Summary
The cellular mechanisms controlling infection-induced emergency granulopoiesis are poorly defined. Here we found that reactive oxygen species (ROS) concentrations in the bone marrow (BM) were elevated during acute infection in a phagocytic NADPH oxidase-dependent manner in myeloid cells. Gr1+ myeloid cells were uniformly distributed in the BM, and all c-Kit+ progenitor cells were adjacent to Gr1+ myeloid cells. Inflammation-induced ROS production in the BM played a critical role in myeloid progenitor expansion during emergency granulopoiesis. ROS elicited oxidation and deactivation of phosphatase and tensin homolog (PTEN), resulting in up-regulation of PtdIns(3,4,5)P3 signaling in BM myeloid progenitors. We further revealed that BM myeloid cell-produced ROS stimulated proliferation of myeloid progenitors via a paracrine mechanism. Taken together, our results establish that phagocytic NADPH oxidase-mediated ROS production by BM myeloid cells plays a critical role in mediating emergency granulopoiesis during acute infection.
Keywords: Infection and inflammation; emergency granulopoiesis; neutrophils; myeloid cells; reactive oxygen species; NADPH oxidase; PTEN; PtdIns(3,4,5)P3
Introduction
Neutrophils are important participants in the innate immune system. They are mobilized from the bone marrow (BM) in response to acute infection or inflammation, and they protect their host by phagocytosing, killing, and digesting bacterial and fungal pathogens. Neutrophil mobilization results in an immediate reactive neutrophilia, followed by accelerated “emergency” granulopoiesis in the BM. Emergency granulopoiesis is a critical host response to restore neutrophil homeostasis after acute infection and inflammation (Manz and Boettcher, 2014).
The mechanisms that regulate emergency granulopoiesis remain incompletely defined. Recent studies suggest that emergency granulopoiesis can be regulated by granulopoietic factors, such as interleukin-6 (IL-6), IL-3, granulocyte colony-stimulating factor (G-CSF), and granulocyte-macrophage colony-stimulating factor (GM-CSF), which are often upregulated during acute infection (Hirai et al., 2006; Manz and Boettcher, 2014; Walker et al., 2008). In vitro, IL-6, IL-3, G-CSF, and GM-CSF promote proliferation and granulocytic differentiation of myeloid progenitors (Caracciolo et al., 1989; Donahue et al., 1988; Koike et al., 1986). However, mice deficient for G-CSF, the G-CSF receptor (G-CSF-R), G-CSF and IL-6, or G-CSF and GM-CSF displayed normal emergency granulopoiesis elicited by sterile inflammation (Hibbs et al., 2007). In addition, both steady-state and inflammation-induced emergency granulopoiesis are intact in mice deficient for the common β-chain of the IL-3R, GM-CSF receptor and IL-5R (Nishinakamura et al., 1996). Taken together, these results indicate that the granulopoietic factors may be dispensable for inflammation-elicited emergency granulopoiesis, and there may be other factors that control the process.
It is well established that reactive oxygen species (ROS) can play a regulatory role in hematopoiesis (Haneline, 2008; Ito et al., 2004; Ito et al., 2006; Jang and Sharkis, 2007; Lewandowski et al., 2010). It has been reported that ROS can prime Drosophila hematopoietic progenitors for differentiation (Owusu-Ansah and Banerjee, 2009). ROS induced by oncogenic Ras are able to promote growth factor-independent proliferation in human CD34+ hematopoietic progenitors (Hole et al., 2010). In addition, recent studies suggest that the regulation of hematopoiesis by Akt and G-CSF is at least partially mediated by ROS (Juntilla et al., 2010; Zhu et al., 2006). Culturing mouse BM in the presence of catalase dramatically alters hematopoiesis; after two to three weeks, there are over 200-fold more LSK cells (Lin−Sca-1+c-Kit− cells; primitive HSCs) in catalase treated cultures than in controls, suggesting that, protected from H2O2, hematopoietic progenitors multiply and become quiescent (Gupta et al., 2006). Physiologic oxidative stress in the BM needs to be controlled in order to maintain the quiescence and survival of the HSC compartment, a function that is required for its long-term regenerative potential. The FoxO proteins play essential roles in the response to oxidative stress, and it has been shown that FoxO-deficient BM has defective long-term repopulating activity that correlates with increased cell cycling and apoptosis of HSCs (Tothova et al., 2007). Jang and Sharkis recently reported that HSCs can be fractioned into two major subpopulations based on the cellular content of ROSs: the ROSlo population has a higher self-renewal potential, while the ROShi population undergoes significant HSC exhaustion following serial transplantation, which is restored with treatment with an antioxidant or rapamycin (Jang and Sharkis, 2007).
Here we examined the role of ROS in emergency granulopoiesis using heat-inactivated E.coli. We show that ROS concentrations in the BM are significantly elevated during acute infection, which is dependent on the expression of NADPH oxidase in myeloid cells. We further demonstrate that NADPH oxidase-dependent ROS production by BM myeloid cells is essential for acute infection-induced myeloid progenitor expansion. In addition, using a BM transplantation model, we reveal that ROS produced by BM myeloid cells regulate myeloid progenitor expansion in emergency granulopoiesis via a paracrine mechanism.
Results
Acute inflammation induces emergency granulopoiesis
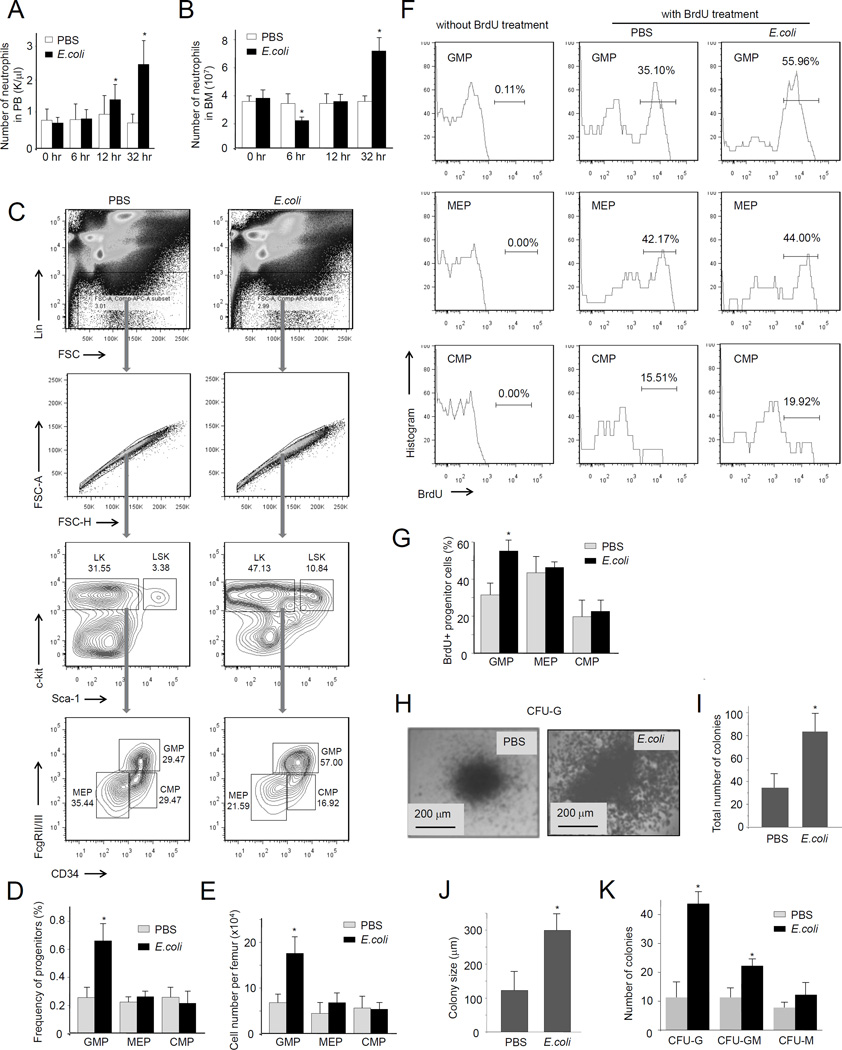
In order to examine microbial infection-driven emergency granulopoiesis, we utilized heat-inactivated E.coli to induce peritonitis (Jia et al., 2007; Subramanian et al., 2007). The use heat-inactivated E.coli rather than live bacteria eliminates the effect of variable host bactericidal capability. E.coli-induced acute peritoneal inflammation elicited instant neutrophil mobilization from the bone marrow, leading to an increased peripheral blood neutrophil count and a reduced BM neutrophil count (Figure 1A). However, at the late stage of acute inflammation (32 h after E.coli injection), the BM neutrophil count was consistently elevated compared to unchallenged mice due to inflammation-induced emergency granulopoiesis (Figure 1B).
Figure 1. Acute inflammation leads to increased progenitor cell proliferation in the bone marrow (BM).
(A) WT mice were intraperitoneally injected with PBS or 1×107 heat inactivated E.coli. Peripheral blood was collected at the indicated times after E.coli injection. The number of neutrophils in the PB was measured using a Hemavet-950FS Hematology system. Data shown are means ± SD of n=5 mice. *p<0.01 versus time 0. (B) The BM was flushed out from the femurs and tibia at the indicated times after E.coli injection. The number of neutrophils in the BM was measured using the Wright-Giemsa staining method. Data shown are means ± SD of n=5 mice. * p<0.01 versus control (0 hr). (C) Flow cytometry-based lineage analysis of the BM cells. The experiments were conducted 36 hr after the E.coli injection. (D) The percentage of each cell population among BM-derived mononuclear cells (BMMCs). (E) The absolute cell number per femur. Data shown are means ± SD of n=5 mice. * p<0.01 versus control (PBS treated mice). (F) Measurement of cycling cells in each progenitor population by incorporation of bromodeoxyuridine (BrdU). Mice were sacrificed 36 hr after the E.coli injection. BrdU was administrated by intraperitoneal injection as a single dose 24 hr before sacrifice. (G) The percentages of BrdU+ cells in each progenitor compartment are shown. Data shown are means ± SD of n=5 mice. * p<0.01 versus control. (H) The number of myeloid progenitors analyzed using an in vitro CFU-GM colony-forming assay. BMMCs were prepared 36 hr after the E.coli injection and cultured in semisolid medium containing rm SCF, rm IL-3, or rh IL-6 for 7 days. Representative pictures of cell clusters/colonies are shown. (I) Total colony numbers from 20,000 BMMCs. (J) The size of colony was analyzed at day 7. (K) The number of indicated colonies from 20,000 BMMCs. Data are means ± SD of n=5 mice. Also see Figure S1.
