Abstract
Background
Stem cell factor (SCF), a ligand of the c-kit receptor, is a critical cytokine which contributes to cell migration, proliferation, and survival. It has been shown that SCF expression increases after myocardial infarction (MI) and may be involved in cardiac repair. The aim of this study was to determine whether gene transfer of membrane-bound human SCF improves cardiac function in a large animal model of MI.
Methods and Results
A transmural MI was created by implanting an embolic coil in the left anterior descending artery in Yorkshire pigs. One week after the MI, the pigs received direct intramyocardial injections of either a recombinant adenovirus encoding for SCF, (Ad.SCF, n=9) or β-gal (Ad.β-gal, n=6) into the infarct border area. At three months post-MI, ejection fraction increased by 12% relative to baseline after Ad.SCF therapy, whereas it decreased by 4.2% (P=0.004) in pigs treated with Ad.β-gal. Preload-recruitable stroke work was significantly higher in pigs after SCF treatment (Ad.SCF, 55.5±11.6 mmHg vs Ad.β-gal, 31.6±12.6 mmHg, P=0.005), indicating enhanced cardiac function. Histological analyses confirmed the recruitment of c-kit+ cells as well as a reduced degree of apoptosis one week after Ad.SCF injection. In addition, increased capillary density compared to pigs treated with Ad.β-gal was found at three months and suggests an angiogenic role of SCF.
Conclusions
Local over-expression of SCF post-MI induces the recruitment of c-kit+ cells at the infarct border area acutely. In the chronic stages, SCF gene transfer was associated with improved cardiac function in a pre-clinical model of ischemic cardiomyopathy.
Keywords: gene therapy, myocardial infarction, angiogenesis, paracrine factor
Ischemic cardiomyopathy is one of the major causes of HF1. It is characterized by poor perfusion, chronic loss of cardiomyocytes, scar formation, and adverse ventricular remodeling. Recently, cell therapy has received significant attention due to its potential for regenerating cardiomyocytes and replacing scar tissue with new cardiomyocytes. Despite the initial expectations that cardiomyogenesis would occur by introducing exogenous stem cells into the ischemic heart, most studies have failed to show trans-differentiation of injected cells into cardiomyocytes2, 3. It is now accepted that paracrine effects play a major role in the documented functional improvements following cell therapy in HF patients4, 5. Pre-clinical investigations have identified several paracrine factors that contribute positively to cardiac repair after MI, such as vascular endothelial growth factor, stromal cell-derived factor-1 (SDF-1), insulin like growth factor-1, hepatocyte growth factor, and stem cell factor (SCF)6. SCF is a ligand of c-kit, and c-kit is a receptor tyrosine kinase. SCF binding to c-kit leads to receptor dimerization and activation of multiple downstream signaling pathways related to cell recruitment7–9, differentiation10, 11, angiogenesis7, 9, 11, 12, and survival7, 11, 12.
More recently, Bolli et al. showed that injecting c-kit+ stem cells in patients with ischemic cardiomyopathy resulted in dramatic improvement of left ventricular (LV) function in these patients13. This study demonstrated the clinical importance of this cell type to the reparative process. While their approach is capable of using the c-kit+ cells from the same patient (using the tissue obtained during the bypass surgery or from biopsy samples), ex-vivo harvesting process limits its utility for acute to sub-acute disease applications. In these settings, gene delivery may be useful as gene expression can be up-regulated relatively quickly.
Previous studies in rodents have provided evidence that SCF increases the number of c-kit+ stem cells in the injured heart and contributes to improved cardiac function and survival6, 7, 12, 14, 15. These studies have also identified valuable insights including the interaction between SCF and c-kit+ stem cells, mechanism of cardiac repair, and origin as well as type of the c-kit+ cells involved. The important question remaining is whether SCF treatment is also efficacious in human ischemic heart diseases since there are large physiological and structural differences between rodents and humans. Therefore, the goal of our study was to validate the therapeutic efficacy of SCF gene transfer in a clinically relevant animal model of ischemic HF. We hypothesized that local over-expression of membrane-bound human SCF at the infarct border zone following MI will improve cardiac function in a swine model of MI. Using invasive and non-invasive means of LV functional and structural assessments, we report the beneficial effects of SCF gene transfer on post-MI heart with focus on the LV hemodynamics.
Methods
Study protocol
The experimental protocols complied with the National Institutes of Health, Guide for the Care and Use of Laboratory Animals and standards of United States regulatory agencies as well as Position of the American Heart Association on Research Animal Use16. Protocols were approved by the Institutional Animal Care and Use Committee at the Icahn School of Medicine at Mount Sinai. A total of 22 female Yorkshire pigs were enrolled in our chronic study. An MI was created at day 0, and the gene transfer was performed 1 week after the MI creation (1Wk). The animals were cage housed in the Mount Sinai animal facility and underwent cardiac function assessments at 6 weeks (6Wk) and 3 months (3Mo) post-MI. The study was performed in a randomized manner and 3-dimensional echocardiographic (3DE) images were analyzed by a blinded investigator. We aimed to show improved LV ejection fraction (EF) in the SCF treatment group assessed by left ventriculography, which we have previously shown to accurately estimate systolic dysfunction after MI17. Accordingly, hemodynamic measurements with a pressure catheter, left ventriculography, and 3DE measurements were performed at 1Wk (immediately prior to gene transfer), 6Wk, and 3Mo. Additionally, pressure-volume loop relationships were assessed at 1Wk and 3Mo. In addition to the main chronic study, adenovirus encoding for SCF with green fluorescent protein (GFP) (Ad.SCF, 2.0x1012 viral genomes (vg), n=4) and β-gal with GFP (Ad.β-gal 2.0x1012 vg, n=2) injected pigs were euthanized at 1week after the virus injection to evaluate the efficacy of adenoviral gene transfer.
Animal model and gene transfer
An MI was created as previously described with minor modification18. Briefly, pigs (18-21 Kg) were sedated with Telazol (tiletamine/zolazepam) (8.0 mg/kg) and Buprenorphine (0.6 mg) and anesthetized with propofol (8 to 10 mg/Kg/hr). Arterial access was obtained by puncturing the femoral artery and a coronary balloon was delivered to the proximal left anterior descending artery (LAD). Complete occlusion of the LAD was confirmed by angiogram and maintained for 60 minutes. The balloon was then deflated and an embolic coil was implanted just distal to the first diagonal branch. Animals were allowed to recover after the confirmation of hemodynamic stability. Gene transfer was performed 1week after the MI creation by direct intramyocardial injection. Under sterile conditions and inhalational isoflurane (2.0% to 2.5%) anesthesia, a left thoracotomy was performed in the fourth intercostal space. Ad.SCF or Ad.β-gal was delivered into the border area of the LV infarct and normal tissues through an opened pericardium where a total of 15-20 injections (50 μl/site) were made approximately 0.5 cm apart, while avoiding the epicardial vessels. After virus injections, the chest was closed and blood and air were removed via a vacuumed thoracic drain.
