. It has also been reported that supplementation with vitamin D has modest effects on ventricular function and N-terminal brain natriuretic peptide (NT-pro BNP) levels among HF patients6.
The role of calcium in HF pathogeneses is not clear. There are no existing data on the direct effect of calcium supplementation on HF. However, results from two meta-analyses suggest calcium may be associated with increased risk of some cardiovascular diseases (CVD). Bolland et al11 reported calcium (alone) supplementation to be associated with a significant 27% increased risk of myocardial infarction in a pooled sample of 12,000 trial participants, while Wang et al12 reported a 14% elevated risk of a composite of CVD associated with calcium (alone) supplementation. However, combined supplementation of calcium and vitamin D had a null effect on CVD incidence12.
Hsia et al (2007) previously reported that vitamin D plus calcium (CaD) supplementation did not increase the risk of CVD, including HF, in the Women’s Health Initiative (WHI) among CaD trial population13. Their analyses involved participants with preexisting HF at baseline; hence, it is not clear what the effect of the intervention was on women free of HF at baseline. The present analyses therefore evaluate the effect of randomized assignment to CaD supplementation in the prevention of HF among post-menopausal women free of HF and participating in the Women’s Health Initiative (WHI) CaD trial. Secondary pre-specified goals for the present analyses were to examine the results in high-risk versus low-risk groups and according to baseline intake of personal vitamin D and calcium supplements. Hypertension, diabetes, and CVD, associated with HF population attributable risks of 59%, 12%, and 26%, respectively, among U.S women aged 40 to 80 years old14 are also associated with low serum vitamin D levels6, 12, 14–17. Hence vitamin D supplementation may have HF benefits in populations with these preexisting chronic conditions. Pre-specified sensitivity analysis included per-protocol analysis and estimation of CaD effects through inverse probability of censored weights (IPCW) methods18.
Methods
Design Overview: The Calcium and Vitamin D (CaD) Trial
This is a secondary analysis of HF outcomes from a previously completed RCT testing the effects of CaD on fractures and cancer outcomes as primary endpoints, however, CVD including HF were also ascertained. The CaD trial design and methodology has been published19, 20. This study was approved by an institutional review committee and all subjects gave informed consent.
Randomization and Intervention
The 36,282 postmenopausal women, aged 50 – 79 years old, who met the inclusion criteria and consented to participate in the CaD trial were randomized in a 1:1 ratio double-blinded fashion to receive either the intervention or placebo. The intervention group received a total dosage of 1,000 mg elemental calcium and 400 IU vitamin D3 per day19, 20. This dose, according to the IOM, was sufficient to meet the recommended dietary allowance (RDA) of both vitamin D and calcium in postmenopausal women. Participants randomized to the placebo group were provided ‘inactive’ tablets that looked exactly like the ‘active’ tablets and instructed to take these tablets in the same format as those in the intervention group to preserve the blinding of the randomization to both study staff and participants19.
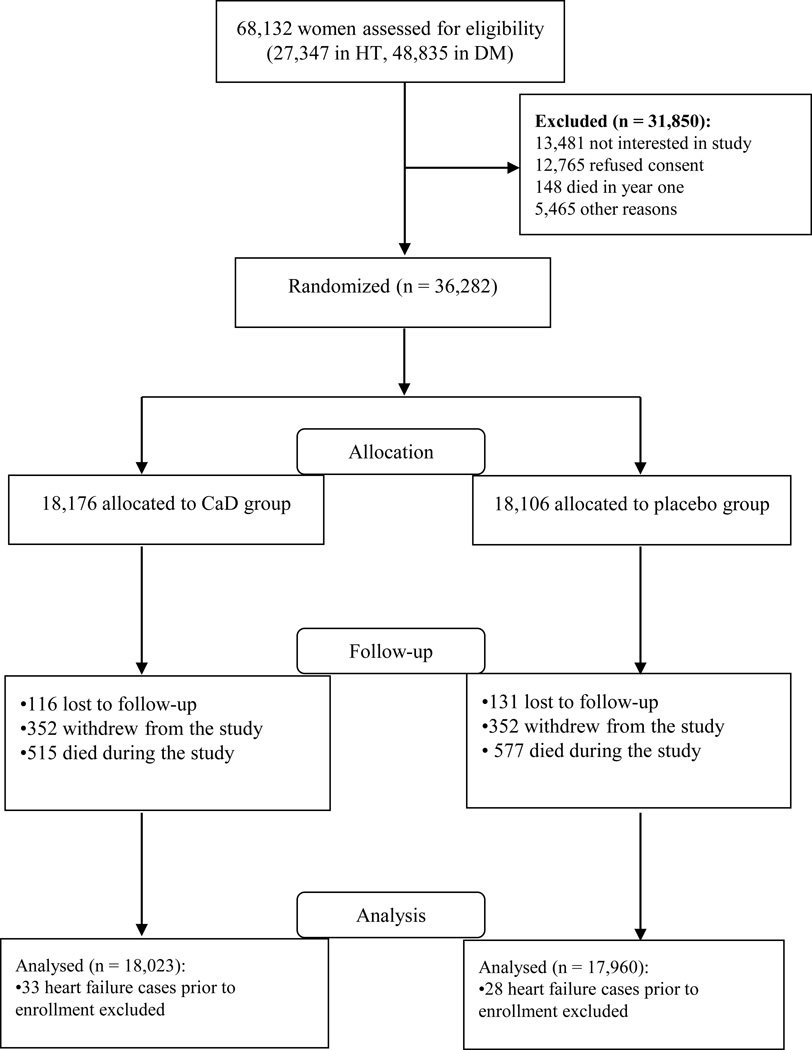
Participants who reported use of personal vitamin D (48.4%) and/or calcium (54.9%) supplements were allowed to continue taking them even after enrolling in the CaD trial. The personal vitamin D supplement consumption was later raised to an upper limit of 1,000 IU/day after the IOM published new recommendations on vitamin D intake in 199921. Details of participant selection for the CaD trial and the present analyses are reported in Figure 1.