We next measured the number and type of hematopoietic progenitor cells using fluorescence-activated cell sorting (FACS) analysis. The number of BM granulocyte/macrophage progenitors (GMPs), as measured by the percentage of Lin−Sca-1loc-kit+CD34+FcγRhi cells in the BM, increased gradually in response to E.coli-induced acute inflammation. Since the common myeloid progenitor (CMP) (Lin−Sca-1loc-kit+CD34+FcγRlo) population was unchanged, the increase in GMPs suggested enhancement of cell differentiation/proliferation of myeloid progenitor cells in E.coli-treated mice (Figure 1C–E). In addition, E.coli treatment did not alter the number of megakaryocyte/erythroid progenitors (MEPs) (Lin−Sca-1loc-kit+CD34−FcγR−) in the BM (Figure 1C–E), suggesting that E.coli-induced acute inflammation specifically modulates myelopoiesis. However, similar to previously reported (King and Goodell, 2011), E.coli-elicited inflammation either directly or indirectly modulated the HSC properties and led to a drastic expansion of LSK cells (Figure S1). We examined the proportion of cycling cells by measuring incorporation of bromodeoxyuridine (BrdU), a pyrimidine analog of thymidine that is incorporated into DNA in the S-phase of the cell cycle. BrdU injected intraperitoneally is only incorporated into nuclei when DNA is being actively replicated. E.coli treatment specifically augmented proliferation of GMPs, but not MEPs or CMPs (Figure 1F–G). To further confirm E.coli-induced elevation of myelopoiesis, we used a quantitative granulocyte–monocyte colony-forming unit (CFU-GM) assay to functionally assess the number of committed myeloid progenitors in the BM (Figure 1H–K). As expected, BM from E.coli-treated mice contained more CFUGM (63/20000 BM cells) than untreated controls (42/20000 BM cells), and the size of the colonies originating from E.coli-treated animals was also larger. Taken together, our results demonstrate that E.coli-induced acute peritoneal inflammation can efficiently elicit emergency granulopoiesis.
ROS concentrations in the BM are elevated during acute inflammation
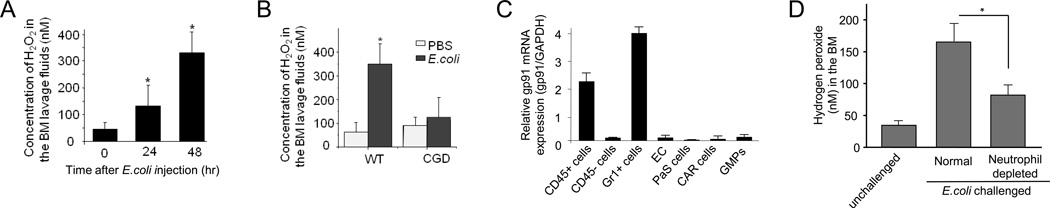
To explore the role of ROS in emergency granulopoiesis, we first measured the level of ROS in the BM using the Amplex® Red reagent, a colorless substrate that reacts with hydrogen peroxide (H2O2) with a 1:1 stoichiometry to produce highly fluorescent resorufin (excitation/emission maxima=570/585). During E.coli-induced acute inflammation, the concentration of H2O2 in the BM extracellular space increased gradually, reaching a peak by 48 hr (Figure 2A).
Figure 2. Hydrogen peroxide (H2O2) concentrations increase in the BM during E.coli-elicited acute inflammation.
(A) The BM was flushed out from the femurs and tibia at the indicated times after E.coli injection. The extracellular ROS were measured using the Amplex® Red assay. Data shown are means ± SD of n=5 mice. * p<0.01 versus time 0. (B) E.coli -elicited elevation of ROS production in the BM was abolished in CGD mice (48 hr after E.coli injection). Data shown are means ± SD of n=3 mice. * p<0.01 versus control (PBS treated mice). (C) gp91phox (encoding NOX2) expression in hematopoietic cells in the BM. Bone marrow CD45+ hematopoietic cells, CD45− nonhematopoietic cells, Gr1+ myeloid cells, endothelial cells (EC) (Sca-1+CD31+CD45−Ter119−), CXCL12-abundant reticular (CAR) cells (PDGFR-b+Sca-1−CD31−CD45−Ter119−), and PaS multipotent stromal cells (CD45−Ter119−CD31− PDGFRa+Sca-1+) were obtained by flow cytometry sorting using specific antibodies. gp91 mRNA expression was measured by quantitative real time RT-PCR and normalized to GAPDH. Data shown are means ± SD of n=3 mice. (D) Acute inflammation-elicited ROS production in mice depleted of neutrophils. Two days after injection of anti-Gr1 antibody, peritonitis was induced by E.coli. The concentration of H2O2 in the BM was measured 24 hr after the E.coli injection. Data shown are means ± SD of n=5 mice. *p<0.01. Also see Figure S2.
Acute inflammation-elicited ROS production is mediated by phagocytic NADPH oxidase
During infection, myeloid cells produce a large amount of ROS to kill invading pathogens. In the BM, over half of the cells are myeloid cells, in which ROS are mainly produced by phagocytic NADPH oxidase (NOX2), a multisubunit enzyme (Subramanian and Luo, 2009). During cell activation, the cytosolic components of the enzyme, p47phox, p67phox, Rac2, and p40phox, are recruited to the membrane to form a complex with its membrane components, p22phox and gp91 (gp91phox or cytochrome-b 558 complex) (Dinauer, 2005). Assembly of the oxidase complex (holoenzyme) catalyzes the conversion of molecular oxygen to superoxide. To elucidate the role of NADPH oxidase in emergency granulopoiesis, we investigated peritonitis-induced granulopoiesis in the chronic granulomatous disease (CGD) mouse, in which the gp91 subunit of NADPH oxidase holoenzyme is deleted (Pollock et al., 1995), and therefore chemokine-elicited superoxide production is completely abolished (Hattori et al., 2010). Disruption of NOX2 abolished E.coli-elicited ROS production in the BM (Figure 2B). Since phagocytic NADPH oxidase NOX2 is mainly expressed in myeloid cells (Henderson and Chappel, 1996; Segal et al., 2000) (Figure 2C), our result suggests that acute inflammation-elicited myeloid progenitor expansion mainly relies on NOX2-mediated ROS production by myeloid cells. In further support of this hypothesis, depletion of neutrophils with anti-Gr1 antibody inhibited acute inflammation-elicited ROS production (Figure 2D and Figure S2).
NADPH oxidase-dependent ROS production is required for emergency granulopoiesis
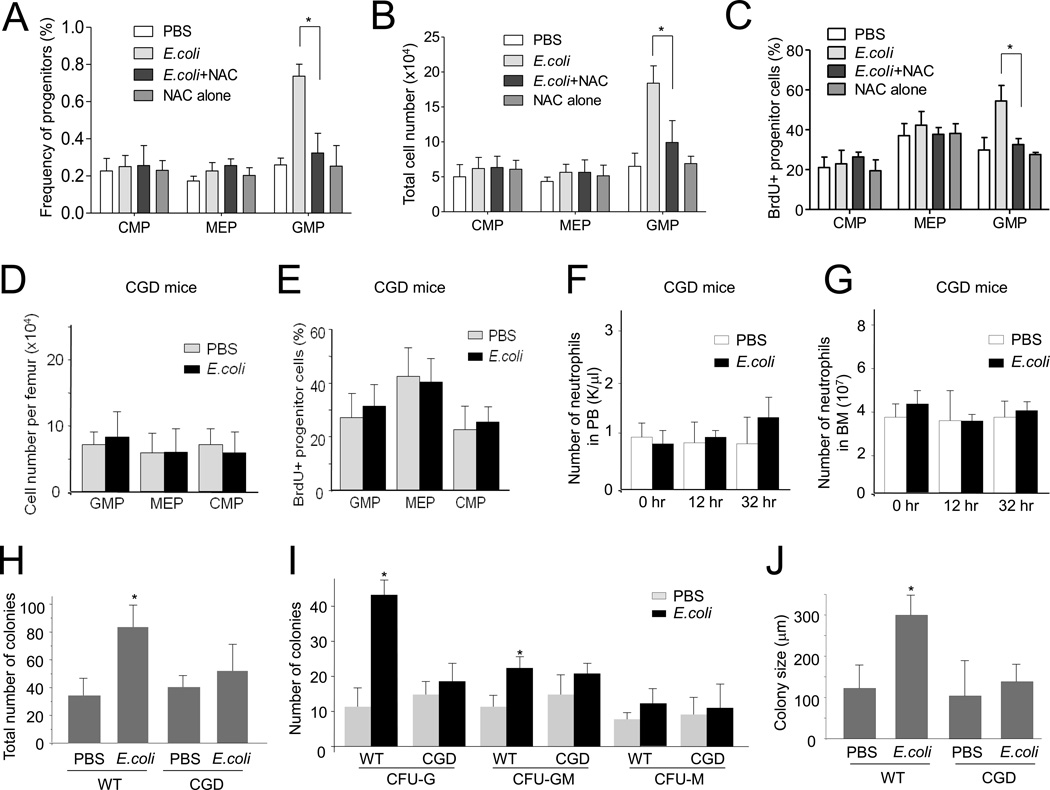
To explore whether ROS are critical for the proliferation of myeloid progenitor cells in emergency granulopoiesis, we used the ROS scavenger N-acetyl-cysteine (NAC) to reduce ROS concentrations. Culture of myeloid progenitor cells with NAC reduced their colony forming capability (Figure S3A). NAC was also able to reduce ROS in vivo. The reduction of ROS in the BM was confirmed using the Amplex assay (Figure S3B). Treatment with NAC suppressed E.coli-elicited expansion of GMPs (Figure 3A–C) and LSK cells (Figure S3C). Similar results were observed in the CGD mice, in which NADPH oxidase-mediated ROS accumulation in the BM was abolished (Figure 3C and Figure S3D). In addition, there was a lower percentage of cycling cells over time in the GMP population of E.coli-challenged CGD mice compared to WT mice (Figure 3E) measured by BrdU incorporation. E.coli-induced neutrophilia in PB and the BM were also abolished in the CGD mice (Figure 3F–G). Consistent with this, inhibition of NADPH oxidase-dependent ROS production also reduced the number of committed myeloid progenitors in the BM in CFU-GM assays (Figure 3H–J). Despite its critical role in emergency granulopoiesis, phagocyte NADPH oxidase is not absolutely required for homeostatic hematopoiesis, since unchallenged CGD mice had normal granulopoiesis, BM cellularity, and peripheral blood counts (Figure 3D–G, Figure S3E and Table S1).
