Abstract
Activating mutations of the interleukin-7 receptor (IL7R) occur in approximately 10% of patients with T-cell acute lymphoblastic leukaemia (T-ALL). Most mutations generate a cysteine at the transmembrane domain leading to receptor homodimerization through disulfide bond formation and ligand-independent activation of STAT5. We hypothesized that the reducing agent N-acetylcysteine (NAC), a well-tolerated drug used widely in clinical practice to treat acetaminophen overdose, would reduce disulfide bond formation, and inhibit mutant IL7R-mediated oncogenic signalling. We found that treatment with NAC disrupted IL7R homodimerization in IL7R-mutant DND-41 cells as assessed by non-reducing Western blot, as well as in a luciferase complementation assay. NAC led to STAT5 dephosphorylation and cell apoptosis at clinically achievable concentrations in DND-41 cells, and Ba/F3 cells transformed by an IL7R-mutant construct containing a cysteine insertion. The apoptotic effects of NAC could be rescued in part by a constitutively active allele of STAT5. Despite using doses lower than those tolerated in humans, NAC treatment significantly inhibited the progression of human DND-41 cells engrafted in immunodeficient mice. Thus, targeting leukaemogenic IL7R homodimerization with NAC offers a potentially effective and feasible therapeutic strategy that warrants testing in patients with T-ALL.
INTRODUCTION
Coordinated signalling through the JAK and STAT pathways is essential for normal lymphopoiesis (Higuchi, et al 1997, Igaz, et al 2001). This dependence is exemplified by the development of severe combined immunodeficiency when integral components of this pathway, including interleukin-7 receptor alpha (IL7R), are inactivated through loss-of-function genetic lesions (Kalman, et al 2004). Conversely, constitutive activation of JAK-STAT signalling has been implicated in the development of acute lymphoblastic leukaemia (ALL), with activating mutations in JAK1, JAK2, JAK3, and more recently STAT5B and TYK2, all identified in T-ALL (Flex, et al 2008, Kontro, et al 2013, Sanda, et al 2013, Zhang, et al 2012).
Several groups recently reported the presence of somatically acquired activating IL7R mutations occurring in approximately 10% of T-ALL cases (Shochat, et al 2011, Zenatti, et al 2011, Zhang, et al 2012). The vast majority of such mutations are short in-frame insertions that result in the introduction of a novel cysteine just extracellular to the transmembrane domain. These unpaired cysteine residues result in inter-molecular disulfide bond formation leading to ligand-independent IL7R homodimerization, and constitutive JAK1 activation with phosphorylation and activation of STAT5 (Shochat, et al 2011, Zenatti, et al 2011, Zhang, et al 2012). Furthermore, inhibition of JAK-STAT signalling results in apoptosis of IL7R-mutant cells, suggesting that these tumours depend on this pathway for survival (Porcu, et al 2012, Shochat, et al 2011, Zenatti, et al 2011, Zhang, et al 2012).
We hypothesized that leukaemogenic activation of this pathway by cysteine mutations in IL7R could be blocked with the reducing agent N-acetylcysteine (NAC), which is able to reduce disulfide bond formation in vitro and in vivo (Cartwright, et al 1977, Chen, et al 2011). NAC is an approved drug that has been used extensively and safely in clinical practice as an antidote for acetaminophen overdose for over three decades (Peterson and Rumack 1977, Smilkstein, et al 1988). Here we use biochemical, genetic and in vivo studies to show that NAC treatment inhibits mutant IL7R-mediated oncogenic signalling by disrupting disulfide bond formation, potentially offering an effective, affordable and well-tolerated therapeutic strategy for T-ALL patients with IL7R cysteine insertions.
MATERIALS and METHODS
Cell culture, IL7R sequencing and NAC treatment in vitro
T-ALL cell lines were maintained as previously described (Sanda, et al 2013). Exon 6 of IL7R was Sanger sequenced from T-ALL cell lines using the published protocol (Shochat, et al 2011). For drug assays, cells were grown at a density of 1 × 105/ml in 96-well format and treated with NAC (Sigma-Aldrich). Due to the acidity of NAC in culture, both control and NAC treated wells were buffered with 20 μM HEPES (Invitrogen, Grand Island, NY, USA).
Retroviral transductions
The pMSCV-IL7R-243insPPCL-IRES-GFP and pMSCV-IL7R-V253G-IRES-GFP were created by site directed mutagenesis from pMSCV-IL7R-IRES-GFP wild-type vector (Stratagene/Agilent, Santa Clara, CA, USA) (Shochat, et al 2011). pMSCV-BCR-ABL-puro has been previously described (Yoda, et al 2010). The pMX-Stat5b1*6-IRES-GFP constitutively active murine STAT5B mutant (aStat5b) and control pMX-IRES-GFP retroviral vectors were kind gifts from Professor Toshio Kitamura (University of Tokyo, Tokyo, Japan), and were used in DND-41 rescue experiments (Onishi, et al 1998). The pMSCV-cS5F-IRES-eGFP encoding a constitutively active murine STAT5A mutant (S710F=cS5) and control pMSCV-IRES-eGFP were cloned and validated previously (Moriggl, et al 2005). These constructs were used in the Ba/F3 rescue experiments because their high GFP expression allowed sorting of a Ba/F3-IL7R-PPCL population co-expressing cS5. The generation of retroviral supernatants, viral transductions and cell selection have been described previously (Sanda, et al 2013).
Western blots
Immunoblotting was carried out with the following antibodies: anti-IL7Rα (clone-40131, R&D Systems, Minneapolis, MN, USA) and anti-pY-STAT5 (Y694) (Cell Signaling Technology, Danvers, MA, USA), both diluted 1 in 1000; anti-β-actin (ACTB); Sigma-Aldrich, St. Louis, MO, USA) diluted 1 in 5000 and secondary horseradish peroxidase (HRP)-linked antibody to mouse or rabbit (Cell Signaling Technology) diluted 1 in 10,000.
Cell viability and apoptosis assays
Cell viability in vitro was determined at 48 h after the initiation of treatment with NAC using the Cell Titer Glo assay (Promega, Madison, WI, USA). For Annexin V staining, cells were washed twice in phosphate-buffered saline (PBS) at 24 h after drug treatment, labelled with Annexin V fluorescein isothiocyanate (FITC) antibody (BD Pharmingen, San Jose, CA, USA) according to the manufacturer’s recommendations and assessed by flow cytometry.