Figure 1.
Participant recruitment and selection for the CaD trial and analyses.
Outcome: Ascertainment of Heart Failure Cases
Trained staff abstracted medical records annually for self-reported HF hospitalization and any two-day hospitalization that reported a CVD outcome. The abstracted HF cases were then classified by an adjudication committee of physicians at local clinical sites; only a subset of HF cases were adjudicated by a central adjudication physician committee since HF was a secondary endpoint for the CAD trial. The local and central adjudication methods had an excellent 79% agreement rate (k)22. The local physician committee used the following method: Hospitalized HF (HF requiring and/or occurring during hospitalization) required physician diagnosis of new-onset or worsened congestive HF on the reported hospital admission and 1 or more of the following 4 criteria: 1) HF diagnosed by physician and receiving medical treatment for HF; 2) above plus documentation in the current medical record of a history of an imaging procedure showing impaired left ventricular (LV) systolic or diastolic function; 3) pulmonary edema/congestion on chest X-ray on the current admission; 4) dilated ventricle(s) or “poor” LV or right ventricular (RV) function by echocardiogram, multi gated acquisition (MUGA), radionuclide ventriculogram (RVG), or other contrast ventriculography, or evidence of LV diastolic dysfunction.
Covariates and Effect Modifiers
We stratified the study sample into two subgroups by baseline status of medical histories of major cardiovascular precursors of HF to estimate the effect of CaD intervention in these subgroups. For the pre-specified subgroup analyses, we defined “high-risk” using American College of Cardiology criteria, based on the presence of hypertension, diabetes, CHD or CVD23.Women who reported any histories of medically diagnosed hypertension, diabetes, CHD, or CVD before randomization were grouped as high-risk of HF while those without any of these conditions were considered to be at low-risk of HF.
Self-reported histories of physician-diagnosed cardiovascular conditions were obtained from all participants during trial enrollment. Details of the description of these baseline factors and other baseline potential confounders listed in Table 1 have been previously reported19. Hypertension was defined as a self-reported physician diagnosis of hypertension with or without current use of antihypertensive medications or high blood pressure (BP) (measured systolic BP ≥ 140 mmHg and/or diastolic BP ≥ 90 mmHg) during enrollment into the CaD trial. CVD was defined as self-reported physician diagnosis of problems with the heart, blood circulation or clots. CHD was defined as a composite of self-reported cardiac arrest, angina, coronary artery bypass graft surgery (CABG), and/or percutaneous transluminal coronary angioplasty (PTCA). Diabetes was defined based on self-reported physician diagnosis of diabetes and current prescription of diabetic medications.
Table 1.
Baseline Characteristics of Participants Stratified by Baseline Risk Status of Heart Failure and by Treatment Group - the Vitamin D and Calcium (CaD) Trial of the Women’s Health Initiative (WHI) Study, 1995 – 2005
| Baseline characteristics | Baseline Risk Status | |||
|---|---|---|---|---|
| Low-risk group (N = 18,534) |
High-risk group (N = 17,449) |
|||
| CaD (n = 9,307) |
Placebo (n = 9,227) |
CaD (n = 8,716) |
Placebo (n = 8,733) |
|
| No. (%) with data | ||||
| Age (years) | ||||
| 49 – 59 | 44 | 44 | 30 | 30 |
| 60 – 69 | 43 | 43 | 48 | 48 |
| 70 – 81 | 13 | 13 | 22 | 22 |
| Race/Ethnicity | ||||
| White | 86 | 87 | 80 | 80 |
| Black | 6 | 6 | 12 | 12 |
| Hispanic | 5 | 4 | 4 | 4 |
| Income ($) | ||||
| <25,000 | 18 | 18 | 24 | 25 |
| 25, 000 – 50,000 | 43 | 44 | 47 | 46 |
| 50,000 – 70,000 | 21 | 20 | 17 | 18 |
| 75,000 – 100,000 | 10 | 10 | 6 | 7 |
| >100,000 | 9 | 9 | 5 | 6 |
| Physical activity level (total METs/week) | ||||
| Low | 37 | 37 | 40 | 40 |
| Moderate | 12 | 13 | 14 | 13 |
| High | 51 | 50 | 47 | 47 |
| Smoking status | ||||
| None | 52 | 53 | 53 | 54 |
| Past | 40 | 39 | 40 | 40 |
| Current | 8 | 8 | 7 | 7 |
| Family history of CVD | 62 | 62 | 70 | 70 |
| Personal calcium (alone) supplements | 50 | 50 | 50 | 50 |
| Personal vitamin D (alone) supplements | 50 | 50 | 50 | 50 |
Abbreviations: SD, standard deviation; BMI, body mass index; MET, metabolic equivalent of task; CVD, cardiovascular disease.