Animals
Four of the 22 pigs for chronic study died within 24 hours after the MI creation. Three pigs with a large amount of bloody pericardial effusion were excluded due to the major influence for assessing baseline cardiac function and difficulty to differentiate infarct border because of abundant fibrocollagenous tissue around the infarct. Therefore, 9 pigs injected with Ad.SCF and 6 pigs with Ad.β-gal were included in the chronic study. During the follow up, one pig each from Ad.SCF group (11%) and Ad.β-gal group (17%) died due to HF.
Statistical analysis
All values are reported as mean ± standard deviation except where indicated. Study sample size was determined by using an alpha value of 0.05, a power of 80% to detect a 5% absolute increase of LVEF in the assumption of 3% standard deviation with 0.67 controls per treatment animal. Comparison between the 2 groups was performed by Student t test. Comparisons between repeated values at 1Wk and at 3Mo were done by repeated measures analysis of variance (ANOVA) to test the statistical significance of the treatment*time interaction. Intra-class correlation coefficient was analyzed to assess the agreement of 2 repeated measurements for 3DE volume analysis. A P value of < 0.05 was considered statistically significant.
Results
Gene transfer efficacy and c-kit+ cell recruitment
To examine the sub-acute effects of SCF gene transfer within the myocardium, 6 extra pigs were euthanized 1 week after the virus injection aside from the chronic study. Successful gene transfer was inferred from GFP expression along the needle track after the injections (Figure 1A). In the pigs injected with Ad.SCF, clusters of c-kit+ cells were found at the injection sites (median 340 cells/mm2, 25th to 75th percentile; 131-1277 cells/mm2) (Figure 1B), whereas almost no c-kit+ cells were detected in the control pig heart. Western blot analysis confirmed the increased expression of SCF at the injection sites in the pigs treated with Ad.SCF (Figure 1C).
Figure 1. Gene transfer efficacy and recruitment of c-kit+ cells 1 week after gene transfer.
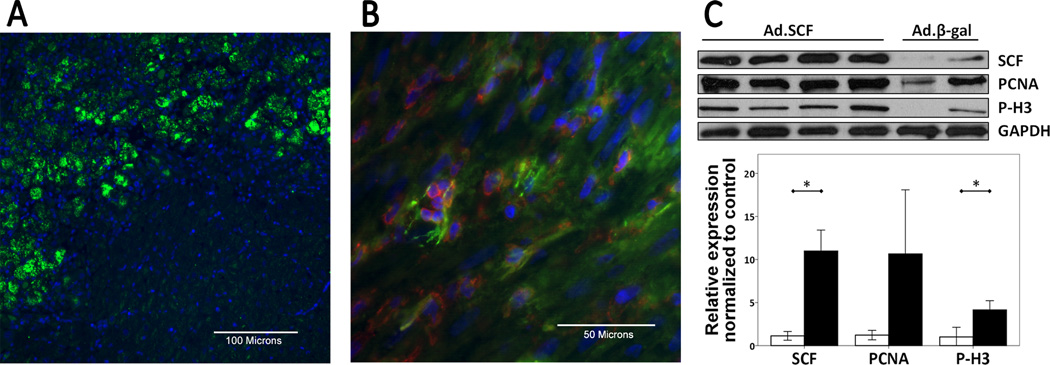
A. Infected area is clearly distinguishable by GFP expression. Blue; DAPI-stained nuclei, green; GFP. B. Confocal image represents c-kit membrane staining (Red). Clusters of c-kit+ cells were found at the injection sites after SCF gene transfer. Blue; DAPI-stained nuclei, red; c-kit, green; α-sarcomeric actin. C. SCF, PCNA, and P-H3 expression by Western blotting. Expression of SCF (P=0.001) as well as proliferation proteins (PCNA: P=0.08, P-H3: P=0.008) were increased in the SCF treated pigs.
*; P<0.05, Abbreviations: GFP = Green fluorescent protein, SCF = stem cell factor, PCNA = proliferation cell nuclear antigen, P-H3 = phospho-histone h3.
Improvement of cardiac function
There was a 12% relative increase in LVEF at 3Mo (absolute change +4.9%) in the Ad.SCF group compared to before gene transfer, whereas it decreased 4.2% (absolute change −1.6%, P = 0.004) in the Ad.β-gal group (Figure 2, Table 1). With respect to the 3DE analysis, image quality was suboptimal in 13% of the data sets due to the lung overlap presumably from post-surgical lung adhesions. However, volume data were assessable in all the measurements with intra-class correlation coefficient of 0.992. Repeated measures ANOVA revealed statistically significant improvements of LVEF after Ad.SCF therapy (Table 2) in 3DE analysis as well. Furthermore, stroke volume index assessed by 3DE also increased significantly after SCF treatment (Table 2). Pressure-volume loop analyses exhibited similar end-systolic pressure-volume relationship between the groups at 1Wk, however the slopes in the Ad.SCF group were steeper at 3Mo (Table 3, Figure 3). Though the slope of the end-systolic pressure-volume relationship did not reach a statistically significant difference, preload-recruitable stroke work (PRSW), another load-independent measure of cardiac contractility, was found to be significantly higher after Ad.SCF treatment (Table 3). Other parameters of systolic function were consistent with these findings including higher change in dP/dt maximum (Figure 2), higher LV maximum pressure, and increased cardiac index (Table 2), but they did not reach statistical significance. In contrast, diastolic functional parameters including dP/dt minimum, tau, and end-diastolic pressure-volume relationship did not exhibit any noteworthy trends between the treatment groups (Table 2).
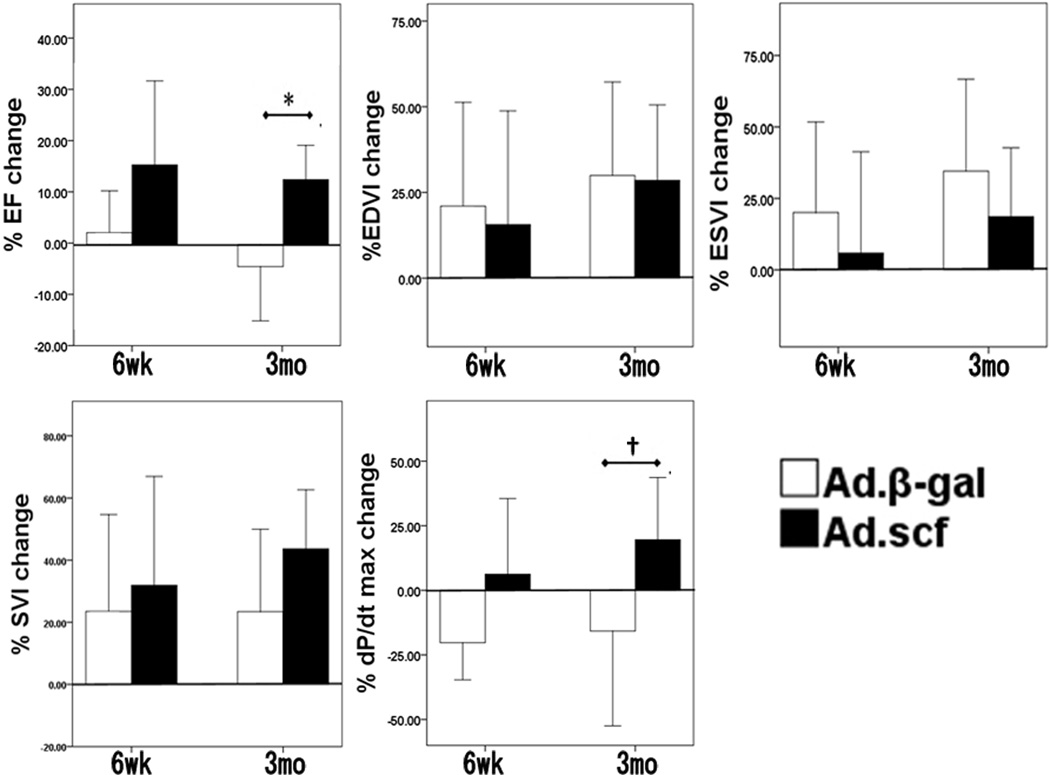
Figure 2. Percent change of functional and volumetric parameters from 1 week (before gene transfer) to 6 weeks and to 3 months.