Figure 3. NADPH oxidase-mediated ROS production is essential for E.coli-induced granulopoiesis.
(A) Flow cytometry-based lineage analysis. Mice were treated with NAC (100 mg/kg, ip) 3 hr before E.coli injection. Thirty-six hr after E.coli injection, BM cells were isolated and the percentage of each cell population among BMMCs was analyzed. (B) Statistical analysis of absolute cell number per femur of different cell populations based on lineage analysis (n=5 for each group). (C) Measurement of cycling cells in each progenitor population by incorporation of BrdU. (D–G) E.coli-induced granulopoiesis in CGD mice. The CGD mice were intraperitoneally injected with PBS or 1×107 heat inactivated E.coli. (D) Flow cytometry-based lineage analysis of the BM cells. Data shown are means ± SD of n=3 mice. * p<0.01 versus control (PBS treated mice). (E) Measurement of cycling cells in each progenitor population by incorporation BrdU. The percentages of BrdU+ cells in each progenitor compartment are shown. Data shown are means ± SD of n=3 mice. * p<0.01 versus control (PBS treated mice). (F) The number of neutrophils in PB. Data shown are means ± SD of n=5 mice. *p<0.01 versus time 0. (G) The number of neutrophils in the BM. Data shown are means ± SD of n=5 mice. * p<0.01 versus time 0. (H–J) The number of myeloid progenitors analyzed using an in vitro CFU-GM colony-forming assay. Data shown are mean ± SD of n=5 mice. * p<0.01 versus CGD. (H) Total colony numbers from 20,000 BMMCs. (I) The size of colony was analyzed at day 7. (J) The number of indicated colonies from 20,000 BMMCs. Data are means ± SD of n=5 mice. Also see Figure S3, Figure S4, and Table S1.
ROS and G-CSF regulate emergency granulopoiesis parallelly
Both ROS and G-CSF can elevate granulopoiesis. However, ROS- and G-CSF- mediated pathways appear to be independent from each other in infection-elicited emergency granulopoiesis. Inhibition of NADPH oxidase-dependent ROS production did not alter E.coli-elicited elevation of G-CSF expression in Gr1+ myeloid cells (Figure S4A) and serum G-CSF concentrations (Figure S4B), suggesting that the effect of ROS was not mediated by G-CSF. Blocking G-CSF signaling with an anti-G-CSF antibody inhibited G-CSF-elicited neutrophil mobilization from the BM (Figure S4C) as well as G-CSF-elicited expansion of myeloid progenitors (Figure S4D). However, the same treatment did not inhibit acute infection-elicited ROS production (Figure S4E), and did not inhibit E.coli-elicited emergency granulopoiesis (Figure S4F). These results are consistent with the published findings that G-CSF is not indispensable for emergency myelopoiesis, although mice deficient for G-CSF show markedly reduced and delayed kinetics of neutrophilia (Basu et al., 2000; Hibbs et al., 2007; Nishinakamura et al., 1996; Walker et al., 2008). G-CSF can induce granulopoiesis in the absence of acute inflammation. However, treatment with G-CSF did not significantly elevate ROS concentrations in the BM (Figure S4G). Consistently, G-CSF-induced neutrophilia was not altered in the CGD mice (Figure S4H). Thus NADPH oxidase-mediated ROS production by the BM Gr1+ cells is not a required mechanism for G-CSF-induced granulopoiesis.
G-CSF has been demonstrated in multiple clinical studies to be elevated upon sepsis/severe bacterial infection, and is clinically used to increase the production of granulocytes. The published and our own result that blocking G-CSF does not abolish emergency granulopoiesis is somewhat surprising. This could be simply caused by compensation from other granulopoietic cytokines and/or growth factors in the absence of G-CSF. Alternatively, it could be due to the relatively low concentration (about 3 ng/ml serum) of endogenously produced G-CSF during acute inflammation in our E.coli induced system. Indeed, when emergency granulopoiesis was induced in mice by higher dose of heat inactivated E.coli, a much higher concentration of G-CSF is detected in the serum (Figure S4B). As a result, the infection-elicited neutrophilia became less dependent on neutrophil ROS production; since significant elevation of neutrophil count in PB (Figure S4I) and BM (Figure S4J) was still detected in the CGD mice. Interestingly, the increase of myeloid progenitor number (Figure S4K) and elevated proliferation of progenitor cells (Figure S4L) in the BM was completely abolished in the same CGD mice, suggesting that the increased myeloid progenitor proliferation and measurably increased neutrophil production might be regulated independently. This notion was also supported by a recent seminal study from Boettcher et al. (Boettcher et al., 2014; Boettcher et al., 2012).
Elevated ROS is sufficient to promote proliferation of myeloid progenitor cells
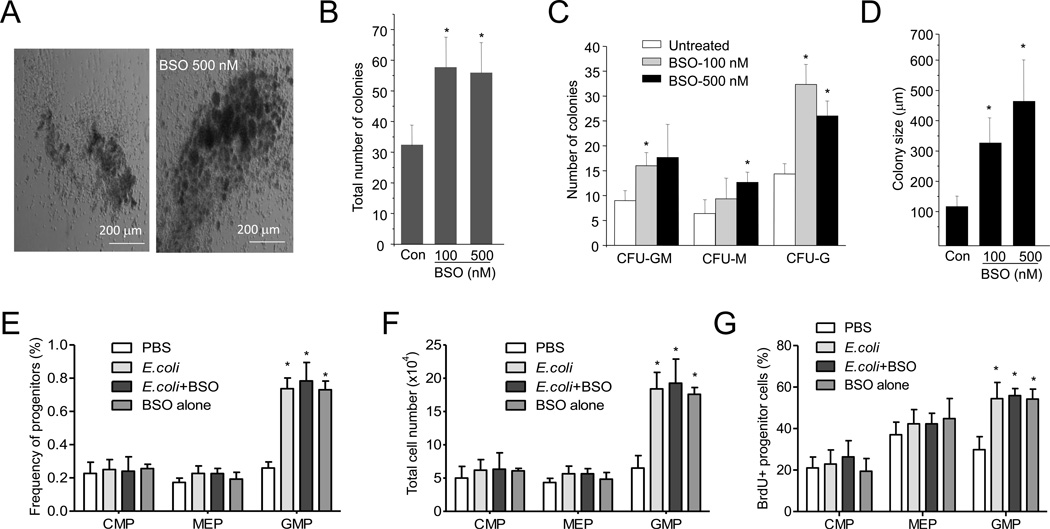
We first augmented intracellular ROS concentrations in the cultured myeloid progenitor cells by treating them with L-buthionine-S,R-sulfoximine (BSO), a GSH biosynthesis inhibitor. This led to increased CFU-GM colony number, accompanied by a drastic increase in the number of cells per colony (Figure 4A–D). After seven days of culturing, the average diameter of the colonies growing in the presence of 500 nM BSO was 450 µm, compared to less than 150 µm for colonies growing in the absence of BSO. These results indicate that proliferation of myeloid progenitor cells is enhanced in the presence of high ROS concentrations. We next examined whether BSO treatment can elevate granulopoiesis in vivo. Treatment with BSO alone increased ROS levels in the BM more than three fold, which is comparable to inflammation-elicited ROS elevation in the BM (Figure S5A). The same treatment specifically expanded the GMP (Figure 4E–G) and LSK (Figure S5B–C) populations in the BM, even in the absence of inflammation. These results demonstrate that elevating BM ROS concentrations alone is sufficient to increase proliferation of myeloid progenitor cells.
Figure 4. ROS promotes granulopoiesis both in vitro and in vivo.
(A) BM cells isolated from WT mice were cultured in semisolid medium in the presence or absence of BSO (500 nM) for 7 days. Representative pictures of cell clusters/colonies are shown. (B) Statistical analysis of CFU-GM colony number from 20,000 BMMCs. BM cells isolated from WT mice were cultured in the presence of indicated amount of BSO for 7 days. (C) The number of indicated colonies from 20,000 BMMCs. (D) The size of colony was analyzed at day 7. Data shown are means ± SD of n=5 mice. * p<0.01 versus untreated. (E–G) Granulopoiesis in vivo. Mice were treated with BSO (10 mg/kg, ip) 3 hr before E.coli injection. Flow-cytometry based lineage analysis of the BM cells was conducted 36 hr after E.coli injection. The percentage of each cell population among BM-derived mononuclear cells (E), the absolute cell number per femur (F), and the percentages of BrdU+ cells in each progenitor compartment (G) are shown. Data represent the means ± SD of n = 5 mice per group. * p<0.01 versus PBS control. Also see Figure S5.
