Abstract
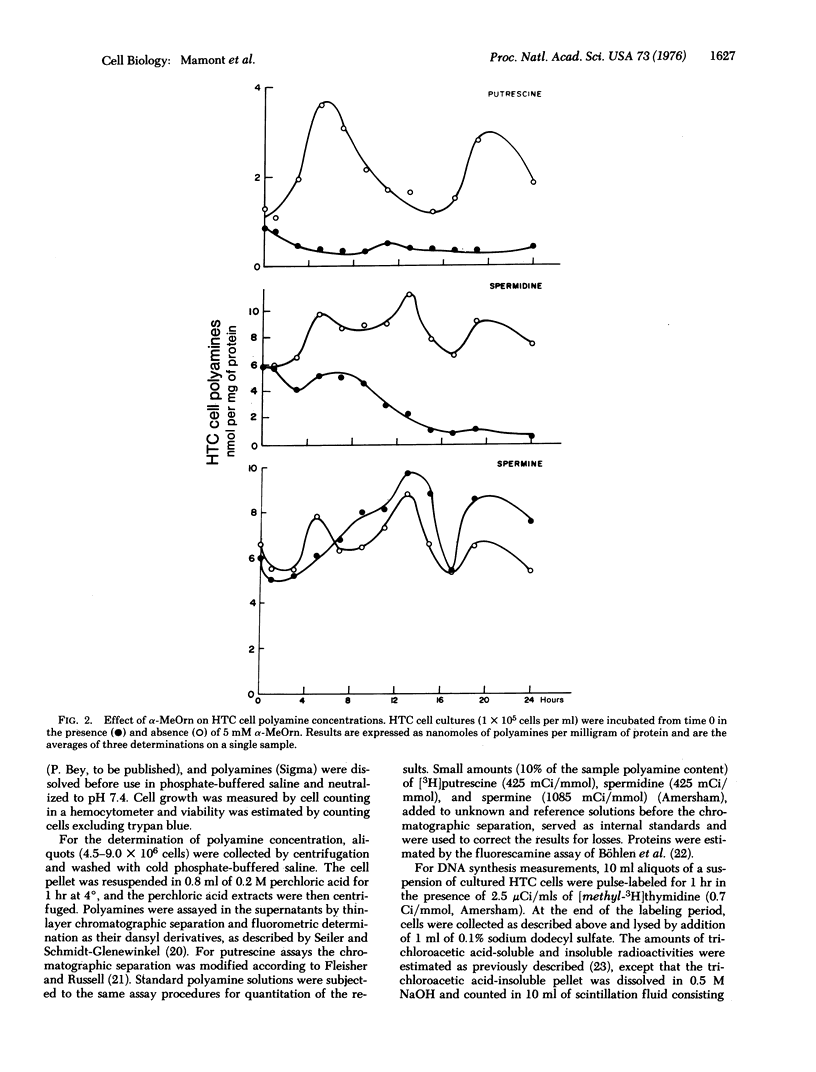
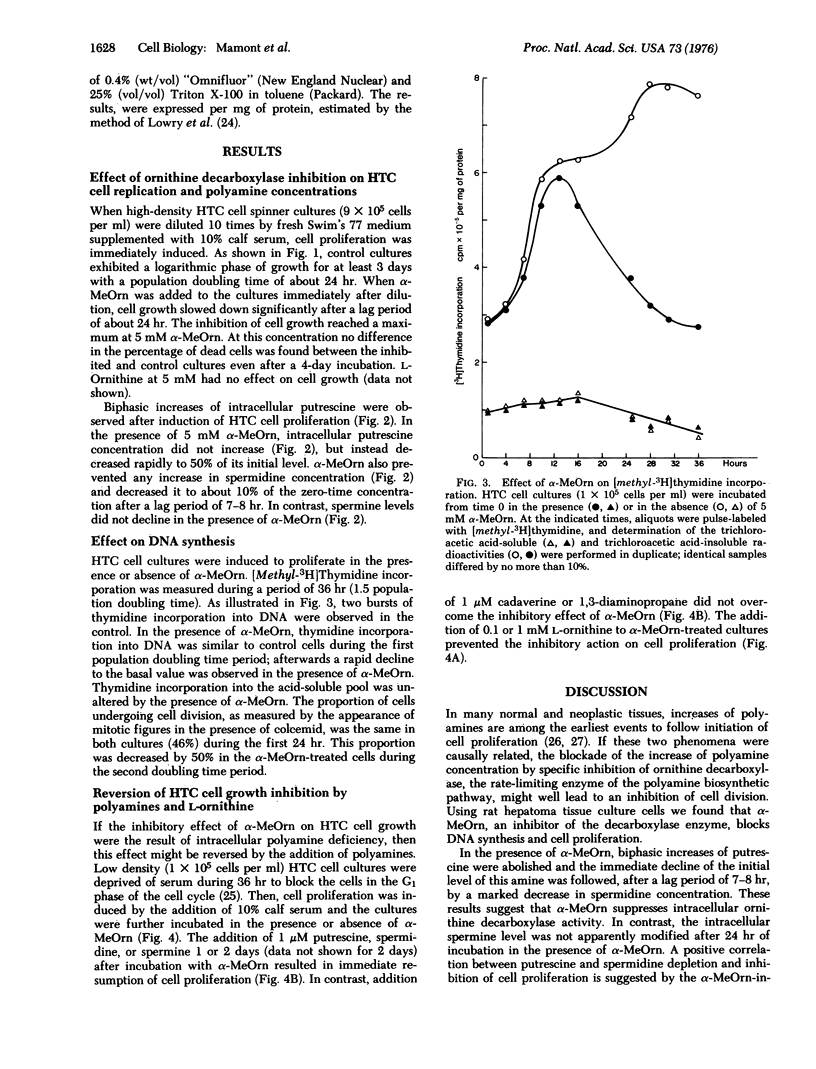
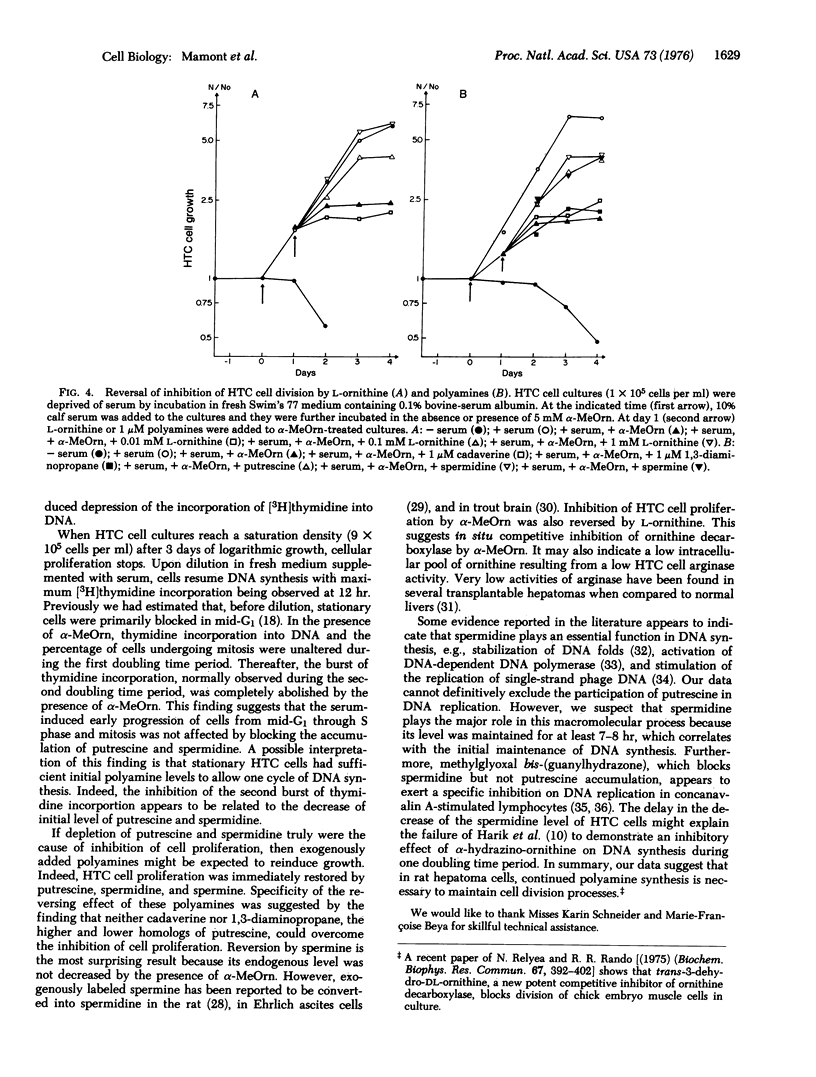
A biphasic increase of putrescine concentration occurs in rat hepatoma tissue culture cells induced to proliferate. DL-alpha-Methyl ornithine, a competitive inhibitor of ornithine decarboxylase ( L-ornithine carboxylyase, EC 4.1.1.7) of hepatoma tissue culture cells, blocks the usual increases of putrescine and spermidine concentrations in these cells, and causes a rapid fall in the levels of putrescine which is followed by a striking decrease of spermidine.In parallel with the depletion of these amines, incorporation of [3H]thymidine into DNA and cell proliferation are inhibited. Addition of putrescine, spermidine, or spermine results in an immediate resumption of cell proliferation. Cell proliferation is also restored by L-ornithine presumably due to in situ competitive inhibition of ornithine decarboxylase. These findings of hepatoma tissue culture cells support the concept that polyamines play an essential function in the cell division processes.
