Abstract
Background
Post-percutaneous coronary intervention (PCI) bleeding complications are an important quality metric. We sought to characterize site-level variation in post-PCI bleeding and explore the influence of patient and procedural factors on hospital bleeding performance.
Methods and Results
Hospital-level bleeding performance was compared pre- and post-adjustment using the newly-revised CathPCI Registry® bleeding risk model (c-index 0.77) among 1,292 NCDR® hospitals performing >50 PCIs from 7/2009–9/2012 (n=1,984,998 procedures). Using random effects models, outlier sites were identified based on 95% confidence intervals around the hospital’s random intercept. Bleeding 72 hours post-PCI was defined as: arterial access site, retroperitoneal, gastrointestinal, or genitourinary bleeding; intracranial hemorrhage; cardiac tamponade; non-bypass surgery-related blood transfusion with pre-procedure hemoglobin ≥8 g/dl; or absolute decrease in hemoglobin value ≥3g/dl with pre-procedure hemoglobin ≤16 g/dl. Overall, the median unadjusted post-PCI bleeding rate was 5.2% and varied among hospitals from 2.6%–10.4% (5th, 95th percentiles). Center-level bleeding variation persisted after case-mix adjustment (2.8%–9.5%; 5th, 95th percentiles). While hospitals’ observed and risk-adjusted bleeding ranks were correlated (Spearman’s rho 0.88), individual rankings shifted after risk-adjustment (median Δ rank order ± 91.5; IQR 37.0, 185.5). Outlier classification changed post-adjustment for 29.3%, 16.1%, and 26.5% of low-, non-, and high-outlier sites, respectively. Hospital use of bleeding avoidance strategies (bivalirudin, radial access, or vascular closure device) was associated with risk-adjusted bleeding rates.
Conclusions
Despite adjustment for patient case-mix, there is wide variation in rates of hospital PCI-related bleeding in the United States. Opportunities may exist for best performers to share practices with other sites.
Keywords: percutaneous coronary intervention, bleeding rates, quality metrics
Each year, approximately 600,000 percutaneous coronary intervention (PCI) procedures are performed in the United States,1 yet there are few outcomes-based quality indicators of PCI performance. Currently used performance measures include in-hospital PCI mortality and risk-standardized 30-day readmissions after PCI2; however, one of the challenges of these quality improvement metrics is whether they can be modified by alterations in care processes and consequently improved upon.3–6 Another limitation of in-hospital PCI mortality is that the rates are low,7 limiting the variation across hospitals, as well as the usefulness of this metric to judge performance.8
Recent attention has focused on PCI-related bleeding as a potential hospital quality indicator. Bleeding is the most common non-cardiac complication of PCI and is associated with increased morbidity, mortality, and cost.9–12 Since bleeding after PCI has been consistently associated with known patient characteristics such as older age, female sex, and renal insufficiency,13–15 bleeding risk models have been developed and validated to provide accurate estimates of post-PCI bleeding risk and, therefore, guide therapy and improve patient outcomes.15,16 PCI-related bleeding risk can be modified by provider factors, such as use of bivalirudin and radial access,17–21 and vascular closure devices may potentially reduce bleeding complications in certain populations, but have not been definitively tested.22–24 However, data suggest that the use of these approaches (collectively termed bleeding avoidance strategies [BAS]), is variable.24 Recently, the National Cardiovascular Data Registry® CathPCI Registry® began including hospital risk-adjusted post-PCI bleeding rates in its provider reports. Furthermore, PCI bleeding has been designated as a quality metric in the Centers for Medicare & Medicaid Services Acute Care Episode Demonstration program.25
Although there is interest in the adoption of post-PCI bleeding as a site performance measure, evidence to support it has been limited. To date, overall variability in hospital rates of post-PCI bleeding has not been reported, and the influence of patient or procedural factors on hospital bleeding rates has not been examined. Therefore, we sought to: (1) characterize hospital-level variation in post-PCI bleeding rates; (2) assess the contribution of patient case-mix to variation in bleeding rates among sites; and (3) explore whether hospital factors, including use of BAS, are associated with post-PCI bleeding.
Methods
The CathPCI Registry is a national quality improvement program jointly sponsored by the American College of Cardiology and the Society for Cardiovascular Angiography and Interventions. This registry provides in-hospital data on patients undergoing cardiac catheterization and PCI from approximately 1400 hospitals in the United States. Details about the CathPCI Registry have been previously published.26
Study Sample
We included all PCI procedures performed between July 2009 and September 2012 with data reported using version 4 of the CathPCI Registry data collection form. From a total of 2,024,161 index PCI procedures performed at 1,383 participating sites, we excluded patients with missing bleeding data (n=1,097) and sites that reported no bleeding events or did not record any hemoglobin values (n=13,141 patients from 73 sites). We also excluded patients undergoing coronary artery bypass grafting during the index hospitalization (n=24,380 procedures) and sites with <50 PCIs (n=545 procedures from 18 sites). Our final analysis population consisted of 1,984,998 PCI procedures performed at 1,292 sites (Figure 1).
Figure 1. Selection of Analysis Population.
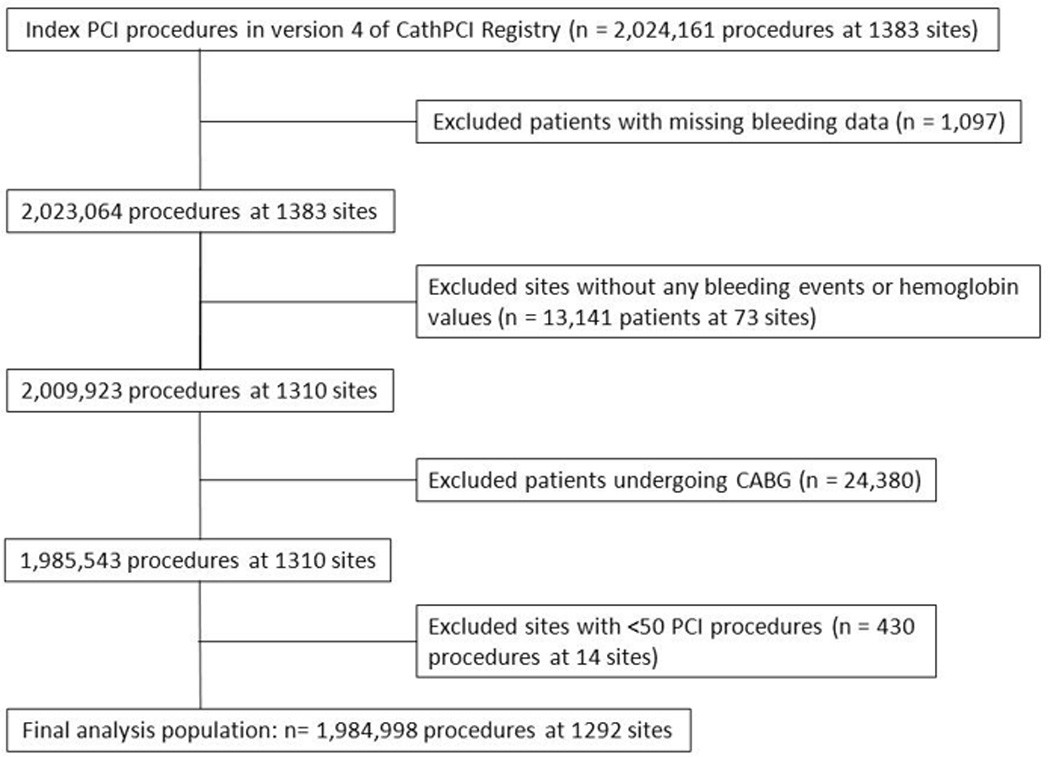
This figure displays the selection of the analysis population, from index PCI procedures, followed by exclusions, followed by the final study population.