Luciferase complementation assay
The luciferase complementation assay was performed according to the protocol of Cassonnet et al. (2011). Wild-type and mutant (p.L242_L243ins PPCL) IL7R sequences were amplified from MSCV-IL7R-IRES-GFP plasmid templates (Shochat, et al 2011) using primers IL7R-GW-F 5′-GGGGACAACTTTGTACAAAAAAGTTGGCATGACAATTCTAGGTACA and IL7R-GW-R 5′-GGGGACAACTTTGTACAAGAAAGTTGAGAGACTGGGCCATACGATAGG, containing attB1 and attB2 Gateway cloning sites. Polymerase chain reaction (PCR) products were purified and transferred by BP recombination into pDONR223 vector. Generated entry clones were used in a LR recombination reaction with destination vectors pSPICA-C1 and pSPICA-C2 (kindly provided by Dr. Yves Jacob, Unité de Génétique, Papillomavirus et Cancer Humain (GPCH), Institut Pasteur, Paris - France) allowing C-terminal fusion of IL7R sequences with the amino acids 18 to 109 or amino acids 110 to 185 of the humanized Gaussia princeps luciferase, respectively. All clones were verified by Sanger sequencing.
HEK-293T cells were seeded at 5×104 cells per well in Dulbecco’s modified Eagle medium (DMEM) with 10% fetal calf serum (FCS) without antibiotics in a 24-well plate format, and transfected at 24 h using Fugene reagent and 250 ng of total DNA/well. Cells were cultured at 37°C for 48 h, after which the media was changed with fresh DMEM containing 10% FCS buffered with 20 μM HEPES (to maintain pH after the addition of NAC) and then treated with or without NAC or β-mercaptoethanol (BME 2 mM) for 90 min. Gaussia princeps luciferase activity was measured using the Promega kit (E2810) according to the manufacturer’s instructions. Luciferase activity was measured on a FLUOstar Omega plate reader.
Xenograft model
1 × 106 luciferase-expressing DND-41 cells were injected into the tail vein of 7-week-old female NOD-SCID-IL2Rcgnull (NSG) mice (The Jackson Laboratory, Bar Harbor, ME, USA). Tumour burden was assessed by bioluminescence imaging (BLI) using an IVIS Spectrum system (Caliper Life Sciences, Santa Clara, CA, USA), every 3–5 days until leukaemia was established, generally 2–3 weeks after intravenous injection, at which time NAC therapy was initiated. For each BLI time point, cancer bioluminescence was visualized by intraperitoneal (IP) injection of d-luciferin (Promega) in PBS at 75 mg/kg. In the pilot study, NAC-treated mice received the drug (Sigma-Aldrich) both in drinking water (10 mg/ml, supplemented with 45 mg/ml dextrose for palatability) and by IP injection 1 g/kg bid (twice daily) for 7 days, because continuous intravenous (IV) administration is not feasible in mice and osmotic administration using implanted pumps is precluded by the need to achieve micromolar concentrations.
In the follow-up experiment, mice either received NAC by IP injection (1 g/kg) bid, or IP injections of PBS. For IP injections, NAC was made up with water and brought to pH=5 with NaOH and filter-sterilized. Mice treated with this approach for 19 days displayed no detectable toxicity or weight loss upon daily monitoring. Femurs were fixed in 10% formalin, sectioned, paraffin-embedded and stained with anti-human-CD45 antibody (Clone 2B11+PD7/26, diluted 1 in 50; Dako/Agilent, Santa Clara, CA, USA). Stained slides were photographed using an Olympus BX41 microscope and Q-colour5 digital camera (Olympus, Center Valley, PA, USA) and staining scores were calculated using Aperio software (Leica, Newcastle, UK).
Statistical analyses
Dose-response curves were fitted using least squares (ordinary) fit as log (inhibitor) versus response using GraphPad Prism software (GraphPad Software, La Jolla, CA, USA). Differential sensitivities were calculated by non-linear regression. Two-tailed Student’s t-test was used to calculate statistical differences between continuous variables, with P<0.05 considered as statistically significant.
RESULTS
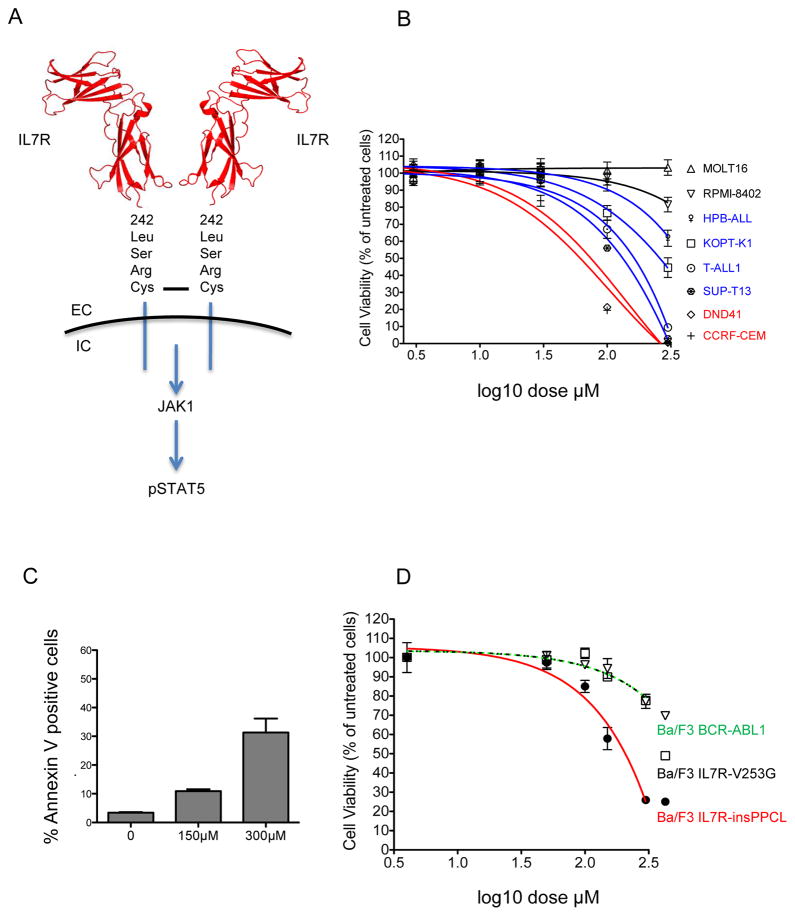
To identify a cell model of mutant IL7R signalling, we first sequenced exon 6 of IL7R in 21 T-ALL cell lines. We identified one somatic mutation; a 12 bp insertion in DND-41 cells resulting in a 4-amino-acid insertion (p.L242_L243insLSRC), as described previously (Table I) (Porcu, et al 2012). Modelling the insertion on the crystal structure of IL7R using the PyMOL software (Schrodiner, New York, NY, USA) predicted that it would reside just extracellular to the transmembrane domain and form homodimers through disulfide bond formation with the unpaired cysteine from the other mutant IL7R molecules (Figure 1A) (McElroy, et al 2009, McElroy, et al 2012). Of note, we had previously detected particularly high levels of phospho-STAT5 (pSTAT5) in this cell line by Western blotting [see Supplementary Figure 3 in (Sanda, et al 2013)], consistent with its activation by mutant IL7R signalling.
