Abstract
In mammals, circadian rhythms are essential for coordinating the timing of various metabolic processes. The Clock gene regulates diurnal plasma triglyceride fluctuation through nuclear receptor small heterodimer partner (Shp, Nr0b2). Given that SHP is a critical regulator of metabolism in the liver, it is unknown whether SHP is necessary to coordinate metabolism and circadian rhythms.
Methods
Shp+/+ and Shp−/− mice on a C57BL/6 background (n=3–5/group) were fed a standard chow diet and water ad libitum. Serum and livers were collected at zeitgeber time (ZT) 2, 6, 10, 14, 18 and 22. In vivo and in vitro assays include: RNA-sequencing (RNA-seq), qPCR, VLDL production, adenovirus overexpression and siRNA knockdown, serum parameters, circadian locomotor activity, oil-red O staining, transient transfection, luciferase reporter assay, ChIP assay, gel-shift assay, Co-IP, Western blots.
Results
Shp-deficiency had a robust global impact on major liver metabolic genes. Several components of the liver clock including Pgc-1α, Npas2 and Rorα/γ were sharply induced in Shp−/− liver. At the molecular level, SHP inhibited Npas2 gene transcription and promoter activity through interaction with Rorγ to repress Rorγ transactivation and by interacting with Rev-erbα to enhance its inhibition of Rorα activity. Conversely, Npas2 controlled the circadian rhythm of Shp expression by binding rhythmically to the Shp promoter, which was enhanced by NADH, but not NADPH. Phenotypically, Npas2-deficiency induced severe steatosis in Shp−/− mice, which was attributed to the dysregulation of lipoprotein metabolism.
Conclusion
Shp and Npas2 crosstalk is essential to maintain hepatic lipid homeostasis.
Keywords: nuclear receptor, gene regulation, circadian clock, metabolism, gene knockout
The primary mammalian circadian clock is located within the suprachiasmatic nucleus (SCN) of the anterior hypothalamus and is necessary for light entrainment of the sleep–wake cycle and locomotor activity (1). The core clock system is the end result of alternating actions of specific transcriptional activators and repressors (2). The positive transcriptional regulator, brain and muscle ARNT-like protein1 (BMAL1), forms a heterodimer with circadian locomotor output cycles kaput (CLOCK), or neuronal PAS domain protein 2 (NPAS2). These dimers activate many transcripts, most notably the period (Per1 and Per2) and cryptochrome (Cry1 and Cry2) genes, by binding to the E-box regulatory elements on their promoters (3). The translated PER and CRY proteins then heterodimerize and repress Clock/Bmal1 or Npas2/Bmal1 transcription (4). Additionally, a secondary feedback loop consisting of nuclear hormone receptors adds another level of control to the transcriptional output of the primary loop (5).
Endogenous autonomous circadian clocks exist in various peripheral tissues (6). Multiple local mediators of both core clock genes and clock-controlled rhythmic transcripts respond to stimuli originating from the SCN as well as local input signals related to metabolic states (7). Rev-erbα was initially identified as a clock controlling and clock-regulated gene (8), which has crucial regulatory functions in hepatic metabolism (9). Retinoic acid-related orphan nuclear receptor α/γ (RORα/γ) competes with REV-ERBα to bind the ROR element of the Bmal1 promoter and activate its transcription (10). RORγ directly regulates Npas2 transcription by binding two ROREs in its proximal promoter (11) and plays an important role in glucose and lipid metabolism (12). Peroxisome proliferator-activated receptor alpha (PPARα) binds to the Bmal1 promoter and regulates its expression, while the CLOCK/BMAL1 heterodimer in turn regulates Pparα, generating a positive feedback loop (13). The PPARα coactivator-1α (PGC-1α) activates the expression of Bmal1 and Rev-erbα through co-activation of RORs (14), is a part of the SIRT1 histone deacetylase complex, and may directly sense the cellular metabolic state.
Although Npas2 and Clock display overlapping functions (15, 16), Npas2-deficient mice show particular impairment in their adaptability to food restriction (17). The circadian rhythm of Npas2 transcription is in phase with that of Bmal1, strongly indicating a joint mechanism for efficient activation of target genes (18). Both NADH and NADPH enhance the DNA-binding activity of the NPAS2/BMAL1 heterodimer, suggesting that the redox state regulates molecular clock activity (19).
Small heterodimer partner (Shp, Nr0b2) functions as a transcriptional repressor of genes critical to hepatic metabolism (20–25). The circadian regulation of triglyceride metabolism by the Clock gene is mediated by Shp (22). However, the role of Shp in controlling the rhythmicity of metabolites and liver clock machinery remains elusive. In this study, we employed transcriptomics analysis, which identified Shp as an integral component of the liver circadian network through crosstalk with Npas2, Rorα, Rorγ, Rev-erbα, and Pgc-1α.
Materials and Methods
Mice
Shp+/+ (C57BL/6J, WT) and Shp−/− (C57BL/6J, SKO), SHP non-transgenic control (NC) and hepatocyte specific SHP transgenic (STG) mice were described previously (20, 25, 26). Mice were fed a standard rodent chow (Harlan No. 2020X) with free access to water and maintained in a 12h/12h light/dark (LD) cycle (light on 6 AM to 6 PM), temperature-controlled (23°C), and virus-free facility. Experiments on mice were performed on males at the age of 8 weeks unless stated otherwise. Hepatocyte isolation was performed as described (27). Protocols for animal use were approved by the Institutional Animal Care and Use Committee at the University of Utah.