CABG indicates coronary artery bypass graft surgery; PCI, percutaneous coronary intervention
Definitions and Outcomes
CathPCI Registry data definitions can be found online at: www.ncdr.com/webncdr/cathpci/home/datacollection. The use of BAS was defined as the use of any of the following during PCI: radial artery access, bivalirudin, or vascular closure device. Academic or teaching status was defined as the presence of an internship, residency, or fellowship program at an institution.
Bleeding after PCI was the primary outcome for our study. In 2009, the CathPCI Registry implemented a new data collection form with more detailed data variables related to bleeding events. Using these data elements, the CathPCI Registry post-procedure bleeding definition was recently revised to capture: (1) potentially unreported bleeding events using more objective laboratory data; and (2) important bleeding complications, such as intracranial hemorrhage and cardiac tamponade, that were not available in prior versions.16 Bleeding was reported by trained site data collectors and defined based on any of the following events occurring within 72 hours of the procedure: arterial access site bleeding, either overt external bleeding or a hematoma >10 cm for femoral access, >5 cm for brachial access, or >2 cm for radial access; retroperitoneal, gastrointestinal, or genitourinary bleeding; intracranial hemorrhage; cardiac tamponade; decrease of ≥3 g/dl in hemoglobin post-PCI in patients with pre-procedure hemoglobin ≤16 g/dl; or post-procedure non-bypass surgery-related blood transfusion in patients with a pre-PCI hemoglobin of ≥8 g/dl.
CathPCI Registry Bleeding Risk Adjustment Model
The CathPCI Registry bleeding model was recently updated to include bleeding events not captured in previous CathPCI Registry data collection forms and to be more comparable to bleeding definitions used in other studies.16 This new standard CathPCI Registry bleeding model was developed using data from PCI procedures performed between February 2008 and April 2011. Factors significantly associated with bleeding complications within 72 hours of PCI were identified using multivariable logistic regression. The final model includes the following clinical factors: demographic variables (female sex, age, body mass index), comorbidities (diabetes, prior congestive heart failure, prior PCI, cerebrovascular disease, peripheral vascular disease, chronic lung disease, chronic kidney disease), clinical presentation characteristics (ST-segment elevation myocardial infarction, New York Heart Association heart failure class, ejection fraction, cardiac arrest within 24 hours, cardiogenic shock, baseline hemoglobin), and procedural variables (pre-procedure Thrombolysis in Myocardial Infarction flow, number of diseased vessels, Society for Cardiovascular Angiography and Interventions lesion class, lesion segment). The model had good discrimination (c-index 0.77) and was well-calibrated.
Statistical Analysis
Patient, procedure, and hospital characteristics were described across tertiles (low, average, high) of unadjusted hospital PCI bleeding rates. Categorical variables were presented as frequencies and percentages, and continuous variables were summarized as medians with interquartile ranges. Comparisons among categorical and continuous variables were performed using Pearson Chi-Square and Wilcoxon rank-sum tests, respectively. Unadjusted hospital bleeding rates were calculated with hospital included as a random effect variable, whereas adjusted bleeding rates were determined after accounting for all variables in the CathPCI Registry bleeding model in addition to the hospital. Models incorporating hospital as a random effect allow for formal statistical testing of whether any observed variation in outcome is due to differences among hospitals (variance parameter estimates greater than zero and with p-values <0.05) versus simple sampling variation. Hospitals were classified as outliers using the 95% confidence interval (CI) around the hospital’s random intercept, which is a shrunken estimator representing the log odds of bleeding for each hospital (Supplemental Material).27 Hospitals for which the lower 95% CI limit was greater than one were considered to have high outlier status; hospitals for which the upper 95% CI limit was less than one were considered to have low outlier status; and hospitals whose 95% CI included one were considered to be non-outliers. We assessed the relationship between unadjusted and adjusted bleeding rates by calculating the absolute values of change in rank order for hospitals and by quantifying the association between unadjusted and adjusted hospital rankings using Spearman’s rank correlation coefficient.
We performed sensitivity analyses. We increased the threshold for site exclusion from <50 PCIs to <150 PCIs to explore the stability of bleeding rate estimates. Although there has been increasing acceptance of random effects modeling to compare hospital outcomes, current methodology used to assess participating CathPCI Registry hospital performance in site reports employs use of non-random effects models (Supplemental Material).27,28 Therefore, analyses were repeated using these same fixed effect models. We calculated observed bleeding rates for hospitals by dividing the observed number of bleeds by the total number of admissions. The expected number of bleeds for each hospital was determined using the validated CathPCI Registry bleeding model to tabulate the sum of the predicted probabilities of bleeding for each patient at that hospital. Hospital adjusted rates were then obtained by multiplying the ratio of observed to expected number of events (a measure commonly used to assess hospital performance) by the population bleeding rate. Hospital outlier status for observed rates was defined using the 95% CI for a hospital’s observed bleeding rate divided by the population bleeding rate, using the same definitions of outlier status as in the main analysis. Hospital outlier status for adjusted rate was defined similarly using the 95% CI for a hospital’s ratio of observed-to-expected bleeding rates. Standard errors were based on the binomial distribution rather than Poisson, as this is also the statistical methodology used by the CathPCI Registry. Finally, we determined Spearman’s correlation coefficients to explore a limited number of hospital and procedural factors that might be associated with hospital-level post-PCI bleeding. For all analyses, statistical tests were two-sided, and a p-value <0.05 was considered statistically significant. Analyses were performed at the Duke Clinical Research Institute using SAS software (version 9.2, SAS Institute, Cary, NC) and STATA (Release 11, StataCorp, College Station, TX). This study was approved by the Duke University Medical Center Institutional Review Board and is determined to qualify for a waiver of informed consent.
Results
Hospital-level Variation in Bleeding
Among the 1,984,998 PCI procedures at 1292 sites, the overall median unadjusted rate of post-PCI bleeding was 5.2%. As shown in Figure 2a, there was wide variation in bleeding rates across hospitals. Center-level variation in composite bleeding rates ranged as follows: 5th, 10th, 25th, 75th, 90th, and 95th percentiles were 2.6%, 3.0%, 3.9%, 6.9%, 8.8%, and 10.4%, respectively. We also assessed the rates of the individual bleeding components that make up this composite (Table 1). We found that the individual bleeding endpoint rates generally followed the composite results across hospital-level bleeding tertiles. Since the need for red blood cell transfusion remains somewhat subjective, we also investigated whether differences in the composite endpoint were due to differential thresholds in transfusion. We found that hospital pre-transfusion hemoglobin values were highly consistent across all three bleeding tertiles (Table 1).
Figure 2. Distribution of Post-PCI Bleeding Among Hospitals.
