Abstract
Objective
The purpose of this prospective study was to investigate whether poor oral health predicted eight-year cognitive function change in predominantly late middle adults in the Atherosclerosis Risk in Communities (ARIC) study.
Methods
Participants included a subset of ARIC participants aged 52–75 years at 1996–1998 from two study sites: Forsyth County NC and Jackson MS. All subjects completed cognitive function assessments both in 1996–1998 and 2004–2006, and the same subjects received a dental examination at the initial visit. Cognitive assessment consisted of Delayed Word Recall (DWR), Digit Symbol Substitution (DSS), and Word Fluency (WF) tests. In the analysis, cognitive function for 911 dentally screened participants was evaluated, and 558 of 785 dentate participants received comprehensive oral examinations, including periodontal probing. Measures of oral health included dental status, number of teeth, and periodontal disease classified by the Biofilm-Gingival Interface (BGI) index. The generalized estimating equations (GEE) method was used to analyze repeated measures of cognitive scores with adjustment for socio-demographic characteristics and cardiovascular risk factors.
Results
Of 911 study participants, 13.8% were edentulous. About 13 % of dentally examined participants had periodontal pockets (≥4 mm) with severe bleeding. At the follow-up visit, DWR and WF scores were lower in edentulous compared to dentate people, whereas other oral health measures were not associated with cognitive function. Mean values declined over time for all three cognitive measures, although poor oral health conditions were not associated with greater degree of decline in cognitive function.
Conclusions
In these late-middle aged adults, complete tooth loss was significantly associated with lower cognitive performance. However, neither edentulism, number of teeth, nor periodontal disease predicted greater subsequent cognitive decline.
Keywords: Cognitive function, periodontal disease, tooth loss
Introduction
Cognitive functions change throughout one’s life. The changes can be physiological or pathological and can occur in one or multiple cognitive domains (1,2). Emerging evidence has linked tooth loss and periodontal disease to a greater age-related cognitive decline and to neurodegenerative diseases, such as Alzheimer’s disease (AD) (3–10). An inflammatory model, based on the fundamental theory that chronic periodontal disease is a complex interaction between bacterial pathogens and the host inflammatory response, has been proposed to explain the observed associations (11).
However, there is considerable uncertainty concerning the mechanisms that could lead to associations between poor oral health and cognitive performance. Since most studies have been cross-sectional and conducted primarily in older adults (4,8,9,12–18), the possibility exists that poor nutritional status resulting from tooth loss may negatively affect cognitive ability (18,19). Cognitive dysfunction may lead to poorer self-care, thereby worsening oral health status (20). Low cognitive ability in early life may lead to socioeconomic inequalities in oral health (21).
Whereas many studies report associations of fewer teeth and complete tooth loss with poorer cognitive function (3,5,14,22–24), evidence regarding the association between actual periodontal disease and cognitive ability is mixed (3,6–8,14,15). Furthermore, periodontal disease exposure in earlier studies has been assessed with measures, such as the community periodontal index (CPI), which are based on clinical signs that do not provide much information about the underlying biology of periodontal disease (7).
The purpose of the present study was to determine whether tooth loss and current inflammatory state of periodontal disease, as classified by the Biofilm-Gingival Interface (BGI) index (25), predicted eight-year changes in cognitive function among community-dwelling, late middle-aged adults in the Atherosclerosis Risk in Communities (ARIC) study. We hypothesized that multiple tooth loss and severity of periodontal disease would predict low cognitive performance and subsequent cognitive decline.
Methods
Design and study population
This present study analyzed data obtained from the ARIC study, a prospective investigation of the etiology and natural history of atherosclerosis and clinical cardiovascular disease of middle- aged adults (aged 45–64 years at the inception) enrolled between 1987 and 1989 (Visit 1) in four U.S. communities (Forsyth County, NC; Washington County, MD; suburban Minneapolis, MN; and Jackson, MS). The Jackson cohort was comprised exclusively of African Americans. In this study, we used data from ARIC Visit 4 (1996–1998) and two ARIC ancillary studies: a) the Dental ARIC study (1996–1998); and b) the Brain Magnetic Resonance Imaging (MRI) study (2004–2006). Dental ARIC was a cross-sectional study conducted at Visit 4. Cognitive function of ARIC participants was initially assessed between 1990 and 1992 (Visit 2), and approximately six years later (Visit 4) by ARIC interviewers. Only a subset of participants from two study sites (Forsyth County NC and Jackson MS) received another cognitive function assessment as part of the Brain MRI study in 2004–2006 (Fig 1).
Figure 1.
Data collection time points
For the present study, analyses were restricted to subjects who participated in the dental study at Visit 4 and completed two cognitive assessments separated by eight years (between 1996–1998 and 2004–2006). Sampling and data collection procedures used in ARIC and its ancillary studies have been described elsewhere (26–28).
At Visit 4, 4,737 participants from the Forsyth County and Jackson study sites completed three cognitive functional assessments and a dental screening questionnaire, and 15.5% (n = 733) of these subjects reported complete tooth loss. In 2004–2006, the cognitive function of 985 participants was reevaluated. After excluding participants with missing covariates, the final analytic samples were 911 subjects, and 126 (13.8%) were edentulous. Of 785 dentate participants, 558 (71.1%) underwent a comprehensive dental examination (Supplemental Fig 1, Supplemental Table 2).
Oral health measures
The cross-sectional Dental ARIC study (at Visit 4) consisted of a comprehensive dental examination, which included periodontal probing; collection of gingival crevicular fluid (GCF), dental plaque, and serum; and an interview. Persons requiring antibiotic prophylaxis for periodontal probing were excluded from the Dental ARIC study. Periodontal probing depth (PPD) and bleeding on probing (BOP) were assessed at six sites per tooth on all teeth by trained examiners.