Ad.SCF group showed significant improvement in EF (P=0.004) and trends towered improved dP/dt max (P=0.06) at 3 month. There were no statistically significant differences in EDVI (P=0.95), ESVI (P=0.35), and SVI changes (0.11) evaluated by left ventriculogram.
*; P<0.05, †; P=0.06, Abbreviations: EF = left ventricular ejection fraction, EDVI = end-diastolic volume index, ESVI = end-systolic volume index, SVI = stroke volume index, dP/dt max = dP/dt maximum
Table 1.
Baseline left ventriculogram data and dP/dt max
| Ad.-βgal | Ad.SCF | |
|---|---|---|
| EF (%) | 42.4±6.2 | 40.2±5.1 |
| EDVI (ml/m2) | 115.9±30.7 | 106.6±14.8 |
| ESVI (ml/m2) | 67.4±22.6 | 64.2±15.1 |
| SVI (ml/m2) | 48.3±10.2 | 42.3±3.0 |
| dP/dt max (mmHg/s) | 1666±373 | 1778±147 |
dP/dt max = dP/dt maximum, EF = ejection fraction, EDVI = end diastolic volume index, ESVI = end systolic volume index, SVI = stroke volume index
Table 2.
Temporal transition of hemodynamic and 3D echocardiographic parameters
| 1 Wk |
6 Wk |
3 Mo |
|||||
|---|---|---|---|---|---|---|---|
| Ad.-βgal | Ad.SCF | Ad.-βgal | Ad.SCF | Ad.-βgal | Ad.SCF | P | |
| Body weight (Kg) | 20.8±1.3 | 20.8±1.2 | 28.2±2.3 | 28.0±2.0 | 36.4±1.5 | 36.9±4.7 | 0.86 |
| Pressure catheter | |||||||
| Pmax (mmHg) | 93.1±10.4 | 91.3±10.5 | 99.1±3.9 | 105.5±23.0 | 108.2±24.9 | 126.4±17.3 | 0.08 |
| EDP (mmHg) | 19.6±5.0 | 19.7±6.8 | 18.5±6.1 | 14.8±3.9 | 19.1±6.1 | 18.0±5.4 | 0.73 |
| dP/dtmin (mmHg/s) | −1196±232 | −1174±338 | −1395±419 | −1475±620 | −1619±654 | −1853±377 | 0.57 |
| tau (ms) | 64.5±17.3 | 61.0±18.7 | 73.3±8.3 | 71.3±17.7 | 69.3±21.6 | 61.3±7.3 | 0.80 |
| HR (bpm) | 90.9±23 | 82.6±26 | 61.3±12.5 | 72.3±18.8 | 75.3±18.2 | 67.5±4.0 | 0.96 |
| Right heart catheter | |||||||
| CI (l/min/m2) | 4.3±1.0 | 3.6±0.9 | 3.3±0.4 | 3.5±0.8 | 3.6±1.2 | 3.9±0.7 | 0.34 |
| 3D echocardiography | |||||||
| EF (%) | 50.1±9.5 | 44.7±2.7 | 47.4±9.5 | 48.7±8.7 | 45.0±10.6 | 51.8±7.9 | 0.05 |
| EDVI (ml/m2) | 124.8±12.1 | 117.2±14.9 | 160.8±18.5 | 141.4±34.9 | 151.2±21.7 | 145.7±37.1 | 0.89 |
| ESVI (ml/m2) | 61.5±8.8 | 65.0±8.9 | 84.4±16.6 | 74.2±28.8 | 84.0±25.5 | 72.0±29.6 | 0.29 |
| SVI(ml/m2) | 62.3±7.8 | 52.7±7.8 | 78.7±22.4 | 66.8±13.1 | 68.4±14.9 | 74.2±12.0 | 0.05 |
Pmax = maximum pressure, EDP = end diastolic pressure, dP/dt min = dP/dt minimum, HR = heart rate, CI = cardiac index, EF = ejection fraction, EDVI = end diastolic volume index, ESVI = end systolic volume index, SVI = stroke volume index, P values are from repeated measures ANOVA
Table 3.
Pressure-volume loop relationships
| 1 Wk |
3 Mo |
||||
|---|---|---|---|---|---|
| Ad.-βgal | Ad.SCF | Ad.-βgal | Ad.SCF | P | |
| ESPVR | |||||
| Slope (mmHg/ml) | 2.24±0.90 | 2.07±0.84 | 1.66±0.45 | 2.16±0.59 | 0.33 |
| V0 (ml) | −27.3±23.2 | −22.1±27.7 | −16.3±17.1 | −16.8±17.7 | 0.72 |
| PRSW | |||||
| Slope (mmHg) | 35.4±12.4 | 31.5±5.9 | 31.6±12.6 | 55.5±11.6 | 0.005 |
| EDPVR | |||||
| Slope (mmHg/ml) | 0.69±0.46 | 0.88±0.42 | 0.51±0.19 | 0.63±0.22 | 0.74 |
ESPVR = end systolic pressure volume relationship, PRSW = preload recruitable stroke work, EDPVR = end diastolic pressure volume relationship, P values are from repeated measures ANOVA
Figure 3. Representative pressure-volume loops during inferior vena cava occlusion at 3 months post myocardial infarction.