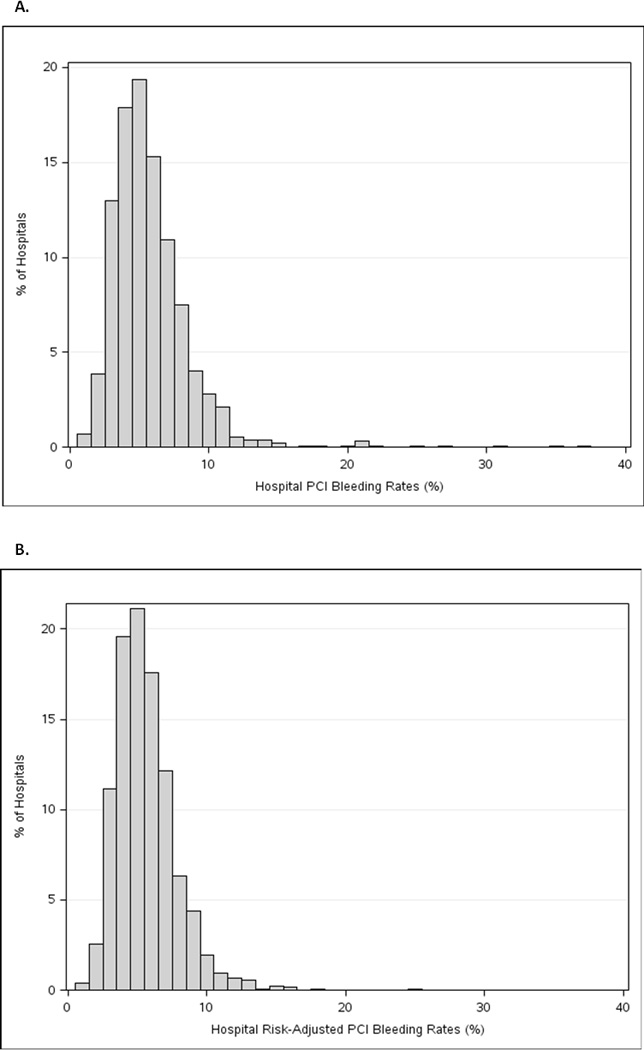
This figure displays the distribution of post-PCI bleeding according to: (a) unadjusted bleeding rates; and (b) risk-adjusted bleeding rates using the revised CathPCI Registry bleeding model.
PCI indicates percutaneous coronary intervention
Table 1.
Bleeding Outcomes by Tertile of Hospital-level Unadjusted Bleeding Rates
| Variable | Low (n=430 sites) |
Average (n=431 sites) |
High (n=431 sites) |
p-value |
|---|---|---|---|---|
| Median overall unadjusted bleeding rate (IQR), % | 3.5 (2.9, 3.9) | 5.2 (4.8, 5.7) | 7.7 (6.9, 9.1) | <0.01 |
| Bleeding components | ||||
| Median bleeding event within 72 hours (IQR), %* | 0.9 (0.6, 1.3) | 1.6 (1.1, 2.1) | 2.4 (1.6, 3.4) | <0.01 |
| Median bleeding at access site (IQR), % | 0.2 (0.1, 0.4) | 0.4 (0.2, 0.7) | 0.5 (0.2, 1.1) | <0.01 |
| Median access site hematoma (IQR), % | 0.4 (0.2, 0.6) | 0.6 (0.4, 1.0) | 0.9 (0.6, 1.5) | <0.01 |
| Median retroperitoneal bleed (IQR), % | 0.1 (0.0, 0.2) | 0.2 (0.1, 0.3) | 0.2 (0.1, 0.4) | <0.01 |
| Median gastrointestinal bleed (IQR), % | 0.1 (0.0, 0.3) | 0.3 (0.1, 0.4) | 0.4 (0.2, 0.6) | <0.01 |
| Median genitourinary bleed (IQR), % | 0.0 (0.0, 0.0) | 0.0 (0.0, 0.1) | 0.1 (0.0, 0.2) | <0.01 |
| Median other/unknown site bleed (IQR), % | 0.1 (0.0, 0.2) | 0.2 (0.1, 0.4) | 0.3 (0.1, 0.6) | <0.01 |
| Median pericardial tamponade (IQR), % | 0.0 (0.0, 0.1) | 0.0 (0.0, 0.1) | 0.0 (0.0, 0.1) | <0.01 |
| Median hemorrhagic stroke (IQR), % | 0.0 (0.0, 0.0) | 0.0 (0.0, 0.0) | 0.0 (0.0, 0.1) | 0.20 |
| Median transfusion with pre-PCI hemoglobin >8 g/dl and no CABG during index hospitalization (IQR), % | 0.5 (0.1, 0.9) | 1.0 (0.6, 1.5) | 1.8 (1.2, 2.5) | <0.01 |
| Median hemoglobin decrease ≥3 g/dl with pre-hemoglobin ≤16 g/dl (IQR), % | 1.4 (1.0, 1.9) | 2.2 (1.7, 2.8) | 3.4 (2.5, 4.5) | <0.01 |
| Median pre-transfusion hemoglobin among transfused patients (IQR), % | 11.3 (10.8, 11.8) | 11.2 (10.8, 11.6) | 11.3 (10.9, 11.7) | 0.20 |
Includes arterial access site, retroperitoneal, gastrointestinal, or genitourinary bleeding
IQR indicates interquartile range
Patient, Procedural, and Hospital Characteristics Among Hospital Bleeding Tertiles
Patient, procedure, and hospital characteristics according to tertiles of unadjusted hospital-level bleeding (low: <4.41%, average: 4.41%–6.23%, and high: ≥6.24%) are shown in Tables 2 and 3. Compared with patients in the low and average tertiles, patients treated at hospitals in the high tertile were more often of non-white race and more often had a history of prior myocardial infarction and prior congestive heart failure, but were less likely to have undergone prior coronary revascularization. Patients in the high tertile also more frequently presented with ST-segment-elevation myocardial infarction and heart failure and more frequently underwent PCI for emergency and salvage indications than patients in the lower two tertiles. As shown in Table 3, hospitals in the high versus low and average tertiles of hospital-level bleeding were more often teaching hospitals and had lower median annual PCI volumes. Compared with PCI procedures in the lower two tertiles, procedures at centers in the high tertile of hospital-level bleeding were performed more often with unfractionated heparin and glycoprotein (GP) IIb/IIIa inhibitors. In contrast, bivalirudin, radial access, and vascular closure devices were used most frequently during PCI procedures performed at hospitals in the low tertile of post-PCI bleeding, as reflected in the highest proportion of hospital use of any BAS in this tertile.
Table 2.
