Abstract
Background
Despite the application of proven adult heart failure (HF) therapies to children with idiopathic dilated cardiomyopathy (IDC), prognosis remains poor. Clinical experience with phosphodiesterase 3 inhibitors (PDE3i) in pediatric IDC patients, however, demonstrates improved HF symptoms without the increased incidence of sudden death seen in adults treated with PDE3i. We sought to determine age-related differences in PDE activity and associated intracellular signaling responsible for the efficacy and relative safety of chronic PDE3i in pediatric HF.
Methods and Results
Cyclic adenosine monophosphate (cAMP) levels, PDE activity, and phospholamban phosphorylation (pPLB) were determined in explanted human left ventricular myocardium (pediatric n=41, adult n=88). Adults and children with IDC (not treated with PDE3i) had lower cAMP and pPLB compared with non-failing controls. In contrast to their adult counterparts, pediatric IDC patients chronically treated with PDE3i had elevated cAMP (p=0.0403) and pPLB (p=0.0119). Additionally, total PDE and PDE3 specific activities were not altered in pediatric IDC patients on PDE3i, while adult IDC patients on PDE3i demonstrated higher total PDE (74.6 pmol/mg/min ± 13.8) and PDE3 (48.2 pmol/mg/min ± 15.9) specific activities in comparison to those of non-failing controls (59.5 pmol/mg/min ± 14.4 and 35.5 pmol/mg/min ± 12.8, respectively).
Conclusions
Elevated cAMP and higher pPLB may contribute to sustained hemodynamic benefits in pediatric IDC patients treated with PDE3i. In contrast, higher total PDE and PDE3 activities in adult IDC patients treated with PDE3i may perpetuate lower myocardial cAMP and pPLB levels, limiting the potential benefits of PDE3i therapy.
Keywords: phosphodiesterase activity, idiopathic dilated cardiomyopathy, cyclic adenosine monophosphate, pediatric heart failure, phospholamban
Dilated cardiomyopathy is the most common cause of heart failure (HF) in children, and it carries a poor clinical prognosis. Within one year of diagnosis, nearly one third of pediatric patients with dilated cardiomyopathy either die or undergo heart transplantation.1 Surprisingly, no substantial improvement in survival has been observed in children with dilated cardiomyopathy over the past three decades, and five-year freedom from death or transplant remains low at 54%-63%.1-4 The treatment of children with idiopathic dilated cardiomyopathy (IDC) has largely mirrored that of adults, though a recent review suggests that pediatric patients with IDC do not benefit from angiotensin-converting enzyme inhibitor (ACEi) and β-blocker (BB) therapies to the same extent as adults.2,5 Furthermore, differential adaptation of β-adrenergic receptors and adrenergic signaling pathways in children with HF when compared with adults suggest that age-related differences may influence response to therapy.6 This differential response to pharmacotherapy suggests that the pathophysiology of IDC is different in children and adults, highlighting the need for age-specific investigation and treatment.
Milrinone, a phosphodiesterase 3 inhibitor (PDE3i), is often used as a bridge to transplant or recovery in children with HF due to its ability to improve myocardial performance without increasing afterload.7 PDE3i in children with HF improves symptoms and decreases signs of HF on examination.8, 9 Numerous studies have documented beneficial, short-term hemodynamic effects of PDE3i in adult HF patients10-13, thus milrinone has been used in children with IDC and severe HF. Nevertheless, despite acute hemodynamic benefits, clinical trials of PDE3i in adults with severe HF have not shown improvements in major clinical outcomes14, 15 and demonstrate a 34% relative increase in cardiovascular mortality.16 Several adult trials document trends toward increased transient ventricular arrhythmias associated with the use of milrinone, ranging in incidence from 12.2% - 16%.12, 17 While pediatric studies of PDE3i have primarily focused on milrinone use for low cardiac output following congenital heart surgery,18-22 clinical experience suggests that arrhythmias and sudden death are extremely rare in children treated with PDE3i’s, and that its use is safe on a chronic basis.9
PDEs are enzymes that hydrolyze the second messengers cAMP and/or cGMP, with PDE1 and PDE3 being the major cAMP-hydrolyzing PDE families in human cardiomyocytes.23, 24 Downstream effects of cAMP are classically attributed to the phosphorylation of proteins that affect excitation/contraction coupling, including the sarcoplasmic reticulum ATPase 2 (SERCA2) regulatory protein phospholamban (PLB).25 cAMP-mediated signaling is compartmentalized intracellularly and PDE3A is localized in a microdomain with SERCA2 and PLB.26 cAMP levels are markedly decreased in failing adult human myocardium.27, 28 Thus, PDE3i potentially increases cAMP levels, resulting in protein kinase A (PKA)-mediated PLB phosphorylation, which decreases its inhibition of SERCA2. Increased SERCA2 activity increases sarcoplasmic reticulum calcium uptake, contributing to increased inotropy.
We hypothesized that differences in age-related responses may underlie the clinical differences seen in children and adults with HF treated with PDE3i’s. Here we show that PDE activity and associated intracellular signaling are differentially regulated in adult and pediatric IDC patients with HF treated chronically with PDE3i’s.