In vivo and in vitro Studies
Serum and liver tissues were harvested at ZT2, ZT6, ZT10, ZT14, ZT18, and ZT22. A dim red light at intensity of 1 μmol/m2s was used to collect tissues in dark condition (28). For in vivo adenoviral transduction, male mice were injected via tail vein with purified adenoviruses at 1×1011 virus particles per mouse. Gene expression analysis were performed 3 days or 14 days after tail vein injection. Standard methods were used for transient transfection, luciferase reporter assay, ChIP assay, gel-shift assay, Co-IP, and Western blots (27, 29). Total and 5′ capped RNA purification from mouse liver and the PCR libraries used for RNA sequencing were as previously described (30). Detailed methods for histological analysis of liver sections can be found in our previous publication (20, 27).
Statistics Analysis
All the experiments were done in triplicate and repeated at least three times. The data are presented as the mean values ± standard error of the mean (SEM). Statistical analysis was carried out using Student’s t test for unpaired data to compare the values between the two groups; P < .05 was considered statistically significant.
RESULTS
Cyclic Patterns of Liver Metabolic Genes Were Drastically Altered in Shp−/− Mice
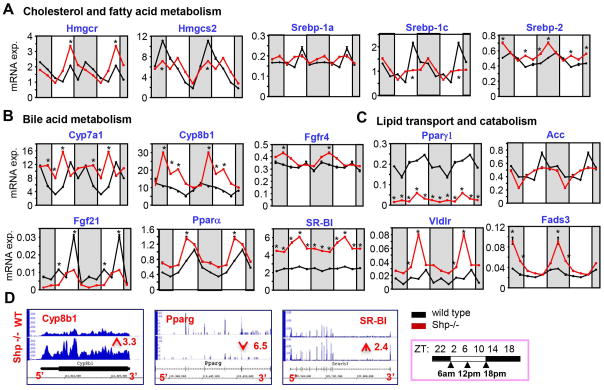
Transcriptomics (RNA-seq) and qPCR analysis of mRNA of key genes involved in cholesterol, fatty acid, bile acid, and lipid metabolism in livers of wild-type (WT) and Shp−/− mice collected over a 12:12 hr light/dark (LD) cycle showed drastic disruption of cyclic patterns (rhythmicity or amplification) in Shp−/− mice. The peak level of HMG-CoA reductase (Hmgcr) was increased, whereas there was a decreased amplification of mitochondrial HMG-CoA synthase2 (Hmgcs2) (Figure 1A). The sterol regulatory element binding protein (SREBP) family member SREBP-1c’s peak expression was decreased, whereas Srebp-2 was largely increased in Shp−/− mice. Consistent with our previous report (21, 25), cholesterol 7 alpha-hydroxylase (Cyp7a1) and sterol 12 alpha-hydroxylase (Cyp8b1), the critical enzymes in bile acid biosynthesis, showed a shift in phase or exhibited overly increased rhythmic expression in Shp−/− mice (Figure 1B). Despite lacking obvious rhythmicity in WT mice, FGF receptor Fgfr4 displayed a noticeable cyclic induction in Shp−/− mice similar to Cyp8b1.
Figure 1. Shp-Deficiency Drastically Alters the Rhythmicity of Liver Metabolic Genes.
(AC) qPCR analysis of the circadian rhythmic expression of genes involved in cholesterol and fatty acid synthesis, bile acid and lipid metabolism in wild-type and Shp−/− mice across the assayed time points. (D) Integrated genome browser visualization of RNA-Seq read coverage for Cyp8b1, Pparγ, and Sr-bI in wild-type and Shp−/− mice. Data are mean ± s.d. from n=5 mice for each time point.
Expression of many other lipid metabolic genes also showed distinct changes (Figure 1C). Pparγ1 (lipid uptake) and fibroblast growth factor 21 (Fgf21) were drastically downregulated in Shp−/− mice, whereas acetyl-CoA carboxylase (Acc, provides malonyl-CoA for FA synthesis) showed a moderate decrease in expression. In contrast, the rhythmic expression of Pparα (FA oxidation), scavenger receptor class B member 1 (Sr-b1, uptake of cholesteryl ester in reverse cholesterol transport), and very low density lipoprotein receptor (Vldlr, cholesterol uptake) was markedly upregulated in Shp−/− mice. Fatty acid desaturase 3 (Fads3) with relatively unknown function also displayed substantial elevation by Shp-deficiency. The up- and down-regulation of selected genes (Cyp8b1, Sr-b1 and Pparγ1) was further confirmed by RNA-seq in livers collected at ZT6 (Figure 1D).
SHP Inhibited Npas2 Expression via Crosstalk with the RORα, γ/REV-ERBα Network
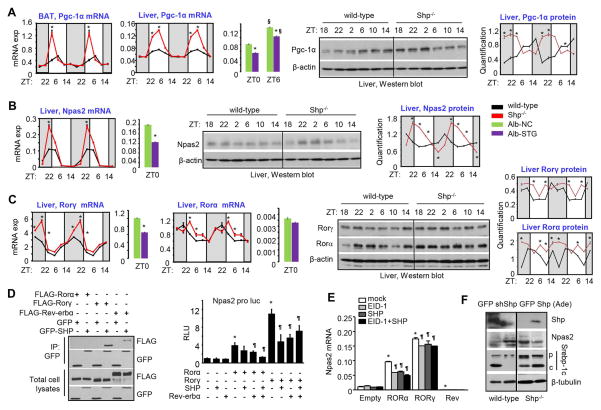
We analyzed core clock genes and nuclear receptors in the interlocking feedback loop (Figure S1). Consistent with SHP as a repressor of Pgc-1α in brown fat (BAT) (24), the amplification of Pgc-1α mRNA was highly induced in BAT of Shp−/− mice at ZT2 (Figure 2A, 1st panel). A striking increase in peak Pgc-1α mRNA was also observed in Shp−/− liver (2nd panel), which was decreased in Alb-Shp-transgenic (STG) liver (3rd panel). This was accompanied by a shift in the circadian phase of its protein expression (4th & 5th panels).