Patient and Procedure Characteristics by Tertile of Hospital-level Unadjusted Bleeding Rates
| Variable | Low (n=695,590 patients) |
Average (n=711,141 patients) |
High (n=578,267 patients) |
|---|---|---|---|
| Unadjusted bleeding rate, % | <4.41 | 4.41–6.23 | ≥6.24 |
| Patient characteristics | |||
| Median age, years (IQR) | 65.0 (56.0, 74.0) | 65.0 (56.0, 74.0) | 64.0 (56.0, 74.0) |
| Female sex, % | 32.7 | 32.2 | 32.8 |
| Non-white race, % | 11.2 | 11.7 | 13.3 |
| Median BMI, kg/m2 (IQR) | 29.1 (25.7, 33.3) | 29.1 (25.7, 33.3) | 29.0 (25.7, 33.3) |
| Medical history, % | |||
| Hypertension* | 81.8 | 82.2 | 81.8 |
| Diabetes | 36.3 | 36.4 | 36.9 |
| Prior MI | 28.9 | 30.7 | 31.1 |
| Prior PCI | 41.7 | 41.1 | 39.2 |
| Prior CABG | 19.2 | 19.2 | 17.5 |
| Prior CHF | 11.1 | 12.5 | 12.7 |
| CVD | 12.0 | 15.0 | 12.6 |
| Chronic lung disease | 15.0 | 27.3 | 15.6 |
| Current/recent smoker | 27.1 | 2.4 | 28.5 |
| Chronic renal failure | 2.1 | 15.7 | 2.7 |
| Presentation with STEMI, % | 13.8 | 10.0 | 18.7 |
| HF within 2 weeks, % | 8.3 | 2.7 | 11.3 |
| Cardiogenic shock w/in 24 hrs, % | 2.3 | 13.7 (12.3, 14.9) | 3.2 |
| Median pre-procedure hemoglobin, g/dl (IQR) | 13.7 (12.4, 14.9) | 13.6 (12.3, 14.9) | |
| Procedure characteristics | |||
| PCI status, % | 42.8 | ||
| Elective | 48.9 | 39.6 | 38.95 |
| Urgent | 35.9 | 17.3 | 40.2 |
| Emergency/salvage | 14.9 | 10.7 | 20.4 |
| Bifurcation lesion, % | 10.6 | 1.3 | 12.4 |
| Dissection, % | 1.00.3 | 0.4 | 1.3 |
| Perforation, % | 71.8 | 71.2 | 0.4 |
| Any DES use, % | 1.8 | 2.1 | 70.2 |
| IABP insertion, % | 2.9 |
All p-values <0.05 except where denoted by an asterisk.
BMI indicates body mass index; CABG, coronary artery bypass graft surgery; CHF, congestive heart failure; CVD, cerebrovascular disease; DES, drug-eluting stent; HF, heart failure; IABP, intra-aortic balloon pump; MI, myocardial infarction; PCI, percutaneous coronary intervention; STEMI, ST-segment elevation myocardial infarction; All other abbreviations can be found in Table 1.
Table 3.
Hospital Characteristics by Tertile of Hospital-level Unadjusted Bleeding Rates
| Variable | Low (n=430 sites) |
Average (n=431 sites) |
High (n=431 sites) |
|---|---|---|---|
| Unadjusted bleeding rate, % | <4.41 | 4.41–6.23 | ≥6.24 |
| Hospital characteristics | |||
| Median number of beds (IQR) | 299.5 (182.0, 426.0) | 319.0 (204.0, 469.0) | 321.5 (218.0, 453.0) |
| Teaching hospital, % | 33.3 | 41.3 | 44.1 |
| Median annual PCI volume, cases (IQR) | 421.9 (215.4, 719.2) | 430.8 (230.8, 692.1) | 310.3 (176.0, 554.8) |
| Intraprocedural medications, % | |||
| Unfractionated heparin | 45.6 | 51.4 | 60.1 |
| Low molecular weight heparin* | 10.7 | 10.5 | 10.7 |
| Bivalirudin | 61.8 | 58.3 | 48.4 |
| GP IIb/IIIa inhibitor | 24.0 | 30.7 | 39.5 |
| Clopidogrel* | 72.1 | 73.3 | 73.6 |
| Prasugrel* | 14.9 | 15.1 | 14.4 |
| Radial access,* % | 9.8 | 9.4 | 7.2 |
| Vascular closure device,* % | 47.8 | 44.3 | 46.4 |
| Use of any BAS, % | 83.4 | 79.2 | 74.6 |
Impact of Risk-adjustment on Hospital Bleeding Performance Rates and Ranks
After applying the CathPCI Registry risk model to adjust for case-mix, variation remained in hospital bleeding rates (Figure 2b). Hospital risk-adjusted bleeding rates ranged with 5th, 10th, 25th, 50th, 75th, 90th, and 95th percentiles of 2.8%, 3.2%, 4.1%, 5.3%, 6.7%, 8.3%, and 9.4%, respectively. We examined the impact of risk-adjustment on hospital rank and outlier status. Figure 3 depicts the relationship between hospital rank based on unadjusted versus risk-adjusted rates of bleeding. In general, the values were well-correlated (Spearman’s correlation coefficient = 0.88). However, individual hospital rankings among the 1292 sites shifted with risk-adjustment; the median change in rank order observed was ± 91.5 (interquartile range [IQR] 37.0, 185.5). Next, hospital outlier status was determined based on either unadjusted or adjusted bleeding rates (Table 4). Prior to adjustment for case-mix, 300 sites were classified as low outliers (lower than expected bleeding rates), and 370 sites were considered high outliers (higher than expected bleeding rates). Overall, risk-adjustment shifted outlier status for 22.1% (n=286) of sites, with 29.3% (n=88) of low outlier, 16.1% (n=100) of non-outlier, and 26.5% (n=98) of high outlier sites changing classification (Table 4).
Figure 3. Correlation Between Unadjusted and Risk-adjusted Hospital Rankings for Post-PCI Bleeding.
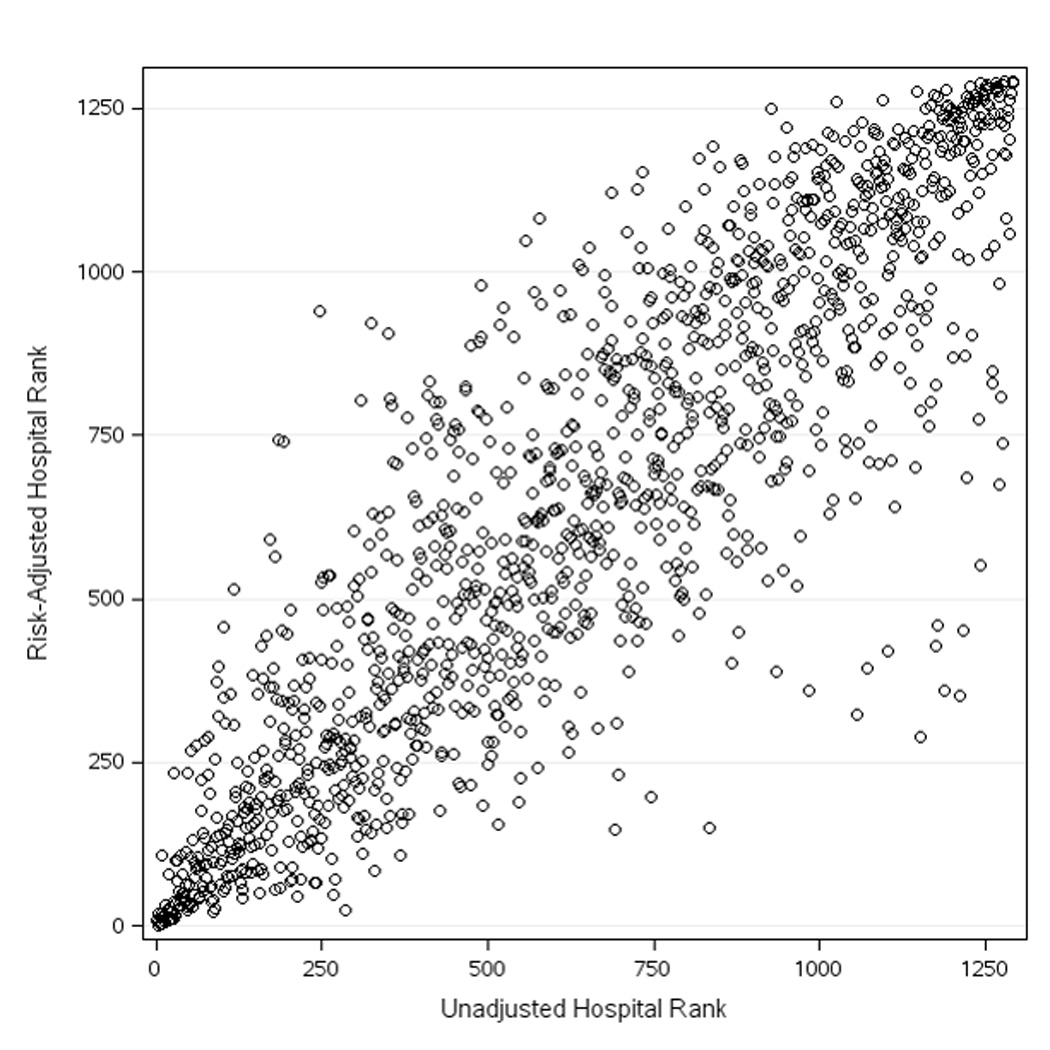
Hospitals are ranked by unadjusted bleeding rates on the x-axis and by risk-adjusted bleeding rates on the y-axis (Spearman’s correlation coefficient = 0.88).