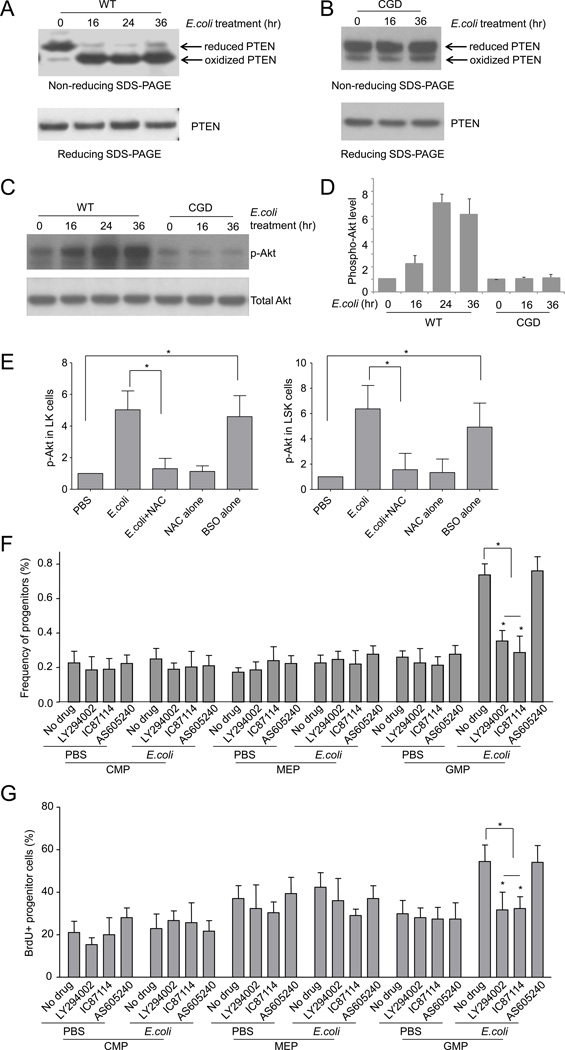
ROS-elicited PTEN oxidation and deactivation participates in emergency granulopoiesis
It has been reported that ROS can enhance PtdIns(3,4,5)P3 signal transduction by inhibiting the lipid phosphatase activity of PTEN in some cell types (Connor et al., 2005; Covey et al., 2007; Kwon et al., 2004; Lee et al., 2002; Leslie et al., 2003; Silva et al., 2008), and the role of PTEN in hematopoiesis is well documented (Yilmaz et al., 2006; Zhang et al., 2006). We therefore investigated whether PTEN oxidation and activation is altered in inflammation-induced emergency granulopoiesis. PTEN oxidation was assessed in sorted LK progenitor cells by measuring accelerated mobilization of oxidized PTEN protein using non-reducing SDS-PAGE (Silva et al., 2008). Consistent with the elevated ROS production in emergency granulopoiesis, almost all PTEN molecules were converted to their oxidized form 16 hr after intraperitoneal E.coli injection. Separating proteins on a reducing SDS-PAGE gel in the presence of dithiothreitol (DTT) abolished the inflammation-induced electrophoretic mobility shift, consistent with the decrease in oxidized PTEN (Figure 5A). Since emergency granulopoiesis-associated ROS are mainly produced by NADPH oxidase, we next measured PTEN oxidation in LK progenitor cells isolated from CGD mice. As expected, disruption of NOX2 prevented PTEN oxidation during acute inflammation. Progenitor cells isolated from CGD mice only showed background concentrations of oxidized PTEN after intraperitoneal E.coli injection (Figure 5B). Taken together, these results show that NADPH oxidase-dependent ROS production induces PTEN oxidation in inflammation-induced emergency granulopoiesis.
Figure 5. Inflammation-induced granulopoiesis is mediated by ROS-elicited deactivation of PTEN and subsequent Akt activation.
(A) E.coli-elicited PTEN oxidation in hematopoietic progenitor cells. WT mice were intraperitoneally injected with PBS or E.coli. The experiments were conducted at each indicated time points after the E.coli injection. LK cells were sorted and the protein lysates from 0.5×106 LK cells (from 3 mice) were resolved using non-reducing SDS-PAGE. PTEN protein was detected using a PTEN specific antibody. Reduced and oxidized forms of PTEN are indicated. Data shown are representative of multiple experiments with similar results. (B) E.coli-elicited PTEN oxidation in CGD hematopoietic progenitor cells. (C) E.coli-elicited Akt activation in hematopoietic progenitor cells. Protein lysates were resolved on a reducing SDS-PAGE gel. Phosphorylated and total Akt were detected by western blotting analysis. (D) Akt phosphorylation was expressed as ratio of phospho-Akt to total Akt. Data represent the means ± SD of n=5 mice per group. (E) Mice were treated with NAC (100 mg/kg, ip) 3 hr before E.coli injection. Akt phosphorylation in hematopoietic progenitor cells (LK) and HSC (LSK) was analyzed 36 hr after E.coli injection. For BSO treatment, Akt phosphorylation was analyzed 27 hr after the BSO (10 mg/kg, ip) injection. Data represent the means ± SD of n=5 mice per group. * p<0.01. (F) Mice were either untreated or treated with PI3 kinase inhibitors LY294002 (i.p. 50 mg/kg body weight), IC87114 (i.p. 25 mg/kg body weight), or AS605240 (i.p. 50 mg/kg body weight) and then challenged with E.coli for 36 hr. Shown are the percentage of each cell population among BMMCs. Data represent the means ± SD of n=5 mice per group. * p<0.01 versus PBS control. (G) The percentages of BrdU+ cells in each progenitor compartment. Data shown are means ± SD of n=5 mice. * p<0.01 versus control. Also see Figure S6.
We next measured PtdIns(3,4,5)P3 signaling in LK progenitor cells using Akt phosphorylation as a reporter (Figure 5C–D). Akt phosphorylation in WT progenitor cells was elevated over three fold (30 hr) by E.coli-induced acute inflammation. In contrast, acute inflammation-elicited augmentation of Akt phosphorylation was abolished in CGD progenitor cells (Figure 5C–D). Treatment of mice with the antioxidant NAC also suppressed inflammation-elicited augmentation of Akt phosphorylation in the progenitor cells, further supporting the role of ROS in regulating PtdIns(3,4,5)P3 signaling in emergency granulopoiesis. On the other hand, elevation of ROS appeared to be sufficient to induced Akt phosphorylation, since BSO treatment induced a greater than four-fold increase in Akt phosphorylation in the absence of any inflammation (Figure 5E). These results are consistent with the role of PTEN as a PtdIns(3,4,5)P3 phosphatase that is negatively regulated by ROS, and suggests that PTEN is a major mediator of inflammation-induced emergency granulopoiesis.
To further investigate whether the upregulation of PtdIns(3,4,5)P3 signaling during acute inflammation is essential for emergency granulopoiesis, we suppressed PtdIns(3,4,5)P3 signaling using several specific inhibitors of PI3 kinases (PI3Ks). It is well documented that PI3Kδ and PI3Kγ operate as partners in distinct signaling pathways in hematopoietic cells (Rommel et al., 2007). The pan-PI3K inhibitor LY294002, and a specific PI3Kδ inhibitor IC87114, suppressed inflammation-induced expansion of GMP (Figure 5F–G) and LSK (Figure S6) populations, while AS605240, a specific inhibitor of PI3Kγ, was essentially ineffective. These results demonstrate that PtdIns(3,4,5)P3 signaling is critical for emergency granulopoiesis, and that PtdIns(3,4,5)P3 signaling in the progenitor cells is mainly maintained by PI3Kδ. It is noteworthy that a catalytically inactive PI3Kδ mutant (p110-deltaD910A) mouse has been generated. However, B and T cell maturation is significantly impaired and immune responses attenuated in this mouse (Okkenhaug et al., 2002). This mutant mouse also developed inflammatory bowel disease (Koyasu, 2003; Okkenhaug and Vanhaesebroeck, 2003), making it difficult to use in the current experiment. Thus here we used isoform-specific PI3K inhibitors to minimize the long-term effect caused by PI3K inhibition. PI3K inhibitors are emerging as a new generation of therapeutics and are being used in clinical trials. The inhibitors used in current study are of proven high specificity and low toxicity.