Figure 2. SHP Inhibits Npas2 Expression via Crosstalk with the Rorα, γ/Rev-ERBα Network.
(A) Left: qPCR analysis of circadian rhythmic expression of Pgc-1α in brown adipose tissue (1st panel) and liver (2nd panel) from wild-type and Shp−/− mice, and in liver of hepatocyte specific Shp transgenic (Alb-STG) and non-transgenic control mice (Alb-NC) (3rd panel). * P < .01 vs. corresponding control. § P < .01 vs. ZT0. Right: Western blot (4th panel) and quantitative analysis (5th panel) of Pgc-1α protein from liver extracts in wild-type and Shp−/− mice. (B) Left: qPCR analysis of circadian rhythmic expression of Npas2 in liver of wild-type and Shp−/− mice (1st panel) and in liver of Alb-NC and Alb-STG at ZT0 (2nd panel). * P < .01 vs. control. Right: Western blot (3rd panel) and quantitative analysis (4th panel) of Npas2 protein in liver of wild-type and Shp−/− mice. (C) Left 4: qPCR analysis of Rorα and Rorγ in in liver of wild-type and Shp−/− mice, and in liver of Alb-NC and Alb-STG at ZT0. Right 3: Western blot and quantitative analysis of Rorα and Rorγ protein in liver of wild-type and Shp−/− mice. Data are mean ± s.d. from n=5 mice for each time point. (D) Left: Co-immunoprecipitation (IP) followed by Western blot to determine proteins interaction of Shp with Rorα, Rorγ, or Rev-erbα. HEK293T cells were transfected with expression plasmids and cultured for 48 hr. Right: Luciferase reporter assay in HEK293T cells transfected with Npas2-Luc and expression plasmids for Rorα, Rorγ, Shp and Rev-erbα alone or in combination. (E) qPCR analysis of Npas2 expression in stable Rorα, Rorγ, or Rev-erbα Hepa1-6 cells transiently transfected with Shp and Eid1 alone or in combination. * P < .01 vs. control cell line. ¶ P < .01 vs. mock transfection. (F) Western blot of hepatic protein in WT mice subjected with Shp knockdown or Shp−/− mice subjected with Shp overexpression by adenovirus through tail vein injection.
Among all the core clock genes we analyzed (not shown), Npas2 mRNA was strongly upregulated in Shp−/− liver and downregulated in Alb-STG liver (Figure 2B, 1st & 2nd panels), as was its protein (3rd & 4th panels), suggesting a direct inhibition by SHP. Interestingly, Rorγ mRNA had an almost identical expression pattern as Npas2 mRNA in Shp−/− liver, while Rorα mRNA expression showed a shift in phase (Figure 2C, left 4 panels). As expected, both RORγ and RORα proteins were elevated by Shp-deficiency (right 3 panels).
Because Npas2 was activated by RORα and RORγ but repressed by REV-ERBα (10), we reasoned that SHP might inhibit Npas2 transcription by binding to RORα, RORγ, or REV-ERBα. SHP interacted with RORγ and REV-ERBα but not with RORα protein (Figure 2D, left), and inhibited the activation of the Npas2 promoter (11) by RORγ with little effect on RORα-mediated activation (right). However, co-expression of SHP with REV-ERBα further inhibited RORα activity, suggesting that SHP acts as a co-repressor of REV-ERBα. SHP overexpression also repressed the induction of Npas2 mRNA by RORα and RORγ in Hepa-1 cells stably expressing either protein (Figure 2E). No synergistic inhibition of Npas2 by SHP and its co-repressor EID-1 was observed. In vivo knockdown of Shp in WT liver by shShp-ade increased NPAS2 protein, whereas Shp-ade reduced NAPS2 protein in Shp−/− liver (Figure 2F). Surprisingly, despite a higher basal level of SREBP-1c precursor (p) protein, the level of cleaved SREBP-1c protein (c) was much lower in Shp−/− liver versus WT liver. SREBP-1c cleavage was largely impaired by Shp knockdown (shShp-ade) in WT liver but was markedly enhanced by Shp overexpression (Shp-ade) in Shp−/− liver. These results suggest that SHP is a bona-fide transcriptional repressor of Npas2 through crosstalk with RORα,γ and REV-ERBα (Figure S2).
NPAS2 Activated Shp Gene Expression in a Feedback Regulatory Loop
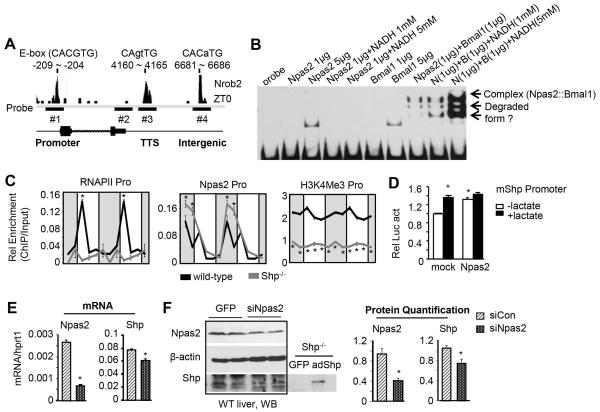
A recent ChIP-seq analysis (31) revealed an oscillatory recruitment of RNA pol II, NPAS2 and BMAL1 protein to the Shp promoter, transcription termination site (TTS) or intergenic region (Figure S3). Of note, the circadian rhythmicity of NPAS2 binding imitated the rhythmic expression of Shp, suggesting that NPAS2 may activate Shp transcription.