Table I.
Sanger sequencing results for IL7R exon 6 from 21 T-ALL cell lines together with STAT5 phosphorylation status.
| T-ALL cell line | IL7R exon 6 | Phospho-STAT5 status* (Y964) | IC50 to NAC (μM) |
|---|---|---|---|
| ALL-SIL | WT | + | |
| Be13 | WT | 91.2 | |
| CCRF-CEM | WT | − | 61.7 |
| DND-41 | L242_L243 ins LSRC | ++ | 74.1 |
| HPB-ALL | WT | − | >300 |
| H-SB2 | WT | + | |
| JURKAT | WT | − | 177.8 |
| K3P | WT | ||
| KOPT-K1 | WT | ++ | 257.0 |
| LOUCY | WT | − | |
| MOLT-3 | WT | ||
| MOLT-4 | WT | − | |
| MOLT-16 | WT | − | >300 |
| P12-ICHIKAWA | WT | − | |
| PEER | WT | + | |
| PF382 | WT | − | |
| RPMI-8402 | WT | − | >300 |
| SKW-3/KE-37 | WT | − | |
| SUP-T1 | WT | ||
| SUP-T11 | WT | − | |
| SUP-T13 | WT | + | 141.3 |
| T-ALL1 | WT | 162.2 |
As determined by Western blot. Data from (Sanda, et al 2013).
−, No pYSTAT5 detectable; +, detectable pYSTAT5; ++, strong pYSTAT5
IC50, 50% inhibitory concentration; WT, wild-type.
Figure 1. NAC induces apoptosis in IL7R mutant DND-41 T-ALL cells and Ba/F3 cells transformed by a cysteine-containing IL7R mutant allele.
A) Structural model of a mutant IL7R homodimer and schematic of downstream signalling events. The disulfide bond between unpaired cysteines of the LSRC insertion of IL7R-mutant DND-41 cells is shown as a black bar. Images were created with PyMOL software based on the published crystal structure (Protein Databank ID 3DI2) (McElroy, et al 2009, McElroy, et al 2012). EC extracellular. IC intracellular. B) Viability of T-ALL cell lines determined by the Cell Titer Glo assay after 48 h of treatment with NAC. C) Apoptosis measured as the percentage of Annexin V-FITC positive DND-41 cells 24 h after treatment with NAC, as determined by flow cytometry. D) Ba/F3 cells were transformed to IL3 independence with retroviral constructs encoding BCR-ABL, IL7R-insPPCL or IL7R-V253G. Cell viability was tested using the Cell Titer Glo assay after 48 h of NAC treatment.
We next tested the effects of NAC on the viability of cells from a panel of ten TALL cell lines over a broad dose range. Cell lines could be broadly categorized as resistant (MOLT-16, RPMI-8402; 50% inhibitory concentration [IC50]>300 μM), moderately sensitive (for example, KOPT-K1 and SUP-T13; IC50 257 μM and 141 μM respectively) and sensitive (for example, DND-41 and CCRF-CEM; IC50 74 μM and 62 μ respectively; Figure 1B and Table I). IL7R-mutant DND-41 cells were among the most sensitive of the cell lines tested. We hypothesized that the efficacy of NAC in DND-41 cells was mediated, at least in part, through disruption of mutant IL7R homodimers, while the sensitivity identified in cell lines such as CCRF-CEM was probably mediated through disruption of disulfide bonds in other cell surface receptors required for cell survival. The reduction in cell viability in DND-41 cells occurred through apoptosis as determined by Annexin V staining (Figure 1C).
Given that we were able to find only a single T-ALL cell line harbouring an IL7R mutation, we recapitulated oncogenic IL7R signalling in Ba/F3 cells using two different IL7R mutations previously described in patients: IL7R-ins243PPCL and IL7R-V253G (Shochat, et al 2011). The IL7R-ins243PPCL mutation is similar to the DND-41 IL7R-243insLSRC mutation in both its insertion position and size, and is predicted to lead to receptor homodimerization through disulfide bond formation from the novel cysteine. By contrast, the IL7R-V253G resides deep within the membrane, and mediates constitutive STAT5 activation through an alternative allosteric mechanism that does not involve the formation of disulfide bonds (Shochat, et al 2014). Both mutations transformed Ba/F3 cells to IL3-independence. In support of our hypothesis, Ba/F3 cells transformed by IL7R-ins243PPCL were significantly more sensitive to NAC than Ba/F3 cells transformed by IL7R-V253G or BCR-ABL1 (P<0.001, Figure 1D).