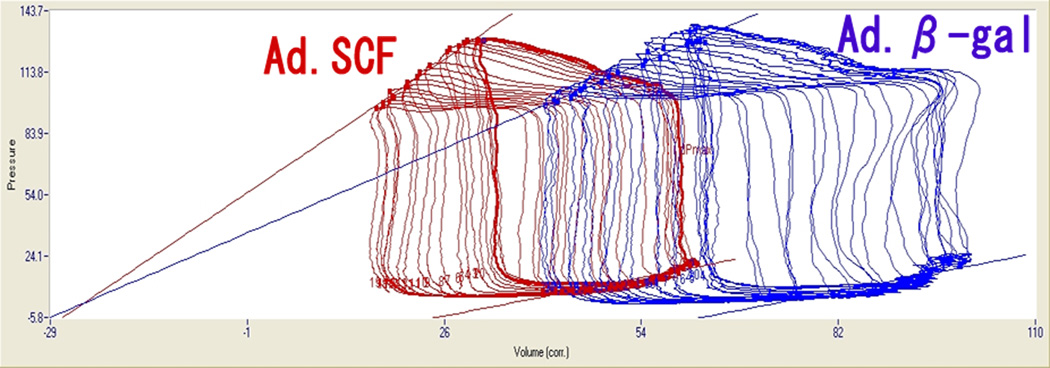
Left loop is from SCF treated pig and shows steeper end-systolic pressure-volume relationship with left ward shift relative to the right loop (Ad.β-gal).
Remodeling and infarct size
The growth of the animals was similar in both experimental groups. The size of the heart was not different between the groups at the time of gene transfer or at follow-up (Table 1, 2). Reflecting the size of the heart, ventricular weight to body weight ratios at 3Mo did not differ significantly between the groups. Likewise, the scar size was similar in both groups (Figure 4A). Wall motion score index, the best available estimate of infarct size in vivo with echocardiography19, showed a modest correlation to the actual scar size at 3Mo (r=0.66, P=0.007). Wall motion score index was similar between the groups at 1Wk which suggests similar scar size before the gene transfer (Figure 4B).
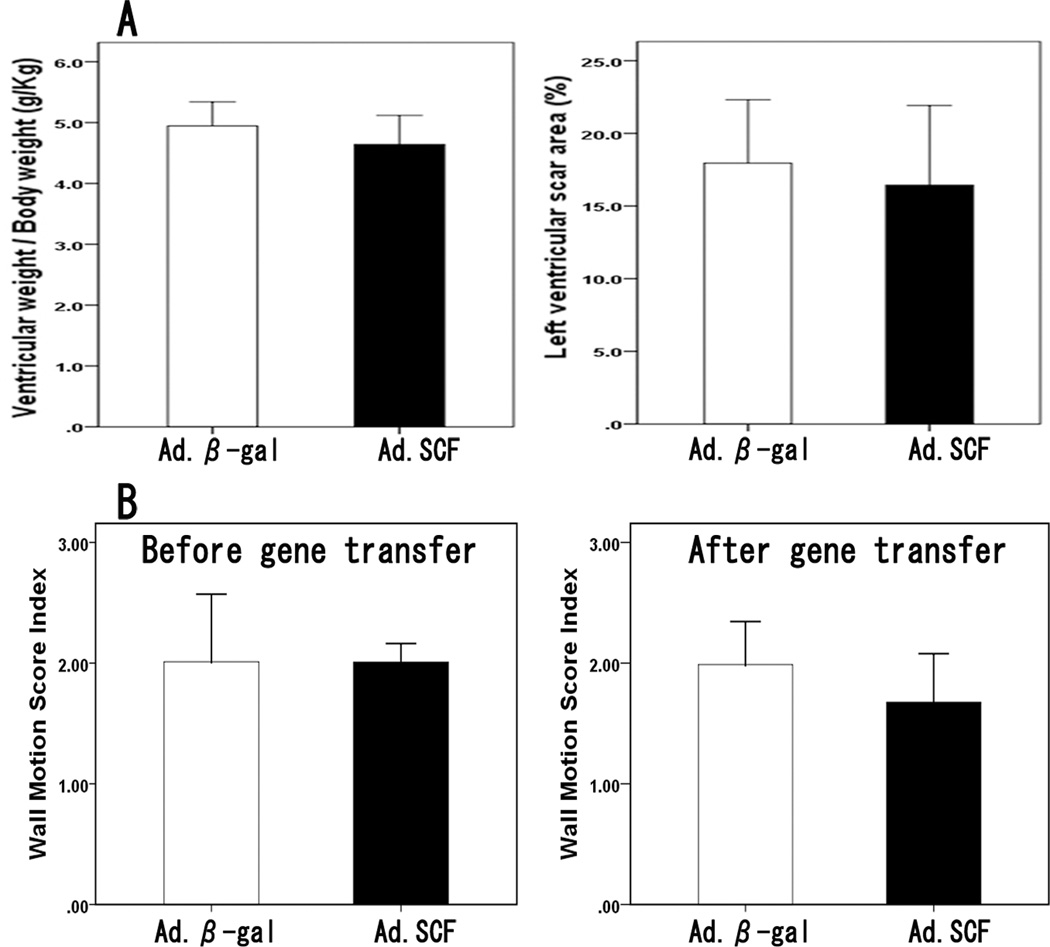
Figure 4. Weight of the heart and the scar size.
A. Ventricular weight/ body weight (g/Kg) was not different between the groups at 3 months (P=0.26). Left ventricular scar size was also similar after Ad.SCF (16.4 ± 5.5%) and Ad.β-gal injection (18.0 ± 4.4%, P = 0.61). B. Wall motion score index, an echocardiographic estimate of infarct size, was similar between the groups at 1 week which suggests similar scar size before the gene transfer (Ad.SCF, 2.01±0.16 vs Ad.β-gal, 2.01±0.56, P=0.98), whereas it was lower in the Ad.SCF group 3 months after the gene delivery without statistical significance (Ad.SCF, 1.68±0.40 vs Ad.β-gal, 1.99±0.36, P=0.18).
Angiogenesis
Co-staining of α-SMA and CD31 indicated a significant increase of angiogenesis after SCF treatment at 3Mo (Figure 5, P<0.001). Vasculogenesis was also assessed by isolectin IB4 staining. The density and the number of small vessels less than 10 μm in diameter were found to be increased after SCF gene transfer (Figure 5C).