Sample Size and Power Calculations
Women (153 intervention; 146 placebo) with prior medical diagnosis of HF at trial enrollment were excluded from this analysis to create a primary prevention cohort of 35,983 (18,023 intervention; 17,960 control) post-menopausal women free of HF. When stratified by baseline risk status of HF, 17,449 (8,716 intervention; 8,733 control) were considered to be at high-risk while 18,534 (9,307 intervention; 9,227 control) were considered to be at low-risk. A total of 744 HF cases (high-risk: 587 [302 intervention; 285 control] vs low-risk subgroup: 157 [61 intervention; 96 control]) during a total follow up of about 9.8 years. Based on these data and an alpha level of 0.05, this study had 80% statistical power to detect protective effects (hazard ratios) of CaD supplementation of at least 0.86, 0.64, and 0.78 in the overall CaD cohort, the low-risk and high-risk subgroups respectively24. Given a two-tailed a priori hypothesis testing, the study was powered at 80%, type I error of 0.05, to detect interaction effect sizes of ≥ 1.52 when CaD effect is stronger in the high-risk compared to the low-risk group or ≤ 0.66 when CaD effect is stronger in the low-risk compared to the high-risk group25.
Statistical Analysis
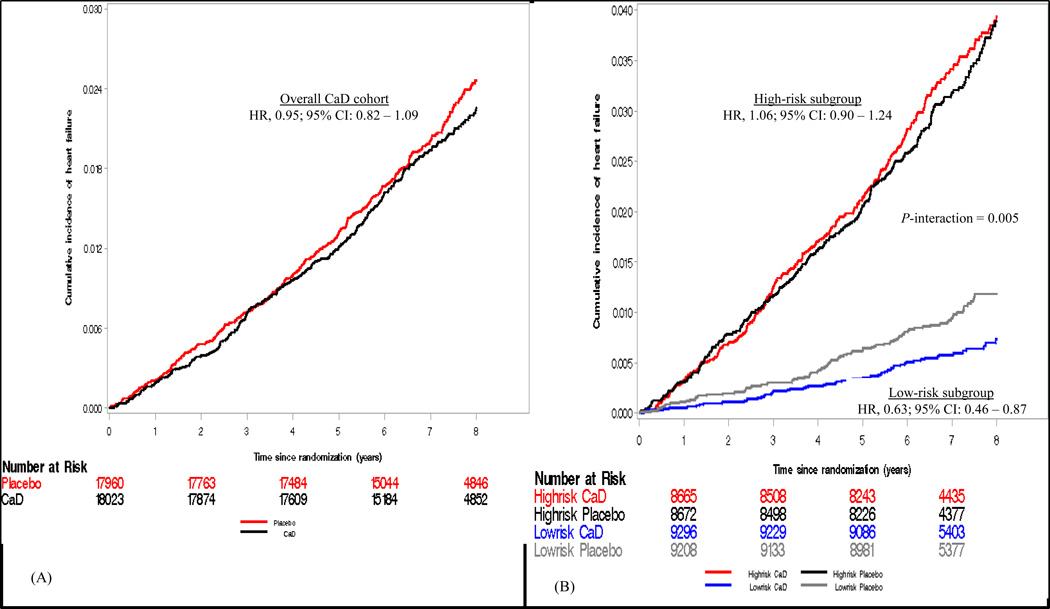
All main analyses were performed based on an intention-to-treat approach. Chi-square and Student’s t-test were performed to assess the balance of potential confounders between trial arms for the entire CaD cohort and by stratified subgroups. Cox proportional hazard (CPH) regression models were used to estimate the effect (hazard ratios, HR) of the intervention on HF. Both graphical, Kaplan-Meier curves (Figure 2) and Schoenfeld residuals plots (Supplemental Figure 1) and time-dependent proportionality tests (study cohort [P for interaction]; overall cohort (0.45), low-risk [0.80], high-risk [0.44]) were used to evaluate whether the proportionality assumption was violated by the intervention variable. Formal test of interaction between the randomization status and a binary indicator of baseline HF risk status was performed by including a product term between the two variables in the CPH models. All HRs were estimated from unadjusted CPH models since all potential covariates evaluated were balanced between the two arms of the study.
Figure 2.
Kaplan-Meier curves comparing the cumulative incidence of HF between the CaD and placebo arms during follow-up period in the overall CaD cohort (A) and stratified baseline subgroups (B).
We also evaluated whether personal consumption of vitamin D or calcium supplements at baseline modified the effect of CaD on HF incidence since participants were allowed to continue consuming their personal calcium and vitamin D supplements after enrolling in the CaD trial. Additionally, we tested whether total (diet plus supplements) vitamin D or calcium intake modified the effect of CaD on HF incidence. We also estimated a potential effect modification by self-reported postmenopausal hormone therapy and randomization to receive intervention in the hormone replacement therapy (HRT) trial.
Sensitivity analyses were performed by restricting the analyses to only participants who achieved 80% adherence rate to study medication (n = 23,601, 65.6%). To estimate CaD effects (HRs) independent of censoring information, the IPCW method was implemented18, 26. The IPCW model was adjusted for some of the factors previously reported as strong predictors of adherence to study medication in the CaD trial – age, education level, use of personal calcium, vitamin D or multivitamin supplements, history of HF risk factors, family history of CVD, and enrollment in other clinical trials27.