BGI classification, in contrast to traditional definitions of periodontal status, uses clinical signs to classify periodontal status into five levels. The classification, based on two clinical parameters, PPD (≤3 mm or ≥ 4 mm) and extent of BOP (low, <10%; moderate, 10–<50%; and severe, ≥50%), has been associated with distinct microbial, inflammatory, and immunologic parameters that reflect different biologic phenotypes of periodontal disease (25). Subjects with PPD ≤ 3 mm at all sites were defined as periodontal healthy if BOP was less than 10% or gingivitis if BOP was 10% or more. Subjects with one or more periodontal pockets or PPD ≥ 4 mm (deep lesion or periodontitis) were divided into low, moderate, or severe bleeding. The number of teeth present was also recorded during the dental examination. An individual’s dental status was obtained from answers to the following items on a self-administered questionnaire: “Do you have any natural teeth?” and “Do you have any dental implants”? Participants who had only dental implants (n = 12) were excluded from the study.
Cognitive function
Outcomes of interest were scores from the following cognitive tests: a) Delayed Word Recall (DWR); b) Digit Symbol Substitution (DSS); and c) Word Fluency (WF). The DWR tests verbal learning and recent memory (29). The DSS, a test of concentration and psychomotor speed (30), and the WF, a test of expressive language, assess executive function (31). Higher scores in each of the three tests indicate better cognitive ability. All cognitive tests were administered by trained examiners. Cognitive test protocols for ARIC have been reported elsewhere (26).
Covariates
Covariates included socio-demographic factors (age, race, sex, educational level, income, and study sites), cardiovascular risk factors, apolipoprotein E (APOE) genotype, stroke, and coronary heart disease (CHD). Educational levels were classified as less than high school (<12 years), high school completion (12–16 years), or post-secondary education (17–21 years). Household income was coded as <$25,000, $25,000–$50,000, > $50,000, or not report (1996–1998 U.S dollars). A nominal variable representing race and ARIC field centers was created to control for the racial, regional, and examiner differences in the ARIC cohort (levels: Forsyth/White, Forsyth/Black, and Jackson/Black). Cardiovascular risk factors included smoking and alcohol use (each recorded as never, former, or current), diabetes, hypertension, hyperlipidemia, and body mass index (BMI). APOE genotype was dichotomized as presence or absence APOE ε4 allele (Supplemental Methods).
Statistical analyses
Race- and sex-specific descriptive and bivariate analyses were conducted. We used generalized estimating equations (GEE) to analyze eight-year changes in the three cognitive scores. The dependent variables were repeated measures of the DWR, DSS, and WF scores. Since time intervals between baseline and follow-up were slightly different among study participants (mean ± s.d. = 7.6 ± 1.0 years; median = 8 years), we used an indicator variable (time (t)) to identify whether the scores represented a baseline (t = 0) or follow-up (t = 1) measurement rather than using actual time intervals. The unstructured working correlation matrix was used to correct within-subject correlations in the analysis.
The hypothesis that oral health predicted cognitive decline involved testing the interaction between time and oral health measures, i.e., a model of E (Yit |Oral health predictorit) = β0 + β1*Oral health predictor + β2*t + β3*(Oral health predictor*t), where the hypothesis H0: β3 = 0 was tested. If p-value for β3 was greater than 0.10, we concluded that oral health measures did not significantly predict cognitive decline over time.
Potential confounders were identified based on previous literature and bivariate analyses assessing the association between exposures and outcomes. We used directed acyclic graphs (DAGs) and a change-in-estimate procedure to select the adjustment variables in this study. The minimally sufficient set for adjustment included socio-demographic factors, smoking, alcohol use, and diabetes (i.e., the reduced model). Fully adjusted models consisted of variables from the reduced model, BMI, hyperlipidemia, hypertension, and APOE ε4. All covariates were included in the GEE models as time-independent factors. If regression coefficients of the reduced models did not differ from those for the fully adjusted models by greater than 10% or ± 0.1, the regression coefficients from the reduced models are presented in table results. Supplementary analyses addressed questions concerning cross-sectional associations between oral health measures and baseline cognitive scores in this study sample, study center-specific associations of oral health indicators and the 8-year change in cognitive scores, as well as impact of including participants with history of stroke at Visit 4 in the analysis. All statistical analyses were performed using SAS 9.3 (Cary, NC).
Result
Characteristics of study participants
The final analytic sample contained 911 individuals, with an average age of 64.7 ± 4.3 at baseline. Forty-nine percent of participants were African American; 61% were female. Nearly 90% of the African American participants were recruited at the Jackson study site. About half of study participants had never smoked, and one-third had never used alcohol. There were notable differences between racial and sex groups in socio-demographic characteristics and in the prevalence of hypertension, diabetes, CHD, and stroke. About one-third of African American participants, compared to about 10% of whites, had less than 12 years of education. African Americans also had lower income and a higher prevalence of diabetes and hypertension. CHD and stroke were more prevalent among African American males compared to the other three race-gender groups. Overall, African American subjects had poor oral health as indicated by fewer teeth, higher prevalence of complete tooth loss, or severe periodontal disease. However, gingivitis was more common among white subjects (Table 1). About 40–60 % of participants with periodontitis, versus only 7% of those classified as periodontal healthy or having gingivitis based on the BGI index, had attachment loss 6 mm. or more (Supplemental Fig 2).
Table 1.