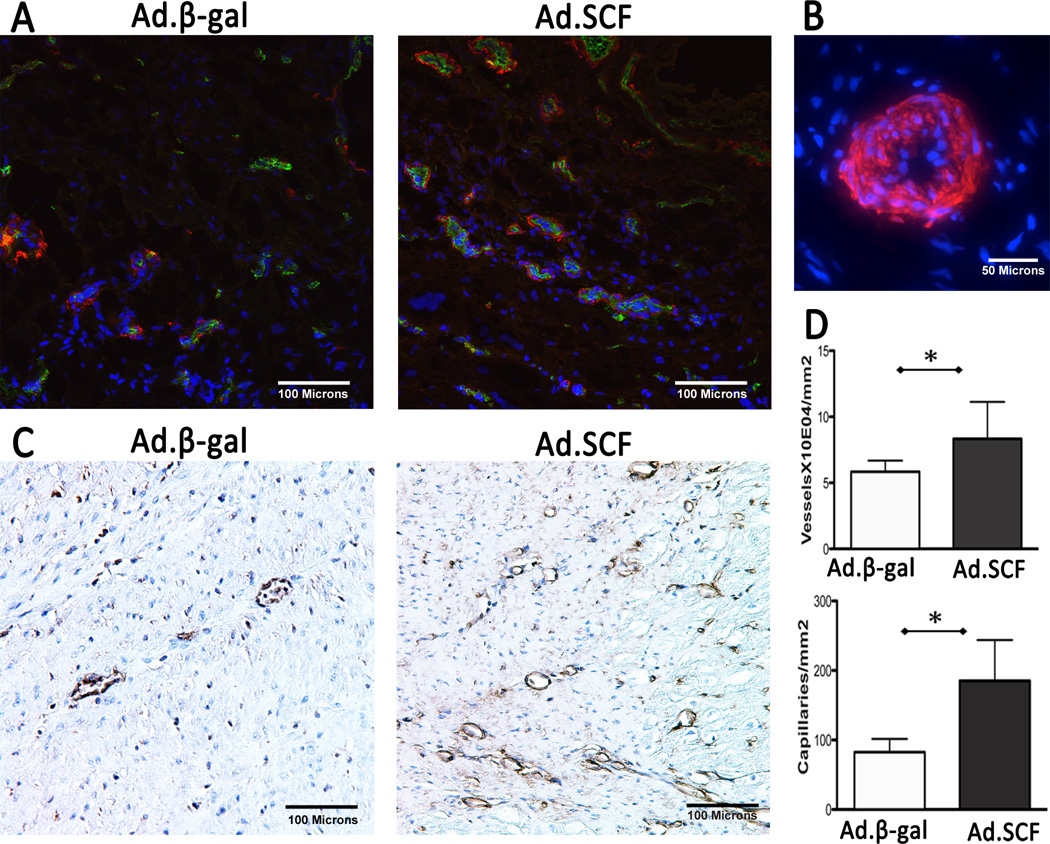
Figure 5. Angiogenesis and vasculogenesis at 3 months after myocardial infarction.
A. Increased numbers of vasculatures co-stained with α-SMA and CD31 were found after Ad.SCF injection compared to the Ad.β-gal. Blue; DAPI-stained nuclei, red; α-SMA, green; CD31. B. Presumably functional vessel stained with α-SMA. Multiple layers of nuclei are found in the vessel wall suggesting that this is a functional arteriole. C. A significant increase in capillaries co-expressing isolectin IB4 (brown) was found at the infarct border after SCF gene transfer. D. Quantification of vessels (P=0.01) and capillaries (P=0.003) suggests angiogenic role of SCF.
*; P<0.05 Abbreviations: α-SMA = α-smooth muscle actin, SCF = stem cell factor
Characterization of c-kit+ cells and sub-acute effects of SCF gene transfer
To further characterize the sub-acute effect of SCF gene transfer, we performed immunohistochemical analyses on infarct border tissue 1 week after the gene transfer. To inquire whether the c-kit+ cells are of bone marrow origin, these cells were co-stained with CD45 (Figure 6A). There were a few isolated cells both positive for c-kit and CD45, however most of the clustered c-kit+ cells were negative for CD45 as well as vascular endothelial growth factor receptor 2 (VEGFR2) (Supplementary Figure 1). Because Western Blot analyses revealed increased proliferation markers in the Ad.SCF group, we utilized double labeling techniques to identify specific cell types undergoing cell cycling. Figure6B clearly shows that a few cardiomyocytes are undergoing DNA replication. Proliferating cell nuclear antigen (PCNA) staining revealed that approximately one out of five PCNA positive cells were cardiomyocytes. To evaluate the effect of SCF on cell survival, TUNEL staining was performed. A significant decrease of TUNEL positive cardiomyocytes was detected after Ad.SCF therapy (Figure 6C, P<0.001).
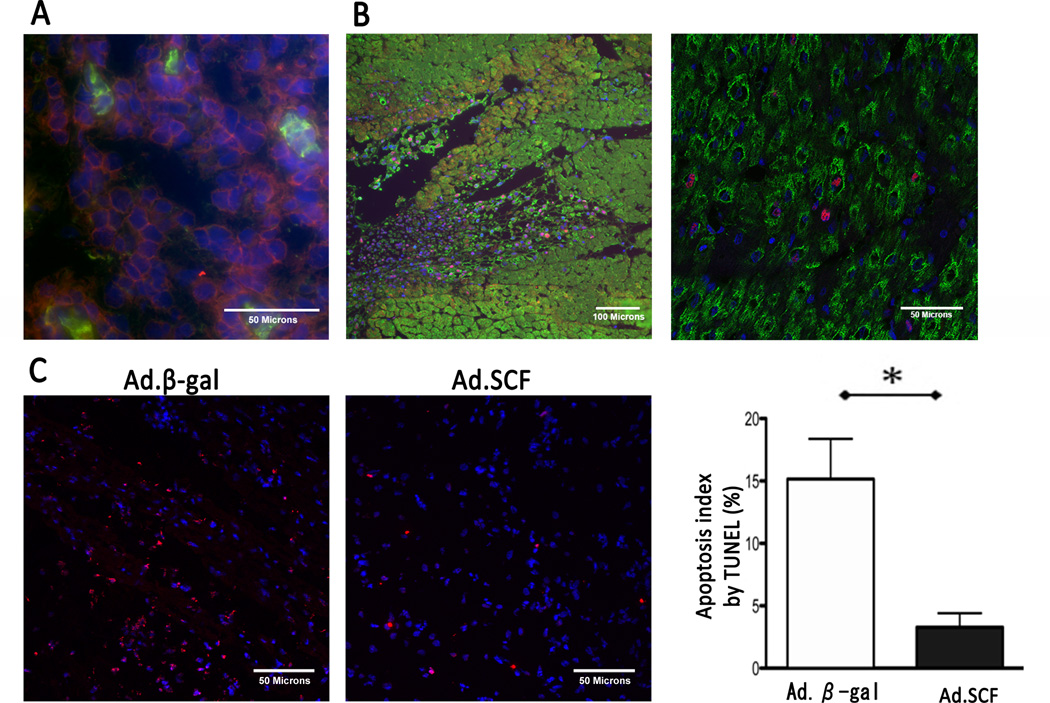
Figure 6. Characterization of infarct border zone 1 week after the gene transfer.
A. A few isolated c-kit+/CD45+ cells were found in clusters of c-kit+/CD45− cells. Blue; DAPI-stained nuclei, red; c-kit, green; CD45. B. Immunohistochemistry revealed increased PCNA expression at the injection site 1 week after gene transfer. Higher magnification image (right) shows that PCNA was expressed in both cardiomyocytes and non-myocytes. Blue; DAPI-stained nuclei, red; PCNA, green; α-sarcomeric actin. C. Representative examples of TUNEL staining and apoptosis index. Decreased apoptotic cells were found at the infarct border area after Ad.SCF injection (Apoptosis index: P<0.001). Blue; DAPI-stained nuclei, red; apoptotic nuclei. *; P<0.05
Discussion
The present study demonstrated that the over-expression of membrane-bound human SCF by adenoviral gene transfer in the sub-acute phase of MI improves cardiac systolic function three months following MI. Using a porcine model of MI induced LV dysfunction, we validated the efficacy of SCF gene therapy in a clinically relevant model of HF. Our results are consistent with the positive effects of SCF treatment on cardiac function observed in small animals, and supports the possible translation of this therapeutic approach to the clinic.