Results
Baseline socio-demographic, physical/lifestyle, and clinical factors were proportionally distributed between the two study arms for the entire CaD cohort (Supplemental Table 1) and in the two stratified subgroups of participants with and without major precursors of HF (Supplemental Table 2). Baseline known CVD risk factors were more prevalent in the high-risk group compared to the low-risk group, however, personal calcium and vitamin D supplements consumption was proportional between these subgroups (Table 1). There were 744 HF cases (29.0/10,000 person-years) during a median follow-up years of 7.06 (interquartile range: 1.61); 363 (28.2/10,000 person-years) of these occurred in the intervention arm versus 381 (29.8/10,000 person-years) in the placebo arm. When stratified by baseline risk status, more HF cases occurred in the high-risk subgroup (587 [302 intervention; 285 control]) than in the low-risk subgroup of women (157 [61 intervention; 96 control]), P value of difference <0.001.
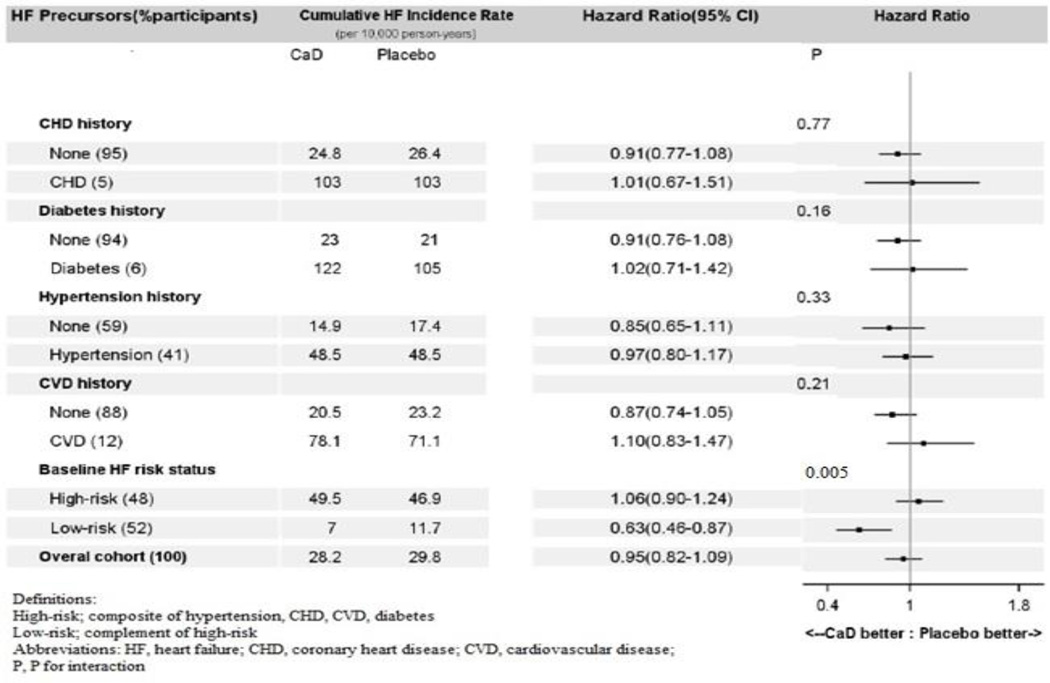
Supplementation with CaD was not associated with risk of HF hospitalization in the overall cohort, HR, 0.95 [95% CI, 0.82 to 1.09]; P = 0.46. The effect of CaD, however, was modified (P for interaction = 0.005) by baseline risk status of HF (defined by the presence or absence of CHD, other CVD, hypertension, or diabetes) at baseline. When stratified by baseline risk status, CaD was associated with a statistically significant 37% lower risk of HF, HR, 0.63 [95% CI, 0.46 to 0.87]; P = 0.005 (number needed-to-treat, 255 [95%CI, 174 to 723] in the low-risk subgroup, but not in the high-risk subgroup (HR, 1.06 [95% CI, 0.90 to 1.24]) (Figure 3). Consumption of personal vitamin D (P for interaction = 0.33) or calcium (P for interaction = 0.11) supplements did not modify the effect of CaD on HF incidence. The effects of CaD were also not modified by baseline self-reported total (diet plus supplements) consumption of vitamin D (P for interaction = 0.26) or calcium (P for interaction = 0.81) (Table 2). Neither assignment to receive the intervention (estrogen alone or estrogen plus progestin) nor self-reported use of postmenopausal hormone therapy at baseline modified the effect of CaD on HF incidence (Table 2). Baseline covariates also did not confound these associations in multivariable-adjusted CPH regression models.
Figure 3.
Forest plot of hazard ratios for effect of vitamin D plus calcium supplementation on HF.
Table 2.