A canonical E-Box (CACGTG), a binding site for BMAL1, NPAS2, and CLOCK (32), was present in the Shp promoter, and non-canonical E-box sequences were found around NPAS2 binding peak in TTS and intergenic region (Figure 3A). We designed three probes (#1-promoter, #3-TTS, #4-intergenic) and one negative control probe (#2) for gel-shift assays using purified His-Npas2 or His-Bmal1 protein (Figure S4). NPAS2 or BMAL1 protein alone at a higher concentration (5 μg), or NPAS2 and BMAL1 heterodimer (4) at a lower concentration (1 μg) all bound to the promoter probe; the latter was dose-dependently enhanced by NADH(19) (Figure 2B) but not by NADPH (Figure S5). Unfortunately, we could not confirm NPAS2 or BMAL1 binding to Shp TSS and intergenic region (not shown).
Figure 3. NPAS2 Activates Shp Gene Expression in a Feedback Regulatory Loop.
(A) ChIP-seq signal showing binding of Npas2 to the E-box sequence (CANNTG) on Shp gene at ZT0 and the location of probes designed for gel-shift assay. Probe #1: the Shp promoter. Probe #2: 3′-untranlated region and worked as a negative control. Probe #3: transcription termination site (TTS). Probe #4: intergenic region. (B) NPAS2 and BMAL1 proteins were subjected to gel-shift assays with probe #1 in the presence of varying amounts of NADH. (C) ChIP assay and qPCR to determine the relative enrichment of RNAPII, NPAS2, and H3K4ME3 to the promoter region on Shp gene in the liver of wild-type and Shp−/− mice. (D) Luciferase reporter assay to determine the transactivation of Npas2 on the Shp promoter in the presence of lactate. *P < .01 vs. mock control. (E-F) qPCR (E) and Western blot (F) of Npas2 and Shp expression in the liver of wild-type mice subjected with adenovirus siRNA targeting Npas2 (ZT2). *P < .01 vs. siRNA Control.
We next designed ChIP assay primers (Figure S6) and validated the specificity of antibodies against RNAPII, NPAS2 and H3K4M3 (Figure S7); the latter served as an active histone modification marker (33, 34). Several surprising results were observed. RNAPII displayed a profound rhythmic binding to the Shp promoter of WT liver, which was blunted in Shp−/− liver (Figure 3C, left). The recruitment of NPAS2 appeared as a “dual-peak” oscillation in WT liver similar to Shp expression (Figure S3), which was enhanced in Shp−/− liver (middle). The binding of H3K4Me3 to the Shp promoter was less rhythmic in WT liver; however, its enrichment was abolished over the LD cycle in Shp−/− liver (right). In addition, treatment of cells with lactate to modulate intracellular NADH levels (19) enhanced Shp promoter reporter activity (Figure 3D), but not the activity of reporter containing TTS or intergenic region (Figure S8, bottom left). However, liver Shp mRNA (Figure 3E) and protein (Figure 3F) expression was only moderately decreased by siNpas2, suggesting that additional factors may be involved in NPAS2 mediated Shp activation in vivo. Overall, the interplay between NPAS2 and Shp represents a new component of NPAS2 signaling that is likely to dictate NPAS2 activity and function.
Knockdown of Npas2 in Shp−/− Liver Induced Steatosis by Impeding Lipoprotein Homeostasis
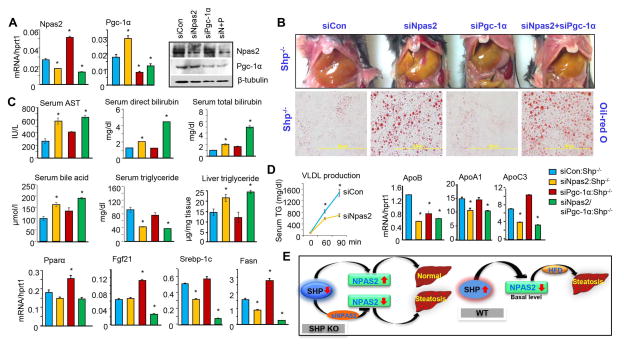
Shp−/− mice are protected against the development of fatty liver (20, 24). The strong induction of Npas2 and Pgc-1α in Shp−/− liver prompted us to examine their potential involvement in the regulation of steatosis under Shp-deficient condition. Both Npas2 and Pgc-1α remained highly induced in Shp−/− liver at ZT2 (Figure 2B), thus ZT2 was chosen as the time point to knockdown both genes. The siNpas2-ade that we generated had a 30~40% efficiency in decreasing hepatic Npas2 mRNA, whereas siPgc-1α-ade (35) resulted in a 50~60% reduction of Pgc-1α mRNA in Shp−/− liver (Figure 4A, left and middle). The protein expression of NPAS2 and PGC-1α was similarly diminished by their respective siRNAs (right). Intriguingly, Npas2 mRNA was highly induced by siPgc-1α, and Pgc-1α mRNA was markedly elevated by siNpas2. An induction of Pgc-1α was also observed in Npas2−/− liver (36), while PGC-1α suppressed Bmal1 expression by activating Rev-erbα (37). Thus, NPAS2 and PGC-1α exhibit mutual inhibition of mRNA expression.