PCI indicates percutaneous coronary intervention
Table 4.
Random Effects Hospital Outlier Status Based on Unadjusted Versus Risk-adjusted Bleeding Rates
| Unadjusted Rates | Adjusted Rates | ||
|---|---|---|---|
| Low Outlier (n=260) | Non-outlier (n=706) | High Outlier (n=304) | |
| Low outlier (n=300) | 212 (81.5%) | 86 (12.2%) | 2 (0.6%) |
| Non-outlier (n=622) | 48 (18.5%) | 522 (73.9%) | 52 (16.0%) |
| High outlier (n=370) | 0 (0.0%) | 98 (13.9%) | 272 (83.4%) |
Factors Associated with Variation in Hospital-level Bleeding
In addition to patient case-mix, we explored hospital and procedural factors that might be associated with hospital-level post-PCI bleeding. Of 1292 total sites, 39.6% (n=511) were teaching hospitals. The median bleeding rates at academic versus non-academic hospitals were similar (5.4% and 5.1%, respectively). Overall, the median annual hospital PCI volume was 391.2 cases (IQR 203.7, 656.8). Hospital annual PCI volume was not correlated with center-level risk-adjusted bleeding (Spearman’s correlation coefficient = 0.02). In contrast, bleeding rates were associated with several procedural factors. On average, hospitals used heparin and GP IIb/IIIa inhibitors in 52.4% and 31.4% of PCI cases, respectively. Increasing hospital use of each medication was correlated with greater bleeding (Spearman’s rho 0.27 for heparin and 0.40 for GP IIb/IIIa inhibitor, p<0.0001 for both). The average hospital-level percentage use of any BAS was 79.1%. We found a correlation between higher hospital percentage use of BAS and lower risk-adjusted bleeding (Spearman correlation coefficient = −0.26).
Sensitivity Analyses
To ensure stability of hospital bleeding estimates, we performed a sensitivity analysis after raising the threshold for exclusion from this study from <50 to <150 PCIs per site. Using this criterion, 60 sites were excluded. Among the remaining 1,236 hospitals, the 5th and 95th percentiles for bleeding rates were 2.6% and 10.3%, respectively, which were similar to the rates of 2.6% and 10.4% from the original analysis.
We also repeated our analyses employing the non-random effects methodology currently used to generate CathPCI Registry site performance reports. We found similar results with respect to residual variation among hospital post-PCI bleeding rates, even after application of the CathPCI Registry bleeding model. Using fixed effect models, risk-adjustment resulted in a median rank order change of ± 86.0 (IQR 35.8, 182.0). After adjustment, there was a reclassification of 25.2% (n=325) of hospitals with respect to outlier status (Table 5). There was 88.8% overall agreement between fixed and random effects strategies to identify hospital outliers (Supplemental Material). Post-PCI bleeding was again correlated with hospital use of BAS (Spearman’s correlation coefficient = −0.26), but not PCI volume or academic status.
Table 5.
Non-random Effects Hospital Outlier Status Based on Observed Versus Risk-adjusted Bleeding Rates
| Observed Rates | Adjusted Rates | ||
|---|---|---|---|
| Low Outlier (n=349) | Non-outlier (n=665) | High Outlier (n=278) | |
| Low outlier (n=480) | 319 (91.4%) | 156 (23.5%) | 5 (1.8%) |
| Non-outlier (n=559) | 30 (8.6%) | 452 (68.0%) | 77 (27.7%) |
| High outlier (n=253) | 0 (0.0%) | 57 (8.6%) | 196 (70.5%) |
Discussion
Post-PCI bleeding, an important procedural complication associated with poor prognosis, has recently been targeted as a site quality metric, but has not been well-characterized among hospitals nationwide. In our analysis of almost 2 million PCI procedures performed at 1,292 United States hospitals, we observed that hospital bleeding rates varied from 2.1% to 10.3% (5th and 95th percentiles, respectively). From a policy perspective, we found that adjustment for patient clinical characteristics changed hospital outlier classification for more than 25% of sites and is necessary for appropriate provider comparisons. However, wide variation in hospital bleeding rates persisted after risk-adjustment. Procedural approaches, such as hospital use of BAS, were associated with reduced rates of bleeding, thereby indicating the potential for provider interventions to mitigate PCI bleeding complications.
Assessment of healthcare quality requires an appropriate measure by which to judge provider performance. Consensus criteria for proper selection of performance measures have been previously described.29 These principles first include the selection of a measure that either represents or is associated with a meaningful outcome to patients and society. Second, the measure must be valid and reliable in its assessment of the process or outcome of interest, and use of this measure to evaluate provider performance must be practically feasible. Finally, consideration of a proper performance measure must account for both patient variability (with the ability to adjust for this variation) and the potential to modify the measure through improvements in processes of care.
Accordingly, post-PCI bleeding may represent an appropriate hospital performance indicator. Bleeding after PCI is associated with increased morbidity, mortality, and cost.9–12 Use of bleeding as a performance measure is feasible due to the efficient data collection capabilities of registries such as the CathPCI Registry, and bleeding rates can be adjusted for patient variability through the application of the CathPCI Registry bleeding model, as was performed in our study. Importantly, the last criterion for selection of a suitable performance measure—whether the measure can be modified by changes in processes of care—necessarily depends on: (1) the presence of persistent variation after adjustment for patient differences; and (2) the availability of strategies to improve performance. In our study, we demonstrated significant residual variation in PCI-related bleeding among hospitals after risk-adjustment, as well as evidence that provider-level alterations in care processes (use of BAS) may reduce bleeding rates.