Methods
Human samples
All subjects gave informed consent and donated their hearts to the Institutional Review Board-approved Pediatric or Adult Cardiac Transplant Tissue Bank at the University of Colorado Denver. To minimize the possible confounding issues of pubertal transitions and heterogeneities within HF populations, only children and adults with IDC were included in the study, and all children were pre-pubertal (≤12 years). All adult patients with IDC had non-ischemic cardiomyopathy without any definitive contributing comorbidity. Patients with IDC were divided into two groups: (1) IDC patients not treated with PDE3i (F) and (2) IDC patients treated with PDE3i (FT). Both adult and pediatric patients in the FT groups are classified as having had “chronic” PDE3i therapy of greater than 48 hours (“short-term” PDE3i treatment has been previously defined as 48 hours of treatment or less14). F and FT groups were also compared to non-failing (NF) controls. NF tissues were from organ donors with normal heart function, whose hearts could not be placed for technical reasons (size or blood type mismatch). At the time of cardiac transplantation or donation, the left ventricle (LV) was rapidly dissected, flash frozen and stored at −80°C until further use.
cAMP quantitation
cAMP levels were measured by ELISA in the core facility at Children’s Hospital Colorado, Aurora, CO using the R&D Parameter immunoassay kit (R&D Systems, Minneapolis, MN) according to manufacturer’s recommendations.
Preparation of subcellular fractions from human LV myocardium
Approximately 150 mg of LV myocardium was homogenized and separated into nuclear, cytosolic and sarcoplasmic reticulum-enriched microsomal fractions by differential sedimentation (protocol adapted from previously published methods).29 Briefly, the tissue was homogenized using a Kinematica Polytron homogenizer for two 5-second cycles in 5 volumes of 0.29 mol/L sucrose, 10 mmol/L 3-[N-morpholino]propanesulfonic acid, 1 mmol/L benzamidine, 2 mmol/L EGTA, phosphatase inhibitor, and protease inhibitor (“sucrose buffer”). The sarcoplasmic reticulum-enriched microsomal fraction, containing SERCA2, PLB and PDE3, was isolated by differential sedimentation at 7,700 × g and then at 113,000 × g as previously described.29 The sarcoplasmic reticulum-enriched microsomal fraction was used in experiments to measure both total PDE and PDE3 specific activities.
Measurement of cAMP-hydrolytic activity
cAMP-hydrolytic activity was quantified at 30°C by the two-step snake-venom method with [3H]cAMP (1 μmol/L) as substrate.30 Total cAMP-hydrolytic activity was quantified by measuring activity without addition of PDE inhibitor. PDE3 activity was quantified by measuring activity in the absence and presence of 0.1 μmol/L cilostazol, a concentration that inhibits rtPDE3A submaximally (IC44). PDE3 activity was calculated by dividing the difference in activity in the presence and absence of cilostazol by the fractional inhibition of PDE3 activity at this concentration.31 The amount of protein used per assay and the incubation times were adjusted to ensure that no more than 20% of the total cAMP was hydrolyzed during the assay.
PLB Western blot
Western blots were performed as described previously.32 Protein was isolated from 10- to 25-mg frozen LV tissue in isoelectric focusing buffer homogenized at 4°C as described.33 Serine 16 (Ser16) PLB phosphorylation (pPLB) (A010-12 - Badrilla) and total PLB (05-205 - Millipore) were quantified on separate blots and normalized to GAPDH (Santa Cruz Biotechnology). PLB is phosphorylated at the Ser16 residue by protein kinase A, which is activated by cAMP. Blots were quantified using ImageJ version 1.46r.
Data analysis and statistics
Statistical analyses were performed using StatView version 5.0 (SAS Institute Inc., Cary, NC, USA). Statistical significance was set a priori at p<0.05 and all data are presented as mean±SEM in the figures. Normality of data was confirmed and non-normally distributed data were log transformed prior to statistical testing. Comparison of the three groups was conducted using one-way ANOVA and, if the overall comparison reached significance, Fisher’s PLSD post hoc tests were performed. Simple linear regression was performed to investigate any relationship between non-PDE3i inotrope usage and PDE activity, as well as determine any association between duration of PDE3i treatment and PDE activity.
Results
Subject characteristics
Pediatric and adult subject characteristics and analyses performed on each sample are listed in Supplemental Table S1 and S2, respectively. Median age at tissue collection for pediatric NF subjects was 7.5 years with an interquartile range (IQR) of 6.9 years; for pediatric F subjects, 3.5 years with an IQR of 3.9 years: and, for pediatric FT subjects, 3.0 years with an IQR of 8.6 years. Median age at tissue collection for adult NF subjects was 54 years with an IQR of 14 years; for adult F subjects, 48 years with an IQR of 26 years: and, for adult FT subjects, 46 years with an IQR of 18 years. Mean duration of milrinone therapy in pediatric patients was 51 days, with a median of 44 days (range 3-122 days). No association was found between duration of PDE3i treatment and PDE activity (total and PDE3) in the pediatric FT group. There were no significant differences between the pediatric groups based on sex, age, BB usage or anti-arrhythmic usage. Non-PDEi inotropes (epinephrine, norepinephrine, dopamine and dobutamine) were used more frequently in the NF group, when compared to the F (p=0.03) or FT (p=0.05) groups. As expected, ACEi and diuretics were used more commonly in pediatric IDC patients (F and FT) compared to the NF group (p<0.0001 for all). Importantly, there were no statistically significant differences between the pediatric F and FT groups based on non-PDEi inotrope usage or other HF therapies. Additional regression analysis did not demonstrate a significant association between non-PDEi inotrope usage and cAMP levels, PDE activity, or pPLB, though it is possible that our study did not have adequate power to demonstrate differences based on inotrope usage due to small sample size. Statistical comparison of the adult groups demonstrated that the FT group had more males compared with the NF group (p=0.005).