ROS regulate proliferation of myeloid progenitor cells via a paracrine mechanism
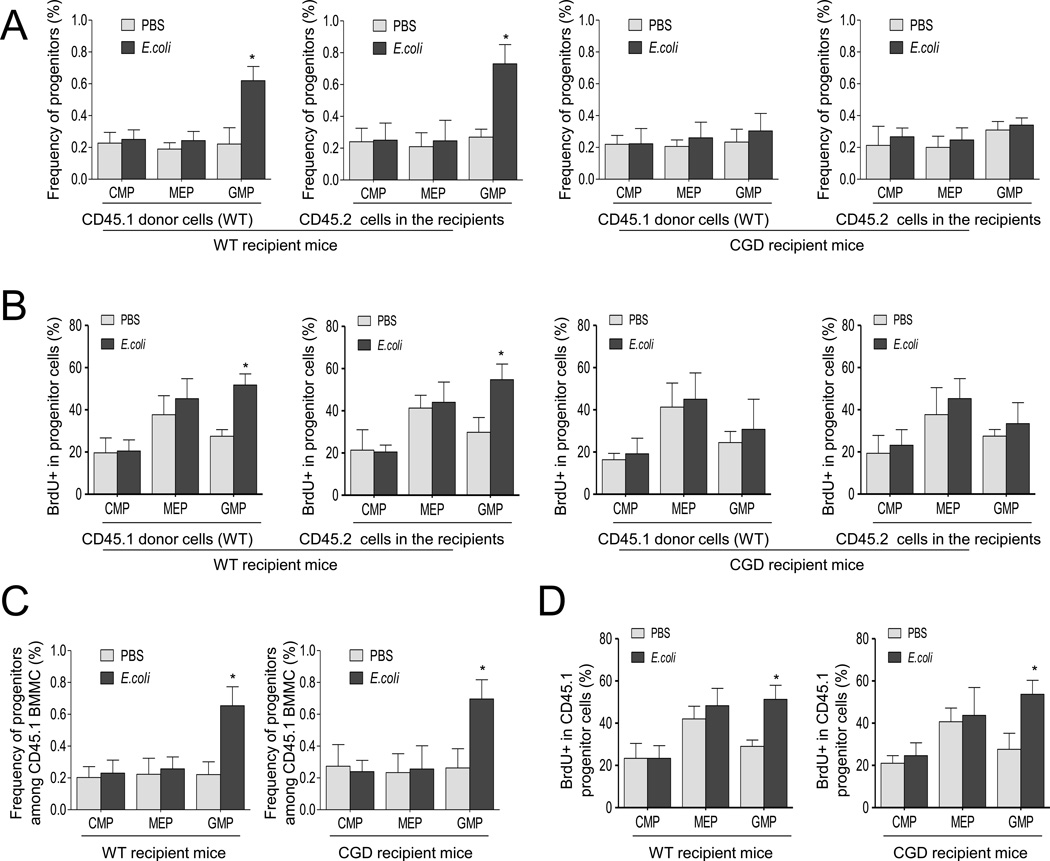
Our results show that phagocytic NADPH oxidase, which is predominately expressed in the myeloid lineage, mainly mediates the elevation of ROS levels during acute inflammation. Components of the NOX2 complex are gradually expressed during myeloid cell differentiation and maturation. The ROS produced by the differentiating myeloid progenitors or immature myeloid cells could either control granulopoiesis in an autonomous manner or via a paracrine mechanism (Figure S7A). In order to test whether the ROS effect was autonomous or paracrine, we conducted a BM transplantation experiment, in which proliferation of transplanted WT progenitors was examined in CGD recipient mice during acute inflammation (Figure S7B). If the ROS produced by the differentiating myeloid progenitors or immature neutrophils control granulopoiesis autonomously, the proliferation of transplanted WT progenitors should still be augmented by acute inflammation in the CGD recipient mice.
BM LK cells, isolated and sorted from WT C57BL/6.CD45.1 mice, were transplanted into congenic C57BL/6.CD45.2 CGD or WT (as control) mice to generate mixed chimeric mice. Since the purpose of this experiment was to test the effect of myeloid cells on the proliferation of transplanted progenitor cells in the recipient BM, and irradiation leads to myeloid depletion in the BM, we used non-irradiated mice as recipients. Donor and recipient cells were distinguished by CD45.1 and CD45.2 expression. CD45.1 LK cells successfully engrafted non-irradiated mice with stable chimerism of 0.4% over six weeks (Figure S7B). Engraftment efficiency was slightly lower than, but comparable to, other reported similar BM transplantation systems(Bhattacharya et al., 2009; Takizawa et al., 2011). E.coli-induced peritonitis was induced six weeks after BM transplantation, and lineage analysis was conducted for both donor (CD45.1+) and recipient (CD45.2+) populations. Inflammation-induced expansion of the GMP population appeared solely dependent on NADPH oxidase in the recipient mice. In these CGD recipient mice, transplanted WT progenitor cells did not produce the phenotype seen in WT mice, and these NADPH oxidase-intact donor progenitors did not expand in CGD recipient mice (Figure 6A). This result was further confirmed using the BrdU incorporation assay (Figure 6B); acute inflammation-induced cell cycling of transplanted WT progenitors was suppressed in CGD recipient mice. Taken together, these results suggest that the ROS produced by the BM myeloid cells regulate proliferation and differentiation of myeloid progenitors via a paracrine mechanism.
Figure 6. ROS regulate inflammation-induced granulopoiesis via a paracrine mechanism.
(A) Flow cytometry-based lineage analysis of the CD45.1 (donor) and CD45.2 (recipient) BM cells. To investigate the proliferation of transplanted WT progenitors in CGD recipient mice during acute inflammation, the sorted LK cells of CD45.1 WT mice were transplanted into non-irradiated CD45.2 CGD mice or WT mice (as a positive control). The BM cells were prepared and analyzed 36 hr after the E.coli injection. The percentage of each cell population among bone marrow-derived CD45.1 or CD45.2 mononuclear cells are shown. Data shown are mean ± SD of n=5 mice. * p<0.01 versus control (PBS treated mice). (B) Measurement of cycling cells in each progenitor population by incorporation of BrdU. BrdU was administrated by i.p. injection 24 hr before sacrificing the mice. The percentages of BrdU+ cells in each CD45.1 or CD45.2 progenitor compartment are shown. Data shown are means ± SD of n=5 mice. * p<0.01 versus control (PBS treated mice). (C–D) The effect of ROS produced by BM mesenchymal cells on emergency granulopoiesis. (C) Flow cytometry-based lineage analysis of the CD45.1+ (donor) BM cells. Data shown are means ± SD of three experiments. * p<0.01 versus control (PBS treated mice). (D) Measurement of cycling cells in each CD45.1+ progenitor population by incorporation of BrdU. Data shown are means ± SD of n=3 mice. * p<0.01 versus control. Also see Figure S7.
Since gp91 is predominately expressed in the myeloid lineage and disruption of gp91 abolished inflammation-elicited ROS production (Figure 2), we hypothesize that acute inflammation-elicited emergency granulopoiesis mainly relies on NOX2-mediated ROS production by myeloid cells. However, to definitively rule out the possibility that gp91-mediated ROS production by BM mesenchymal cells is also involved in emergency granulopoiesis, we conducted another BM transplantation experiment, in which proliferation of transplanted WT progenitors was examined in lethally irradiated WT and CGD recipient mice during acute inflammation 6 weeks after the BM transplantation (Figure S7C). In this setup, the proliferation of WT GMP progenitors still increased during acute inflammation in the CGD recipient mice, suggesting that gp91-mediated ROS production by BM non-hematopoietic cells is not critical for emergency granulopoiesis (Figure 6C–D).
c-Kit+ progenitor cells are adjacent to Gr1+ myeloid cells
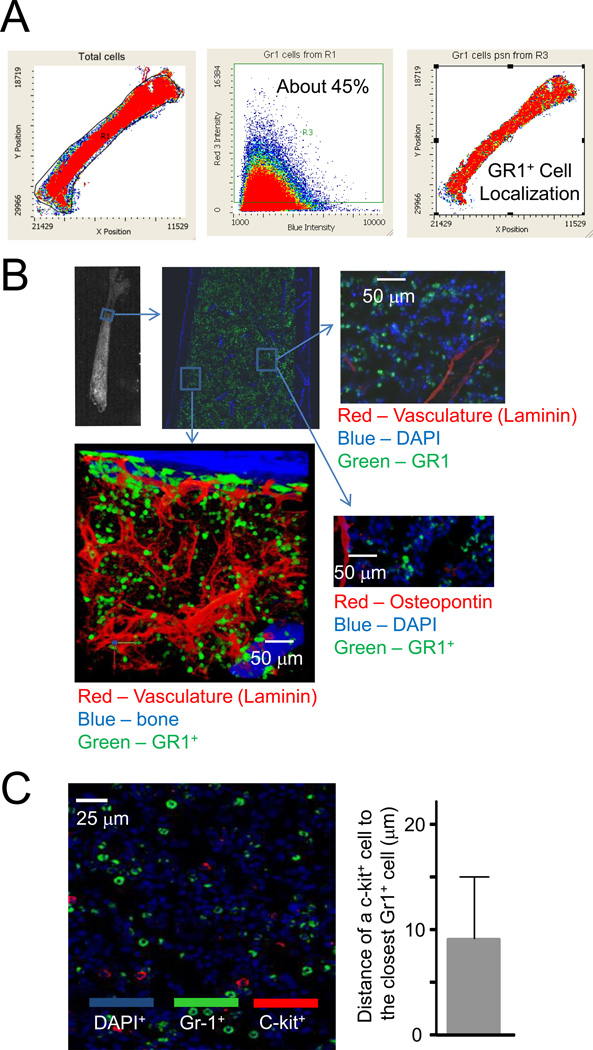
To further explore the role of BM myeloid cells, in particular Gr1+ cells in emergency granulopoiesis, we next examined the localization of Gr1+ myeloid cells in the BM using a laser scanning cytometer (LSC). Gr1+ myeloid cells were evenly distributed in the BM and accounted for 45% of BM cellularity (Figure 7A). We co-stained bone sections with an anti-Gr1 antibody and antibodies against the vasculature marker laminin and the osteoblastic marker osteopontin. Consistent with the uniform distribution of Gr1+ cells in the BM, these cells were not preferentially localized in either the perivascular or periendosteal regions (Figure 7B).
Figure 7. Gr1+ myeloid cells are uniformly distributed in the BM and all c-Kit+ progenitor cells are adjacent to Gr1+ myeloid cells.
(A) Distribution of Gr1+ cells in the BM analyzed by laser scanning cytometry (LSC). Data shown are representative of multiple experiments with similar results. (B) LSC imaging of the trabecular region of a femur from a WT mouse. BM sections were costained with anti-Gr1, anti-osteopontin (a marker of osteoblasts), or anti-laminin (marker of vasculature) antibodies. (C) Gr1+ myeloid cells (green) and c-kit+ cells (red) were stained using specific antibodies against Gr1 and c-kit, respectively. Slides were counterstained with DAPI (blue). The distance of a cKit+ cell to the closest Gr1+ cell was quantified. Data shown are means ± SD of n=5 mice.