Full text
PDF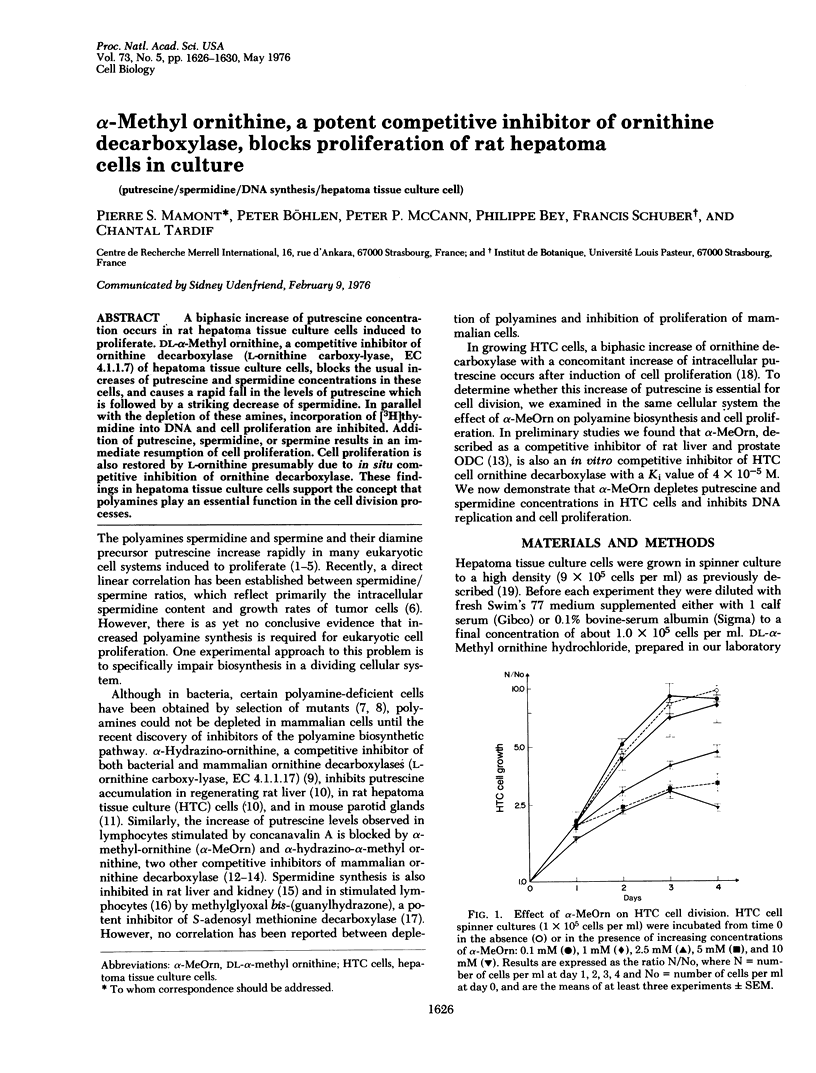

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdel-Monem M. M., Newton N. E., Ho B. C., Weeks C. E. Potential inhibitors of polyamine biosynthesis. 2. alpha-Alkyl- and benzyl-(+/-)-ornithine. J Med Chem. 1975 Jun;18(6):600–604. doi: 10.1021/jm00240a015. [DOI] [PubMed] [Google Scholar]
- Abdel-Monem M. M., Newton N. E., Weeks C. E. Inhibitors of polyamine biosynthesis. 1. Alpha-methyl-(plus or minus)-ornithine, an inhibitor of ornithine decarboxylase. J Med Chem. 1974 Apr;17(4):447–451. doi: 10.1021/jm00250a016. [DOI] [PubMed] [Google Scholar]
- Abdel-Monem M. M., Newton N. E., Weeks C. E. Inhibitors of polyamine biosynthesis. 3. (+/-)-5-Amino-2-hydrazine-2-methylpentanoic acid, an inhibitor of ornithine decarboxylase. J Med Chem. 1975 Sep;18(9):945–948. doi: 10.1021/jm00243a017. [DOI] [PubMed] [Google Scholar]
- Böhlen P., Stein S., Dairman W., Udenfriend S. Fluorometric assay of proteins in the nanogram range. Arch Biochem Biophys. 1973 Mar;155(1):213–220. doi: 10.1016/s0003-9861(73)80023-2. [DOI] [PubMed] [Google Scholar]
- Chiu J. F., Sung S. C. Effect of spermidine on the activity of DNA polymerases. Biochim Biophys Acta. 1972 Nov 9;281(4):535–542. doi: 10.1016/0005-2787(72)90154-2. [DOI] [PubMed] [Google Scholar]
- Echandi G., Algranati I. D. Protein synthesis and ribosomal distribution in a polyamine auxotroph of Escherichia coli: studies in cell-free systems. Biochem Biophys Res Commun. 1975 Jan 20;62(2):313–319. doi: 10.1016/s0006-291x(75)80140-9. [DOI] [PubMed] [Google Scholar]
- Fillingame R. H., Jorstad C. M., Morris D. R. Increased cellular levels of spermidine or spermine are required for optimal DNA synthesis in lymphocytes activated by concanavalin A. Proc Natl Acad Sci U S A. 1975 Oct;72(10):4042–4045. doi: 10.1073/pnas.72.10.4042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillingame R. H., Morris D. R. Polyamine accumulation during lymphocyte transformation and its relation to the synthesis, processing, and accumulation of ribonucleic acid. Biochemistry. 1973 Oct 23;12(22):4479–4487. doi: 10.1021/bi00746a028. [DOI] [PubMed] [Google Scholar]
- Fleisher J. H., Russell D. H. Estimation of urinary diamines and polyamines by thin-layer chromatography. J Chromatogr. 1975 Jul 16;110(2):335–340. doi: 10.1016/0021-9673(75)85014-x. [DOI] [PubMed] [Google Scholar]
- Flink I., Pettijohn D. E. Polyamines stabilise DNA folds. Nature. 1975 Jan 3;253(5486):62–63. doi: 10.1038/253062a0. [DOI] [PubMed] [Google Scholar]