Western blotting of endogenous IL7R in DND-41 cells under non-reducing conditions demonstrated disruption of the IL7R homodimer at the same doses as those required to induce apoptosis in vitro, with associated loss of STAT5 phosphorylation (Figure 2A). We also tested IL7R homodimerization quantitatively in a luciferase complementation assay in HEK-293T cells using constructs encoding the N- or C-terminus of Gaussia princeps luciferase fused to the intracellular domain of IL7R, such that functional luciferase activity occurs on IL7R protein-protein interaction. When expressed individually, the N- or C-terminal luciferase constructs alone were unable to generate a luciferase signal (Figure 2B). However, a robust luciferase signal was obtained when the N- and C-terminal IL7R constructs were expressed concurrently, which was significantly stronger (P<0.001) with IL7R-PPCL than the wild-type (WT) IL7R constructs (notably, WT-IL7R has previously been shown to homodimerize in an inactive configuration through an N-terminal hydrogen bond) (McElroy, et al 2012). NAC had no effect on WT IL7R constructs but, consistent with what we had observed through Western blotting, treatment with NAC significantly inhibited homodimerization of IL7R-PPCL (P<0.001, Figure 2B). In this assay, NAC had the same effect as β-mercaptoethanol (BME), a reducing agent commonly used in vitro, which has been previously shown to inhibit mutant IL7R signalling in vitro (Zenatti, et al 2011). However, given the reported toxicity of BME in vivo compared to the excellent tolerability of NAC, we chose not to pursue BME as a therapeutic agent for T-ALL (White, et al 1973).
Figure 2. NAC disrupts mutant IL7R homodimerization, inhibits STAT5 signalling and its apoptotic effects can be partially rescued by activated STAT5.
A) Western blotting under non-reducing conditions of lysates extracted from DND-41 cells 24 h after treatment with NAC or control medium. B) A luciferase complementation assay to quantify IL7R homodimerization. The assay is based on the principle that expression of two separate inactive fragments of luciferase will produce a functionally active luciferase protein only after significant protein-protein interaction (Cassonnet, et al 2011). Either the N- or C-terminal fragments of luciferase (LUC) were fused to the intracellular domain of wild-type and mutant IL7R (ins PPCL) constructs and expressed in HEK-293T cells for 48 h, treated with or without NAC or β-mercaptoethanol (BME 2 mM) for 90 min, before measurement of Gaussia princeps luciferase activity. Of note, homodimerization of wild-type IL7R has been previously described by crystallography, where the IL7R homodimers are held together by an intermolecular hydrogen bond at the N-terminus, maintaining the dimer in a non-active conformation (McElroy, et al 2012). C) Expression of a constitutively active mutant of Stat5b partially rescues DND-41 cells from apoptosis induced by NAC. DND-41 cells were transduced with either a control pMX-IRES-GFP or pMX-Stat5B*-IRES-GFP retroviral vector encoding a constitutively active Stat5b construct, selected by fluorescence-activated cell sorting (FACS) and then treated with NAC for 48 h before cell viability was measured by the Cell Titer Glo assay. The P value was calculated using non-linear regression. D) Ba/F3 cells transformed by IL7R-insPPCL were infected with retroviral vectors expressing a constitutively active murine Stat5a mutant (S710F) or control pMSCV-IRES-eGFP. Cells were sorted by FACS for high GFP expression, then treated with NAC for 48 h before cell viability was measured by the Cell Titer Glo assay. The P value was calculated using non-linear regression.
To determine whether loss of viability in DND-41 cells from NAC treatment was mediated predominantly through inhibition of STAT5 signalling, we retrovirally transduced DND-41 cells with a constitutively active STAT5 construct (termed aStat5b) (Onishi, et al 1998), such that signalling downstream of STAT5 was no longer dependent on mutant IL7R homodimerization. Consistent with our proposed mechanism, DND-41-aStat5 cells were significantly more resistant to NAC treatment than control transduced DND-41 cells (P<0.001, Figure 2C). Similarly, when IL7R-PPCL transformed Ba/F3 cells were engineered to co-express constitutively active STAT5 (aStat5a) (Moriggl, et al 2005), they demonstrated increasing resistance to NAC therapy (P<0.05, Figure 2D), suggesting the effects of NAC on cell viability were mediated at least in part through STAT5 signalling. The lack of a complete rescue may be attributable to the fact that the IL7R pathway also activates PI3K-AKT signalling in a STAT5 independent fashion (Barata, et al 2004, Dadi and Roifman 1993, Sharfe, et al 1995, Silva, et al 2011).
Given that NAC concentrations of 150–300 μM are required to kill DND-41 cells in vitro, we examined published pharmacokinetic studies to determine if therapeutic levels of NAC are potentially achievable in human subjects. A single oral dose of 600 mg of NAC results in plasma levels of approximately 30–60 μM (Chen, et al 2007, De Caro, et al 1989, Holdiness 1991), with pharmacokinetics influenced by poor bioavailability, extensive first-pass metabolism and short half-life (Table II) (Borgstrom, et al 1986, Hong, et al 2005). Consequently, a widely adopted approach of treating acetaminophen overdose is continuous IV infusion of NAC (Smilkstein, et al 1991), which produces steady-state NAC plasma levels up to 930 μM (Table II), well within the therapeutic range required to induce apoptosis of DND-41 cells (Borgstrom, et al 1986, Brown, et al 2004, Chen, et al 2007). This suggests that the therapeutic concentrations of NAC required to treat IL7R-mutant leukaemias are readily achievable in humans.
Table II.
Summary of reported pharmacokinetic studies assessing steady state N-acetylcysteine concentrations in humans.
| Reference | Patient group | Route of administration | Steady state plasma concentration (μM) |
|---|---|---|---|
| Ahola et al (1999) | Pre-term newborns | IV | 510 |
| Borgstrom et al (1986) | Pediatric | IV | 400 |
| Adult | IV | 200 | |
| Brown et al (2004) | Adult | IV | 930 |
| Jones et al (1997) | Adult | IV | 210 |
| Olsson et al (1988) | Adult | IV | 37 |
| Prescott et al (1989) | Adult | IV | 230 |
| De Caro et al (1989) | Adult | PO (single 600 mg dose) | 33 |
| Holdiness (1991) | Adult | PO | 65 |
IV Intravenous; PO Oral. Adapted from Chen, et al (2007), with permission.
We then tested the efficacy of NAC in vivo using a murine xenograft model. NOD-SCID-IL2Rcgnull (NSG) mice were injected with 1 × 106 luciferase-expressing IL7R-mutant DND-41 cells and were treated with NAC once tumour engraftment was established by BLI. In an attempt to achieve therapeutic levels of NAC, and limited by the difficulty in administering continuous IV infusions to NSG mice due to their small size, we opted to administer NAC both in drinking water (10 mg/ml) and by IP injection (1 g/kg bid) in our initial pilot study (n=4 per group), starting after leukaemia was established, generally 2–3 weeks after intravenous injection of leukaemic cells. After 7 days of treatment, we observed striking responses in 2 of 4 mice, with few if any detectable leukaemic cells by BLI, as compared to widespread disease in all 4 control treated animals (Figure 3A). However, one of the mice died of dehydration because of refusal to drink the NAC-containing drinking water, which has the foul odor of rotten eggs.