Race- and sex-specific characteristics at baseline (1996–1998) of study participants
| Characteristics | African American | White | All (n = 911) | ||
|---|---|---|---|---|---|
|
| |||||
| Female (n = 291) | Male (n = 151) | Female (n = 268) | Male (n = 201) | ||
| Age at Visit 4, mean ± SD | 64.0 ± 4.3 | 63.8 ± 4.5 | 65.4 ± 4.3 | 65.5 ± 4.1 | 64.7 ± 4.3 |
| Study sites, % | |||||
| Forsyth | 10.0 | 13.2 | 100 | 100 | 56.9 |
| Jackson | 90.0 | 86.8 | 0 | 0 | 43.1 |
| Education, % | |||||
| Less than high school | 32.0 | 31.8 | 8.6 | 9.0 | 20.0 |
| High school completion | 27.5 | 19.2 | 51.5 | 34.8 | 34.8 |
| Post-secondary education | 40.5 | 49.0 | 39.9 | 56.2 | 45.2 |
| Income, % | |||||
| Refused | 3.1 | 0.7 | 3.0 | 2.0 | 2.4 |
| <$25,000 | 62.9 | 45.7 | 26.5 | 13.9 | 38.5 |
| $25–<$50,000 | 23.7 | 29.1 | 38.0 | 35.3 | 31.4 |
| $50,000 or more | 10.3 | 24.5 | 32.5 | 48.8 | 27.7 |
| Cigarette use, % | |||||
| Current | 12.0 | 15.9 | 16.0 | 8.5 | 13.1 |
| Former | 27.8 | 47.0 | 26.5 | 65.2 | 38.8 |
| Never | 60.2 | 37.1 | 57.5 | 26.3 | 48.1 |
| Alcohol use, % | |||||
| Current | 18.9 | 34.4 | 40.3 | 55.2 | 35.8 |
| Former | 35.1 | 48.3 | 25.4 | 33.3 | 34.0 |
| Never | 46.0 | 17.2 | 34.3 | 11.4 | 30.2 |
| Diabetes mellitus, % | 25.1 | 21.9 | 7.1 | 13.4 | 16.7 |
| Hypertension, % | 67.0 | 51.7 | 32.8 | 39.8 | 48.4 |
| Coronary heart disease1 % | 3.1 | 3.4 | 1.9 | 11.6 | 4.7 |
| Stroke, % | 2.8 | 0.7 | 0.8 | 3.0 | 1.9 |
| Hyperlipidemia, % | 35.0 | 31.8 | 35.1 | 36.3 | 34.8 |
| Body mass index (kg/m2), mean ± SD | 30.6 ± 5.4 | 28.2 ± 4.5 | 26.1 ± 4.7 | 27.0 ± 3.6 | 28.1 ± 5.0 |
| APOE ε4, % | 37.5 | 37.1 | 25.0 | 23.4 | 30.6 |
| Oral health condition | |||||
| Edentulous, % | 23.4 | 15.2 | 7.1 | 8.0 | 13.8 |
| Number of teeth2, mean ± SD | 16.3 ± 7.3 | 18.9 ± 7.9 | 23.7 ± 5.5 | 22.1 ± 7.3 | 20.6 ± 7.5 |
| Periodontal disease (BGI)2, % | |||||
| Had periodontal pockets | |||||
| BOP > 50% | 12.2 | 27.8 | 3.7 | 19.0 | 13.3 |
| BOP 10- ≤50% | 27.9 | 38.0 | 29.5 | 35.9 | 31.9 |
| BOP < 10% | 6.8 | 8.9 | 8.4 | 6.3 | 7.5 |
| No periodontal pockets | |||||
| BOP ≥ 10% | 24.5 | 13.9 | 39.5 | 25.4 | 28.3 |
| BOP < 10% | 28.6 | 11.4 | 18.9 | 13.4 | 19.0 |
n, total number of study group; SD, standard deviation; APOE, Apolipoprotein E; BGI, Biofilm-gingival interface
BGI classified periodontal disease based on probing pocket depth and the extent of bleeding on probing (BOP).
Missing coronary heart disease variable (n = 16); Missing stroke variable (n= 1)
Only among dentate participants who received periodontal examination (n = 558)
Cognitive function
Over a median interval of eight years between the two examinations, there was a modest decline in mean cognitive function, with substantial variability across subject. Mean changes in DWR, DSS, and WF scores were, respectively, −0.7, −3.6, and −1.8 points, with 53.5%, 67.8%, and 58.2% declining and 23.7%, 6.5%, and 5.2% unchanged. In general, white females tended to have a greater decline in DSS scores while African Americans (both males and females) exhibited a greater decrease in WF scores. The decline in DWR scores was similar across race- and gender-specific groups (Table 2).
Table 2.