cAMP levels
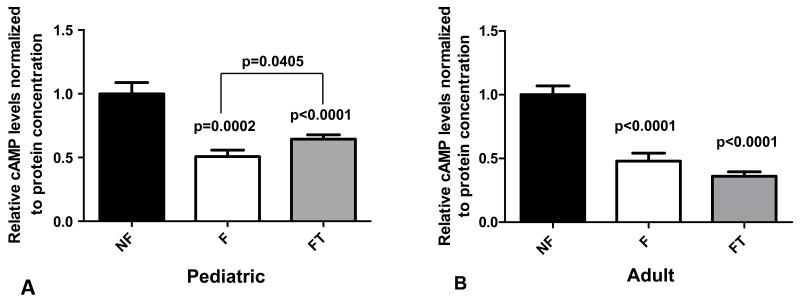
As shown in Figure 1A, cAMP levels were lower in failing pediatric myocardium than in nonfailing myocardium regardless of PDE3i treatment (p<0.0001 for F versus NF, p=0.0002 for FT versus NF, Figure 1A). This was also seen in failing adult myocardium, as described previously (p<0.0001, Figure 1B and 27). However, cAMP levels were significantly higher in failing pediatric myocardium chronically treated with PDE3i compared to failing pediatric myocardium without PDE3i treatment (p<0.05, Figure 1A). In contrast, cAMP levels were similarly low in failing adult myocardium with or without PDE3i treatment (Figure 1B). Thus, when comparing failing myocardium with and without PDE3i treatment, a rise in cAMP in response to PDE3i was seen only in pediatric myocardium.
Figure 1.
cAMP levels in left ventricular adult and pediatric myocardium (quantitated by ELISA). (A) Relative cAMP levels in pediatric myocardium. (B) Relative cAMP levels in adult myocardium. P-values correspond to comparisons with non-failing unless otherwise noted in the figure. NF, non-failing; F, failing; FT, failing treated with PDE3i; cAMP, cyclic adenosine monophosphate.
Total PDE activity
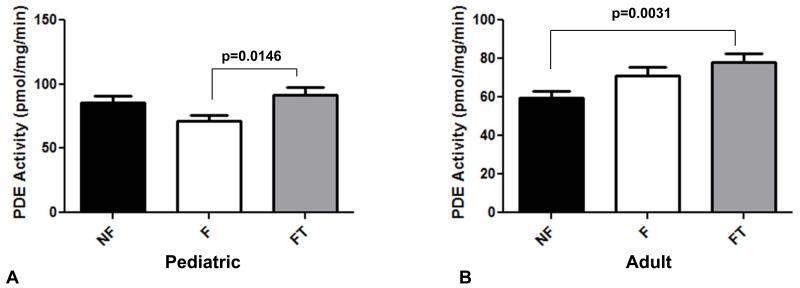
Total PDE activity was determined in myocardium from NF and pediatric IDC patients. While there was no change in total PDE activity between NF and untreated failing hearts in both adults and children, there was an age-related difference in response to PDE3i treatment. As shown in Figure 2A, total PDE activity in children with IDC chronically treated with PDE3i was similar to that of non-failing controls, and significantly higher than that of the failing group without PDE3i treatment (p=0.01). In contrast, total PDE activity in adults with IDC chronically treated with PDE3i was significantly higher than that of non-failing controls (p=0.003), and similar to that of the failing group without PDE3i treatment (Figure 2B).
Figure 2.
Total PDE activity in (A) pediatric myocardium, and (B) adult myocardium. PDE, phosphodiesterase; NF, non-failing; F, failing; FT, failing treated with PDE3i.
PDE3 activity
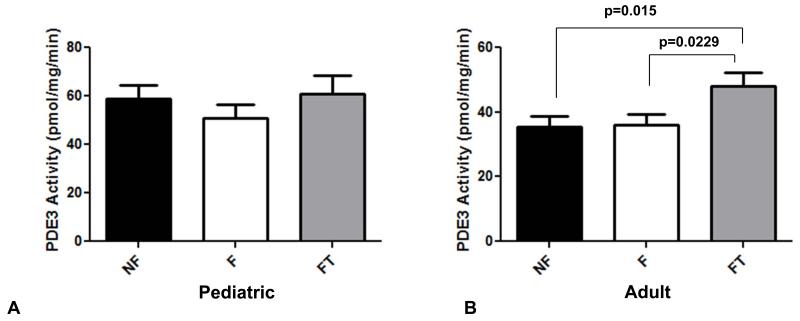
As described above, PDE3A is compartmentalized with SERCA2 and PLB, and is one of the main cAMP-hydrolyzing PDEs in human myocardium. As shown in Figure 3A, there were no differences in PDE3-specific activity in pediatric myocardium, regardless of disease or treatment with PDE3i. In contrast, PDE3 activity was higher in adults treated with PDE3i when compared to either NF controls (p=0.02) or to adults with IDC not treated with PDE3i (p=0.02, Figure 3B).