Figure 3. NAC inhibits the growth of DND-41 leukaemic cells in vivo in NSG mice.
A) Bioluminescent images of NSG mice in the pilot study when treated with a combination of oral (10 mg/ml in drinking water) and IP injected NAC (1 g/kg bid) compared to vehicle for 7 days (n=4 per group). B) Schematic showing the timeline for the follow-up in vivo study. 1 × 106 luciferase-expressing DND-41 cells were injected into the tail vein of 7-week-old female NOD-SCID-IL2Rcgnull mice. After determination of tumour engraftment by bioluminescent imaging (BLI), mice received treatment with either IP injected NAC 1g/kg bid or vehicle (n=8 per group). C) In vivo monitoring of tumour burden of DND-41 cells as assessed by BLI. The P value at day 19 was calculated using two-tailed Student’s t test. D) Bioluminescent images of NSG mice after 19 days of treatment with IP NAC (1 g/kg bid) compared to vehicle. Four representative animals from each group are shown. E) Immunohistochemistry of human CD45 from representative paraffin-embedded femur sections from NSG mice treated with either control or NAC, photographed at 40x magnification. F) Score for human CD45 staining of femurs from NSG mice treated with either control or NAC, as calculated with Aperio software, based on the abundance of positively staining cells over a set area of magnification (n=3 per group). The P value was calculated using two-tailed Student’s t test.
To avoid the difficulty with oral administration, we gave mice free access to drinking water and administered NAC by IP injection 1 g/kg twice per day (Figure 3B). Despite the suboptimal pharmacokinetics of IP NAC (in vivo half-life in mice, 9–11 minutes) (Neuwelt, et al 2004), NAC treatment nonetheless significantly delayed tumour progression in this model (Figure 3C and 3D): mean bioluminescence on day 19 was 13.9×109 for control versus 7.75×109/photons(ph)/s/cm2/steradian (sr) for NAC-treated animals (P<0.001; n=8 in each group). We confirmed these findings by analysing bone marrow biopsies of mice for the presence of human leukaemia cells, as measured by immunohistochemistry against human CD45 (Figure 3E). We consistently found a marked reduction of human T-ALL burden in mice treated with NAC as compared to control animals (Figure 3F, P<0.005).
DISCUSSION
Peterson & Rumack (1997) initially reported the clinical use of NAC to reverse the severe hepatic toxicity associated with acetaminophen overdose. Since then, this antidote has saved countless lives and to this day remains the mainstay of effective treatment for this indication (Ferner, et al 2011). Given that acetaminophen overdose is the commonest form of poisoning worldwide, with over 50,000 cases per annum in the USA alone, the experience of using NAC in the clinic is extensive (Nourjah, et al 2006). Despite the remarkably high steady-state plasma concentrations achievable on standard treatment protocols, it has an exceptionally favourable tolerability profile.
As predicted based on the known reducing properties of NAC, it was able to disrupt cysteine-bond-mediated homodimers of mutant IL7R, resulting in reduced pSTAT5 expression and the induction of apoptosis in IL7R mutant DND-41 human TALL cells. Unfortunately, we found only a single human T-ALL cell line that harbours an IL7R mutation. For this reason, we also analysed Ba/F3 cells immortalized with a cysteine-mutated activated IL7R, and these cells also responded to treatment with NAC. We also observed a partial rescue in cell viability when Ba/F3 cells were transduced with an activated STAT5 construct, suggesting that a significant proportion of the effect of NAC was mediated through loss of IL7R-mediated STAT5 activation.
NAC also showed effects on cell viability in IL7R-WT T-ALL cell lines, such as SUP-T13, when the cells were treated at high concentrations. We are not sure of the mechanism of activity, although one could surmize that intramolecular cysteine bonds in key cellular proteins might also be required for T-ALL cell viability. For example, many cell surface receptors contain reactive cysteines involved in the formation of intra- and inter-molecular disulfide bond formation (Metcalfe, et al 2011), the reduction of which are likely to have consequences on receptor configuration and affect ‘outside-in’ signalling; increased dependency on such signals in leukaemic cells compared to normal cells may offer the therapeutic window predicted from our data. Further studies will be required to investigate the cytotoxic effects of NAC in T-ALLs lacking IL7R mutations. The fact that patients can tolerate continuous NAC infusions for over 24 h, where steady state plasma levels are greater than 300 μM, without undergoing catastrophic organ failure, suggests that this leukaemia-specific cytotoxicity may prove clinically useful in a subset of T-ALL cases that lack IL7R mutations. Other mutations leading to aberrant disulfide bond formation have been shown to activate different oncogenic receptors in several other cancers – F232C mutation of CRLF2 in B-ALL (Hertzberg, et al 2010, Yoda, et al 2010), RET mutations in thyroid cancers (Asai, et al 1995, Santoro, et al 1995) and HER2 R896C mutation in breast cancer (Bose, et al 2013) – also suggesting that NAC may have application beyond IL7R mutant T-ALL.
One limitation of our study was the inability to adequately dose NSG mice to levels that are comparable to those that are achievable in humans. The most striking responses occurred in mice that were treated with both oral and IP NAC in our pilot study, suggesting that efficacy was dose-dependent, but oral NAC was repulsive to mice and several mice became dehydrated. With an in vivo half-life of NAC in mice of approximately 10 min (Neuwelt, et al 2004), a continuous IV infusion schedule would have been optimal, but we were technically unable to deliver the drug by this route in such small animals. Nonetheless, we observed significant responses, particularly with respect to leukaemic infiltration of the bone marrow.