Cogniti ve scores at ARIC Visit 4 (1996–1998) and eight-year changes in cognitive scores
| Cognitive scores, mean ± SD | African American | White | All (n = 911) | ||
|---|---|---|---|---|---|
|
| |||||
| Female (n = 291) | Male (n = 151) | Female (n = 268) | Male (n = 201) | ||
| Cognitive scores at 1996–1998 | |||||
| Delayed word recall | 6.5 ± 1.5 | 5.8 ± 1.6 | 7.2 ± 1.3 | 6.8 ± 1.5 | 6.7 ± 1.6 |
| Digit symbol substitution | 34.3 ± 12.5 | 30.0 ± 12.7 | 50.1 ± 9.9 | 44.6 ± 40.5 | 40.5 ± 14.0 |
| Word fluency | 30.8 ±12.6 | 28.9 ± 14.4 | 37.0 ± 11.4 | 35.1 ± 12.1 | 33.3 ± 12.9 |
| Cognitive scores at 2004–2006 | |||||
| Delayed word recall | 5.8 ± 1.7 | 5.2 ± 1.7 | 6.6 ± 1.6 | 6.0 ± 1.6 | 6.0 ± 1.7 |
| Digit symbol substitution | 31.2 ± 11.8 | 26.6 ± 11.6 | 45.2 ± 9.9 | 41.7 ± 11.0 | 36.9 ± 13.2 |
| Word fluency | 28.3 ± 12 | 26.4 ± 12.6 | 35.4 ± 10.8 | 34.4 ± 12.1 | 31.4 ± 12.3 |
| Change in cognitive scores1 | |||||
| Delayed word recall | −0.7 ± 1.8 | −0.6 ± 1.7 | −0.6 ± 1.7 | −0.8 ± 1.6 | −0.7 ± 1.7 |
| Digit symbol substitution | −3.1 ± 9.1 | −3.4 ± 6.9 | − 4.9 ± 6.7 | −2.9 ± 6.8 | −3.6 ± 7.6 |
| Word fluency | −2.5 ± 7.1 | −2.5 ± 9.4 | −1.6 ± 7.4 | −0.7 ± 6.9 | −1.8 ± 7.6 |
n, total number of study group; SD, standard deviation
Changes in cognitive scores = Scores at follow-up (2004–2006) – Scores at baseline (1996–1998)
Older age, male sex, low education, low income, diabetes, hypertension, current smoking, CHD, stroke, APOE ε4, and poor oral health (complete tooth loss, few teeth, and periodontitis with severe bleeding) were associated with a low cognitive profile. The differences in cognitive scores were generally greater for race, educational attainment, and income. There was, as expected, a strong association between dental status and overall cognitive performance. On average, individuals with complete tooth loss had cognitive scores 0.62, 9.08, and 8.30 points lower than dentate participants for the DWR, DSS, and WF tests, respectively. The associations of other covariates with repeated measures of cognitive scores are summarized in the Supplemental Table 3.
Oral health measures and cognitive function
Table 3 shows the regression coefficients for oral health measures, time, and their interactions; parameter estimates for the first-order effects of each oral health measures can be interpreted as the magnitude of association between the Visit 4 oral health measures and cognitive function, while interaction terms indicate the difference between oral health status groups (edentulous vs. dentate, BGI-DL/SB, DL/MB, DL/LB, or G vs. BGI-H, and one tooth increase in number of teeth) in degree of change in cognitive function over eight years (follow-up vs. baseline). After controlling for covariates, negative values of “time” regression coefficients indicated a significant decline in cognitive test scores. At Visit 4, lower levels of all three cognitive function test scores were seen in subjects with complete tooth loss when compared to dentate subjects, although the differences did not reach statistical significance for the DSS test in the fully adjusted model. In contrast, periodontal disease and the number of teeth were not significantly associated with cognitive performance, either as first-order effects or as interactions with time (Fig 2–4).
Table 3.
Regression coefficients for the effects of time, oral health measures, and their interaction on three cognitive scores
| n | Delayed word recall | Digit symbol substitution | Word fluency | ||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| b (SE) | P-value1 | b (SE) | P-value1 | b (SE) | P-value1 | ||
| Dental status2,3 | 911 | ||||||
| Time (F/U vs. Baseline) | −0.74 (0.06) | <0.0001 | −3.76 (0.26) | <0.0001 | −1.91 (0.28) | <0.0001 | |
| Dental status (Edentulous vs. Dentate) | −0.42 (0.16) | 0.0357 | −1.47 (1.08) | 0.2438 | −3.03 (0.02) | 0.0014 | |
| Dental status x Time | 0.29 (0.17) | 0.0855 | 0.89 (0.93) | 0.3389 | 0.46 (0.60) | 0.4512 | |
| Periodontal disease2 | 558 | ||||||
| Time (F/U vs. Baseline) | −0.98 (0.18) | <0.001 | −3.38 (0.84) | <0.0001 | −1.32 (0.80) | <0.0001 | |
| BGI-DL/SB | −0.16 (0.21) | 0.6040 | −0.28 (1.56) | 0.5165 | −0.50 (1.79) | 0.6225 | |
| BGI-DL/MB | 0.0015 (0.16) | 0.88 (1.18) | 1.50 (1.44) | ||||
| BGI-DL/LB | 0.15 (0.24) | 2.38 (1.56) | 1.75 (2.15) | ||||
| BGI-G | 0.12 (0.16) | 0.91 (1.22) | −0.75 (1.40) | ||||
| BGI-DL/SB x Time | 0.45 (0.27) | 0.5655 | 0.55 (1.14) | 0.7636 | −0.96 (1.17) | 0.2173 | |
| BGI-DL/MB x Time | 0.23 (0.22) | −0.33 (0.96) | −1.36 (1.00) | ||||
| GI-DL/LB x Time | 0.10 (0.29) | 0.12 (1.32) | −2.18 (1.45) | ||||
| BGI-G x Time | 0.22 (0.22) | −0.64 (0.99) | 0.17 (0.98) | ||||
| Number of teeth2 | 558 | ||||||
| Time (F/U vs. Baseline) | −0.67 (0.23) | 0.0048 | −3.38 (0.86) | 0.0001 | −2.72 (0.90) | 0.0028 | |
| 1-tooth increase | 0.0031 (0.0086) | 0.7129 | 0.046 (0.056) | 0.3959 | −0.0071 (0.073) | 0.9233 | |
| 1-tooth increase x Time | −0.0053 (0.01) | 0.6109 | −0.0094 (0.039) | 0.8071 | 0.035 (0.0042) | 0.4100 | |
b, regression coefficient; SE, standard error; BGI, Biofilm-Gingival Interface
BGI index classified periodontal disease based on probing pocket depth and the extent of bleeding on probing.