Figure 3.
PDE3-specific activity in (A) pediatric myocardium, and (B) adult myocardium. PDE, phosphodiesterase; NF, non-failing; F, failing; FT, failing treated with PDE3i.
PLB phosphorylation
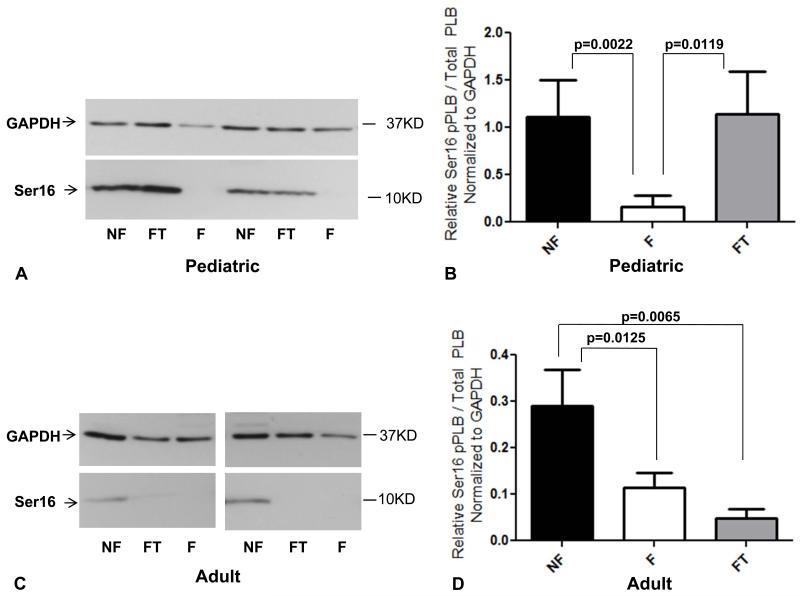
In adults, the level of pPLB at Ser16 was significantly lower in failing myocardium than in non-failing myocardium, irrespective of treatment with PDE3i (Figures 4C and 4D, p=0.01 and p=0.007). Our findings in children treated with PDE3i were markedly different (Figures 4A and 4B). While pPLB levels were low in the myocardium of children with IDC not treated with PDE3i (p=0.002), myocardial pPLB levels in children treated with PDE3i were significantly higher (p=0.01) and similar to those of NF controls. Total PLB levels were unchanged in both pediatric and adult IDC patients (not shown and 34).
Figure 4.
Phospholamban phosphorylation at the serine 16 residue in pediatric (A and B) and adult (C and D) myocardium; representative Western blot and quantitation for each are shown. GAPDH was used as a loading control. Ser16, serine 16 residue; pPLB, phospholamban phosphorylation; PLB, phospholamban; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; NF, non-failing; F, failing; FT, failing treated with PDE3i.
Discussion
Clinical differences exist in both efficacy and adverse events between adult and pediatric patients treated with PDE3i for end-stage HF. In a review of the pediatric patients who underwent heart transplantation at the Children’s Hospital Colorado since the year 2000, 94 children were on milrinone as a bridge to transplant, 56% (n=53) of whom were receiving milrinone infusions at home (outpatient therapy). In contrast to the adult experience, there were no sudden, unexpected deaths among the pediatric patients on milrinone.35 We hypothesized that clinical differences in response to PDE3i therapy are due to age-related variations in cellular adaptation and signaling in cardiac myocytes, and present several novel findings.