The improvement in LVEF was significantly better after Ad.SCF treatment assessed by left ventriculography, and this observation was confirmed by blinded analyses of 3DE. Although they did not reach statistical significance, higher dP/dt maximum and steeper slope of ESPVR after Ad.SCF treatment support the conclusion of improved cardiac function through this gene therapy based cytokine therapy. Moreover, PRSW increased significantly in the Ad.SCF group. PRSW is shown to be independent of heart size20, more reproducible compared to ESPVR, and afterload independent21. Thus, we believe it is valid to rely on PRWS as a key indicator of SCF gene transfer efficacy, since the heart size differs between individual animals. Furthermore, afterload may differ depending on the severity of HF as well as sensitivity to the anesthetic agents between the animals.
We postulate that the functional improvement in our study is in part due to enhanced angiogenesis and increased cell survival. Pigs treated with SCF showed increased angiogenesis at 3Mo. The presence of small vessels with multiple layers of smooth muscle cells suggests functional angiogenesis. Increased vasculogenesis at 3Mo after Ad.SCF treatment also indicates the important role of SCF in the formation of capillaries. In addition, while keeping in mind a small sample number, higher relative coronary flow at the infarct border (Supplementary Figure 2) supports functional angiogenesis by SCF treatment and the potential for increased cardiac perfusion. SCF over-expression also resulted in decreased apoptotic cardiomyocytes 1 week after the gene transfer as demonstrated by decreased TUNEL positive cells.
To our knowledge, this is the first demonstration of SCF gene transfer efficacy in a large animal model of heart failure. Beneficial effects of SCF treatment have been previously shown in small animal models of MI. Systemic injection of SCF and granulocyte colony-stimulating factor (G-CSF) after MI improved cardiac function together with other beneficial effects in mice22, 23. A single local injection of SCF protein into the peri-infarct with intravenous injections of c-kit+ cells increased homing of c-kit+ cells in a mouse MI model, however failed to show functional improvement24. In that study, the amount of c-kit+ cells found at the SCF injected sites was small, and the authors concluded that it was not enough to improve cardiac function. In contrast, repeated administration of SCF and SDF-1 gene using a microbubble destruction method in rats improved cardiac function, increased angiogenesis, and decreased infarct size8. Likewise, sustained expression of SCF by gene transfer7, 12, 14, 25, or by injection of SCF–overproducing mesenchymal stem cells successfully improved cardiac function in rodents6. Our study is consistent with these previous reports, establishing the translation of SCF treatment from rodents to large animals. However, despite the dramatic reduction of infarct size detected in the smaller animals25, we did not find a significant difference in the infarct size nor in the weight of the heart after Ad.SCF injection in swine. This may derive from the disparity in size; the pig heart is about a thousand times heavier, whereas the amount of virus used was only five to ten times more than in the rat study.
Gene therapy and stem cell therapy are both new emerging fields in cardiac treatment. Our study allows for comparison of gene therapy to recent reports of cell-based SCF delivery. Although increased SCF levels and improved cardiac function were confirmed after stem cell injection into the myocardium6, we chose gene transfer as a means of treatment in our study, because augmenting the level of a specific paracrine factor can be achieved by gene therapy. The combination of various paracrine factors in cell therapy may provide synergic effects by involving multiple pathways, however this complicates interpretation of the results, and the effects can sometimes be deleterious. For example, intracoronary stem cell injection with G-CSF improved cardiac function, but was accompanied by an increased risk of restenosis after coronary stenting26. In contrast, gene transfer activates only the pathways involving the specified factor. Moreover, gene therapy has an advantage of potentially achieving a higher level of targeted protein expression compared to stem cell injection. Previously, Fazel et al. reported tumor development after the injection of mesenchymal stem cells engineered to over-express SCF27. The authors concluded that the combination of mesenchymal stem cells with SCF over-expression was the probable cause, although a prolonged cell culture period may have affected the cell quality. Our method does not require exogenous cell injection and may be able to circumvent such complications with natural or modified exogenous cells.
Despite the improvement in systolic function, diastolic function did not differ between Ad.SCF and Ad.β-gal treatments. This may be attributed to the delivery method and the choice of vector. We found adhesions of the pericardium in all of the pigs, which may have impeded ventricular expansion, and varying degrees of inflammatory response were found at the infarct in both groups. Both adenovirus and direct intramyocardial injection have been reported to induce inflammation. Thus, while we have demonstrated the beneficial effects of SCF gene therapy for ischemic HF, a different route of gene delivery as well as a different vector may minimize the inflammatory response and maximize the effect of SCF gene transfer that we detected.
Encouraging results in early clinical trials that used c-kit+ cells have been recently reported. In the SCIPIO study, 20 patients those received intracoronary injections of c-kit+ cells had striking improvement in LVEF as well as significant reduction of infarct size up to 12 months28. The CADUCEUS trial used cardiosphere-derived cells that contain a mixed population including c-kit+ progenitors, and also showed significant reduction in infarct size together with improved regional contractility29. Furthermore, Hare et al. reported a combination of c-kit+ cells and mesenchymal stem cells enhanced the effect of therapeutic efficacy30. These studies highlight the promising potential of c-kit+ cells over other types of stem cells. Previous studies in rodents reported the migration of bone marrow derived c-kit+ cells after SCF treatment in cardiac repair22, 24, 31. Similarly, Fazel et al demonstrated that c-kit+ cells are increased after MI accompanied by increased SCF expression, and majority of c-kit+ cells found in the infarcted myocardium were from bone marrow using a bone marrow chimeric mouse9. In contrast, our results do not support these findings, since most of c-kit+ cells found at the injection sites were negative for CD45 as well as VEGFR2. The discrepancy may be explained by the differences in studied time points or type of species. This also highlights the importance of present study in large animals which have much closer physiological profiles to human. Although the actual origin of c-kit+ cells in our study remains to be elucidated, these cells could be of cardiac origin as suggested by others23, 25. Thus, in addition to the recruitment of bone marrow derived stem cells, SCF therapy may be activating the cardiac resident c-kit+ cells and enhancing the endogenous repair.