Five categories of BGI included BGI-DL/SB (deep lesion/severe bleeding), BGI-DL/MB (deep lesion/moderate bleeding), BGI-DL/LB (deep lesion/low bleeding), BGI-G (gingivitis), and BGI-H (healthy). In regression models, BGI-H was the reference group.
Type III p-value
Adjusting for age, sex, race-center, education, income, smoking, alcohol use, and diabetes
Digit symbol substitution: Adjusting for age, sex, race-center, education, income, smoking, alcohol use, diabetes, hypertension, hyperlipidemia, body mass index, and APOE ε4
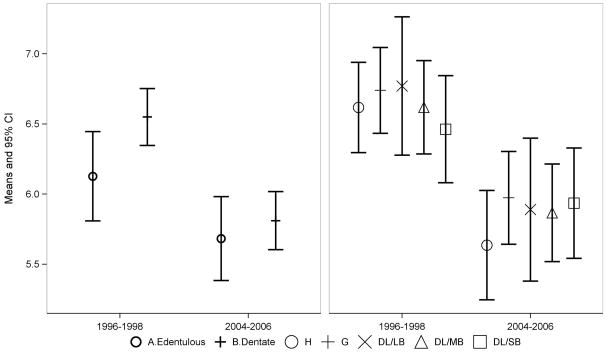
Figure 2. Adjusted means and 95% confidence intervals for the Delayed Word Recall test scores at baseline and follow-up.
Abbreviation: H, healthy; G, gingivitis; SB, severe bleeding; MB, moderated bleeding; LB, low bleeding
Figure 4. Adjusted means and 95% confidence intervals for the Word Fluency test scores at baseline and follow-up.
Abbreviation: H, healthy; G, gingivitis; SB, severe bleeding; MB, moderated bleeding; LB, low bleeding
Generally, cognitive decline among edentulous participants tended to be slightly smaller than that among dentate subjects (positive values of interaction coefficients). However, dental status did not significantly modify the time-related degree of cognitive decline (Fig 2–4), except for a small association between edentulism and DWR scores (p-value 0.0855, Table 3). Individuals with complete tooth loss had adjusted DWR scores that declined from 6.1 (95% CI 5.8, 6.4) at baseline to 5.7 (95% CI 5.4, 6.0) at the follow-up visit compared with 6.6 (95% CI 6.3, 6.7) at baseline and 5.8 (95% CI 5.6, 6.0) at the follow-up visit for dentate subjects (Fig 2).
Supplemental analyses showed that dental status was associated with lower DWR and WF at baseline (Supplemental Table 4). Neither fewer teeth nor periodontal disease was associated with baseline cognitive scores on any of the three tests. Associations between edentulism and change in cognitive scores for the Forsyth study center were slightly stronger than those for the Jackson study center. The results were generally unchanged after excluding participants with history of stroke at Visit 4 (Data not shown).
Discussion
Four possible mechanisms have been proposed for the relation between poor oral health and lower cognitive function: (a) residual confounding by socio-demographic factors or other environmental factors (23); (b) nutritional deficiency resulting from tooth loss (tooth loss is often a consequence of severe periodontal disease)(18); (c) increased systemic inflammatory response (11,16,17); and (d) an adverse impact of cognitive decline on oral hygiene (12).
Our study found complete tooth loss, though not periodontal disease and number of teeth, to be associated with low performance on two cognitive tests (DWR and WF) at baseline (Visit 4). However, although all three cognitive scores declined over time, we did not find that complete tooth loss, periodontal disease, and few teeth at baseline predicted a greater cognitive decline. We also observed a smaller decline in memory function among edentulous participants compared to dentate subjects.
The most important strengths of the present study are using data from the population-based cohort of community-dwelling, late middle-aged adults, and the quality of examination data. Cognitive assessment and periodontal examination, using a full-mouth protocol, were carried out by trained examiners. Several limitations are also relevant. Our study followed participants in only two of the four ARIC sites (Forsyth County and Jackson), and these differed greatly in regard to racial composition, socioeconomic characteristics, edentulism, and baseline cognitive scores (especially DSS and WF test scores) (Supplemental Table 2).
Another limitation of our study is that oral health indicators were measured only at Visit 4, so that information was unavailable on the trajectory of oral health (i.e., additional tooth loss or periodontal disease progression). Thus, we cannot assess whether low cognitive performance influences oral health. Furthermore, assessments at two time points provide limited ability to differentiate true change from changes due to learning effects, random fluctuations, and measurement errors. Lastly, our study examined only three cognitive function tests, evaluating two cognitive domains. Thus, our failure to observe more rapid cognitive decline in participants with worse oral health provides limited evidence against the existence of a relationship.
Complete tooth loss
Our findings of lower scores on Visit 4 DWR and WF for edentulous participants are consistent with previous studies which found that people with few teeth or complete tooth loss have lower cognitive function in the memory or executive function domains (3,6,14). We also observed a marginally-significantly lower score on the DSS for edentulous participants in the Forsyth County site but no association among Jackson site participants, whose cognitive function, education, and oral health measures were all markedly lower.
Our findings are not consistent, however, with previous studies that found edentulism to predict more rapid cognitive decline and greater incidence of dementia (6,24). In fact, our study found a slower cognitive decline in edentulous participants. The many differences between the communities studied in the previous studies and in ours could conceivably account for the different findings. However, when we carried out separate analyses in the Forsyth and Jackson sites, which differ substantially in numerous characteristics, we observed a smaller decline in memory function in edentulous participants in both sites; it was, if anything, stronger in Forsyth (Data not shown). The previous studies followed older participants, who experienced substantial cognitive declines, whereas our study population was predominantly late middle-aged adults, who had only modest cognitive declines during the follow-up period. The smaller overall decline may have limited the opportunity to see a difference by oral health status.