Several factors may account for the wide variation in hospital-level bleeding after PCI and hospital outliers reported in our study. First, we demonstrate that patient case-mix largely contributes and should be considered when evaluating bleeding rates. Second, differential provider use of BAS may also help to explain differences in post-PCI hospital bleeding. Third, there is likely variability in both ascertainment and reporting of bleeding events. Low bleeding rates may be a result of low site interest in surveillance for bleeding and under-reporting of events. Conversely, sites interested in quality improvement are more likely to report bleeding events and may appear to have higher than average bleeding rates. While the updated CathPCI Registry bleeding definition tries to address some of these differences in reporting thresholds among sites, accurate assessment of PCI-related bleeding and its use as a performance measure may ultimately require more than participation in a registry and may depend on standardized collection of “critical” variables at each institution. Finally, some have suggested that the statistical methodology used to create provider reports may be a source of bias.27,30 Although there are multiple statistical approaches that can be used to perform site-specific analyses,27,30 random effects modeling was chosen for our main analysis because this method employs shrinkage estimators designed to produce estimates that better reflect true hospital effects; furthermore, random effects modeling is becoming widely accepted for profiling of hospital outcomes.27,31,32 We repeated analyses with the non-random effects modeling used in the CathPCI Registry site reports.28 Risk-adjustment via both approaches resulted in re-classification of outlier status for a large proportion of hospitals (22%–25%). Despite inherent limitations, current CathPCI Registry site performance reports may be useful to incentivize motivated sites to improve practices.
Evaluation of quality improvement strategies from other areas may inform efforts to implement post-PCI bleeding as a hospital performance measure and to reduce bleeding rates among sites. A study by Mehta et al. found that implementation of the Guidelines Applied in Practice Initiative, a multi-faceted program consisting of caregiver and patient education about key quality indicators, site visits, and guideline-based practice tools, increased adherence to guideline-recommended treatment in the acute myocardial infarction population.33 Greater adherence to treatment guidelines for acute myocardial infarction patients was also achieved among hospitals routinely using standardized care tools, such as order sets, chart stickers, and discharge checklists.34 Institutional education and incorporation of BAS and post-procedural bleeding variables into standardized PCI order sets and patient care algorithms might similarly increase awareness of this important quality indicator and help to reduce bleeding rates. Furthermore, although we found an association of hospital BAS use with reduced bleeding after PCI, identification of additional methods for providers to reduce this complication is important and could be achieved through hospital surveys, followed by national dissemination of the most effective approaches. This hospital survey strategy has been successfully employed to reduce door-to-balloon times in treating acute myocardial infarction patients.35
Limitations
Our study has several limitations. First, hospital participation in the CathPCI Registry is voluntary, and self-selected sites may have greater interest in quality improvement, potentially precluding generalization of our results to non-CathPCI Registry-participating hospitals. Second, we could not account for institutional variability in the reporting of post-procedure bleeding, although we tried to reduce the effect of underreporting by excluding sites without any submitted bleeding events. Third, there may be concerns over the inclusion of subjective site-reported variables and blood transfusions in the revised bleeding definition resulting in reporting bias and counting of non-PCI-related transfusions as bleeding events. Nevertheless, the revised bleeding definition does include objective measures of bleeding, and we found consistently higher proportions of all bleeding components at hospitals in the top tertile, as well as similar transfusion thresholds among hospitals across all tertiles. Finally, analyses of hospital factors associated with post-PCI bleeding were hypothesis-generating, and our results should be further investigated. The lack of correlation in our analysis between hospital PCI volume and bleeding rates in contrast to prior studies36,37 may be a result of reporting bias, with lower volume sites less likely to report bleeding complications. The modest correlation between hospital BAS use and reduced post-PCI bleeding suggests that other strategies may be important to reduce post-PCI bleeding, but may also be explained by a relatively insensitive hospital-level measure resulting in an underestimation of the true impact of BAS. More accurate assessment of the impact of BAS on bleeding requires further investigation at the patient-level, as strategic application of BAS in the highest-risk patients may be the best approach to reduce bleeding.
Conclusions
Post-PCI bleeding is associated with adverse patient outcomes and has recently been adopted as a quality of care metric. We demonstrated in a large national data registry that rates of bleeding vary significantly among United States hospitals, even after accounting for patient case-mix using a recently revised CathPCI Registry PCI bleeding model. We also found that procedural factors may be important to further reduce bleeding risk. Taken together, our findings support the CathPCI Registry’s use of PCI-related bleeding as a site performance measure and potential incorporation of this metric into other PCI registries. Our results also suggest that provider decisions regarding procedure methods, such as BAS, may be useful to reduce PCI bleeding. Ultimately, quality improvement initiatives to reduce post-PCI bleeding, perhaps through wide implementation of BAS and sharing of practices from best-performing sites, might lead to improved PCI outcomes, though further investigation is needed.
Supplementary Material
Acknowledgements
We thank Erin Hanley for her editorial contributions to this manuscript. Ms. Hanley did not receive compensation for her contributions, apart from her employment at the institution where this study was conducted.
Sources of Funding
This research was supported by the American College of Cardiology Foundation’s National Cardiovascular Data Registry (NCDR). The views expressed in this manuscript represent those of the author(s), and do not necessarily represent the official views of the NCDR or its associated professional societies identified at www.ncdr.com. Connie N. Hess received support from the National Institutes of Health (grant number 5T32HL069749-09).
JA Spertus: Dr. Spertus reports research grants/contracts from the National Heart, Lung, and Blood Institute, the American Heart Association, and the American College of Cardiology Foundation (all significant); consulting for United Healthcare (modest); and equity in Health Outcomes Sciences, Inc. (significant)
ED Peterson: Dr. Peterson reports research funding from Eli Lilly & Company, Ortho-McNeil-Janssen Pharmaceuticals, Inc., Society of Thoracic Surgeons, American Heart Association, American College of Cardiology (all significant); consulting for AstraZeneca, Boehringer Ingelheim, Genentech, Johnson & Johnson, Ortho-McNeil-Janssen Pharmaceuticals, Inc., Pfizer, Sanofi-Aventis, and WebMD (all modest).
Footnotes
Disclosures
CN Hess: Dr. Hess has no relevant disclosures to report.
SV Rao: Dr. Rao reports consulting for The Medicines Company and Terumo (modest)
LA McCoy: Ms. McCoy has no relevant disclosures to report.
ML Neely: Dr. Neely has no relevant disclosures to report.
M Singh: Dr. Singh has no relevant disclosures to report.
RJ Krone: Dr. Krone has no relevant disclosures to report.
WD Weaver: Dr. Weaver has no relevant disclosures to report.