Given that optimal and safe dosing schedules to achieve high micromolar plasma concentrations are well-established in humans, our findings suggest that NAC use in IL7R-mutant T-ALL could afford a safe and effective new therapeutic strategy warranting testing in clinical trials. Lastly, it is worth noting that 11 of the 12 US Food and Drug Administration (FDA)-approved cancer drugs in 2012 cost over $100,000 per annum (Experts in Chronic Myeloid Leukemia 2013). In the UK, a 2-g ampoule of NAC for IV use costs £1.96 (equivalent to approximately $3.20), meaning that a 24-h- infusion on a typical NAC protocol for a 70 kg adult for acetaminophen overdose costs £21.56 (~$34.50). Given the fact that NAC is well tolerated in clinical use by continuous infusion, we recommend that it be evaluated in clinical trials as an approach to targeted therapy for a subset of T-ALL patients, the economical implications of which may be particularly relevant to healthcare in the developing world.
Acknowledgments
This research was supported by the Kay Kendall Leukaemia Fund of the UK (M.R.M), the Claudia Adams Barr Innovative Basic Science Research Program (M.R.M), grants from the National Cancer Institute (5P01CA109901, 5P01CA68484 and 5K08CA160660) by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research and by the ISF, ICRF and WLBH foundation (to SI). We would like to thank John Gilbert for editorial advice.
Footnotes
Authorship contributions
M.R.M. and A.K. designed, performed and analysed research and wrote the paper; C.R., A.E., J-C.T., S.D., A.Y., N.T., R.M., A.B., and J.T. performed experiments and/or provided crucial reagents; A.L.K., D.J.D., D.M.W., S.I. and S.E.S. designed and analysed research; S.J.R. performed and analysed research; and A.T.L. supervised research, analysed data and co-wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
References
- Ahola T, Fellman V, Laaksonen R, Laitila J, Lapatto R, Neuvonen PJ, Raivio KO. Pharmacokinetics of intravenous N-acetylcysteine in pre-term newborn infants. Eur J Clin Pharmacol. 1999;55:645–650. doi: 10.1007/s002280050687. [DOI] [PubMed] [Google Scholar]
- Asai N, Iwashita T, Matsuyama M, Takahashi M. Mechanism of activation of the ret proto-oncogene by multiple endocrine neoplasia 2A mutations. Mol Cell Biol. 1995;15:1613–1619. doi: 10.1128/mcb.15.3.1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barata JT, Silva A, Brandao JG, Nadler LM, Cardoso AA, Boussiotis VA. Activation of PI3K is indispensable for interleukin 7-mediated viability, proliferation, glucose use, and growth of T cell acute lymphoblastic leukemia cells. J Exp Med. 2004;200:659–669. doi: 10.1084/jem.20040789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgstrom L, Kagedal B, Paulsen O. Pharmacokinetics of N-acetylcysteine in man. Eur J Clin Pharmacol. 1986;31:217–222. doi: 10.1007/BF00606662. [DOI] [PubMed] [Google Scholar]
- Bose R, Kavuri SM, Searleman AC, Shen W, Shen D, Koboldt DC, Monsey J, Goel N, Aronson AB, Li S, Ma CX, Ding L, Mardis ER, Ellis MJ. Activating HER2 mutations in HER2 gene amplification negative breast cancer. Cancer Discov. 2013;3:224–237. doi: 10.1158/2159-8290.CD-12-0349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M, Bjorksten A, Medved I, McKenna M. Pharmacokinetics of intravenous N-acetylcysteine in men at rest and during exercise. Eur J Clin Pharmacol. 2004;60:717–723. doi: 10.1007/s00228-004-0862-9. [DOI] [PubMed] [Google Scholar]
- Cartwright T, Senussi O, Grady D. Reagents which inhibit disulphide bond formation stabilize human fibroblast interferon. J Gen Virol. 1977;36:323–327. doi: 10.1099/0022-1317-36-2-323. [DOI] [PubMed] [Google Scholar]
- Cassonnet P, Rolloy C, Neveu G, Vidalain PO, Chantier T, Pellet J, Jones L, Muller M, Demeret C, Gaud G, Vuillier F, Lotteau V, Tangy F, Favre M, Jacob Y. Benchmarking a luciferase complementation assay for detecting protein complexes. Nat Methods. 2011;8:990–992. doi: 10.1038/nmeth.1773. [DOI] [PubMed] [Google Scholar]
- Chen J, Reheman A, Gushiken FC, Nolasco L, Fu X, Moake JL, Ni H, Lopez JA. N-acetylcysteine reduces the size and activity of von Willebrand factor in human plasma and mice. J Clin Invest. 2011;121:593–603. doi: 10.1172/JCI41062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen N, Aleksa K, Woodland C, Rieder M, Koren G. Prevention of ifosfamide nephrotoxicity by N-acetylcysteine: clinical pharmacokinetic considerations. Can J Clin Pharmacol. 2007;14:e246–250. [PubMed] [Google Scholar]
- Dadi HK, Roifman CM. Activation of phosphatidylinositol-3 kinase by ligation of the interleukin-7 receptor on human thymocytes. J Clin Invest. 1993;92:1559–1563. doi: 10.1172/JCI116736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Caro L, Ghizzi A, Costa R, Longo A, Ventresca GP, Lodola E. Pharmacokinetics and bioavailability of oral acetylcysteine in healthy volunteers. Arzneimittelforschung. 1989;39:382–386. [PubMed] [Google Scholar]