Figure 4. Npas2-Deficiency Sensitizes Shp−/− Mice to Steatosis.
(A) qPCR and Western blot analysis of Npas2 and Pgc-1a mRNA (left) and protein (right) in liver of Shp−/− mice subjected with adenovirus siRNAs targeting Npas2 or Pgc-1α, alone or in combination (ZT2). Liver tissues were harvested 4 days post adenovirus tail vein injection. Non-targeting siRNA adenovirus was included as control. Data are mean ± s.d. from n=5 mice for each group. * P < .01 vs. control. (B) Histological analysis of liver steatosis in Shp−/− mice subjected with adenovirus siNpas2 and siPgc-1α. Top, gross morphology of liver. Bottom, oil-red O staining of liver sections. (C) Serum parameter analysis and measurement of serum and liver triglyceride levels in Shp−/− mice subjected with adenovirus siRNAs. (D) Left: Measurement of VLDL production. Mice were subjected with adenovirus siRNAs for 7 days followed by overnight-fasting, intravenous injection with tyloxapol, and serum TG levels were examined. Right 7 panels: qPCR analysis of genes involved in lipoprotein and lipid metabolism in liver of Shp−/− mice subjected with adenovirus siRNAs. (E) Proposed regulatory model that elucidates a role of Npas2 in Shp-mediated steatosis.
siNpas2 triggered severe steatosis as revealed by oil-red O staining of neutral lipid in Shp−/− liver, which was not observed with siPgc-1α alone (Figure 4B). Serum AST, bilirubin, and bile acid levels were elevated in siNpas2:Shp−/− mice, which was somewhat exacerbated by a combinational effect of siNpas2 and siPgc-1α in siNpas2/siPgc-1α:Shp−/− mice (Figure 4C). It was noted that serum TG levels were decreased whereas liver TG contents were increased in siNpas2:Shp−/− or siNpas2/siPgc-1α:Shp−/− mice, suggesting a potential disruption of lipid metabolism by siNpas2. On the other hand, siPgc-1α had little effect on TG levels.
VLDL secretion was markedly obstructed by siNpas2 in Shp−/− mice (Figure 4D, 1st panel), which correlated with reduced expression of ApoB, an activator of VLDL secretion (2nd panel). Apolipoprotein A-I (APOA1) is the major protein component of high density lipoprotein (HDL) in plasma, and APOC3 inhibits hepatic uptake of triglyceride-rich particles (38). Both genes were downregulated by siNpas2 (3rd & 4th panels). Unfortunately, ChIP-seq did not detect NPAS2 binding to the ApoB gene (31), and we could not observe NPAS2 and/or BMAL1 activation of the ApoB promoter (not shown). Therefore, ApoB is unlikely a direct NPAS2 target.
On the other hand, the mRNA levels of Pparα and its target Fgf21 (39) were not affected by siNpas2 but were induced by siPgc-1α (5th & 6th panels), suggesting that the PPARα-mediated increase in fatty acid oxidation may play a role in protecting siPgc-1α:Shp−/− mice from developing steatosis compared to siNpas2:Shp−/− mice. The severe steatosis caused by siNpas2 may in turn have inhibited the expression of Srebp-1c and Fasn (7th & 8th panels) to diminish lipid synthesis. The increased Fasn expression by siPgc-1α was in agreement with its induction in Pgc-1α−/− mice (40).
Unfortunately, siNpas2 did not show desirable knockdown efficiency in WT liver (not shown), which may be due to the low basal level of Npas2 in WT relative to Shp−/− liver (Figure 2C). Overall, our results suggest a regulatory model (Figure 4E): 1) Shp−/− mice are resistant to the development of fatty liver (20, 24), which is in part associated with upregulation of Npas2, because Npas2 knockdown in Shp−/− mice reversed this phenotype; 2) WT mice are sensitive to HFD-induced fatty liver, which is associated with high Shp and low Npas2 expression.
DISCUSSION
Shp Is an Integral Component in the Liver Circadian Clock Network
The reversal of SHP-mediated inhibition of expression of Hmgcr (41), Cyp7a1 (25, 42), and Cyp8b1 (43) in Shp−/− mice is consistent with SHP’s known role as a transcriptional repressor. Although Srebp-1c promoter activity was inhibited by Shp (44), decreased Srebp-1c mRNA and increased precursor protein was observed in Shp−/− mice. More surprisingly, Shp overexpression enhanced SREBP-1c cleavage to generate its mature form; the latter is responsible for stimulating lipogenesis. The results suggest a potential new mechanism for post-translational regulation of SREBP-1c protein by Shp. Importantly, most of the metabolic genes analyzed exhibited an oscillatory pattern of expression, consistent with the notion that circadian rhythms and cellular metabolism are intimately linked (45). The overall gene expression profile altered by Shp-deficiency favors a lipid lowering phenotype, suggesting that SHP mainly serves as a modulator of metabolic homeostasis.
At the molecular level, we revealed a feedback regulatory loop between Npas2 and Shp. SHP inhibits Npas2 transcription by repressing Rorγ transactivation of the Npas2 promoter or by enhancing Rev-erbα inhibition. NPAS2 activates Shp gene expression by binding rhythmically to the Shp promoter, complementing an additional layer of control of Shp rhythmic expression by CLOCK (46). Overall, our findings suggest that SHP functions as an integral component of the liver circadian clock network by interfacing with RORα, RORγ, or REV-ERBα pathways to modulate the regulation and function of Npas2.