Limitations
Our study in this large animal model has a number of limitations. The mechanism by which SCF induces functional improvement cannot be exactly assessed in this large animal model since the precise origin of the c-kit+ cells cannot be exactly assessed. Nevertheless, c-kit+ cell recruitment, apoptosis inhibition, and increased angiogenesis are consistent findings with previous studies in small animals, and we believe demonstrating the functional improvement in a large animal study is a critical step in translating SCF therapy to clinical application. Due to our study design and the difficulty in endogenous cell labeling in large animals, the presence of cardiomyocyte regeneration was not demonstrated. Future study is required to determine the regenerative property of SCF gene therapy focusing on the fate of c-kit+ cells. Relatively short follow-up in view of treating patients is another common limitation of large animal studies. van der Spoel et al. performed meta-analysis on the effect of cardiac stem cell therapy in large animals, and reported that the effect of cell therapy declines after 8 weeks32. In contrast, we found a consistent trend of improved systolic function at 6Wk, and at 3Mo. It is notable that at these time points, the SCF over-expression is presumably minimal due to the transient nature of adenoviral gene transfer. However, longer term follow-ups are required to establish the full potential of SCF gene transfer as an efficacious approach to treat heart failure.
Conclusion
In a swine model of MI, gene transfer of SCF at the ischemic border area improved systolic function up to 3 months post-treatment. Our results advance the potential of SCF gene transfer as a future treatment option for ischemic heart failure.
Supplementary Material
Acknowledgements
The authors thank Lauren Leonardson for providing excellent technical assistance and expertise.
Sources of Funding
This work is supported by NIH R01 HL117505, HL 119046, a NHLBI Program of Excellence in Nanotechnology (PEN) Award, Contract # HHSN268201000045C, a P50 HL112324, and a Transatlantic Fondation Leducq grant. J. Aguero was supported by Spanish Society of Cardiology (Ischemic Heart Disease section) and Fundacion Alfonso Martin-Escudero.
Footnotes
Disclosures
None.
References
- 1.Bui AL, Horwich TB, Fonarow GC. Epidemiology and risk profile of heart failure. Nat Rev Cardiol. 2011;8:30–41. doi: 10.1038/nrcardio.2010.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murry CE, Soonpaa MH, Reinecke H, Nakajima H, Nakajima HO, Rubart M, Pasumarthi KB, Virag JI, Bartelmez SH, Poppa V, Bradford G, Dowell JD, Williams DA, Field LJ. Haematopoietic stem cells do not transdifferentiate into cardiac myocytes in myocardial infarcts. Nature. 2004;428:664–668. doi: 10.1038/nature02446. [DOI] [PubMed] [Google Scholar]
- 3.Wagers AJ, Sherwood RI, Christensen JL, Weissman IL. Little evidence for developmental plasticity of adult hematopoietic stem cells. Science. 2002;297:2256–2259. doi: 10.1126/science.1074807. [DOI] [PubMed] [Google Scholar]
- 4.Gnecchi M, He H, Liang OD, Melo LG, Morello F, Mu H, Noiseux N, Zhang L, Pratt RE, Ingwall JS, Dzau VJ. Paracrine action accounts for marked protection of ischemic heart by Akt-modified mesenchymal stem cells. Nat Med. 2005;11:367–368. doi: 10.1038/nm0405-367. [DOI] [PubMed] [Google Scholar]
- 5.Maltais S, Tremblay JP, Perrault LP, Ly HQ. The paracrine effect: pivotal mechanism in cell-based cardiac repair. J Cardiovasc Transl Res. 2010;3:652–662. doi: 10.1007/s12265-010-9198-2. [DOI] [PubMed] [Google Scholar]
- 6.Fazel S, Chen L, Weisel RD, Angoulvant D, Seneviratne C, Fazel A, Cheung P, Lam J, Fedak PW, Yau TM, Li RK. Cell transplantation preserves cardiac function after infarction by infarct stabilization: augmentation by stem cell factor. J Thorac Cardiovasc Surg. 2005;130:1310. doi: 10.1016/j.jtcvs.2005.07.012. [DOI] [PubMed] [Google Scholar]
- 7.Xiang FL, Lu X, Hammoud L, Zhu P, Chidiac P, Robbins J, Feng Q. Cardiomyocyte-specific overexpression of human stem cell factor improves cardiac function and survival after myocardial infarction in mice. Circulation. 2009;120:1065–1074. doi: 10.1161/CIRCULATIONAHA.108.839068. 9 p following 1074. [DOI] [PubMed] [Google Scholar]
- 8.Fujii H, Li SH, Wu J, Miyagi Y, Yau TM, Rakowski H, Egashira K, Guo J, Weisel RD, Li RK. Repeated and targeted transfer of angiogenic plasmids into the infarcted rat heart via ultrasound targeted microbubble destruction enhances cardiac repair. Eur Heart J. 2011;32:2075–2084. doi: 10.1093/eurheartj/ehq475. [DOI] [PubMed] [Google Scholar]
- 9.Fazel S, Cimini M, Chen L, Li S, Angoulvant D, Fedak P, Verma S, Weisel RD, Keating A, Li RK. Cardioprotective c-kit+ cells are from the bone marrow and regulate the myocardial balance of angiogenic cytokines. J Clin Invest. 2006;116:1865–1877. doi: 10.1172/JCI27019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li M, Naqvi N, Yahiro E, Liu K, Powell PC, Bradley WE, Martin DI, Graham RM, Dell'Italia LJ, Husain A. c-kit is required for cardiomyocyte terminal differentiation. Circ Res. 2008;102:677–685. doi: 10.1161/CIRCRESAHA.107.161737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ayach BB, Yoshimitsu M, Dawood F, Sun M, Arab S, Chen M, Higuchi K, Siatskas C, Lee P, Lim H, Zhang J, Cukerman E, Stanford WL, Medin JA, Liu PP. Stem cell factor receptor induces progenitor and natural killer cell-mediated cardiac survival and repair after myocardial infarction. Proc Natl Acad Sci U S A. 2006;103:2304–2309. doi: 10.1073/pnas.0510997103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sun Z, Lee CJ, Mejia-Guerrero S, Zhang Y, Higuchi K, Li RK, Medin JA. Neonatal transfer of membrane-bound stem cell factor improves survival and heart function in aged mice after myocardial ischemia. Human gene therapy. 2012;23:1280–1289. doi: 10.1089/hum.2012.063. [DOI] [PubMed] [Google Scholar]