- Experts in Chronic Myeloid Leukemia. The price of drugs for chronic myeloid leukemia (CML) is a reflection of the unsustainable prices of cancer drugs: from the perspective of a large group of CML experts. Blood. 2013;121:4439–4442. doi: 10.1182/blood-2013-03-490003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferner RE, Dear JW, Bateman DN. Management of paracetamol poisoning. BMJ. 2011;342:d2218. doi: 10.1136/bmj.d2218. [DOI] [PubMed] [Google Scholar]
- Flex E, Petrangeli V, Stella L, Chiaretti S, Hornakova T, Knoops L, Ariola C, Fodale V, Clappier E, Paoloni F, Martinelli S, Fragale A, Sanchez M, Tavolaro S, Messina M, Cazzaniga G, Camera A, Pizzolo G, Tornesello A, Vignetti M, Battistini A, Cave H, Gelb BD, Renauld JC, Biondi A, Constantinescu SN, Foa R, Tartaglia M. Somatically acquired JAK1 mutations in adult acute lymphoblastic leukemia. J Exp Med. 2008;205:751–758. doi: 10.1084/jem.20072182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertzberg L, Vendramini E, Ganmore I, Cazzaniga G, Schmitz M, Chalker J, Shiloh R, Iacobucci I, Shochat C, Zeligson S, Cario G, Stanulla M, Strehl S, Russell LJ, Harrison CJ, Bornhauser B, Yoda A, Rechavi G, Bercovich D, Borkhardt A, Kempski H, te Kronnie G, Bourquin JP, Domany E, Izraeli S. Down syndrome acute lymphoblastic leukemia, a highly heterogeneous disease in which aberrant expression of CRLF2 is associated with mutated JAK2: a report from the International BFM Study Group. Blood. 2010;115:1006–1017. doi: 10.1182/blood-2009-08-235408. [DOI] [PubMed] [Google Scholar]
- Higuchi M, Asao H, Tanaka N, Oda K, Takeshita T, Sugamura K. Regulation of IL-2 signaling. Leukemia. 1997;11(Suppl 3):416–417. [PubMed] [Google Scholar]
- Holdiness MR. Clinical pharmacokinetics of N-acetylcysteine. Clin Pharmacokinet. 1991;20:123–134. doi: 10.2165/00003088-199120020-00004. [DOI] [PubMed] [Google Scholar]
- Hong SY, Gil HW, Yang JO, Lee EY, Kim HK, Kim SH, Chung YH, Lee EM, Hwang SK. Effect of high-dose intravenous N-acetylcysteine on the concentration of plasma sulfur-containing amino acids. Korean J Intern Med. 2005;20:217–223. doi: 10.3904/kjim.2005.20.3.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igaz P, Toth S, Falus A. Biological and clinical significance of the JAK-STAT pathway; lessons from knockout mice. Inflamm Res. 2001;50:435–441. doi: 10.1007/PL00000267. [DOI] [PubMed] [Google Scholar]
- Jones AL, Jarvie DR, Simpson D, Hayes PC, Prescott LF. Pharmacokinetics of N-acetylcysteine are altered in patients with chronic liver disease. Aliment Pharmacol Ther. 1997;11:787–791. doi: 10.1046/j.1365-2036.1997.00209.x. [DOI] [PubMed] [Google Scholar]
- Kalman L, Lindegren ML, Kobrynski L, Vogt R, Hannon H, Howard JT, Buckley R. Mutations in genes required for T-cell development: IL7R, CD45, IL2RG, JAK3, RAG1, RAG2, ARTEMIS, and ADA and severe combined immunodeficiency: HuGE review. Genet Med. 2004;6:16–26. doi: 10.1097/01.GIM.0000105752.80592.A3. [DOI] [PubMed] [Google Scholar]
- Kontro M, Kuusanmaki H, Eldfors S, Pemovska T, Rajala H, Edgren H, Ellonen P, Lagstrom S, Lundan T, Kallioniemi O, Mustjoki S, Porkka K, Heckman C. Novel Activating STAT5B Mutations As Drivers Of T-ALL. Blood. 2013:122. [Google Scholar]
- McElroy CA, Dohm JA, Walsh ST. Structural and biophysical studies of the human IL-7/IL-7Ralpha complex. Structure. 2009;17:54–65. doi: 10.1016/j.str.2008.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElroy CA, Holland PJ, Zhao P, Lim JM, Wells L, Eisenstein E, Walsh ST. Structural reorganization of the interleukin-7 signaling complex. Proc Natl Acad Sci U S A. 2012;109:2503–2508. doi: 10.1073/pnas.1116582109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalfe C, Cresswell P, Ciaccia L, Thomas B, Barclay AN. Labile disulfide bonds are common at the leucocyte cell surface. Open Biol. 2011;1:110010. doi: 10.1098/rsob.110010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriggl R, Sexl V, Kenner L, Duntsch C, Stangl K, Gingras S, Hoffmeyer A, Bauer A, Piekorz R, Wang D, Bunting KD, Wagner EF, Sonneck K, Valent P, Ihle JN, Beug H. Stat5 tetramer formation is associated with leukemogenesis. Cancer Cell. 2005;7:87–99. doi: 10.1016/j.ccr.2004.12.010. [DOI] [PubMed] [Google Scholar]
- Neuwelt EA, Pagel MA, Kraemer DF, Peterson DR, Muldoon LL. Bone marrow chemoprotection without compromise of chemotherapy efficacy in a rat brain tumor model. J Pharmacol Exp Ther. 2004;309:594–599. doi: 10.1124/jpet.103.063347. [DOI] [PubMed] [Google Scholar]
- Nourjah P, Ahmad SR, Karwoski C, Willy M. Estimates of acetaminophen (Paracetomal)-associated overdoses in the United States. Pharmacoepidemiol Drug Saf. 2006;15:398–405. doi: 10.1002/pds.1191. [DOI] [PubMed] [Google Scholar]
- Olsson B, Johansson M, Gabrielsson J, Bolme P. Pharmacokinetics and bioavailability of reduced and oxidized N-acetylcysteine. Eur J Clin Pharmacol. 1988;34:77–82. doi: 10.1007/BF01061422. [DOI] [PubMed] [Google Scholar]
- Onishi M, Nosaka T, Misawa K, Mui AL, Gorman D, McMahon M, Miyajima A, Kitamura T. Identification and characterization of a constitutively active STAT5 mutant that promotes cell proliferation. Mol Cell Biol. 1998;18:3871–3879. doi: 10.1128/mcb.18.7.3871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson RG, Rumack BH. Treating acute acetaminophen poisoning with acetylcysteine. JAMA. 1977;237:2406–2407. [PubMed] [Google Scholar]