The Interplay between SHP and NPAS2 Maintains Triglyceride and Lipoprotein Homeostasis
We discovered a novel interplay between NPAS2 and SHP to maintain triglyceride and lipoprotein homeostasis (Figure S9). Shp−/− mice were protected against fatty liver (24) at least in part due to increased VLDL secretion (20). This phenotype was reversed by knockdown of Npas2, which caused severe steatosis and impaired VLDL production. Concurrently, the expression of numerous genes in lipoprotein metabolism was downregulated by siNpas2. A recent study showed that the primary dysregulated pathways in Npas2−/− mice uniformly converged on lipid metabolism (36). Importantly, dysregulation of NPAS2 was reported in alcohol-induced hepatic steatosis (47). Additional clinical studies also linked genomic variants of NPAS2 to the risk factors of metabolic syndrome (29). Taken together, findings from other groups and ours highlight the importance of NPAS2 in maintaining circadian rhythm mediated lipid homeostasis. Further investigation is needed to explore the role of NPAS2 in human NAFLD and AFL. It is postulated that modulating Npas2 function may open new avenues for therapeutic intervention of fatty liver disease.
In conclusion, our study reinforces the notion that SHP serves as a molecular switch that synchronizes metabolic functions to the liver circadian timing cues through a multiple regulatory modes.
Supplementary Material
Acknowledgments
Financial Support
L.W. is supported by NIH DK080440, AHA 13GRNT14700043, VA Merit Award 1I01BX002634, P30 DK020579 by the Diabetes Research Center at Washington University, and P30 CA042014 from Huntsman Cancer Institute. S.L. is supported by AHA Postdoctoral fellowship 13POST14630070. H.T. is supported by Manpei Suzuki Diabetes Foundation and Mochida Memorial Foundation for Medical and Pharmaceutical Research. R.S. is supported by AHA Predoctoral fellowship 14PRE17930013.
We thank the Microarray and Genomic Analysis Core for RNA-seq analysis.
Footnotes
AUTHOR CONTRIBUTIONS
S.L, Y.Z, H.T, and R.S performed experiments and prepared the manuscript. S.Y helped with mouse circadian behavior study. A.M.J contributed reagents. L.W. conceived and supervised the study, and wrote the manuscript.
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
References
- 1.Saper CB, Scammell TE, Lu J. Hypothalamic regulation of sleep and circadian rhythms. Nature. 2005;437:1257–1263. doi: 10.1038/nature04284. [DOI] [PubMed] [Google Scholar]
- 2.Gachon F, Nagoshi E, Brown SA, Ripperger J, Schibler U. The mammalian circadian timing system: from gene expression to physiology. Chromosoma. 2004;113:103–112. doi: 10.1007/s00412-004-0296-2. [DOI] [PubMed] [Google Scholar]
- 3.Rey G, Reddy AB. Connecting cellular metabolism to circadian clocks. Trends Cell Biol. 2013;23:234–241. doi: 10.1016/j.tcb.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 4.Ko CH, Takahashi JS. Molecular components of the mammalian circadian clock. Hum Mol Genet. 2006;15(Spec No 2):R271–277. doi: 10.1093/hmg/ddl207. [DOI] [PubMed] [Google Scholar]
- 5.Duez H, Staels B. The nuclear receptors Rev-erbs and RORs integrate circadian rhythms and metabolism. Diab Vasc Dis Res. 2008;5:82–88. doi: 10.3132/dvdr.2008.0014. [DOI] [PubMed] [Google Scholar]
- 6.Storch KF, Lipan O, Leykin I, Viswanathan N, Davis FC, Wong WH, Weitz CJ. Extensive and divergent circadian gene expression in liver and heart. Nature. 2002;417:78–83. doi: 10.1038/nature744. [DOI] [PubMed] [Google Scholar]
- 7.Yang X, Downes M, Yu RT, Bookout AL, He W, Straume M, Mangelsdorf DJ, et al. Nuclear receptor expression links the circadian clock to metabolism. Cell. 2006;126:801–810. doi: 10.1016/j.cell.2006.06.050. [DOI] [PubMed] [Google Scholar]
- 8.Preitner N, Damiola F, Lopez-Molina L, Zakany J, Duboule D, Albrecht U, Schibler U. The orphan nuclear receptor REV-ERBalpha controls circadian transcription within the positive limb of the mammalian circadian oscillator. Cell. 2002;110:251–260. doi: 10.1016/s0092-8674(02)00825-5. [DOI] [PubMed] [Google Scholar]
- 9.Yin L, Wu N, Curtin JC, Qatanani M, Szwergold NR, Reid RA, Waitt GM, et al. Rev-erbalpha, a heme sensor that coordinates metabolic and circadian pathways. Science. 2007;318:1786–1789. doi: 10.1126/science.1150179. [DOI] [PubMed] [Google Scholar]
- 10.Takeda Y, Jothi R, Birault V, Jetten AM. RORgamma directly regulates the circadian expression of clock genes and downstream targets in vivo. Nucleic Acids Res. 2012;40:8519–8535. doi: 10.1093/nar/gks630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Takeda Y, Kang HS, Angers M, Jetten AM. Retinoic acid-related orphan receptor gamma directly regulates neuronal PAS domain protein 2 transcription in vivo. Nucleic Acids Res. 2011;39:4769–4782. doi: 10.1093/nar/gkq1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burris TP, Busby SA, Griffin PR. Targeting orphan nuclear receptors for treatment of metabolic diseases and autoimmunity. Chem Biol. 2012;19:51–59. doi: 10.1016/j.chembiol.2011.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Canaple L, Rambaud J, Dkhissi-Benyahya O, Rayet B, Tan NS, Michalik L, Delaunay F, et al. Reciprocal regulation of brain and muscle Arnt-like protein 1 and peroxisome proliferator-activated receptor alpha defines a novel positive feedback loop in the rodent liver circadian clock. Mol Endocrinol. 2006;20:1715–1727. doi: 10.1210/me.2006-0052. [DOI] [PubMed] [Google Scholar]