LSC also allows analyses of whole tissue sections at the single cell level, thereby permitting quantitative statistical analysis of even extremely rare cells (<1%), which has hitherto been very difficult using conventional image analysis techniques. Using LSC, we identified rare cKit+ progenitor cells in the bone marrow. All Kit+ HSC or progenitor cells were in the proximity of evenly distributed Gr1+ myeloid cells (<50µm) (Figure 7C). Considering that H2O2 can diffuse as far as 1500µm from its site of origin in the extracellular space(Winterbourn, 2008), the ROS produced by BM myeloid cells are easily capable of regulating proliferation and differentiation of the myeloid progenitors via the paracrine mechanism.
Discussion
Physiologic numbers of mature neutrophils are maintained by “steady-state” granulopoiesis. Infection and inflammation alter normal leukocyte production by promoting accelerated, or emergency, granulopoiesis over lymphopoiesis (Ueda et al., 2005; Ueda et al., 2004). Steady-state and emergency granulopoiesis appear to be regulated by different cellular mechanisms. For example, steady-state granulopoiesis is absolutely dependent on the C/EBPalpha, but not the C/EBPbeta transcription factor (Hirai et al., 2006; Zhang et al., 1997). In contrast, emergency granulopoiesis is mainly controlled by C/EBPbeta (Hirai et al., 2006; Ueda et al., 2009). In the current study, we provide direct evidence that NADPH oxidase-dependent ROS production by BM myeloid cells is required to initiate acute infection-induced emergency granulopoiesis. Emergency granulopoiesis is generally thought to represent increased GMP proliferation (Hirai et al., 2006; Manz and Boettcher, 2014). Consistent with this, we observed significant increases in GMP numbers two days after E.coli injection, accompanied by increased BrdU uptake and augmented GM colony-forming capability. Depletion of ROS suppressed acute infection-elicited expansion of GMP cells and abolished their GM colony-forming capability.
Enhanced granulopoiesis can be driven by both microbial infection-induced emergency granulopoiesis and sterile inflammation-elicited reactive granulopoiesis (Manz and Boettcher, 2014). How an 'emergency' state, such as acute inflammation, sends the message to the BM to trigger granulopoiesis remains incompletely defined. It was reported that preferential pathogen-mediated stimulation of myeloid differentiation pathways involves toll-like receptor (TLR) signaling, and may provide a means to rapidly replenish the innate immune system during infection (Nagai et al., 2006). TLRs are expressed in some early hematopoietic progenitors, and TLR signaling via the Myd88 adaptor protein can drive differentiation of myeloid progenitors, bypassing some normal growth and differentiation requirements, as well as lymphoid progenitors, to become dendritic cells. A recent study, using tissue-specific Myd88-deficient mice and in vivo LPS administration to model severe bacterial infection, Boettcher et al demonstrated that endothelial cells but not hematopoietic cells, hepatocytes, pericytes, or BM stromal cells, are essential cells for infection-induced emergency granulopoiesis (Boettcher et al., 2014). Another study proposed that inflammation initiates emergency granulopoiesis via a density-dependent feedback mechanism. In an alum-induced inflammation model, IL-1RI-dependent production of G-CSF and G-CSF-G-CSF-R signals are necessary for both the proliferative responses of hematopoietic stem and progenitor cells (HSPCs), and the mobilization of BM neutrophils (Ueda et al., 2009). Depletion of neutrophil reserves during acute inflammation activates a feedback mechanism that further increases G-CSF production/availability and accelerates neutrophil production (Cain et al., 2011). In this way, alum elicits a transient increase in G-CSF production via IL-1RI to mobilize BM neutrophils, but density-dependent feedback sustains G-CSF for accelerated granulopoiesis. It appears that the induction of neutropenia, via depletion with a Gr-1 monoclonal antibody or myeloid-specific ablation of Mcl-1, also triggers G-CSF production and increases HSPC proliferation, leading to emergency granulopoiesis. Finally, clearance of apoptotic neutrophils at the site of inflammation by tissue macrophages and dendritic cells, a process known as efferocytosis, also plays an essential role in regulation of neutrophil production. Efferocytosis leads to reduced phagocyte secretion of IL-23, a cytokine that controls IL-17 production by gamma-delta T cells and unconventional alpha-beta T cells (Smith et al., 2007). Reduction of IL-17 results in reduced G-CSF production and decreased granulopoiesis (Stark et al., 2005).
The role of PTEN in hematopoiesis is well documented (Yilmaz et al., 2006; Zhang et al., 2006). A recent study showed that the effect of PTEN on hematopoiesis may be indirect (Tesio et al., 2013). Disruption of PTEN gene in hematopoietic system causes an up-regulation of the PI(3,4,5)P3 signal in myeloid cells, but not in HSCs. PTEN deficient myeloid cells secret higher amounts of G-CSF, leading to mobilization of HSCs from the bone marrow. Here we show that ROS-induced transient and partial deactivation of PTEN in myeloid progenitors was sufficient to accelerate the proliferation of these cells during acute inflammation. This effect was directly caused by augmentation of PI(3,4,5)P3 signaling in the progenitor cells (Figure 5), since the concentration of G-CSF was not altered in the CGD mice (Figure S6B). Thus, our finding reveal a mechanism by which ROS and PTEN regulate granulopoiesis during acute inflammation, and fills an important gap in our understanding of how ROS regulate granulopoiesis. Although it is well established that ROS can play a regulatory role in hematopoiesis, the underlying mechanism is largely unknown. It has been reported that the FoxO proteins play essential roles in the response to oxidative stress (Tothova et al., 2007). However, the direct downstream target(s) of ROS has not been identified. The PTEN-Akt pathway identified in current study is, by far, the only proven pathway that directly links ROS to hematopoiesis/granulopoiesis.
During emergency granulopoiesis, ROS are predominantly produced by BM myeloid cells; the BM is the major reservoir of myeloid cells, and over 95% of granulocytes are stored in the BM. The signals that relay the remote inflammatory message to the BM and stimulate ROS production during inflammatory responses remain elusive. Proinflammatory chemokines such as keratinocyte chemoattractant (KC) and macrophage inflammatory protein (MIP)-2, as well as certain cytokines such as G-CSF and tumor necrosis factor-α (TNF-α) can all elicit NADPH oxidase activation, leading to elevated ROS production.
CGD is an inherited genetic disorder in which mutations in the genes encoding components of the NADPH oxidase complex cause diminished ROS release by neutrophils (Heyworth et al., 2003). The classical explanation for the severe infection seen in CGD patients is due to a lack of ROS-dependent pathogen clearance by mature neutrophils. Since NADPH oxidase-mediated ROS accumulation also plays a critical role in emergency granulopoiesis, impaired pathogen clearance in CGD might also occur as a result of defective infection-induced granulopoiesis.
This mechanism explaining the pathogenesis of CGD may be helpful for developing alternative therapeutic strategies that specifically target ROS-mediated granulopoiesis. Infection-induced emergency granulopoiesis is a unique process with specific features compared to normal (homeostatic) hematopoiesis. In the current study, we, for the first time, establish a role of NADPH oxidase-mediated ROS production by BM myeloid cells in infection-induced emergency granulopoiesis, and this mechanism does not play any role in homeostatic hematopoiesis. We show that it is the ROS in the microenvironment that contribute to the maintenance and proliferation of stem and progenitor cells emergency granulopoiesis, rather than those within stem or progenitor cells as classically thought. This study provides a mechanism to target in various physiologic and pathologic conditions in which neutrophil homeostasis requires rebalancing, such as immune reconstitution after BM transplantation.
Experimental Procedures
Mice
X-linked CGD mice, mice conditionally expressing EGFP (eGFP loxP/loxP), and the myeloid-specific Cre mice were purchased from Jackson Laboratories. All animal manipulations were conducted in accordance with the Animal Welfare Guidelines of the Children’s Hospital Boston.
E.coli-elicited peritoneal inflammation
CGD or wild-type mice were left uninjected or intraperitoneally injected with 200 µl of heat inactivated E.coli (strain 19138, ATCC) in PBS. At various times after injection, the mice were sacrificed and inflammation induced granulopoiesis was analyzed using the BM cells. To prepare heat inactivated E.coli, bacteria were first cultured in LB broth at 37°C for 16 hours and then washed and resuspended in PBS. E.coli were killed by heating suspensions to 60°C for 1 hr.
BM cell transplantation
Age-matching C57BL/6 and CD45.1 mice were purchased from Jackson Laboratories. Donor whole BM cells (WBM) were prepared by spinning femurs and tibias under sterile conditions, and red blood cells were lysed using ACK lysing buffer. LK progenitor cells were sorted using a FACS AriaII equipped with FACSDiva software (BD Bioscience). The transplantation was conducted using non-irradiated WT (CD45.2) and CGD (CD45.2) recipient mice. The donor LK cells (CD45.1, 2×105) were transplanted into each non-irradiated WT (CD45.2) and CGD (CD45.2) recipient mouse via tail vein injection. To increase the efficiency of engraftment, LK cells were transplanted into recipient mice every 2 days for 1 wk. The acute peritonitis was induced using heat-inactivated E.coli (1×107) 6 weeks after the first BM transplantation. The emergency granulopoiesis elicited by inflammation was assessed 36 hr after the E.coli injection.
Granulocyte/Monocyte Colony forming Unit (CFU-GM) assays
Bone marrow cells (2×104) from WT or CGD mice were seeded in semisolid Methocult GF M3534 medium containing rmSCF, rmIL-3 and rhIL-6 for detection of CFU-GM (Stem Cell Technologies). L-butionine-sulfoxamine (BSO, Sigma-Aldrich) was added to methylcellulose media at the indicated concentrations at the time of plating.
Detection of hydrogen peroxide using Amplex Red
WT and CGD mice were intraperitoneally injected with 1 ml of heat-inactivated E.coli (1×107) in PBS. ROS accumulation in the BM was measured in freshly isolated BM using an Amplex red Hydrogen Peroxide assay Kit (Invitrogen).