Effects of the intervention stratified by baseline supplements (calcium and vitamin D) and postmenopausal hormone therapy use
| Baseline supplement intake | Overall | Low-risk | High-risk | P - interaction | |||
|---|---|---|---|---|---|---|---|
| 95% CI | P | 95% CI | P | 95% CI | P | ||
| Non-protocol calcium supplements | 0.11 | ||||||
| No (n = 16,218) | 1.07 (0.87 – 1.31) | 0.54 | 0.73 (0.46 – 1.16) | 0.18 | 1.19 (0.95 – 1.50) | 0.14 | |
| Yes (n = 19,765) | 0.85 (0.69 – 1.03) | 0.102 | 0.55 (0.36 – 0.87) | 0.01 | 0.94 (0.75 – 1.18) | 0.58 | |
| Non-protocol vitamin D supplements | 0.33 | ||||||
| No (n = 18,558) | 1.01 (0.83 – 1.23) | 0.91 | 0.74 (0.47 – 1.14) | 0.17 | 1.12 (0.90 – 1.39) | 0.33 | |
| Yes (n = 17,425) | 0.88 (0.71 – 1.08) | 0.22 | 0.53 (0.33 – 0.85) | 0.01 | 0.99 (0.78 – 1.26) | 0.93 | |
| Hormone therapy trial assignment | 0.83 | ||||||
| Not randomized or randomized to receive placebo (n=27,929) | 0.94 (0.80 – 1.11) | 0.45 | 0.76 (0.52 – 1.09) | 0.14 | 0.99 (0.82 – 1.19) | 0.88 | |
| Randomized to receive intervention (n = 8,054) | 0.97 (0.73 – 1.30) | 0.86 | 0.36 (0.18 – 0.71) | <0.01 | 1.32 (0.94 – 1.84) | 0.11 | |
| Self-reported postmenopausal hormone therapy use ever | 0.36 | ||||||
| No (n = 17,252) | 0.89 (0.74 – 1.08) | 0.23 | 0.57 (0.37 – 0.89) | 0.01 | 1.01 (0.81 – 1.25) | 0.95 | |
| Yes (n = 18,731) | 1.02 (0.82 – 1.27) | 0.86 | 0.70 (0.44 – 1.13) | 0.14 | 1.12 (0.88 – 1.44) | 0.36 | |
Abbreviations: HR, hazard ratio; CI, confidence interval; P, P value; P – interaction, P value for interaction with intervention
The ITT and per-protocol results were similar (Table 3). In the per-protocol analysis, CaD was not also associated with HF in the overall cohort nor in the high-risk subgroup but was associated with a significantly reduced risk of HF in the low-risk subgroup (Table 3). Similar CaD effects were observed among women who did not report taking personal vitamin D and calcium supplements and were also independent of competing events when estimated with the IPCW methods (Supplemental Table 3). Regardless of baseline risk status of HF, mean total calcium and vitamin D intake and serum 25(OH)D levels (available for only 2,012 women) were relatively lower in the incident HF cases compared to the non-cases (Supplemental Table 4).
Table 3.
Results of Intention-to-treat and Per-protocol Analysis Estimating the Association between CaD Supplementation and Heart Failure Incidence - The Vitamin D and calcium (CaD) Trial of the Women’s Health Initiative (WHI) Study, 1995 – 2005
| Study population |
Intention-to-treat analysis (N = 35,983) |
*Per-protocol analysis (N = 23,601) |
||||
|---|---|---|---|---|---|---|
| Total | HF cases, n (rate/10,000 person-years) |
HR (95% CI) | Total | HF cases, n (rate/10,000 person-years) |
HR (95% CI) | |
| Overall | ||||||
| Control | 17,960 | 381 (29.8) | 1.00 | 11,993 | 190 (21.9) | 1.00 |
| CaD | 18,023 | 363 (28.2) | 0.95 (0.82 – 1.09) | 11,608 | 188 (22.4) | 1.02 (0.84 – 1.25) |
| Low-risk | ||||||
| Control | 9,227 | 96 (11.7) | 1.00 | 6,320 | 51 (11.0) | 1.00 |
| CaD | 9,307 | 61 (7.0) | 0.63 (0.46 – 0.87) | 6,186 | 30 (6.6) | 0.60 (0.38 – 0.94) |
| High-risk | ||||||
| Control | 8,733 | 285 (46.9) | 1.0 | 5,673 | 139 (34.4) | 1.00 |
| CaD | 8,716 | 302 (49.5) | 1.06 (0.90 – 1.24) | 5,422 | 158 (40.9) | 1.19 (0.95 – 1.49) |
Abbreviations: CaD, calcium plus vitamin D trial; HR, hazard ratio; CI, confidence interval; CI, confidence interval; P, P value
Analysis are based on data from women with at least 80% adherence to study protocol
Discussion
Vitamin D and calcium supplementation was associated with lower risk of HF in a subgroup of women without preexisting HF precursors at baseline but had no effect in those with these conditions. These findings were independent of baseline total vitamin D and calcium intake and persisted in per-protocol analysis. Results from a recent meta-analysis of 17 trials (n = 12,440), including the MRC RECORD trial, showed that vitamin D alone (vs placebo) was associated with a 21% lower risk of cardiac failure (HR, 0.79 [95% CI: 0.60 to 0.98]; P = 0.03) among men and women with a mean/median age > 60 years old28. It is not clear whether the observed effect of vitamin D supplementation might have differed between baseline populations stratified by preexisting HF precursors in the meta-analysis population.