Effects of Chronic PDE3i on cAMP and pPLB
Despite low levels of cAMP in both failing adult and pediatric myocardium, a rise in cAMP in response to chronic PDE3i was only evident in the pediatric population. Previous work showed that PDE3i had diminished effectiveness in muscle (trabecular strips) from failing hearts compared with their effectiveness in control muscle; however, in the presence of low-dose forskolin (which increases cAMP levels through direct activation of adenylate cyclase), responsiveness of the failing hearts to PDE3i was restored.36 Thus, higher cAMP levels in pediatric IDC patients treated with PDE3i may contribute to the sustained hemodynamic benefits observed clinically in children. In contrast, others theorized that the increase in intracellular cAMP induced by agents such as milrinone may result in accelerated progression of the underlying disease and provoke development of serious ventricular arrhythmias in adults with HF.16 However there are numerous spatially, temporally and functionally distinct pools of cAMP which regulate cardiac function in a wide variety of capacities (including acute myocardial contractility, remodeling, apoptosis, and arrhythmogenesis) and, despite higher myocardial cAMP levels in pediatric IDC patients treated with PDEi, there were no new ventricular arrhythmias and no increase in sudden deaths in this cohort to suggest adverse consequences. Furthermore, the lack of elevated cAMP levels in adult IDC patients treated with chronic PDEi would argue against global elevations in intracellular cAMP as the singular cause of disease progression or arrhythmogenesis; it may instead contribute to the tachyphylaxis/tolerance phenomenon previously described with chronic PDE3i in adults.37, 38
The elevation in pPLB in PDE3i-treated pediatric patients is considerable, and greater than what would be predicted based on the magnitude of global intracellular cAMP elevation. Our results emphasize the significance of cAMP compartmentation, and suggest that chronic PDE3i treatment in children results in an increase in cAMP in a microdomain localized to the sarcoplasmic reticulum, where SERCA2 and PLB are found. cAMP-dependent phosphorylation of PLB at Ser16 is known to enhance cardiac contractility.39, 40 By de-inhibiting SERCA2, increased PKA-mediated pPLB accelerates calcium re-uptake into the sarcoplasmic reticulum and increases sarcoplasmic reticulum calcium content, contributing to both lusitropic and inotropic effects, respectively.25 Elevated pPLB in children chronically treated with PDE3i may explain the persistent benefit of PDE3i treatment in this population. In contrast, pPLB remained low in PDE3i-treated adults, which may account for the lack of sustained hemodynamic benefit in adults treated with chronic PDE3i. This compartment-specific difference between children and adults chronically treated with PDE3i may contribute to the age-related differences in clinical efficacy of PDE3i.
PDE Activity in Response to Chronic PDE3i Treatment
Our results confirm that PDE activity in the adult heart is not significantly altered by HF alone.29 We have now extended this finding to the pediatric population. Furthermore, we have described the molecular response to chronic PDE3i treatment in both the pediatric and adult HF populations. Thus, low cAMP in HF is likely secondary to decreased adenylate cyclase activity associated with down-regulation and desensitization of the β-adrenergic receptor in HF34, 41 rather than increased PDE activity. Based on the higher cAMP levels in children treated with milrinone, we hypothesized that a decrease in PDE3 activity may account for this relative increase in cAMP. However, we found that chronic PDE3i treatment in children with IDC did not significantly change either total or PDE3 activity. In contrast, adult IDC patients treated with chronic PDE3i had significantly higher myocardial PDE3 activity, with activity above that of adult IDC patients not treated with PDE3i. This likely perpetuates lower myocardial cAMP and pPLB levels and could explain the lack of sustained clinical benefit of chronic PDE3i treatment in some adults. However, we cannot exclude other mechanisms, including the activity of kinases, phosphatases and adenylate cyclases, which may be differentially regulated in an age-specific manner. Our results underscore the importance of dedicated age-specific HF studies, and highlight the weakness of extrapolating from adult studies to the pediatric HF population.
Limitations
There are several limitations to our study. First, although our findings appear to support clinical observations, tissue bank-based studies are cross-sectional and cannot establish causality. Second, due to limitations of medication history in our adult database, concurrent HF medications could not be completely determined, and thus conclusions regarding our adult groups were drawn with these limitations in mind. Lastly, we were unable to control for variability of PDE3i dosing. While pharmacokinetic data suggest that milrinone has a larger volume of distribution and faster clearance in infants and children than in adults,22 PDE3i dosages were determined by physicians treating these patients based on clinical experience and effect. The equivalence of PDE3i dosages between our pediatric and adult cohorts could therefore not be established. Despite these limitations, this study represents the first investigation of the chronic myocardial effects of PDE3i treatment in both children and adults with IDC. The described differences in myocardial response to chronic PDE3i treatment between children and adults with IDC are part of a growing body of literature demonstrating that pediatric myocardial adaptation is unique and could have implications for future clinical treatment paradigms.
Conclusion
Previous observational studies and our own institutional experience suggest a dissimilar clinical response to chronic PDE3i treatment between children and adults with HF.8, 9 Elevated cAMP and higher downstream pPLB may contribute to sustained hemodynamic benefits in pediatric IDC patients treated with PDE3i. In contrast, higher total PDE and PDE3 activities in adult IDC patients on PDE3i treatment may perpetuate lower myocardial cAMP levels and pPLB, limiting the potential benefits of PDE3i therapy. These findings may help explain the age-related differences in clinical response to chronic PDE3i treatment, and suggest that the results of adult chronic PDE3i clinical trials may be limited in their applicability to children with HF.
Supplementary Material
Acknowledgments
Sources of Funding
This work was supported by the National Institutes of Health grants: [T32 HL007822 to S.J.N.], [R21 HL097123 to S.D.M., B.L.S., C.C.S.], [R01 HL107715 to B.L.S.]; National Institutes of Health / National Center for Research Resources, Colorado Clinical and Translational Sciences Institute [UL1 TR000154 to S.J.N. and C.C.S.]; American Academy of Pediatric Section on Cardiology and Cardiac Surgery Research Fellowship Award to S.J.N.; and research grants from the US Department of Veterans Affairs [Merit Review CARA-029-09F and CARA-027-12S] to M.A.M.
