Abstract
Although reagents are available to block mouse complement receptor type 2 and/or type1 (CR2/CR1, CD21/CD35) function in acute or short term models of human disease, a mouse anti-rat antibody response limits their use in chronic models. We have addressed this problem by generating in Cr2−/− mice a mouse monoclonal antibody (mAb 4B2) to mouse CR2/CR1. The binding of murine mAb 4B2 to CR2/CR1 directly blocked C3dg (C3d) ligand binding. In vivo injection of mAb 4B2 induced substantial down regulation of CR2 and CR1 from the B cell surface, an effect that lasted six weeks after a single injection of 2 mg of mAb. The 4B2 mAb was studied in vivo for the capability to affect immunological responses to model antigens. Pre-injection of mAb 4B2 before immunization of C57BL/6 mice reduced the IgG1 antibody response to the T-dependent antigen sheep red blood cells (SRBC) to a level comparable to that found in Cr2−/− mice. We also used the collagen-induced arthritis (CIA) model, a CR2/CR1-dependent autoimmune disease model, and found that mice pre-injected with mAb 4B2 demonstrated substantially reduced levels of pathogenic IgG2a antibodies to both the bovine type II collagen (CII) used to induce arthritis and to endogenous mouse CII. Consistent with this result, mice pre-injected with mAb 4B2 demonstrated only very mild arthritis. This reduction in disease, together with published data in CII-immunized Cr2−/− mice, confirm both that the arthritis development depends on CR2/CR1 receptors and that mAb 4B2 can be used to induce biologically relevant receptor blockade. Thus mAb 4B2 is an excellent candidate for use in chronic murine models to determine how receptor blockage at different points modifies disease activity and autoantibody responses.
Keywords: Monoclonal antibody, B cells, complement receptors, immunological responses, murine arthritis models
1. Introduction
Mouse complement receptors type 2 and type 1 (CR2/CR1, CD21/CD35) are products of alternative splicing of a single gene. In humans, however, these proteins are encoded by linked but distinct genes. Despite the differences in origin, there are great similarities between mice and human in function and expression of these receptors. Moreover human CR2 and mouse CR2 share 67% homology at the nucleotide level and 58% homology in protein sequence (Fingeroth, 1990). Mouse CR2 consists of 15 short consensus repeats (SCR), while mouse CR1 is identical to CR2 but with the addition of six N-terminal SCRs (Fingeroth et al., 1989;Kurtz et al., 1990;Molina et al., 1990). These six SCRs are responsible for unique mouse CR1 activities that go beyond the iC3b/C3d binding activity of CR2, including C3b and C4b binding and factor I cofactor activity for C3b/C4b cleavage (Kinoshita et al., 1985;Ross and Medof, 1985).
Since mouse CR2 has identical sequences as mouse CR1, monoclonal antibodies (mAbs) directed against mouse CR2 recognize mouse CR1 (Kinoshita et al., 1990), and gene targeting of the Cr2 gene results in mice lacking both mouse CR2 and CR1 proteins (Kurtz et al., 1990;Molina et al., 1990). Mouse CR2 and CR1 are expressed on B lymphocytes and follicular dendritic cells, and some populations of activated T cells (Fischer et al., 1991;Kinoshita et al., 1991;Tedder et al., 1984;Molnar et al., 2008b). In humans CR1 is also expressed on erythrocytes where it serves the function of transporting immune complexes to the reticuloendothelial system, and on macrophages and neutrophils where it facilitates the phagocytosis of complement-opsonized particles (Ahearn and Fearon, 1989). Both, human and mouse CR2 proteins are receptors for the C3d fragment of activated complement component C3 (Iida et al., 1983), in addition to the larger C3dg and iC3b fragments (Kalli et al., 1991;Weis et al., 1984).
The CR2-C3dg/C3d binding interaction is a major regulator of immunological responses. Co-ligation of CR2 with the B cell receptor (BCR) through Ag-C3d complexes markedly amplifies the BCR activation signal, lowering by up to 10,000 times the amount of antigen needed to trigger immunological responses (Dempsey et al., 1996;Luxembourg and Cooper, 1994;Lyubchenko et al., 2005). Amplification of BCR activation by CR2 is CD19-dependent and is primarily due to the non-covalent association of CD19 with CR2 (Bradbury et al., 1993;Buhl et al., 1998;Tedder et al., 1994). This association of CR2 with the BCR brings CD19 into proximity of BCR-associated kinases, following which the cytoplasmic tail of CD19 rapidly becomes phosphorylated and subsequently engages several positive signaling pathways (Carter and Fearon, 1992;Dempsey et al., 1996;Fearon and Carroll, 2000). In addition, ligation of CR2 can promote the survival and proliferation of B cells (Molnar et al., 2008a), and CR2 on marginal zone (MZ) B cells also plays an important role in capturing immune complexes (IC) and transferring them to follicular B cells (Phan et al., 2007;Whipple et al., 2007).