Analysis of in vivo cell proliferation by BrdU incorporation
Cell proliferation was determined using a BrdU labeling kit (BD bioscience). Twenty four hours before sacrifice, BrdU was administrated by intraperitoneal injection (2 µg/mouse in 200 µl PBS) as a single dose. At indicated time points, LSK, GMP, CMP, MEP cells were sorted and the mean frequencies of BrdU+ cells in the HSC and each progenitor populations were measured.
Expression of gp91phox (NOX2) in hematopoietic and non-hematopoietic cells
Bone marrow CD45+ hematopoietic cells, CD45− nonhematopoietic cells, Gr1+ myeloid cells, Pα S cells (CD45−Ter119−CD31−PDGFRα+Sca-1+), endothelial cells (Sca-1+CD31+CD45−Ter119−), and CXCL12-abundant reticular (CAR) cells (PDGFR-β+Sca-1−CD31−CD45−Ter119−)(Omatsu et al., 2014) were obtained by flow cytometry sorting. Total RNA was extracted with the TRIzol reagent and the quantitative RT-PCR was performed using a SYBR Green Quantitative RT–PCR Kit (Sigma).
Statistical analysis
Analysis of statistical significance for indicated data sets was performed using the Student’s t-Test capability on Microsoft Excel and GraphPad Prism.
Supplementary Material
Acknowledgments
The authors thank John Lucky and John Manis for helpful discussions. T. Cheng is supported by the grants from the Ministry of Science and Technology of China (2011CB964801) and from the Nature Science Foundation of China (81090411 and 81421002)). Y. Xu is supported by the grants from National Basic Research Program of China (2012CB966403) and Chinese National Natural Sciences Foundation (31271484 and 31471116), and Tianjin Natural Science Foundation (12JCZDJC24600). H. Luo is supported by NIH grants R01AI103142, R01HL092020, P01 HL095489 and a grant from the FAMRI.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions
H. Kwak, P. Liu, B. Bajrami, and H. Zhu designed and carried out experiments, analyzed data and prepared manuscript. Y. Xu, C. Nombela-Arrieta, S. Park, Y. Sun, S. Mondal, L. Chai, L. Silberstein, and T. Cheng helped with designing experiments, analyzing data and evaluating manuscript. H. Luo designed experiments, analyzed data, and wrote paper.
References
- Basu S, Hodgson G, Zhang HH, Katz M, Quilici C, Dunn AR. "Emergency" granulopoiesis in G-CSF-deficient mice in response to Candida albicans infection. Blood. 2000;95:3725–3733. [PubMed] [Google Scholar]
- Bhattacharya D, Czechowicz A, Ooi AG, Rossi DJ, Bryder D, Weissman IL. Niche recycling through division-independent egress of hematopoietic stem cells. J Exp Med. 2009;206:2837–2850. doi: 10.1084/jem.20090778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boettcher S, Gerosa RC, Radpour R, Bauer J, Ampenberger F, Heikenwalder M, Kopf M, Manz MG. Endothelial cells translate pathogen signals into G-CSF-driven emergency granulopoiesis. Blood. 2014;124:1393–1403. doi: 10.1182/blood-2014-04-570762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boettcher S, Ziegler P, Schmid MA, Takizawa H, van Rooijen N, Kopf M, Heikenwalder M, Manz MG. Cutting edge: LPS-induced emergency myelopoiesis depends on TLR4-expressing nonhematopoietic cells. J Immunol. 2012;188:5824–5828. doi: 10.4049/jimmunol.1103253. [DOI] [PubMed] [Google Scholar]
- Cain DW, Snowden PB, Sempowski GD, Kelsoe G. Inflammation triggers emergency granulopoiesis through a density-dependent feedback mechanism. PLoS One. 2011;6:e19957. doi: 10.1371/journal.pone.0019957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caracciolo D, Clark SC, Rovera G. Human interleukin-6 supports granulocytic differentiation of hematopoietic progenitor cells and acts synergistically with GM-CSF. Blood. 1989;73:666–670. [PubMed] [Google Scholar]
- Connor KM, Subbaram S, Regan KJ, Nelson KK, Mazurkiewicz JE, Bartholomew PJ, Aplin AE, Tai YT, Aguirre-Ghiso J, Flores SC, Melendez JA. Mitochondrial H2O2 regulates the angiogenic phenotype via PTEN oxidation. J Biol Chem. 2005;280:16916–16924. doi: 10.1074/jbc.M410690200. [DOI] [PubMed] [Google Scholar]
- Covey TM, Edes K, Fitzpatrick FA. Akt activation by arachidonic acid metabolism occurs via oxidation and inactivation of PTEN tumor suppressor. Oncogene. 2007;26:5784–5792. doi: 10.1038/sj.onc.1210391. [DOI] [PubMed] [Google Scholar]
- Dinauer MC. Chronic granulomatous disease and other disorders of phagocyte function. Hematology Am Soc Hematol Educ Program. 2005:89–95. doi: 10.1182/asheducation-2005.1.89. [DOI] [PubMed] [Google Scholar]
- Donahue RE, Seehra J, Metzger M, Lefebvre D, Rock B, Carbone S, Nathan DG, Garnick M, Sehgal PK, Laston D, et al. Human IL-3 and GM-CSF act synergistically in stimulating hematopoiesis in primates. Science. 1988;241:1820–1823. doi: 10.1126/science.3051378. [DOI] [PubMed] [Google Scholar]
- Gupta R, Karpatkin S, Basch RS. Hematopoiesis and stem cell renewal in long-term bone marrow cultures containing catalase. Blood. 2006;107:1837–1846. doi: 10.1182/blood-2005-03-1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haneline LS. Redox regulation of stem and progenitor cells. Antioxid Redox Signal. 2008;10:1849–1852. doi: 10.1089/ars.2008.2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori H, Subramanian KK, Sakai J, Jia Y, Li Y, Porter TF, Loison F, Sarraj B, Kasorn A, Jo H, et al. Small-molecule screen identifies reactive oxygen species as key regulators of neutrophil chemotaxis. Proc Natl Acad Sci U S A. 2010;107:3546–3551. doi: 10.1073/pnas.0914351107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson LM, Chappel JB. NADPH oxidase of neutrophils. Biochim Biophys Acta. 1996;1273:87–107. doi: 10.1016/0005-2728(95)00140-9. [DOI] [PubMed] [Google Scholar]
- Heyworth PG, Cross AR, Curnutte JT. Chronic granulomatous disease. Curr Opin Immunol. 2003;15:578–584. doi: 10.1016/s0952-7915(03)00109-2. [DOI] [PubMed] [Google Scholar]
- Hibbs ML, Quilici C, Kountouri N, Seymour JF, Armes JE, Burgess AW, Dunn AR. Mice lacking three myeloid colony-stimulating factors (G-CSF, GM-CSF, and M-CSF) still produce macrophages and granulocytes and mount an inflammatory response in a sterile model of peritonitis. J Immunol. 2007;178:6435–6443. doi: 10.4049/jimmunol.178.10.6435. [DOI] [PubMed] [Google Scholar]
- Hirai H, Zhang P, Dayaram T, Hetherington CJ, Mizuno S, Imanishi J, Akashi K, Tenen DG. C/EBPbeta is required for 'emergency' granulopoiesis. Nat Immunol. 2006;7:732–739. doi: 10.1038/ni1354. [DOI] [PubMed] [Google Scholar]
- Hole PS, Pearn L, Tonks AJ, James PE, Burnett AK, Darley RL, Tonks A. Ras-induced reactive oxygen species promote growth factor-independent proliferation in human CD34+ hematopoietic progenitor cells. Blood. 2010;115:1238–1246. doi: 10.1182/blood-2009-06-222869. [DOI] [PubMed] [Google Scholar]
- Ito K, Hirao A, Arai F, Matsuoka S, Takubo K, Hamaguchi I, Nomiyama K, Hosokawa K, Sakurada K, Nakagata N, et al. Regulation of oxidative stress by ATM is required for self-renewal of haematopoietic stem cells. Nature. 2004;431:997–1002. doi: 10.1038/nature02989. [DOI] [PubMed] [Google Scholar]
- Ito K, Hirao A, Arai F, Takubo K, Matsuoka S, Miyamoto K, Ohmura M, Naka K, Hosokawa K, Ikeda Y, Suda T. Reactive oxygen species act through p38 MAPK to limit the lifespan of hematopoietic stem cells. Nat Med. 2006;12:446–451. doi: 10.1038/nm1388. [DOI] [PubMed] [Google Scholar]
- Jang YY, Sharkis SJ. A low level of reactive oxygen species selects for primitive hematopoietic stem cells that may reside in the low-oxygenic niche. Blood. 2007;110:3056–3063. doi: 10.1182/blood-2007-05-087759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia Y, Subramanian KK, Erneux C, Pouillon V, Hattori H, Jo H, You J, Zhu D, Schurmans S, Luo HR. Inositol 1,3,4,5-tetrakisphosphate negatively regulates phosphatidylinositol-3,4,5- trisphosphate signaling in neutrophils. Immunity. 2007;27:453–467. doi: 10.1016/j.immuni.2007.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juntilla MM, Patil VD, Calamito M, Joshi RP, Birnbaum MJ, Koretzky GA. AKT1 and AKT2 maintain hematopoietic stem cell function by regulating reactive oxygen species. Blood. 2010;115:4030–4038. doi: 10.1182/blood-2009-09-241000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King KY, Goodell MA. Inflammatory modulation of HSCs: viewing the HSC as a foundation for the immune response. Nat Rev Immunol. 2011;11:685–692. doi: 10.1038/nri3062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koike K, Stanley ER, Ihle JN, Ogawa M. Macrophage colony formation supported by purified CSF-1 and/or interleukin 3 in serum-free culture: evidence for hierarchical difference in macrophage colony-forming cells. Blood. 1986;67:859–864. [PubMed] [Google Scholar]