Other possible explanations for why our study did not observe greater cognitive decline for participants with poorer oral health – and for our observation of a smaller decrease in DWR scores for edentulous participants than for dentate participants – relate to the narrow time window under observation. Cognitive function of edentulous participants, who were older and had poorer socio-demographic characteristics than dentate participants, may have begun to decline earlier in life, before our study baseline, and had now reached a stable level. Other possible explanations relate to the heterogeneity of age-related cognitive decline, where some cognitive domains decline earlier (i.e., memory function) and more rapidly than others, and inter-individual variability increases during the aging process (32). A longitudinal study in French elderly has reported a lower risk of dementia in lower educated participants with extensive tooth loss. The authors suggested that the observed association was possibly a result of greater tooth extraction in people with lower educational attainment, leading to better periodontal health (7). Such suppression of a source of chronic inflammation could conceivably explain why we observed a smaller decrement in cognitive function among edentulous persons. In this study sample, more than 70% of both edentulous and dentate subjects reported history of tooth loss due to “cavities”. However, 38% of edentulous participants reported tooth loss due to “gum disease”, whereas 6–20% of dentate participants depending on BGI category tooth loss due to “gum disease” (Supplemental Table 5).
Yet another possibility is that the cross-sectional association between poorer oral health and worse cognitive function reflects an adverse effect of low cognitive function on oral health status. One longitudinal study has suggested that the observed association between complete tooth loss and cognitive impairment in older adults resulted from lower cognitive ability in early life predisposing individuals to edentulism (22).
Periodontal disease
Previous findings regarding periodontal disease and cognitive function are mixed. Some studies, including our previous cross-sectional study (33), have found a significant association between periodontal disease and low cognitive ability (3,8,15–17), whereas others have not (5–7,14). Interpretation of these findings is complicated by differences in the definition and measurement of periodontal disease. A wide range of periodontal disease measures have been used, including biological markers (15,34), attachment loss (17), periodontal pocket depth (3,14), and self-reported periodontal status (6). Also, many study populations are highly selected, and results from them may not be indicative of what would be seen in other populations (3,5,7,8,14).
In our study, BGI index was selected based on the concept that if periodontal infection and inflammation truly affect cognitive decline, we should observe a dose-response trend in the association because BGI reflects the underlying biology of periodontal disease (25). However, the expected dose-response pattern might be masked by the nature of the clinical periodontal measures and effects of past treatments, none of which can be discerned given that periodontal measurements were recorded on only one visit. Furthermore, although different bacterial species are linked to gingivitis and severe periodontitis, both conditions have been correlated with elevated levels of gingival bleeding, inflammatory markers, and plaque scores.
We found some indication that participants with a greater extent of bleeding at baseline (Visit 4) had lower cognitive function than those who were healthy or had a lesser extent of bleeding, but we did not observe differences in the rate of cognitive decline to be related to the value of the BGI index. Our study may have been underpowered to detect an association between BGI and cognitive function because the mean differences between the two extreme BGI groups were relatively small, and cognitive change during the eight-year follow-up was modest (Supplemental material). However, it is also possible that subtle changes in cognitive function occurring prior to study baseline resulted in poor oral hygiene care, thereby increasing plaque level and the extent of bleeding on probing at baseline (35).
Conclusion
We have not observed a relationship between edentulism, number of teeth, or periodontal disease in middle-aged adults and cognitive decline over the subsequent 8 years. Our study may have had insufficient statistical power given the low rate of observed cognitive decline and the limited sample size, or the relationship may manifest only at older ages and/or in relation to more severe levels of impaired cognition.
Supplementary Material
Supplemental Fig 1: Study participants
Abbreviation: AA, African American; W, white
Supplemental Fig 2: Extent of attachment loss by five levels of Biofilm-Gingival Interface classification
Abbreviation: BOP, bleeding on probing; AL, attachment loss
Supplemental Fig 3: Crude and adjusted means and 95% confidence intervals for the Delayed Word Recall test scores at baseline and follow-up
Abbreviation: H, healthy; G, gingivitis; SB, severe bleeding; MB, moderated bleeding; LB, low bleeding
Supplemental Fig 4: Crude and adjusted means and 95% confidence intervals for the Digit Symbol Substitution test scores at baseline and follow-up
Abbreviation: H, healthy; G, gingivitis; SB, severe bleeding; MB, moderated bleeding; LB, low bleeding
Supplemental Fig 5: Crude and adjusted means and 95% confidence intervals for the Word Fluency test scores at baseline and follow-up
Abbreviation: H, healthy; G, gingivitis; SB, severe bleeding; MB, moderated bleeding; LB, low bleeding
Figure 3. Adjusted means and 95% confidence intervals for the Digit Symbol Substitution test scores at baseline and follow-up.
Abbreviation: H, healthy; G, gingivitis; SB, severe bleeding; MB, moderated bleeding; LB, low bleeding
Acknowledgments
The ARIC study was carried out as a collaborative study supported by the National Heart, Lung and Blood Institute (contracts N01-HC55015, N01-HC 55016, N01-HC 55018, N01-HC 55019, N01-HC 55020, N01-HC 55021, N01-HC 55022). The collection and analysis of dental data were supported by the National Institute of Dental and Craniofacial Research (grants DE 13807-01A1 and DE1 1551). Brain MRI examinations were funded by R01-HL70825. We thank the staff and participants of the ARIC study for their important contributions.
Footnotes
Human participants protection
No protocol approval was necessary because this study involved the analysis of secondary data.
Conflict of interest
The authors have no conflicts of interest to report.