- Porcu M, Kleppe M, Gianfelici V, Geerdens E, De Keersmaecker K, Tartaglia M, Foa R, Soulier J, Cauwelier B, Uyttebroeck A, Macintyre E, Vandenberghe P, Asnafi V, Cools J. Mutation of the receptor tyrosine phosphatase PTPRC (CD45) in T-cell acute lymphoblastic leukemia. Blood. 2012;119:4476–4479. doi: 10.1182/blood-2011-09-379958. [DOI] [PubMed] [Google Scholar]
- Prescott LF, Donovan JW, Jarvie DR, Proudfoot AT. The disposition and kinetics of intravenous N-acetylcysteine in patients with paracetamol overdosage. Eur J Clin Pharmacol. 1989;37:501–506. doi: 10.1007/BF00558131. [DOI] [PubMed] [Google Scholar]
- Sanda T, Tyner JW, Gutierrez A, Ngo VN, Glover J, Chang BH, Yost A, Ma W, Fleischman AG, Zhou W, Yang Y, Kleppe M, Ahn Y, Tatarek J, Kelliher MA, Neuberg DS, Levine RL, Moriggl R, Muller M, Gray NS, Jamieson CH, Weng AP, Staudt LM, Druker BJ, Look AT. TYK2-STAT1-BCL2 Pathway Dependence in T-cell Acute Lymphoblastic Leukemia. Cancer Discov. 2013;3:564–577. doi: 10.1158/2159-8290.CD-12-0504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoro M, Carlomagno F, Romano A, Bottaro DP, Dathan NA, Grieco M, Fusco A, Vecchio G, Matoskova B, Kraus MH, et al. Activation of RET as a dominant transforming gene by germline mutations of MEN2A and MEN2B. Science. 1995;267:381–383. doi: 10.1126/science.7824936. [DOI] [PubMed] [Google Scholar]
- Sharfe N, Dadi HK, Roifman CM. JAK3 protein tyrosine kinase mediates interleukin-7-induced activation of phosphatidylinositol-3′ kinase. Blood. 1995;86:2077–2085. [PubMed] [Google Scholar]
- Shochat C, Tal N, Bandapalli OR, Palmi C, Ganmore I, te Kronnie G, Cario G, Cazzaniga G, Kulozik AE, Stanulla M, Schrappe M, Biondi A, Basso G, Bercovich D, Muckenthaler MU, Izraeli S. Gain-of-function mutations in interleukin-7 receptor-alpha (IL7R) in childhood acute lymphoblastic leukemias. J Exp Med. 2011;208:901–908. doi: 10.1084/jem.20110580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shochat C, Tal N, Gryshkova V, Birger Y, Bandapalli OR, Cazzaniga G, Gershman N, Kulozik AE, Biondi A, Mansour MR, Twizere JC, Muckenthaler MU, Ben-Tal N, Constantinescu SN, Bercovich D, Izraeli S. Novel activating mutations lacking cysteine in type I cytokine receptors in acute lymphoblastic leukemia. Blood. 2014;124:106–110. doi: 10.1182/blood-2013-10-529685. [DOI] [PubMed] [Google Scholar]
- Silva A, Girio A, Cebola I, Santos CI, Antunes F, Barata JT. Intracellular reactive oxygen species are essential for PI3K/Akt/mTOR-dependent IL-7-mediated viability of T-cell acute lymphoblastic leukemia cells. Leukemia. 2011;25:960–967. doi: 10.1038/leu.2011.56. [DOI] [PubMed] [Google Scholar]
- Smilkstein MJ, Knapp GL, Kulig KW, Rumack BH. Efficacy of oral N-acetylcysteine in the treatment of acetaminophen overdose. Analysis of the national multicenter study (1976 to 1985) N Engl J Med. 1988;319:1557–1562. doi: 10.1056/NEJM198812153192401. [DOI] [PubMed] [Google Scholar]
- Smilkstein MJ, Bronstein AC, Linden C, Augenstein WL, Kulig KW, Rumack BH. Acetaminophen overdose: a 48-hour intravenous N-acetylcysteine treatment protocol. Ann Emerg Med. 1991;20:1058–1063. doi: 10.1016/s0196-0644(05)81352-6. [DOI] [PubMed] [Google Scholar]
- White K, Bruckner JV, Guess WL. Toxicological studies of 2-mercaptoethanol. J Pharm Sci. 1973;62:237–241. doi: 10.1002/jps.2600620211. [DOI] [PubMed] [Google Scholar]
- Yoda A, Yoda Y, Chiaretti S, Bar-Natan M, Mani K, Rodig SJ, West N, Xiao Y, Brown JR, Mitsiades C, Sattler M, Kutok JL, DeAngelo DJ, Wadleigh M, Piciocchi A, Dal Cin P, Bradner JE, Griffin JD, Anderson KC, Stone RM, Ritz J, Foa R, Aster JC, Frank DA, Weinstock DM. Functional screening identifies CRLF2 in precursor B-cell acute lymphoblastic leukemia. Proc Natl Acad Sci U S A. 2010;107:252–257. doi: 10.1073/pnas.0911726107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenatti PP, Ribeiro D, Li W, Zuurbier L, Silva MC, Paganin M, Tritapoe J, Hixon JA, Silveira AB, Cardoso BA, Sarmento LM, Correia N, Toribio ML, Kobarg J, Horstmann M, Pieters R, Brandalise SR, Ferrando AA, Meijerink JP, Durum SK, Yunes JA, Barata JT. Oncogenic IL7R gain-of-function mutations in childhood T-cell acute lymphoblastic leukemia. Nat Genet. 2011;43:932–939. doi: 10.1038/ng.924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Ding L, Holmfeldt L, Wu G, Heatley SL, Payne-Turner D, Easton J, Chen X, Wang J, Rusch M, Lu C, Chen SC, Wei L, Collins-Underwood JR, Ma J, Roberts KG, Pounds SB, Ulyanov A, Becksfort J, Gupta P, Huether R, Kriwacki RW, Parker M, McGoldrick DJ, Zhao D, Alford D, Espy S, Bobba KC, Song G, Pei D, Cheng C, Roberts S, Barbato MI, Campana D, Coustan-Smith E, Shurtleff SA, Raimondi SC, Kleppe M, Cools J, Shimano KA, Hermiston ML, Doulatov S, Eppert K, Laurenti E, Notta F, Dick JE, Basso G, Hunger SP, Loh ML, Devidas M, Wood B, Winter S, Dunsmore KP, Fulton RS, Fulton LL, Hong X, Harris CC, Dooling DJ, Ochoa K, Johnson KJ, Obenauer JC, Evans WE, Pui CH, Naeve CW, Ley TJ, Mardis ER, Wilson RK, Downing JR, Mullighan CG. The genetic basis of early T-cell precursor acute lymphoblastic leukaemia. Nature. 2012;481:157–163. doi: 10.1038/nature10725. [DOI] [PMC free article] [PubMed] [Google Scholar]