- Harik S. I., Hollenberg M. D., Snyder S. H. Alpha-hydrazino-ornithine blocks net synthesis of putrescine but not of RNA and DNA. Nature. 1974 May 17;249(454):250–251. doi: 10.1038/249250a0. [DOI] [PubMed] [Google Scholar]
- Harik S. I., Snyder S. H. Ornithine decarboxylase: inhibition by alpha-hydrazinoornithine. Biochim Biophys Acta. 1973 Dec 19;327(2):501–509. doi: 10.1016/0005-2744(73)90433-6. [DOI] [PubMed] [Google Scholar]
- Heby O., Marton L. J., Wilson C. B., Martinez H. M. Polyamines: a high correlation with cell replication. FEBS Lett. 1975 Jan 15;50(1):1–4. doi: 10.1016/0014-5793(75)81026-x. [DOI] [PubMed] [Google Scholar]
- Heby O., Marton L. J., Zardi L., Russell D. H., Baserga R. Changes in polyamine metabolism in WI38 cells stimulated to proliferate. Exp Cell Res. 1975 Jan;90(1):8–14. doi: 10.1016/0014-4827(75)90350-x. [DOI] [PubMed] [Google Scholar]
- Hershko A., Mamont P., Shields R., Tomkins G. M. "Pleiotypic response". Nat New Biol. 1971 Aug;232(33):206–211. [PubMed] [Google Scholar]
- Hershko A., Tomkins G. M. Studies on the degradation of tyrosine aminotransferase in hepatoma cells in culture. Influence of the composition of the medium and adenosine triphosphate dependence. J Biol Chem. 1971 Feb 10;246(3):710–714. [PubMed] [Google Scholar]
- Hirshfield I. N., Rosenfeld H. J., Leifer Z., Maas W. K. Isolation and characterization of a mutant of Escherichia coli blocked in the synthesis of putrescine. J Bacteriol. 1970 Mar;101(3):725–730. doi: 10.1128/jb.101.3.725-730.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue H., Kato Y., Takigawa M., Adachi K., Takeda Y. Effect of DL-alpha-hydrazino-delta-aminovaleric acid, an inhibitor of ornithine decarboxylase, on polyamine metabolism in isoproterenol-stimulated mouse parotid glands. J Biochem. 1975 Apr;77(4):879–893. doi: 10.1093/oxfordjournals.jbchem.a130796. [DOI] [PubMed] [Google Scholar]
- Kay John E., Cooke Anne. Ornithine decarboxylase and ribosomal RNA synthesis during the stimulation of lymphocytes by phytohaemagglutinin. FEBS Lett. 1971 Jul 15;16(1):9–12. doi: 10.1016/0014-5793(71)80671-3. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- McCann P. P., Tardiff C., Mamont P. S., Schuber F. Biphasic induction of ornithine decarboxylase and putrescine levels in growing HTC cells. Biochem Biophys Res Commun. 1975 May 5;64(1):336–341. doi: 10.1016/0006-291x(75)90258-2. [DOI] [PubMed] [Google Scholar]