References
- 1.Tucker AM, Stern Y. Cognitive reserve in aging. Curr Alzheimer Res. 2011;8(4):354–60. doi: 10.2174/156720511795745320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Salthouse TA. When does age-related cognitive decline begin? Neurobiology of Aging. 2009;30(4):507–14. doi: 10.1016/j.neurobiolaging.2008.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaye EK, Valencia A, Baba N, Spiro A, Dietrich T, Garcia RI. Tooth loss and periodontal disease predict poor cognitive function in older men. J Am Geriatr Soc. 2010;58(4):713–8. doi: 10.1111/j.1532-5415.2010.02788.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grabe HJ, Schwahn C, Völzke H, Spitzer C, Freyberger HJ, John U, et al. Tooth loss and cognitive impairment. J Clin Periodontol. 2009;36(7):550–7. doi: 10.1111/j.1600-051X.2009.01426.x. [DOI] [PubMed] [Google Scholar]
- 5.Stein PS, Desrosiers M, Donegan SJ, Yepes JF, Kryscio RJ. Tooth loss, dementia and neuropathology in the Nun study. J Am Dent Assoc. 2007;138(10):1314–22. doi: 10.14219/jada.archive.2007.0046. [DOI] [PubMed] [Google Scholar]
- 6.Batty GD, Li Q, Huxley R, Zoungas S, Taylor BA, Neal B, et al. Oral disease in relation to future risk of dementia and cognitive decline: Prospective cohort study based on the Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified-Release Controlled Evaluation (ADVANCE) trial. European Psychiatry. 2013;28(1):49–52. doi: 10.1016/j.eurpsy.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arrivé E, Letenneur L, Matharan F, Laporte C, Helmer C, Barberger-Gateau P, et al. Oral health condition of French elderly and risk of dementia: a longitudinal cohort study. Community Dent Oral Epidemiol. 2012;40(3):230–8. doi: 10.1111/j.1600-0528.2011.00650.x. [DOI] [PubMed] [Google Scholar]
- 8.Kamer AR, Morse DE, Holm-Pedersen P, Mortensen EL, Avlund K. Periodontal inflammation in relation to cognitive function in an older adult Danish population. J Alzheimers Dis. 2012;28(3):613–24. doi: 10.3233/JAD-2011-102004. [DOI] [PubMed] [Google Scholar]
- 9.Yamamoto T, Kondo K, Hirai H, Nakade M, Aida J, Hirata Y. Association Between Self-Reported Dental Health Status and Onset of Dementia: A 4-Year Prospective Cohort Study of Older Japanese Adults from the Aichi Gerontological Evaluation Study (AGES) Project. Psychosom Med. 2012;74(3):241–8. doi: 10.1097/PSY.0b013e318246dffb. [DOI] [PubMed] [Google Scholar]
- 10.Stewart R, Weyant RJ, Garcia ME, Harris T, Launer LJ, Satterfield S, et al. Adverse oral health and cognitive decline: the health, aging and body composition study. J Am Geriatr Soc. 2013;61(2):177–84. doi: 10.1111/jgs.12094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kamer AR, Craig RG, Dasanayake AP, Brys M, Glodzik-Sobanska L, de Leon MJ. Inflammation and Alzheimer’s disease: possible role of periodontal diseases. Alzheimers Dement. 2008;4(4):242–50. doi: 10.1016/j.jalz.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 12.Syrjälä A-MH, Ylöstalo P, Ruoppi P, Komulainen K, Hartikainen S, Sulkava R, et al. Dementia and oral health among subjects aged 75 years or older. Gerodontology. 2012;29(1):36–42. doi: 10.1111/j.1741-2358.2010.00396.x. [DOI] [PubMed] [Google Scholar]
- 13.Yu Y-H. Association Between Cognitive Function and Periodontal Disease in Older Adults. J Am Geriatr Soc. 2008;56:1693–7. doi: 10.1111/j.1532-5415.2008.01847.x. [DOI] [PubMed] [Google Scholar]
- 14.Okamoto N, Morikawa M, Okamoto K, Habu N, Iwamoto J, Tomioka K, et al. Relationship of tooth loss to mild memory impairment and cognitive impairment: findings from the fujiwara-kyo study. Behav Brain Funct. 2010;6(1):1–8. doi: 10.1186/1744-9081-6-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Noble JM, Borrell LN, Papapanou PN, Elkind MSV, Scarmeas N, Wright CB. Periodontitis is associated with cognitive impairment among older adults: analysis of NHANES-III. J Neurol Neurosurg Psychiatry. 2009;80(11):1206–11. doi: 10.1136/jnnp.2009.174029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stewart R, Hirani V. Dental health and cognitive impairment in an English national survey population. J Am Geriatr Soc. 2007;55(9):1410–4. doi: 10.1111/j.1532-5415.2007.01298.x. [DOI] [PubMed] [Google Scholar]
- 17.Stewart R, Sabbah W, Tsakos G, D’Aiuto F, Watt RG. Oral health and cognitive function in the Third National Health and Nutrition Examination Survey (NHANES III) Psychosom Med. 2008;70(8):936–41. doi: 10.1097/PSY.0b013e3181870aec. [DOI] [PubMed] [Google Scholar]
- 18.Kim J-M, Stewart R, Prince M, Kim S-W, Yang S-J, Shin I-S, et al. Dental health, nutritional status and recent-onset dementia in a Korean community population. Int J Geriatr Psychiatry. 2007;22(9):850–5. doi: 10.1002/gps.1750. [DOI] [PubMed] [Google Scholar]
- 19.Shimazaki Y, Soh I, Saito T, Yamashita Y, Koga T, Miyazaki H, et al. Influence of Dentition Status on Physical Disability, Mental Impairment, and Mortality in Institutionalized Elderly People. J Dent Res. 2001;80(1):340–5. doi: 10.1177/00220345010800010801. [DOI] [PubMed] [Google Scholar]