The basis for the differential effects of CaD intervention on HF incidence by baseline risk status is uncertain. Data from animal models suggest vitamin D may regulate cardiac functions, at least partially, via interaction with the VDR in cardiaomyocytes2, 3. Through a receptor-mediated mechanism, 1,25(OH)2D regulates intracellular calcium homeostasis and calcium ion uptake in ventricular cardiac muscle cells to modify cardiac contractility29. An intermediate pathophysiological model involving the up-regulation of the renin-angiotensin-aldosterone system (RAAS) has been observed in both human and animal studies. RAAS plays a major role in HF pathogenesis by regulating blood pressure, cardiac contractility, electrolyte homeostasis, and eccentric hypertrophy of the myocardium30,31. It has been demonstrated that a knock-out model of mice without the VDR develop high blood pressure, cardiac enlargement, and experience increased activation of the RAAS32. The activation of RAAS by low vitamin D status has recently been suggested in humans based on observational studies33, 34. However, a meta-analysis of 10 trials did not show an association between vitamin D (alone or with calcium) supplementation and reduction in either systolic or diastolic blood pressures16.
Hyperparathyroidism has also been postulated as an intermediate process in the vitamin D-HF pathophysiology. Data from end-stage renal disease (ESRD) patients suggests secondary hyperparathyroidism and elevated parathyroid hormone (PTH) levels were associated with low vitamin D levels caused by failure of the kidneys to convert 25(OH)D to the metabolically active form of 1,25(OH)D22, 8–10. Chronic exposure to PTH has been reported to be associated with poor myocardial structure and functioning as well as elevated blood pressure and accelerated atherosclerosis. It is therefore plausible the lower risk of HF observed in the low-risk subgroup is a result of the ability of the vitamin D supplements to prevent or mitigate the process of hyperparathyroidism.
We expected the high-risk subgroup would benefit more from the intervention, based on evidence that vitamin D deficiency is relatively higher in this subgroup in the general population, but our data did not confirm this hypothesis. One plausible explanation for the lack of an association between CaD and HF incidence in the high-risk subgroup is that HF pathogenesis was already too advanced to be influenced by the CaD supplements. Thus, in this high-risk population, any potential benefit related to HF prevention may have been diminished. Our data suggest that the high-risk group may indeed have poorer health status compared to those free of these comorbidities; baseline factors associated with poor health and risk of HF, such as older age, obesity, and hypercholesterolemia, were predominantly prevalent in this high-risk subgroup (Table 1).
It is also plausible that medical therapy for management of the cardiovascular risk factors in the high-risk group may have modified the effect of CaD intervention on HF. We therefore evaluated whether the use of CVD medications such as angiotensin concerting enzymes (ACE) inhibitors, angiotensin II receptor blockers (ARB), statins, beta-blockers, or calcium channel blockers were effect modifiers. Individually and collectively (composite of CVD medications), CVD medications did not significantly interact with CaD in relation to HF except ARB (P for interaction = 0.04) (Supplemental material). This supports the possibility that CaD supplementation may interact with medications used to treat comorbidities, thus making any direct effect of the intervention on HF outcome more difficult to detect. Additional studies on this topic are warranted.
This study contributes to the growing literature on the association between vitamin D and lower incidence of HF. This study is probably the first to demonstrate that CaD supplementation may be associated with lower risk of HF among postmenopausal women without preexisting hypertension, diabetes, CHD, or other CVD but not in those with these conditions. The study had adequate statistical power to perform the pre-specified subgroup analysis. The randomized trial design nature of the data minimized the effect of potential confounders.
Despite the strengths of this randomized trial, limitations of the study also warrant consideration. First, as a result of combining calcium with vitamin D as the intervention, it is unclear whether the observed associations are due to one or both of these dietary supplements. However, based on the meta-analysis26 report that suggested an association between vitamin D (alone) supplementation and lower risk of HF and other reports that suggest calcium (alone) may be associated with increased risk of CVD events, it can be inferred that vitamin D was the active agent responsible for the prevention of HF in the WHI trial. Second, the CaD trial was limited to postmenopausal women, 50 – 79 years old, which may preclude generalizability of the findings to men or to younger women. Lastly, for ethical reasons, participants were allowed to continue consumption of both vitamin D and calcium supplements within the recommended dietary allowance guidelines put forth by the Institute of Medicine. However, consumption of personal vitamin D and calcium supplements did not modify the effect of CaD on HF incidence.
Our findings suggest that a very low cost daily supplementation with vitamin D (400 IU) plus calcium (1,000 mg) may be an effective primary prevention strategy for HF in postmenopausal women free of preexisting cardiovascular conditions. However, it appears to be of little value in preventing HF in the overall trial population and the subgroup of postmenopausal women who already have CVD conditions, including CHD, diabetes, and hypertension. These findings, if confirmed by other research, may have important public health and clinical implications.
Supplementary Material
Acknowledgements
The authors thank the Women’s Health Initiative study investigators, staff, and study participants for their outstanding dedication and commitment. The Women’s Health Initiative study investigators and National Institutes of Health sponsors all contributed to the design and execution of the study. A list of key investigators involved in this research follows: A full listing of WHI investigators can be found at the following Web site: https://cleo.whi.org/researchers/Documents%20%20Write%20a%20Paper/WHI%20Investigator%20Long%20List.pdf.
Macarius Donneyong affirms that everyone who significantly contributed to this work has been listed in the Acknowledgments.
Sources of Funding
The Women’s Health Initiative study program is funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, U.S. Department of Health and Human Services through contracts HHSN268201100046C, HHSN268201100001C, HHSN268201100002C, HHSN268201100003C, HHSN268201100004C, and HHSN271201100004C. The active study drug and placebo were supplied by GlaxoSmithKline Consumer Healthcare (Pittsburgh).
Footnotes
Disclosures
None.