References
- 1.Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Soliman EZ, Sorlie PD, Sotoodehnia N, Turan TN, Virani SS, Wong ND, Woo D, Turner MB. American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics--2012 update: a report from the American Heart Association. Circulation. 2012;125:e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cardiovascular endorsement maintenance 2010 technical report. [Accessed May 2013];National Quality Forum web site. http://www.qualityforum.org/Publications/2012/03/Cardiovascular_Endorsement_Maintenance_2010_Technical_Report.aspx. Updated March 2012. [Google Scholar]
- 3.Oddone EZ, Weinberger M. Hospital readmission rates: are we measuring the right thing? Ann Intern Med. 2012;157:910–911. doi: 10.7326/0003-4819-157-12-201212180-00013. [DOI] [PubMed] [Google Scholar]
- 4.Yeh RW, Rosenfield K, Zelevinsky K, Mauri L, Sakhuja R, Shivapour DM, Lovett A, Weiner BH, Jacobs AK, Normand SL. Sources of hospital variation in short-term readmission rates after percutaneous coronary intervention. Circ Cardiovasc Interv. 2012;5:227–236. doi: 10.1161/CIRCINTERVENTIONS.111.967638. [DOI] [PubMed] [Google Scholar]
- 5.Axon RN, Williams MV. Hospital readmission as an accountability measure. JAMA. 2011;305:504–505. doi: 10.1001/jama.2011.72. [DOI] [PubMed] [Google Scholar]
- 6.Vaduganathan M, Bonow RO, Gheorghiade M. Thirty-day readmissions: the clock is ticking. JAMA. 2013;309:345–346. doi: 10.1001/jama.2012.205110. [DOI] [PubMed] [Google Scholar]
- 7.Peterson ED, Dai D, DeLong ER, Brennan JM, Singh M, Rao SV, Shaw RE, Roe MT, Ho KK, Klein LW, Krone RJ, Weintraub WS, Brindis RG, Rumsfeld JS, Spertus JA, Participants NR. Contemporary mortality risk prediction for percutaneous coronary intervention: results from 588,398 procedures in the National Cardiovascular Data Registry. J Am CollCardiol. 2010;55:1923–1932. doi: 10.1016/j.jacc.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thomas JW, Hofer TP. Accuracy of risk-adjusted mortality rate as a measure of hospital quality of care. Med Care. 1999;37:83–92. doi: 10.1097/00005650-199901000-00012. [DOI] [PubMed] [Google Scholar]
- 9.Rao SV, Kaul PR, Liao L, Armstrong PW, Ohman EM, Granger CB, Califf RM, Harrington RA, Eisenstein EL, Mark DB. Association between bleeding, blood transfusion, and costs among patients with non-ST-segment elevation acute coronary syndromes. Am Heart J. 2008;155:369–374. doi: 10.1016/j.ahj.2007.10.014. [DOI] [PubMed] [Google Scholar]
- 10.Rao SV, Jollis JG, Harrington RA, Granger CB, Newby LK, Armstrong PW, Moliterno DJ, Lindblad L, Pieper K, Topol EJ, Stamler JS, Califf RM. Relationship of blood transfusion and clinical outcomes in patients with acute coronary syndromes. JAMA. 2004;292:1555–1562. doi: 10.1001/jama.292.13.1555. [DOI] [PubMed] [Google Scholar]
- 11.Doyle BJ, Rihal CS, Gastineau DA, Holmes DR., Jr Bleeding, blood transfusion, and increased mortality after percutaneous coronary intervention: implications for contemporary practice. J Am CollCardiol. 2009;53:2019–2027. doi: 10.1016/j.jacc.2008.12.073. [DOI] [PubMed] [Google Scholar]
- 12.Eikelboom JW, Mehta SR, Anand SS, Xie C, Fox KA, Yusuf S. Adverse impact of bleeding on prognosis in patients with acute coronary syndromes. Circulation. 2006;114:774–782. doi: 10.1161/CIRCULATIONAHA.106.612812. [DOI] [PubMed] [Google Scholar]
- 13.Kinnaird TD, Stabile E, Mintz GS, Lee CW, Canos DA, Gevorkian N, Pinnow EE, Kent KM, Pichard AD, Satler LF, Weissman NJ, Lindsay J, Fuchs S. Incidence, predictors, and prognostic implications of bleeding and blood transfusion following percutaneous coronary interventions. Am J Cardiol. 2003;92:930–935. doi: 10.1016/s0002-9149(03)00972-x. [DOI] [PubMed] [Google Scholar]
- 14.Subherwal S, Bach RG, Chen AY, Gage BF, Rao SV, Newby LK, Wang TY, Gibler WB, Ohman EM, Roe MT, Pollack CV, Jr, Peterson ED, Alexander KP. Baseline risk of major bleeding in non-ST-segment-elevation myocardial infarction: the CRUSADE (Can Rapid risk stratification of Unstable angina patients Suppress ADverse outcomes with Early implementation of the ACC/AHA Guidelines) Bleeding Score. Circulation. 2009;119:1873–1882. doi: 10.1161/CIRCULATIONAHA.108.828541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mehran R, Pocock SJ, Nikolsky E, Clayton T, Dangas GD, Kirtane AJ, Parise H, Fahy M, Manoukian SV, Feit F, Ohman ME, Witzenbichler B, Guagliumi G, Lansky AJ, Stone GW. A risk score to predict bleeding in patients with acute coronary syndromes. J Am CollCardiol. 2010;55:2556–2566. doi: 10.1016/j.jacc.2009.09.076. [DOI] [PubMed] [Google Scholar]
- 16.Rao SV, Kaltenbach LA, Spertus J, Krone RJ, Singh M, Peterson ED. An updated bleeding model to predict the risk of post-procedure bleeding among patients undergoing percutaneous coronary intervention: A report using an expanded bleeding definition from the National Cardiovascular Data Registry CathPCI Registry. J Am CollCardiolIntv. 2013;6:897–904. doi: 10.1016/j.jcin.2013.04.016. [DOI] [PubMed] [Google Scholar]
- 17.Stone GW, Witzenbichler B, Guagliumi G, Peruga JZ, Brodie BR, Dudek D, Kornowski R, Hartmann F, Gersh BJ, Pocock SJ, Dangas G, Wong SC, Kirtane AJ, Parise H, Mehran R. Bivalirudin during primary PCI in acute myocardial infarction. New Engl J Med. 2008;358:2218–2230. doi: 10.1056/NEJMoa0708191. [DOI] [PubMed] [Google Scholar]
- 18.Stone GW, McLaurin BT, Cox DA, Bertrand ME, Lincoff AM, Moses JW, White HD, Pocock SJ, Ware JH, Feit F, Colombo A, Aylward PE, Cequier AR, Darius H, Desmet W, Ebrahimi R, Hamon M, Rasmussen LH, Rupprecht HJ, Hoekstra J, Mehran R, Ohman EM ACUITY Investigators. Bivalirudin for patients with acute coronary syndromes. New Engl J Med. 2006;355:2203–2216. doi: 10.1056/NEJMoa062437. [DOI] [PubMed] [Google Scholar]
- 19.Lincoff AM, Bittl JA, Harrington RA, Feit F, Kleiman NS, Jackman JD, Sarembock IJ, Cohen DJ, Spriggs D, Ebrahimi R, Keren G, Carr J, Cohen EA, Betriu A, Desmet W, Kereiakes DJ, Rutsch W, Wilcox RG, de Feyter PJ, Vahanian A, Topol EJ REPLACE-2 Investigators. Bivalirudin and provisional glycoprotein iib/iiia blockade compared with heparin and planned glycoprotein IIb/IIIa blockade during percutaneous coronary intervention: REPLACE-2 randomized trial. JAMA. 2003;289:853–863. doi: 10.1001/jama.289.7.853. [DOI] [PubMed] [Google Scholar]