Footnotes
Disclosures
Stephanie Nakano: None. Shelley Miyamoto: None. Matthew Movsesian: Grants from the US Department of Veterans Affair; patent EP1430140 “N-terminally truncated isoforms of PDE3A cyclic phosphodiesterases’, and USPTO 8722866, ”Isoform-selective inhibitors and activators of PDE3 cyclic nucleotide phosphodiesterases’. Penny Nelson: None. Brian Stauffer: Research support from Forest Laboratories, Inc. Carmen Sucharov: Equity in miRagen, Inc.
References
- 1.Towbin JA, Lowe AM, Colan SD, Sleeper LA, Orav EJ, Clunie S, Messere J, Cox GF, Lurie PR, Hsu D, Canter C, Wilkinson JD, Lipshultz SE. Incidence, causes, and outcomes of dilated cardiomyopathy in children. JAMA. 2006;296:1867–1876. doi: 10.1001/jama.296.15.1867. [DOI] [PubMed] [Google Scholar]
- 2.Kantor PF, Abraham JR, Dipchand AI, Benson LN, Redington AN. The impact of changing medical therapy on transplantation-free survival in pediatric dilated cardiomyopathy. J Am Coll Cardiol. 2010;55:1377–1384. doi: 10.1016/j.jacc.2009.11.059. [DOI] [PubMed] [Google Scholar]
- 3.Daubeney PE, Nugent AW, Chondros P, Carlin JB, Colan SD, Cheung M, Davis AM, Chow CW, Weintraub RG. Clinical features and outcomes of childhood dilated cardiomyopathy: Results from a national population-based study. Circulation. 2006;114:2671–2678. doi: 10.1161/CIRCULATIONAHA.106.635128. [DOI] [PubMed] [Google Scholar]
- 4.Tsirka AE, Trinkaus K, Chen SC, Lipshultz SE, Towbin JA, Colan SD, Exil V, Strauss AW, Canter CE. Improved outcomes of pediatric dilated cardiomyopathy with utilization of heart transplantation. J Am Coll Cardiol. 2004;44:391–397. doi: 10.1016/j.jacc.2004.04.035. [DOI] [PubMed] [Google Scholar]
- 5.Shaddy RE, Boucek MM, Hsu DT, Boucek RJ, Canter CE, Mahony L, Ross RD, Pahl E, Blume ED, Dodd DA, Rosenthal DN, Burr J, LaSalle B, Holubkov R, Lukas MA, Tani LY. Pediatric Carvedilol Study G. Carvedilol for children and adolescents with heart failure: A randomized controlled trial. JAMA. 2007;298:1171–1179. doi: 10.1001/jama.298.10.1171. [DOI] [PubMed] [Google Scholar]
- 6.Miyamoto SD, Stauffer BL, Nakano S, Sobus R, Nunley K, Nelson P, Sucharov CC. Beta-adrenergic adaptation in paediatric idiopathic dilated cardiomyopathy. Eur Heart J. 2014;35:33–41. doi: 10.1093/eurheartj/ehs229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alousi AA, Canter JM, Montenaro MJ, Fort DJ, Ferrari RA. Cardiotonic activity of milrinone, a new and potent cardiac bipyridine, on the normal and failing heart of experimental animals. J Cardiovasc Pharmacol. 1983;5:792–803. doi: 10.1097/00005344-198309000-00014. [DOI] [PubMed] [Google Scholar]
- 8.Price JF, Towbin JA, Dreyer WJ, Moffett BS, Kertesz NJ, Clunie SK, Denfield SW. Outpatient continuous parenteral inotropic therapy as bridge to transplantation in children with advanced heart failure. J Card Fail. 2006;12:139–143. doi: 10.1016/j.cardfail.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 9.Berg AM, Snell L, Mahle WT. Home inotropic therapy in children. J Heart Lung Transplant. 2007;26:453–457. doi: 10.1016/j.healun.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 10.Grose R, Strain J, Greenberg M, LeJemtel TH. Systemic and coronary effects of intravenous milrinone and dobutamine in congestive heart failure. J Am Coll Cardiol. 1986;7:1107–1113. doi: 10.1016/s0735-1097(86)80231-5. [DOI] [PubMed] [Google Scholar]
- 11.Monrad ES, Baim DS, Smith HS, Lanoue AS, Silverman KJ, Gervino EV, Grossman W. Assessment of long-term therapy with milrinone and the effects of milrinone withdrawal. Circulation. 1986;73:III205–212. [PubMed] [Google Scholar]
- 12.Anderson JL. Hemodynamic and clinical benefits with intravenous milrinone in severe chronic heart failure: Results of a multicenter study in the united states. Am Heart J. 1991;121:1956–1964. doi: 10.1016/0002-8703(91)90832-3. [DOI] [PubMed] [Google Scholar]
- 13.Baim DS, McDowell AV, Cherniles J, Monrad ES, Parker JA, Edelson J, Braunwald E, Grossman W. Evaluation of a new bipyridine inotropic agent--milrinone--in patients with severe congestive heart failure. N Engl J Med. 1983;309:748–756. doi: 10.1056/NEJM198309293091302. [DOI] [PubMed] [Google Scholar]