References
- 1.Ramani GV, Uber PA, Mehra MR. Chronic heart failure: contemporary diagnosis and management. Mayo Clin Proc. 2010;85:180–195. doi: 10.4065/mcp.2009.0494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nemerovski CW, Dorsch MP, Simpson RU, Bone HG, Aaronson KD, Bleske BE. Vitamin D and cardiovascular disease. Pharmacotherapy. 2009;29:691–708. doi: 10.1592/phco.29.6.691. [DOI] [PubMed] [Google Scholar]
- 3.Witham MD. Vitamin D in chronic heart failure. Curr Heart Fail Rep. 2011;8:123–130. doi: 10.1007/s11897-011-0048-6. [DOI] [PubMed] [Google Scholar]
- 4.Lavie CJ, Lee JH, Milani RV. Vitamin D and cardiovascular disease will it live up to its hype? J Am Coll Cardiol. 2011;58:1547–1556. doi: 10.1016/j.jacc.2011.07.008. [DOI] [PubMed] [Google Scholar]
- 5.Achinger SG, Ayus JC. The role of vitamin D in left ventricular hypertrophy and cardiac function. Kidney Int Suppl. 2005:S37–S42. doi: 10.1111/j.1523-1755.2005.09506.x. [DOI] [PubMed] [Google Scholar]
- 6.Chung M, Balk EM, Brendel M, Ip S, Lau J, Lee J, Lichtenstein A, Patel K, Raman G, Tatsioni A, Terasawa T, Trikalinos TA. Vitamin D and calcium: a systematic review of health outcomes. Evid Rep Technol Assess (Full Rep) 2009:1–420. [PMC free article] [PubMed] [Google Scholar]
- 7.Drueke TB, McCarron DA. Paricalcitol as compared with calcitriol in patients undergoing hemodialysis. N Engl J Med. 2003;349:496–499. doi: 10.1056/NEJMe038104. [DOI] [PubMed] [Google Scholar]
- 8.Rostand SG, Drueke TB. Parathyroid hormone, vitamin D, and cardiovascular disease in chronic renal failure. Kidney Int. 1999;56:383–392. doi: 10.1046/j.1523-1755.1999.00575.x. [DOI] [PubMed] [Google Scholar]
- 9.Shoji T, Shinohara K, Kimoto E, Emoto M, Tahara H, Koyama H, Inaba M, Fukumoto S, Ishimura E, Miki T, Tabata T, Nishizawa Y. Lower risk for cardiovascular mortality in oral 1alpha-hydroxy vitamin D3 users in a haemodialysis population. Nephrol Dial Transplant. 2004;19:179–184. doi: 10.1093/ndt/gfg513. [DOI] [PubMed] [Google Scholar]
- 10.Teng M, Wolf M, Ofsthun MN, Lazarus JM, Hernán MA, Camargo CA, Jr, Thadhani R. Activated injectable vitamin D and hemodialysis survival: a historical cohort study. J Am Soc Nephrol. 2005;16:1115–1125. doi: 10.1681/ASN.2004070573. [DOI] [PubMed] [Google Scholar]
- 11.Bolland MJ, Avenell A, Baron JA, Grey A, MacLennan GS, Gamble GD, Reid IR. Effect of calcium supplements on risk of myocardial infarction and cardiovascular events: meta-analysis. BMJ. 2010;341:c3691. doi: 10.1136/bmj.c3691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang L, Manson JE, Song Y, Sesso HD. Systematic review: Vitamin D and calcium supplementation in prevention of cardiovascular events. Ann Intern Med. 2010;152:315–323. doi: 10.7326/0003-4819-152-5-201003020-00010. [DOI] [PubMed] [Google Scholar]
- 13.Hsia J, Heiss G, Ren H, Allison M, Dolan NC, Greenland P, Heckbert SR, Johnson KC, Manson JE, Sidney S, Trevisan M. Calcium/vitamin D supplementation and cardiovascular events. Circulation. 2007;115:846–854. doi: 10.1161/CIRCULATIONAHA.106.673491. [DOI] [PubMed] [Google Scholar]
- 14.Kannel WB. Incidence and epidemiology of heart failure. Heart Fail Rev. 2000;5:167–173. doi: 10.1023/A:1009884820941. [DOI] [PubMed] [Google Scholar]
- 15.Bjelakovic G, Gluud LL, Nikolova D, Whitfield K, Krstic G, Wetterslev J, Gluud C. Vitamin D supplementation for prevention of mortality in adults. Cochrane Database Syst Rev. 2011:CD007470. doi: 10.1002/14651858.CD007470.pub2. [DOI] [PubMed] [Google Scholar]
- 16.Pittas AG, Chung M, Trikalinos T, Mitri J, Brendel M, Patel K, Lichtenstein AH, Lau J, Balk EM. Systematic review: Vitamin D and cardiometabolic outcomes. Ann Intern Med. 2010;152:307–314. doi: 10.1059/0003-4819-152-5-201003020-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grandi NC, Breitling LP, Brenner H. Vitamin D and cardiovascular disease: systematic review and meta-analysis of prospective studies. Prev Med. 2010;51:228–233. doi: 10.1016/j.ypmed.2010.06.013. [DOI] [PubMed] [Google Scholar]
- 18.Xu R, O'Quigley J. Estimating average regression effect under non-proportional hazards. Biostatistics. 2000;1:423–439. doi: 10.1093/biostatistics/1.4.423. [DOI] [PubMed] [Google Scholar]
- 19.Jackson RD, LaCroix AZ, Cauley JA, McGowan J. The Women's Health Initiative calcium-vitamin D trial: overview and baseline characteristics of participants. Ann Epidemiol. 2003;13:S98–S106. doi: 10.1016/s1047-2797(03)00046-2. [DOI] [PubMed] [Google Scholar]