- Nelson J. A., Carpenter J. W., Morris H. P. Growth inhibitory and arginase activities in liver and hepatoma extracts. Proc Soc Exp Biol Med. 1975 Sep;149(4):900–902. doi: 10.3181/00379727-149-38923. [DOI] [PubMed] [Google Scholar]
- Otani S., Mizoguchi Y., Matsui I., Morisawa S. Inhibition of DNA synthesis by methylglyoxal bis(guanylhydrazone) during lymphocyte transformation. Mol Biol Rep. 1974 Dec;1(8):431–436. doi: 10.1007/BF00360667. [DOI] [PubMed] [Google Scholar]
- Pegg A. E. Inhibition of spermidine formation in rat liver and kidney by methylglyoxal bis(guanylhydrazone). Biochem J. 1973 Mar;132(3):537–540. doi: 10.1042/bj1320537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Relyea N., Rando R. R. Potent inhibition of ornithine decarboxylase by beta,gamma unsaturated substrate analogs. Biochem Biophys Res Commun. 1975 Nov 3;67(1):392–398. doi: 10.1016/0006-291x(75)90328-9. [DOI] [PubMed] [Google Scholar]
- Russell D., Snyder S. H. Amine synthesis in rapidly growing tissues: ornithine decarboxylase activity in regenerating rat liver, chick embryo, and various tumors. Proc Natl Acad Sci U S A. 1968 Aug;60(4):1420–1427. doi: 10.1073/pnas.60.4.1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiler N., Schmidt-Glenewinkel T. Regional distribution of putrescine, spermidine and spermine in relation to the distribution of RNA and DNA in the rat nervous system. J Neurochem. 1975 Apr;24(4):791–795. [PubMed] [Google Scholar]
- Siimes M. Studies on the metabolism of 1,4-14C-spermidine and 1,4-14C-spermine in the rat. Acta Physiol Scand Suppl. 1967;298:1–66. [PubMed] [Google Scholar]
- Stastny M., Cohen S. Epidermal growth factor. IV. The induction of ornithine decarboxylase. Biochim Biophys Acta. 1970 Apr 15;204(2):578–589. [PubMed] [Google Scholar]
- Steinberg R. A., Levinson B. B., Tomkins G. M. "Superinduction" of tyrosine aminotransferase by actinomycin D: a reevaluation. Cell. 1975 May;5(1):29–35. doi: 10.1016/0092-8674(75)90088-4. [DOI] [PubMed] [Google Scholar]
- Wickner W., Schekman R., Geider K., Kornberg A. A new form of DNA polymerase 3 and a copolymerase replicate a long, single-stranded primer-template. Proc Natl Acad Sci U S A. 1973 Jun;70(6):1764–1767. doi: 10.1073/pnas.70.6.1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams-Ashman H. G., Schenone A. Methyl glyoxal bis(guanylhydrazone) as a potent inhibitor of mammalian and yeast S-adenosylmethionine decarboxylases. Biochem Biophys Res Commun. 1972 Jan 14;46(1):288–295. doi: 10.1016/0006-291x(72)90661-4. [DOI] [PubMed] [Google Scholar]