- 20.Chalmers JM, Carter KD, Spencer AJ. Caries incidence and increments in community-living older adults with and without dementia. Gerodontology. 2002;19(2):80–94. doi: 10.1111/j.1741-2358.2002.00080.x. [DOI] [PubMed] [Google Scholar]
- 21.Sabbah W, Watt RG, Sheiham A, Tsakos G. The Role of Cognitive Ability in Socioeconomic Inequalities in Oral Health. J Dent Res. 2009;88(4):351–5. doi: 10.1177/0022034509334155. [DOI] [PubMed] [Google Scholar]
- 22.Starr JM, Hall RJ, Macintyre S, Deary IJ, Whalley LJ. Predictors and correlates of edentulism in the healthy old people in Edinburgh (HOPE) study. Gerodontology. 2008;25(4):199–204. doi: 10.1111/j.1741-2358.2008.00227.x. [DOI] [PubMed] [Google Scholar]
- 23.Matthews JC, You Z, Wadley VG, Cushman M, Howard G. The association between self-reported tooth loss and cognitive function in the REasons for Geographic And Racial Differences in Stroke study: an assessment of potential pathways. J Am Dent Assoc. 2011;142(4):379–90. doi: 10.14219/jada.archive.2011.0192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stein PS, Kryscio RJ, Desrosiers M, Donegan SJ, Gibbs MB. Tooth loss, apolipoprotein E, and decline in delayed word recall. J Dent Res. 2010;89(5):473–7. doi: 10.1177/0022034509357881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Offenbacher S, Barros SP, Singer RE, Moss K, Williams RC, Beck JD. Periodontal Disease at the Biofilm–Gingival Interface. J Periodontol. 2007;78(10):1911–25. doi: 10.1902/jop.2007.060465. [DOI] [PubMed] [Google Scholar]
- 26.Cerhan JR, Folsom AR, Mortimer JA, Shahar E, Knopman DS, McGovern PG, et al. Correlates of cognitive function in middle-aged adults. Atherosclerosis Risk in Communities (ARIC) Study Investigators. Gerontology. 1998;44(2):95–105. doi: 10.1159/000021991. [DOI] [PubMed] [Google Scholar]
- 27.Mosley TH, Knopman DS, Catellier DJ, Bryan N, Hutchinson RG, Grothues CA, et al. Cerebral MRI findings and cognitive functioning: the Atherosclerosis Risk in Communities study. Neurology. 2005;64(12):2056–62. doi: 10.1212/01.WNL.0000165985.97397.88. [DOI] [PubMed] [Google Scholar]
- 28.Beck JD, Eke P, Heiss G, Madianos P, Couper D, Lin D, et al. Periodontal disease and coronary heart disease: a reappraisal of the exposure. Circulation. 2005;112(1):19–24. doi: 10.1161/CIRCULATIONAHA.104.511998. [DOI] [PubMed] [Google Scholar]
- 29.Knopman DS, Ryberg S. A verbal memory test with high predictive accuracy for dementia of the Alzheimer type. Arch Neurol. 1989;46(2):141–5. doi: 10.1001/archneur.1989.00520380041011. [DOI] [PubMed] [Google Scholar]
- 30.Wechsler D. Manual for the Wechsler Adult Intelligence Scale-Revised. New York: The Psychological Corporation; 1981. [Google Scholar]
- 31.Benton AL, Eslinger PJ, Damasio AR. Normative observations on neuropsychological test performances in old age. J Clin Neuropsychol. 1981 May;3(1):33–42. doi: 10.1080/01688638108403111. [DOI] [PubMed] [Google Scholar]
- 32.Salthouse TA. Selective review of cognitive aging. J Int Neuropsychol Soc. 2010;16(5):754–60. doi: 10.1017/S1355617710000706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Naorungroj S, Schoenbach VJ, Beck J, Mosley TH, Gottesman RF, Alonso A, et al. Cross-sectional associations of oral health measures with cognitive function in late middle-aged adults: A community-based study. J Am Dent Assoc. 2013;144(12):1362–71. doi: 10.14219/jada.archive.2013.0072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stein PS, Steffen MJ, Smith C, Jicha G, Ebersole JL, Abner E, et al. Serum antibodies to periodontal pathogens are a risk factor for Alzheimer’s disease. Alzheimers Dement. 2012;8(3):196–203. doi: 10.1016/j.jalz.2011.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Naorungroj S, Slade GD, Beck JD, Mosley TH, Gottesman RF, Alonso A, et al. Cognitive decline and oral health in middle-aged adults in the ARIC study. J Dental Res. 2013;92(9):795–801. doi: 10.1177/0022034513497960. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Fig 1: Study participants
Abbreviation: AA, African American; W, white
Supplemental Fig 2: Extent of attachment loss by five levels of Biofilm-Gingival Interface classification
Abbreviation: BOP, bleeding on probing; AL, attachment loss
Supplemental Fig 3: Crude and adjusted means and 95% confidence intervals for the Delayed Word Recall test scores at baseline and follow-up
Abbreviation: H, healthy; G, gingivitis; SB, severe bleeding; MB, moderated bleeding; LB, low bleeding
Supplemental Fig 4: Crude and adjusted means and 95% confidence intervals for the Digit Symbol Substitution test scores at baseline and follow-up
Abbreviation: H, healthy; G, gingivitis; SB, severe bleeding; MB, moderated bleeding; LB, low bleeding
Supplemental Fig 5: Crude and adjusted means and 95% confidence intervals for the Word Fluency test scores at baseline and follow-up
Abbreviation: H, healthy; G, gingivitis; SB, severe bleeding; MB, moderated bleeding; LB, low bleeding