- 13.Bolli R, Chugh AR, D'Amario D, Loughran JH, Stoddard MF, Ikram S, Beache GM, Wagner SG, Leri A, Hosoda T, Sanada F, Elmore JB, Goichberg P, Cappetta D, Solankhi NK, Fahsah I, Rokosh DG, Slaughter MS, Kajstura J, Anversa P. Cardiac stem cells in patients with ischaemic cardiomyopathy (SCIPIO): initial results of a randomised phase 1 trial. Lancet. 2011;378:1847–1857. doi: 10.1016/S0140-6736(11)61590-0. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 14.Higuchi K, Ayach B, Sato T, Chen M, Devine SP, Rasaiah VI, Dawood F, Yanagisawa T, Tei C, Takenaka T, Liu PP, Medin JA. Direct injection of kit ligand-2 lentivirus improves cardiac repair and rescues mice post-myocardial infarction. Mol Ther. 2009;17:262–268. doi: 10.1038/mt.2008.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yaniz-Galende E, Chen J, Chemaly E, Liang L, Hulot JS, McCollum L, Arias T, Fuster V, Zsebo KM, Hajjar RJ. Stem cell factor gene transfer promotes cardiac repair after myocardial infarction via in situ recruitment and expansion of c-kit+ cells. Circ Res. 2012;111:1434–1445. doi: 10.1161/CIRCRESAHA.111.263830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Position of the American Heart Association on research animal use. Circulation. 1985;71:849A–850A. [PubMed] [Google Scholar]
- 17.Ishikawa K, Chemaly ER, Tilemann L, Fish K, Ladage D, Aguero J, Vahl T, Santos-Gallego C, Kawase Y, Hajjar RJ. Assessing left ventricular systolic dysfunction after myocardial infarction: are ejection fraction and dP/dt(max) complementary or redundant? Am J Physiol Heart Circ Physiol. 2012;302:H1423–H1428. doi: 10.1152/ajpheart.01211.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ishikawa K, Ladage D, Tilemann L, Fish K, Kawase Y, Hajjar RJ. Gene transfer for ischemic heart failure in a preclinical model. J Vis Exp. 2011;(51):e2778. doi: 10.3791/2778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gjesdal O, Hopp E, Vartdal T, Lunde K, Helle-Valle T, Aakhus S, Smith HJ, Ihlen H, Edvardsen T. Global longitudinal strain measured by two-dimensional speckle tracking echocardiography is closely related to myocardial infarct size in chronic ischaemic heart disease. Clin Sci (Lond) 2007;113:287–296. doi: 10.1042/CS20070066. [DOI] [PubMed] [Google Scholar]
- 20.Burkhoff D, Mirsky I, Suga H. Assessment of systolic and diastolic ventricular properties via pressure-volume analysis: a guide for clinical, translational, and basic researchers. Am J Physiol Heart Circ Physiol. 2005;289:H501–H512. doi: 10.1152/ajpheart.00138.2005. [DOI] [PubMed] [Google Scholar]
- 21.Little WC, Cheng CP, Mumma M, Igarashi Y, Vinten-Johansen J, Johnston WE. Comparison of measures of left ventricular contractile performance derived from pressure-volume loops in conscious dogs. Circulation. 1989;80:1378–1387. doi: 10.1161/01.cir.80.5.1378. [DOI] [PubMed] [Google Scholar]
- 22.Orlic D, Kajstura J, Chimenti S, Limana F, Jakoniuk I, Quaini F, Nadal-Ginard B, Bodine DM, Leri A, Anversa P. Mobilized bone marrow cells repair the infarcted heart, improving function and survival. Proc Natl Acad Sci U S A. 2001;98:10344–10349. doi: 10.1073/pnas.181177898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kanellakis P, Slater NJ, Du XJ, Bobik A, Curtis DJ. Granulocyte colony-stimulating factor and stem cell factor improve endogenous repair after myocardial infarction. Cardiovasc Res. 2006;70:117–125. doi: 10.1016/j.cardiores.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 24.Lutz M, Rosenberg M, Kiessling F, Eckstein V, Heger T, Krebs J, Ho AD, Katus HA, Frey N. Local injection of stem cell factor (SCF) improves myocardial homing of systemically delivered c-kit + bone marrow-derived stem cells. Cardiovasc Res. 2008;77:143–150. doi: 10.1093/cvr/cvm027. [DOI] [PubMed] [Google Scholar]
- 25.Yaniz-Galende E, Chen J, Chemaly ER, Liang L, Hulot JS, McCollum L, Arias T, Fuster V, Zsebo K, Hajjar RJ. Stem Cell Factor Gene Transfer Promotes Cardiac Repair After Myocardial Infarction via In Situ Recruitment and Expansion of c-kit+ Cells. Circ Res. 2012 doi: 10.1161/CIRCRESAHA.111.263830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kang HJ, Kim HS, Zhang SY, Park KW, Cho HJ, Koo BK, Kim YJ, Soo Lee D, Sohn DW, Han KS, Oh BH, Lee MM, Park YB. Effects of intracoronary infusion of peripheral blood stem-cells mobilised with granulocyte-colony stimulating factor on left ventricular systolic function and restenosis after coronary stenting in myocardial infarction: the MAGIC cell randomised clinical trial. Lancet. 2004;363:751–756. doi: 10.1016/S0140-6736(04)15689-4. [DOI] [PubMed] [Google Scholar]
- 27.Fazel SS, Angoulvant D, Butany J, Weisel RD, Li RK. Mesenchymal stem cells engineered to overexpress stem cell factor improve cardiac function but have malignant potential. J Thorac Cardiovasc Surg. 2008;136:1388–1389. doi: 10.1016/j.jtcvs.2007.11.068. [DOI] [PubMed] [Google Scholar]
- 28.Chugh AR, Beache GM, Loughran JH, Mewton N, Elmore JB, Kajstura J, Pappas P, Tatooles A, Stoddard MF, Lima JA, Slaughter MS, Anversa P, Bolli R. Administration of cardiac stem cells in patients with ischemic cardiomyopathy: the SCIPIO trial: surgical aspects and interim analysis of myocardial function and viability by magnetic resonance. Circulation. 2012;126:S54–S64. doi: 10.1161/CIRCULATIONAHA.112.092627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Makkar RR, Smith RR, Cheng K, Malliaras K, Thomson LE, Berman D, Czer LS, Marban L, Mendizabal A, Johnston PV, Russell SD, Schuleri KH, Lardo AC, Gerstenblith G, Marban E. Intracoronary cardiosphere-derived cells for heart regeneration after myocardial infarction (CADUCEUS): a prospective, randomised phase 1 trial. Lancet. 2012;379:895–904. doi: 10.1016/S0140-6736(12)60195-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Williams AR, Hatzistergos KE, Addicott B, McCall F, Carvalho D, Suncion V, Morales AR, Da Silva J, Sussman MA, Heldman AW, Hare JM. Enhanced effect of combining human cardiac stem cells and bone marrow mesenchymal stem cells to reduce infarct size and to restore cardiac function after myocardial infarction. Circulation. 2013;127:213–223. doi: 10.1161/CIRCULATIONAHA.112.131110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dawn B, Guo Y, Rezazadeh A, Huang Y, Stein AB, Hunt G, Tiwari S, Varma J, Gu Y, Prabhu SD, Kajstura J, Anversa P, Ildstad ST, Bolli R. Postinfarct cytokine therapy regenerates cardiac tissue and improves left ventricular function. Circ Res. 2006;98:1098–1105. doi: 10.1161/01.RES.0000218454.76784.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van der Spoel TI, Jansen of Lorkeers SJ, Agostoni P, van Belle E, Gyongyosi M, Sluijter JP, Cramer MJ, Doevendans PA, Chamuleau SA. Human relevance of pre-clinical studies in stem cell therapy: systematic review and meta-analysis of large animal models of ischaemic heart disease. Cardiovasc Res. 2011;91:649–658. doi: 10.1093/cvr/cvr113. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.