- Koyasu S. The role of PI3K in immune cells. Nat Immunol. 2003;4:313–319. doi: 10.1038/ni0403-313. [DOI] [PubMed] [Google Scholar]
- Kwon J, Lee SR, Yang KS, Ahn Y, Kim YJ, Stadtman ER, Rhee SG. Reversible oxidation and inactivation of the tumor suppressor PTEN in cells stimulated with peptide growth factors. Proc Natl Acad Sci U S A. 2004;101:16419–16424. doi: 10.1073/pnas.0407396101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SR, Yang KS, Kwon J, Lee C, Jeong W, Rhee SG. Reversible inactivation of the tumor suppressor PTEN by H2O2. J Biol Chem. 2002;277:20336–20342. doi: 10.1074/jbc.M111899200. [DOI] [PubMed] [Google Scholar]
- Leslie NR, Bennett D, Lindsay YE, Stewart H, Gray A, Downes CP. Redox regulation of PI 3-kinase signalling via inactivation of PTEN. Embo J. 2003;22:5501–5510. doi: 10.1093/emboj/cdg513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewandowski D, Barroca V, Duconge F, Bayer J, Van Nhieu JT, Pestourie C, Fouchet P, Tavitian B, Romeo PH. In vivo cellular imaging pinpoints the role of reactive oxygen species in the early steps of adult hematopoietic reconstitution. Blood. 2010;115:443–452. doi: 10.1182/blood-2009-05-222711. [DOI] [PubMed] [Google Scholar]
- Manz MG, Boettcher S. Emergency granulopoiesis. Nat Rev Immunol. 2014;14:302–314. doi: 10.1038/nri3660. [DOI] [PubMed] [Google Scholar]
- Nagai Y, Garrett KP, Ohta S, Bahrun U, Kouro T, Akira S, Takatsu K, Kincade PW. Toll-like receptors on hematopoietic progenitor cells stimulate innate immune system replenishment. Immunity. 2006;24:801–812. doi: 10.1016/j.immuni.2006.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishinakamura R, Miyajima A, Mee PJ, Tybulewicz VL, Murray R. Hematopoiesis in mice lacking the entire granulocyte-macrophage colony-stimulating factor/interleukin-3/interleukin-5 functions. Blood. 1996;88:2458–2464. [PubMed] [Google Scholar]
- Okkenhaug K, Bilancio A, Farjot G, Priddle H, Sancho S, Peskett E, Pearce W, Meek SE, Salpekar A, Waterfield MD, et al. Impaired B and T cell antigen receptor signaling in p110delta PI 3-kinase mutant mice. Science. 2002;297:1031–1034. doi: 10.1126/science.1073560. [DOI] [PubMed] [Google Scholar]
- Okkenhaug K, Vanhaesebroeck B. PI3K-signalling in B- and T-cells: insights from gene-targeted mice. Biochem Soc Trans. 2003;31:270–274. doi: 10.1042/bst0310270. [DOI] [PubMed] [Google Scholar]
- Omatsu Y, Seike M, Sugiyama T, Kume T, Nagasawa T. Foxc1 is a critical regulator of haematopoietic stem/progenitor cell niche formation. Nature. 2014;508:536–540. doi: 10.1038/nature13071. [DOI] [PubMed] [Google Scholar]
- Owusu-Ansah E, Banerjee U. Reactive oxygen species prime Drosophila haematopoietic progenitors for differentiation. Nature. 2009;461:537–541. doi: 10.1038/nature08313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollock JD, Williams DA, Gifford MA, Li LL, Du X, Fisherman J, Orkin SH, Doerschuk CM, Dinauer MC. Mouse model of X-linked chronic granulomatous disease, an inherited defect in phagocyte superoxide production. Nat Genet. 1995;9:202–209. doi: 10.1038/ng0295-202. [DOI] [PubMed] [Google Scholar]
- Rommel C, Camps M, Ji H. PI3K delta and PI3K gamma: partners in crime in inflammation in rheumatoid arthritis and beyond? Nat Rev Immunol. 2007;7:191–201. doi: 10.1038/nri2036. [DOI] [PubMed] [Google Scholar]
- Segal BH, Leto TL, Gallin JI, Malech HL, Holland SM. Genetic, biochemical, and clinical features of chronic granulomatous disease. Medicine (Baltimore) 2000;79:170–200. doi: 10.1097/00005792-200005000-00004. [DOI] [PubMed] [Google Scholar]
- Silva A, Yunes JA, Cardoso BA, Martins LR, Jotta PY, Abecasis M, Nowill AE, Leslie NR, Cardoso AA, Barata JT. PTEN posttranslational inactivation and hyperactivation of the PI3K/Akt pathway sustain primary T cell leukemia viability. J Clin Invest. 2008;118:3762–3774. doi: 10.1172/JCI34616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith E, Zarbock A, Stark MA, Burcin TL, Bruce AC, Foley P, Ley K. IL-23 is required for neutrophil homeostasis in normal and neutrophilic mice. J Immunol. 2007;179:8274–8279. doi: 10.4049/jimmunol.179.12.8274. [DOI] [PubMed] [Google Scholar]
- Stark MA, Huo Y, Burcin TL, Morris MA, Olson TS, Ley K. Phagocytosis of apoptotic neutrophils regulates granulopoiesis via IL-23 and IL-17. Immunity. 2005;22:285–294. doi: 10.1016/j.immuni.2005.01.011. [DOI] [PubMed] [Google Scholar]
- Subramanian KK, Jia Y, Zhu D, Simms BT, Jo H, Hattori H, You J, Mizgerd JP, Luo HR. Tumor suppressor PTEN is a physiologic suppressor of chemoattractant-mediated neutrophil functions. Blood. 2007;109:4028–4037. doi: 10.1182/blood-2006-10-055319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian KK, Luo HR. In: Non-classical roles of NADPH-oxidase dependent Reactive Oxygen Species in Phagocytes In Granulocytes: Classification, Toxic Materials Produced and Pathology. Kohlund RHaS., editor. Nova Science Publishers, Inc.; 2009. [Google Scholar]
- Takizawa H, Regoes RR, Boddupalli CS, Bonhoeffer S, Manz MG. Dynamic variation in cycling of hematopoietic stem cells in steady state and inflammation. J Exp Med. 2011;208:273–284. doi: 10.1084/jem.20101643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesio M, Oser GM, Baccelli I, Blanco-Bose W, Wu H, Gothert JR, Kogan SC, Trumpp A. Pten loss in the bone marrow leads to G-CSF-mediated HSC mobilization. J Exp Med. 2013;210:2337–2349. doi: 10.1084/jem.20122768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tothova Z, Kollipara R, Huntly BJ, Lee BH, Castrillon DH, Cullen DE, McDowell EP, Lazo-Kallanian S, Williams IR, Sears C, et al. FoxOs are critical mediators of hematopoietic stem cell resistance to physiologic oxidative stress. Cell. 2007;128:325–339. doi: 10.1016/j.cell.2007.01.003. [DOI] [PubMed] [Google Scholar]
- Ueda Y, Cain DW, Kuraoka M, Kondo M, Kelsoe G. IL-1R type I-dependent hemopoietic stem cell proliferation is necessary for inflammatory granulopoiesis and reactive neutrophilia. J Immunol. 2009;182:6477–6484. doi: 10.4049/jimmunol.0803961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda Y, Kondo M, Kelsoe G. Inflammation and the reciprocal production of granulocytes and lymphocytes in bone marrow. J Exp Med. 2005;201:1771–1780. doi: 10.1084/jem.20041419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda Y, Yang K, Foster SJ, Kondo M, Kelsoe G. Inflammation controls B lymphopoiesis by regulating chemokine CXCL12 expression. J Exp Med. 2004;199:47–58. doi: 10.1084/jem.20031104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker F, Zhang HH, Matthews V, Weinstock J, Nice EC, Ernst M, Rose-John S, Burgess AW. IL6/sIL6R complex contributes to emergency granulopoietic responses in G-CSF- and GM-CSF-deficient mice. Blood. 2008;111:3978–3985. doi: 10.1182/blood-2007-10-119636. [DOI] [PubMed] [Google Scholar]
- Winterbourn CC. Reconciling the chemistry and biology of reactive oxygen species. Nat Chem Biol. 2008;4:278–286. doi: 10.1038/nchembio.85. [DOI] [PubMed] [Google Scholar]
- Yilmaz OH, Valdez R, Theisen BK, Guo W, Ferguson DO, Wu H, Morrison SJ. Pten dependence distinguishes haematopoietic stem cells from leukaemia-initiating cells. Nature. 2006;441:475–482. doi: 10.1038/nature04703. [DOI] [PubMed] [Google Scholar]
- Zhang DE, Zhang P, Wang ND, Hetherington CJ, Darlington GJ, Tenen DG. Absence of granulocyte colony-stimulating factor signaling and neutrophil development in CCAAT enhancer binding protein alpha-deficient mice. Proc Natl Acad Sci U S A. 1997;94:569–574. doi: 10.1073/pnas.94.2.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Grindley JC, Yin T, Jayasinghe S, He XC, Ross JT, Haug JS, Rupp D, Porter-Westpfahl KS, Wiedemann LM, et al. PTEN maintains haematopoietic stem cells and acts in lineage choice and leukaemia prevention. Nature. 2006;441:518–522. doi: 10.1038/nature04747. [DOI] [PubMed] [Google Scholar]
- Zhu QS, Xia L, Mills GB, Lowell CA, Touw IP, Corey SJ. G-CSF induced reactive oxygen species involves Lyn-PI3-kinase-Akt and contributes to myeloid cell growth. Blood. 2006;107:1847–1856. doi: 10.1182/blood-2005-04-1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.