- 14.Liu C, Li S, Liu T, Borjigin J, Lin JD. Transcriptional coactivator PGC-1alpha integrates the mammalian clock and energy metabolism. Nature. 2007;447:477–481. doi: 10.1038/nature05767. [DOI] [PubMed] [Google Scholar]
- 15.Reick M, Garcia JA, Dudley C, McKnight SL. NPAS2: an analog of clock operative in the mammalian forebrain. Science. 2001;293:506–509. doi: 10.1126/science.1060699. [DOI] [PubMed] [Google Scholar]
- 16.Rudic RD, Curtis AM, Cheng Y, FitzGerald G. Peripheral clocks and the regulation of cardiovascular and metabolic function. Methods Enzymol. 2005;393:524–539. doi: 10.1016/S0076-6879(05)93027-9. [DOI] [PubMed] [Google Scholar]
- 17.Dudley CA, Erbel-Sieler C, Estill SJ, Reick M, Franken P, Pitts S, McKnight SL. Altered patterns of sleep and behavioral adaptability in NPAS2-deficient mice. Science. 2003;301:379–383. doi: 10.1126/science.1082795. [DOI] [PubMed] [Google Scholar]
- 18.Yamamoto T, Nakahata Y, Soma H, Akashi M, Mamine T, Takumi T. Transcriptional oscillation of canonical clock genes in mouse peripheral tissues. BMC Mol Biol. 2004;5:18. doi: 10.1186/1471-2199-5-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rutter J, Reick M, Wu LC, McKnight SL. Regulation of clock and NPAS2 DNA binding by the redox state of NAD cofactors. Science. 2001;293:510–514. doi: 10.1126/science.1060698. [DOI] [PubMed] [Google Scholar]
- 20.Huang J, Iqbal J, Saha PK, Liu J, Chan L, Hussain MM, Moore DD, et al. Molecular characterization of the role of orphan receptor small heterodimer partner in development of fatty liver. Hepatology. 2007;46:147–157. doi: 10.1002/hep.21632. [DOI] [PubMed] [Google Scholar]
- 21.Zhang Y, Hagedorn CH, Wang L. Role of nuclear receptor SHP in metabolism and cancer. Biochim Biophys Acta. 2011;1812:893–908. doi: 10.1016/j.bbadis.2010.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pan X, Zhang Y, Wang L, Hussain MM. Diurnal regulation of MTP and plasma triglyceride by CLOCK is mediated by SHP. Cell Metab. 2010;12:174–186. doi: 10.1016/j.cmet.2010.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tabbi-Anneni I, Cooksey R, Gunda V, Liu S, Mueller A, Song G, McClain DA, et al. Overexpression of nuclear receptor SHP in adipose tissues affects diet-induced obesity and adaptive thermogenesis. Am J Physiol Endocrinol Metab. 2010;298:E961–970. doi: 10.1152/ajpendo.00655.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang L, Liu J, Saha P, Huang J, Chan L, Spiegelman B, Moore DD. The orphan nuclear receptor SHP regulates PGC-1alpha expression and energy production in brown adipocytes. Cell Metab. 2005;2:227–238. doi: 10.1016/j.cmet.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 25.Wang L, Lee YK, Bundman D, Han Y, Thevananther S, Kim CS, Chua SS, et al. Redundant pathways for negative feedback regulation of bile acid production. Dev Cell. 2002;2:721–731. doi: 10.1016/s1534-5807(02)00187-9. [DOI] [PubMed] [Google Scholar]
- 26.Zhang Y, Xu P, Park K, Choi Y, Moore DD, Wang L. Orphan receptor small heterodimer partner suppresses tumorigenesis by modulating cyclin D1 expression and cellular proliferation. Hepatology. 2008;48:289–298. doi: 10.1002/hep.22342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang Y, Soto J, Park K, Viswanath G, Kuwada S, Abel ED, Wang L. Nuclear receptor SHP, a death receptor that targets mitochondria, induces apoptosis and inhibits tumor growth. Mol Cell Biol. 2010;30:1341–1356. doi: 10.1128/MCB.01076-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shi S, Hida A, McGuinness OP, Wasserman DH, Yamazaki S, Johnson CH. Circadian clock gene Bmal1 is not essential; functional replacement with its paralog, Bmal2. Curr Biol. 2010;20:316–321. doi: 10.1016/j.cub.2009.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Englund A, Kovanen L, Saarikoski ST, Haukka J, Reunanen A, Aromaa A, Lonnqvist J, et al. NPAS2 and PER2 are linked to risk factors of the metabolic syndrome. J Circadian Rhythms. 2009;7:5. doi: 10.1186/1740-3391-7-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smalling RL, Delker DA, Zhang Y, Nieto N, McGuiness MS, Liu S, Friedman SL, et al. Genome-wide transcriptome analysis identifies novel gene signatures implicated in human chronic liver disease. Am J Physiol Gastrointest Liver Physiol. 305:G364–374. doi: 10.1152/ajpgi.00077.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koike N, Yoo SH, Huang HC, Kumar V, Lee C, Kim TK, Takahashi JS. Transcriptional architecture and chromatin landscape of the core circadian clock in mammals. Science. 2012;338:349–354. doi: 10.1126/science.1226339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ripperger JA, Schibler U. Rhythmic CLOCK-BMAL1 binding to multiple E-box motifs drives circadian Dbp transcription and chromatin transitions. Nat Genet. 2006;38:369–374. doi: 10.1038/ng1738. [DOI] [PubMed] [Google Scholar]