- 14.Cuffe MS, Califf RM, Adams KF, Jr., Benza R, Bourge R, Colucci WS, Massie BM, O’Connor CM, Pina I, Quigg R, Silver MA, Gheorghiade M. Short-term intravenous milrinone for acute exacerbation of chronic heart failure: A randomized controlled trial. JAMA. 2002;287:1541–1547. doi: 10.1001/jama.287.12.1541. [DOI] [PubMed] [Google Scholar]
- 15.Felker GM, Benza RL, Chandler AB, Leimberger JD, Cuffe MS, Califf RM, Gheorghiade M, O’Connor CM. Heart failure etiology and response to milrinone in decompensated heart failure: Results from the optime-chf study. J Am Coll Cardiol. 2003;41:997–1003. doi: 10.1016/s0735-1097(02)02968-6. [DOI] [PubMed] [Google Scholar]
- 16.Packer M, Carver JR, Rodeheffer RJ, Ivanhoe RJ, DiBianco R, Zeldis SM, Hendrix GH, Bommer WJ, Elkayam U, Kukin ML, Mallis GI, Sollano JA, Shannon J, Tandon PK, DeMets DL. Effect of oral milrinone on mortality in severe chronic heart failure. The promise study research group. N Engl J Med. 1991;325:1468–1475. doi: 10.1056/NEJM199111213252103. [DOI] [PubMed] [Google Scholar]
- 17.Seino Y, Momomura S, Takano T, Hayakawa H, Katoh K, Japan intravenous milrinone investigators Multicenter, double-blind study of intravenous milrinone for patients with acute heart failure in japan. Crit Care Med. 1996;24:1490–1497. doi: 10.1097/00003246-199609000-00010. [DOI] [PubMed] [Google Scholar]
- 18.Hoffman TM, Wernovsky G, Atz AM, Bailey JM, Akbary A, Kocsis JF, Nelson DP, Chang AC, Kulik TJ, Spray TL, Wessel DL. Prophylactic intravenous use of milrinone after cardiac operation in pediatrics (primacorp) study. Prophylactic intravenous use of milrinone after cardiac operation in pediatrics. Am Heart J. 2002;143:15–21. doi: 10.1067/mhj.2002.120305. [DOI] [PubMed] [Google Scholar]
- 19.Hoffman TM, Wernovsky G, Atz AM, Kulik TJ, Nelson DP, Chang AC, Bailey JM, Akbary A, Kocsis JF, Kaczmarek R, Spray TL, Wessel DL. Efficacy and safety of milrinone in preventing low cardiac output syndrome in infants and children after corrective surgery for congenital heart disease. Circulation. 2003;107:996–1002. doi: 10.1161/01.cir.0000051365.81920.28. [DOI] [PubMed] [Google Scholar]
- 20.Chang AC, Atz AM, Wernovsky G, Burke RP, Wessel DL. Milrinone: Systemic and pulmonary hemodynamic effects in neonates after cardiac surgery. Crit Care Med. 1995;23:1907–1914. doi: 10.1097/00003246-199511000-00018. [DOI] [PubMed] [Google Scholar]
- 21.Duggal B, Pratap U, Slavik Z, Kaplanova J, Macrae D. Milrinone and low cardiac output following cardiac surgery in infants: Is there a direct myocardial effect? Pediatr Cardiol. 2005;26:642–645. doi: 10.1007/s00246-005-0881-z. [DOI] [PubMed] [Google Scholar]
- 22.Ramamoorthy C, Anderson GD, Williams GD, Lynn AM. Pharmacokinetics and side effects of milrinone in infants and children after open heart surgery. Anesth Analg. 1998;86:283–289. doi: 10.1097/00000539-199802000-00011. [DOI] [PubMed] [Google Scholar]
- 23.Vandeput F, Krall J, Ockaili R, Salloum FN, Florio V, Corbin JD, Francis SH, Kukreja RC, Movsesian MA. Cgmp-hydrolytic activity and its inhibition by sildenafil in normal and failing human and mouse myocardium. J Pharmacol Exp Ther. 2009;330:884–891. doi: 10.1124/jpet.109.154468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hambleton R, Krall J, Tikishvili E, Honeggar M, Ahmad F, Manganiello VC, Movsesian MA. Isoforms of cyclic nucleotide phosphodiesterase pde3 and their contribution to camp hydrolytic activity in subcellular fractions of human myocardium. J Biol Chem. 2005;280:39168–39174. doi: 10.1074/jbc.M506760200. [DOI] [PubMed] [Google Scholar]
- 25.Bers DM. Cardiac excitation-contraction coupling. Nature. 2002;415:198–205. doi: 10.1038/415198a. [DOI] [PubMed] [Google Scholar]
- 26.Beca S, Ahmad F, Shen W, Liu J, Makary S, Polidovitch N, Sun J, Hockman S, Chung YW, Movsesian M, Murphy E, Manganiello V, Backx PH. Phosphodiesterase type 3a regulates basal myocardial contractility through interacting with sarcoplasmic reticulum calcium atpase type 2a signaling complexes in mouse heart. Circ Res. 2013;112:289–297. doi: 10.1161/CIRCRESAHA.111.300003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Danielsen W, v der Leyen H, Meyer W, Neumann J, Schmitz W, Scholz H, Starbatty J, Stein B, Doring V, Kalmar P. Basal and isoprenaline-stimulated camp content in failing versus nonfailing human cardiac preparations. J Cardiovasc Pharmacol. 1989;14:171–173. doi: 10.1097/00005344-198907000-00026. [DOI] [PubMed] [Google Scholar]