- 20.Anderson GL, Manson J, Wallace R, Lund B, Hall D, Davis S, Shumaker S, Wang CY, Stein E, Prentice RL. Implementation of the Women's Health Initiative study design. Ann Epidemiol. 2003;13:S5–S17. doi: 10.1016/s1047-2797(03)00043-7. [DOI] [PubMed] [Google Scholar]
- 21.Dietary reference intakes for calcium, phosphorus, magnesium, vitamin D and fluoride. Washington, DC: 1999. Standing Committee on the Scientific Evaluation of Dietary Reference Intakes FaNB. [Google Scholar]
- 22.Heckbert SR, Kooperberg C, Safford MM, Psaty BM, Hsia J, McTiernan A, Gaziano JM, Frishman WH, Curb JD. Comparison of self-report, hospital discharge codes, and adjudication of cardiovascular events in the Women's Health Initiative. Am J Epidemiol. 2004;160:1152–1158. doi: 10.1093/aje/kwh314. [DOI] [PubMed] [Google Scholar]
- 23.Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, Ganiats TG, Jessup M, Konstam MA, Mancini DM, Michl K, Oates JA, Rahko PS, Silver MA, Stevenson LW, Yancy CW. 2009 Focused update incorporated into the ACC/AHA 2005 Guidelines for the Diagnosis and Management of Heart Failure in Adults A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines Developed in Collaboration With the International Society for Heart and Lung Transplantation. J Am Coll Cardiol. 2009;53:e1–e90. doi: 10.1016/j.jacc.2008.11.013. [DOI] [PubMed] [Google Scholar]
- 24.Hintze J. NCSS 9. Kaysville, Utah, USA: NCSS, LLC.; 2013. www.ncss.com. [Google Scholar]
- 25.Weiliang Qiu, Jorge Chavarro, Ross Lazarus, Bernard Rosner, Jing Ma. powerSurvEpi (v 0.06): Power and sample size calculation for survival analysis of epidemiological studies. Boston, MA, USA: 2012. http://cran.r-project.org/web/packages/powerSurvEpi/ [Google Scholar]
- 26.Heinze MKaG. PSHREG: A SAS macro for proportional and nonproportional subdistribution hazards regression with competing risk data. Vienna: Medical University of Vienna; 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brunner R, Dunbar-Jacob J, Leboff MS, Granek I, Bowen D, Snetselaar LG, Shumaker SA, Ockene J, Rosal M, Wactawski-Wende J, Cauley J, Cochrane B, Tinker L, Jackson R, Wang CY, Wu L. Predictors of adherence in the Women's Health Initiative Calcium and Vitamin D Trial. Behav Med. 2009 Winter;34:145–155. doi: 10.3200/BMED.34.4.145-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ford JA, MacLennan GS, Avenell A, Bolland M, Grey A, Witham M. Cardiovascular disease and vitamin D supplementation: trial analysis, systematic review, and meta-analysis. The American journal of clinical nutrition. 2014;100:746–755. doi: 10.3945/ajcn.113.082602. [DOI] [PubMed] [Google Scholar]
- 29.Walters MR, Ilenchuk TT, Claycomb WC. 1,25-Dihydroxyvitamin D3 stimulates 45Ca2+ uptake by cultured adult rat ventricular cardiac muscle cells. J Biol Chem. 1987;262:2536–2541. [PubMed] [Google Scholar]
- 30.Eichhorn EJ, Bristow MR. Medical therapy can improve the biological properties of the chronically failing heart. A new era in the treatment of heart failure. Circulation. 1996;94:2285–2296. doi: 10.1161/01.cir.94.9.2285. [DOI] [PubMed] [Google Scholar]
- 31.Xiang W1, Kong J, Chen S, Cao LP, Qiao G, Zheng W, Liu W, Li X, Gardner DG, Li YC. Cardiac hypertrophy in vitamin D receptor knockout mice: role of the systemic and cardiac renin-angiotensin systems. Am J Physiol Endocrinol Metab. 2005;288:E125–E132. doi: 10.1152/ajpendo.00224.2004. [DOI] [PubMed] [Google Scholar]
- 32.Li YC, Kong J, Wei M, Chen ZF, Liu SQ, Cao LP. 1,25-Dihydroxyvitamin D(3) is a negative endocrine regulator of the renin-angiotensin system. J Clin Invest. 2002;110:229–238. doi: 10.1172/JCI15219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Forman JP, Williams JS, Fisher ND. Plasma 25-hydroxyvitamin D and regulation of the renin-angiotensin system in humans. Hypertension. 2010;55:1283–1288. doi: 10.1161/HYPERTENSIONAHA.109.148619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tomaschitz A, Pilz S, Ritz E, Grammer T, Drechsler C, Boehm BO, März W. Independent association between 1,25-dihydroxyvitamin D, 25-hydroxyvitamin D and the renin-angiotensin system: The Ludwigshafen Risk and Cardiovascular Health (LURIC) study. Clin Chim Acta. 2010;411:1354–1360. doi: 10.1016/j.cca.2010.05.037. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.