- 20.Rao SV, Ou FS, Wang TY, Roe MT, Brindis R, Rumsfeld JS, Peterson ED. Trends in the prevalence and outcomes of radial and femoral approaches to percutaneous coronary intervention: a report from the National Cardiovascular Data Registry. JACC Cardiovasc Interv. 2008;1:379–386. doi: 10.1016/j.jcin.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 21.Jolly SS, Amlani S, Hamon M, Yusuf S, Mehta SR. Radial versus femoral access for coronary angiography or intervention and the impact on major bleeding and ischemic events: a systematic review and meta-analysis of randomized trials. Am Heart J. 2009;157:132–140. doi: 10.1016/j.ahj.2008.08.023. [DOI] [PubMed] [Google Scholar]
- 22.Sanborn TA, Ebrahimi R, Manoukian SV, McLaurin BT, Cox DA, Feit F, Hamon M, Mehran R, Stone GW. Impact of femoral vascular closure devices and antithrombotic therapy on access site bleeding in acute coronary syndromes: the Acute Catheterization and Urgent Intervention Triage Strategy (ACUITY) trial. Circ Cardiovasc Interv. 2010;3:57–62. doi: 10.1161/CIRCINTERVENTIONS.109.896704. [DOI] [PubMed] [Google Scholar]
- 23.Koreny M, Riedmuller E, Nikfardjam M, Siostrzonek P, Mullner M. Arterial puncture closing devices compared with standard manual compression after cardiac catheterization: systematic review and meta-analysis. JAMA. 2004;291:350–357. doi: 10.1001/jama.291.3.350. [DOI] [PubMed] [Google Scholar]
- 24.Marso SP, Amin AP, House JA, Kennedy KF, Spertus JA, Rao SV, Cohen DJ, Messenger JC, Rumsfeld JS. National Cardiovascular Data Registry. Association between use of bleeding avoidance strategies and risk of periprocedural bleeding among patients undergoing percutaneous coronary intervention. JAMA. 2010;303:2156–2164. doi: 10.1001/jama.2010.708. [DOI] [PubMed] [Google Scholar]
- 25.Medicare acute care episode (ACE) demonstration. [Accessed September 9, 2013]; CMS.gov: Centers for Medicare & Medicaid Services web site. http://innovation.cms.gov/initiatives/ACE.
- 26.Brindis RG, Fitzgerald S, Anderson HV, Shaw RE, Weintraub WS, Williams JF. The American College of Cardiology-National Cardiovascular Data Registry (ACC-NCDR): building a national clinical data repository. J Am CollCardiol. 2001;37:2240–2245. doi: 10.1016/s0735-1097(01)01372-9. [DOI] [PubMed] [Google Scholar]
- 27.Glance LG, Dick A, Osler TM, Li Y, Mukamel DB. Impact of changing the statistical methodology on hospital and surgeon ranking: the case of the New York State cardiac surgery report card. Med Care. 2006;44:311–319. doi: 10.1097/01.mlr.0000204106.64619.2a. [DOI] [PubMed] [Google Scholar]
- 28.CathPCI Registry. [Accessed March 28, 2014];National Cardiovascular Data Registry web site. https://wwwncdrcom/webncdr/cathpci/. [Google Scholar]
- 29.Measuring and improving quality of care: a report from the American Heart Association/American College of Cardiology First Scientific Forum on Assessment of Healthcare Quality in Cardiovascular Disease and Stroke. Circulation. 2000;101:1483–1493. doi: 10.1161/01.cir.101.12.1483. [No authors listed]. [DOI] [PubMed] [Google Scholar]
- 30.DeLong ER, Peterson ED, DeLong DM, Muhlbaier LH, Hackett S, Mark DB. Comparing risk-adjustment methods for provider profiling. Stat Med. 1997;16:2645–2664. doi: 10.1002/(sici)1097-0258(19971215)16:23<2645::aid-sim696>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 31.Ash AS, Feinberg SE, Louis TA, Normand ST, Stukel TA, Utts J The COPSS-CMS White Paper Committee. Statistical issues in assessing hospital performance. Commissioned by the Committee of Presidents of Statistical Societies. [Accessed March 28, 2014];Centers for Medicare & Medicaid web site. https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/HospitalQualityInits/Downloads/Statistical-Issues-in-Assessing-Hospital-Performance.pdf. Updated January 27, 2012.
- 32.He Y, Selck F, Normand SL. On the accuracy of classifying hospitals on their performance measures. Stat Med. 2014;33:1081–1103. doi: 10.1002/sim.6012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mehta RH, Montoye CK, Gallogly M, Baker P, Blount A, Faul J, Roychoudhury C, Borzak S, Fox S, Franklin M, Freundl M, Kline-Rogers E, LaLonde T, Orza M, Parrish R, Satwicz M, Smith MJ, Sobotka P, Winston S, Riba AA, Eagle KA GAP Steering Committee of the American College of Cardiology. Improving quality of care for acute myocardial infarction: the Guidelines Applied in Practice (GAP) Initiative. JAMA. 2002;287:1269–1276. doi: 10.1001/jama.287.10.1269. [DOI] [PubMed] [Google Scholar]
- 34.Mehta RH, Montoye CK, Faul J, Nagle DJ, Kure J, Raj E, Fattal P, Sharrif S, Amlani M, Changezi HU, Skorcz S, Bailey N, Bourque T, LaTarte M, McLean D, Savoy S, Werner P, Baker PL, DeFranco A, Eagle KA American College of Cardiology Guidelines Applied in Practice Steering Committee. Enhancing quality of care for acute myocardial infarction: shifting the focus of improvement from key indicators to process of care and tool use: the American College of Cardiology Acute Myocardial Infarction Guidelines Applied in Practice Project in Michigan: Flint and Saginaw Expansion. J Am CollCardiol. 2004;43:2166–2173. doi: 10.1016/j.jacc.2003.08.067. [DOI] [PubMed] [Google Scholar]
- 35.Bradley EH, Herrin J, Wang Y, Barton BA, Webster TR, Mattera JA, Roumanis SA, Curtis JP, Nallamothu BK, Magid DJ, McNamara RL, Parkosewich J, Loeb JM, Krumholz HM. Strategies for reducing the door-to-balloon time in acute myocardial infarction. New Engl J Med. 2006;355:2308–2320. doi: 10.1056/NEJMsa063117. [DOI] [PubMed] [Google Scholar]
- 36.Moscucci M, Share D, Smith D, O'Donnell MJ, Riba A, McNamara R, Lalonde T, Defranco AC, Patel K, Kline Rogers E, D'Haem C, Karve M, Eagle KA. Relationship between operator volume and adverse outcome in contemporary percutaneous coronary intervention practice: an analysis of a quality-controlled multicenter percutaneous coronary intervention clinical database. J Am CollCardiol. 2005;46:625–632. doi: 10.1016/j.jacc.2005.05.048. [DOI] [PubMed] [Google Scholar]
- 37.Post PN, Kuijpers M, Ebels T, Zijlstra F. The relation between volume and outcome of coronary interventions: a systematic review and meta-analysis. Eur Heart J. 2010;31:1985–1992. doi: 10.1093/eurheartj/ehq151. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