- 33.Bernstein BE, Kamal M, Lindblad-Toh K, Bekiranov S, Bailey DK, Huebert DJ, McMahon S, et al. Genomic maps and comparative analysis of histone modifications in human and mouse. Cell. 2005;120:169–181. doi: 10.1016/j.cell.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 34.Heintzman ND, Stuart RK, Hon G, Fu Y, Ching CW, Hawkins RD, Barrera LO, et al. Distinct and predictive chromatin signatures of transcriptional promoters and enhancers in the human genome. Nat Genet. 2007;39:311–318. doi: 10.1038/ng1966. [DOI] [PubMed] [Google Scholar]
- 35.Koo SH, Satoh H, Herzig S, Lee CH, Hedrick S, Kulkarni R, Evans RM, et al. PGC-1 promotes insulin resistance in liver through PPAR-alpha-dependent induction of TRB-3. Nat Med. 2004;10:530–534. doi: 10.1038/nm1044. [DOI] [PubMed] [Google Scholar]
- 36.O’Neil D, Mendez-Figueroa H, Mistretta TA, Su C, Lane RH, Aagaard KM. Dysregulation of Npas2 leads to altered metabolic pathways in a murine knockout model. Mol Genet Metab. 2013;110:378–387. doi: 10.1016/j.ymgme.2013.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Estall JL, Ruas JL, Choi CS, Laznik D, Badman M, Maratos-Flier E, Shulman GI, et al. PGC-1alpha negatively regulates hepatic FGF21 expression by modulating the heme/Rev-Erb(alpha) axis. Proc Natl Acad Sci U S A. 2009;106:22510–22515. doi: 10.1073/pnas.0912533106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mendivil CO, Zheng C, Furtado J, Lel J, Sacks FM. Metabolism of very-low-density lipoprotein and low-density lipoprotein containing apolipoprotein C-III and not other small apolipoproteins. Arterioscler Thromb Vasc Biol. 2010;30:239–245. doi: 10.1161/ATVBAHA.109.197830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Inagaki T, Dutchak P, Zhao G, Ding X, Gautron L, Parameswara V, Li Y, et al. Endocrine regulation of the fasting response by PPARalpha-mediated induction of fibroblast growth factor 21. Cell Metab. 2007;5:415–425. doi: 10.1016/j.cmet.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 40.Leone TC, Lehman JJ, Finck BN, Schaeffer PJ, Wende AR, Boudina S, Courtois M, et al. PGC-1alpha deficiency causes multi-system energy metabolic derangements: muscle dysfunction, abnormal weight control and hepatic steatosis. PLoS Biol. 2005;3:e101. doi: 10.1371/journal.pbio.0030101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Datta S, Wang L, Moore DD, Osborne TF. Regulation of 3-hydroxy-3-methylglutaryl coenzyme A reductase promoter by nuclear receptors liver receptor homologue-1 and small heterodimer partner: a mechanism for differential regulation of cholesterol synthesis and uptake. J Biol Chem. 2006;281:807–812. doi: 10.1074/jbc.M511050200. [DOI] [PubMed] [Google Scholar]
- 42.Goodwin B, Jones SA, Price RR, Watson MA, McKee DD, Moore LB, Galardi C, et al. A regulatory cascade of the nuclear receptors FXR, SHP-1, and LRH-1 represses bile acid biosynthesis. Mol Cell. 2000;6:517–526. doi: 10.1016/s1097-2765(00)00051-4. [DOI] [PubMed] [Google Scholar]
- 43.Zhang M, Chiang JY. Transcriptional regulation of the human sterol 12alpha-hydroxylase gene (CYP8B1): roles of heaptocyte nuclear factor 4alpha in mediating bile acid repression. J Biol Chem. 2001;276:41690–41699. doi: 10.1074/jbc.M105117200. [DOI] [PubMed] [Google Scholar]
- 44.Watanabe M, Houten SM, Wang L, Moschetta A, Mangelsdorf DJ, Heyman RA, Moore DD, et al. Bile acids lower triglyceride levels via a pathway involving FXR, SHP, and SREBP-1c. J Clin Invest. 2004;113:1408–1418. doi: 10.1172/JCI21025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Eckel-Mahan KL, Patel VR, de Mateo S, Orozco-Solis R, Ceglia NJ, Sahar S, Dilag-Penilla SA, et al. Reprogramming of the circadian clock by nutritional challenge. Cell. 2013;155:1464–1478. doi: 10.1016/j.cell.2013.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Oiwa A, Kakizawa T, Miyamoto T, Yamashita K, Jiang W, Takeda T, Suzuki S, et al. Synergistic regulation of the mouse orphan nuclear receptor SHP gene promoter by CLOCK-BMAL1 and LRH-1. Biochem Biophys Res Commun. 2007;353:895–901. doi: 10.1016/j.bbrc.2006.12.131. [DOI] [PubMed] [Google Scholar]
- 47.Zhou P, Ross RA, Pywell CM, Liangpunsakul S, Duffield GE. Disturbances in the murine hepatic circadian clock in alcohol-induced hepatic steatosis. Sci Rep. 2014;4:3725. doi: 10.1038/srep03725. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.