- 28.Movsesian M, Stehlik J, Vandeput F, Bristow MR. Phosphodiesterase inhibition in heart failure. Heart Fail Rev. 2009;14:255–263. doi: 10.1007/s10741-008-9130-x. [DOI] [PubMed] [Google Scholar]
- 29.Movsesian MA, Smith CJ, Krall J, Bristow MR, Manganiello VC. Sarcoplasmic reticulum-associated cyclic adenosine 5′-monophosphate phosphodiesterase activity in normal and failing human hearts. J Clin Invest. 1991;88:15–19. doi: 10.1172/JCI115272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wechsler J, Choi YH, Krall J, Ahmad F, Manganiello VC, Movsesian MA. Isoforms of cyclic nucleotide phosphodiesterase pde3a in cardiac myocytes. J Biol Chem. 2002;277:38072–38078. doi: 10.1074/jbc.M203647200. [DOI] [PubMed] [Google Scholar]
- 31.Ahmad F, Lindh R, Tang Y, Weston M, Degerman E, Manganiello VC. Insulin-induced formation of macromolecular complexes involved in activation of cyclic nucleotide phosphodiesterase 3b (pde3b) and its interaction with pkb. Biochem J. 2007;404:257–268. doi: 10.1042/BJ20060960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sucharov CC, Mariner P, Long C, Bristow M, Leinwand L. Yin yang 1 is increased in human heart failure and represses the activity of the human alpha-myosin heavy chain promoter. J Biol Chem. 2003;278:31233–31239. doi: 10.1074/jbc.M301917200. [DOI] [PubMed] [Google Scholar]
- 33.Sucharov CC, Hijmans JG, Sobus RD, Melhado WF, Miyamoto SD, Stauffer BL. Beta-adrenergic receptor antagonism in mice: A model for pediatric heart disease. J Appl Physiol (1985) 2013;115:979–987. doi: 10.1152/japplphysiol.00627.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miyamoto SD, Stauffer BL, Nakano S, Sobus R, Nunley K, Nelson P, Sucharov CC. Beta-adrenergic adaptation in paediatric idiopathic dilated cardiomyopathy. Eur Heart J. 2014;35:33–41. doi: 10.1093/eurheartj/ehs229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Auerbach SR, Cavanaugh J, Pietra BA, Miyamoto SD. Outpatient Milrinone Therapy as a Bridge to Pediatric Heart Transplantation: A Large Single Center Experience. Circulation. 2013;128:A18457. Abstract: 2013 American Heart Association Scientific Sessions, Dallas, TX, oral presentation Nov. 17, 2013. [Google Scholar]
- 36.Feldman MD, Copelas L, Gwathmey JK, Phillips P, Warren SE, Schoen FJ, Grossman W, Morgan JP. Deficient production of cyclic amp: Pharmacologic evidence of an important cause of contractile dysfunction in patients with end-stage heart failure. Circulation. 1987;75:331–339. doi: 10.1161/01.cir.75.2.331. [DOI] [PubMed] [Google Scholar]
- 37.Maisel AS, Wright CM, Carter SM, Ziegler M, Motulsky HJ. Tachyphylaxis with amrinone therapy: Association with sequestration and down-regulation of lymphocyte beta-adrenergic receptors. Ann Intern Med. 1989;110:195–201. doi: 10.7326/0003-4819-110-3-195. [DOI] [PubMed] [Google Scholar]
- 38.Packer M, Medina N, Yushak M. Failure of low doses of amrinone to produce sustained hemodynamic improvement in patients with severe chronic congestive heart failure. The Am J Cardiol. 1984;54:1025–1029. doi: 10.1016/s0002-9149(84)80138-1. [DOI] [PubMed] [Google Scholar]
- 39.Mundina-Weilenmann C, Vittone L, Ortale M, de Cingolani GC, Mattiazzi A. Immunodetection of phosphorylation sites gives new insights into the mechanisms underlying phospholamban phosphorylation in the intact heart. J Biol Chem. 1996;271:33561–33567. doi: 10.1074/jbc.271.52.33561. [DOI] [PubMed] [Google Scholar]
- 40.Kuschel M, Karczewski P, Hempel P, Schlegel WP, Krause EG, Bartel S. Ser16 prevails over thr17 phospholamban phosphorylation in the beta-adrenergic regulation of cardiac relaxation. The Am J Physiol. 1999;276:H1625–1633. doi: 10.1152/ajpheart.1999.276.5.H1625. [DOI] [PubMed] [Google Scholar]
- 41.Bristow MR, Ginsburg R, Minobe W, Cubicciotti RS, Sageman WS, Lurie K, Billingham ME, Harrison DC, Stinson EB. Decreased catecholamine sensitivity and beta-adrenergic-receptor density in failing human hearts. N Engl J Med. 1982;307:205–211. doi: 10.1056/NEJM198207223070401. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.