Data obtained with Cr2 −/− mice have further revealed the importance of these receptors in immunological responses to antigen. For example, CR2/CR1 deficient mice demonstrate impaired T-dependent and T-independent antibody production (Carroll, 1998;Carroll, 2000;Kaye et al., 2004;Szomolanyi-Tsuda et al., 2006;Wu et al., 2000), as well as a defective natural antibody repertoire (Fleming et al., 2002;Holers, 2005;Reid et al., 2000;Reid et al., 2002). In some settings, CR2 in association with its ligands helps to maintain tolerance to self-antigens, which may be particularly important in control of reactivity to nuclear antigens (Boackle et al., 2001;Prodeus et al., 1998a;Prodeus et al., 1998b). In addition, CR2/CR1 is involved in central nervous system processes, including prevention of neuronal cell death after head injury trauma (Neher et al., 2014) and in adult hippocampal neurogenesis (Moriyama et al., 2011) by either direct interactions or through shaping of the natural antibody repertoire. Finally, we previously published data using human CR2 transgenic mice demonstrating dramatic inhibition, including the lack of development of antigen-specific IgG responses, following pre-injection of a CR2-C3d blocking mAb directed to human CR2 (Kulik et al., 2011).
Rat mAbs against mouse CR1 or CR1/CR2 have been available for many years (Kinoshita et al., 1988;Kinoshita et al., 1990). They have been used in many assays based on the short term blocking of CR2 and CR1 function (Heyman et al., 1990), as well as used as a carrier for antigen delivery to B cells (Prechl et al., 1999;Prechl et al., 2007;Whipple et al., 2007;Whipple et al., 2004;Baiu et al., 1999). Nevertheless, the use of these reagents for recurrent treatment of long term chronic autoimmune diseases is limited (Whipple et al., 2007) due to anti-rat IgG development . Using mAb 7G6, we were likewise unable to utilize this mAb for multiple injections as anti-rat antibodies were emerging as soon as after first injection (data not shown). Similarly, Cr2−/− mice cannot be used to independently study the effects of chronic treatment introduced to animals after the development of the disease phenotypes. To address these problems, herein we report the generation of a new mouse anti-mouse CR2/CR1 mAb, designated mAb 4B2, and demonstrate its inhibitory characteristics and in vivo immunomodulatory activities.
2. Materials and Methods
2.1. Mice
Adult female DBA/1j mice were obtained from Jackson Laboratory (Bar Harbor, ME). Cr2−/− and CR2/CR1 sufficient C57BL/6 mice were bred at the University of Colorado Denver. All animal work was approved and performed under the guidelines established by the University of Colorado Institutional Animal Care and Use Committee.
2.2. Reagents and antibodies
Purified and biotin-conjugated mAb 4B2 and IgG1 isotype control (anti-Ova), as well as rat anti-mouse CR2/CR1 mAbs 7G6 and 7E9 were produced in the laboratory following standard methods. 2.4G2 (anti-mCD16/mCD32, FcBlock), biotin-conjugated 8C12 (rat anti-mouse CR1), alkaline phosphatase (AP-anti-mouse IgG2a), FITC-conjugated RA3-6B2 (anti-mouse CD45R, B220), FITC-conjugated anti-mouse CD24, PE-conjugated anti-mouse CD23, streptavidin (SA)-allophycocyanin, and SA-PE were all obtained from BD PharMingen (San Diego, CA). AP-conjugated goat anti-mouse IgG1, and goat anti-mouse IgM were obtained from Caltag Laboratories (Burlingame, CA). Goat-anti-mouse IgG and biotin-conjugated goat-anti-mouse-IgM F(ab')2 were purchased from SouthernBiotech (Birmingham, AL). Biotin-conjugated C3dg was produced in our laboratory as described (Henson et al., 2001).
2.3. Flow cytometry
Spleens were meshed by frosted glass slides, and splenocytes were isolated. Blood was collected by tail vain bleeding directly into tubes with ACK lysis buffer (0.15M NH4Cl, 1mM KHCO3, 0.1mM NaEDTA pH 7.2–7.4). After RBC lysis by ACK buffer, nucleated cells were washed and then resuspended in 10 μg/ml 2.4G2 mAb to block FcR. After a 15 min incubation on ice, cells were washed in staining buffer (2% BSA/0.02% NaN3/PBS) and suspended in 100 μl staining buffer containing biotinylated mAb (0.1–3 μg/ml) and flourochrome-labeled mAb to specific cell surface markers. Cells were incubated for 30 min on ice in the dark. After incubation, cells were washed in staining buffer two times and then incubated with the appropriate SA-conjugated fluorochrome to detect biotin-labeled mAb. Following incubation, cells were washed as above and then resuspended in PBS containing 1% formaldehyde. Flow cytometry was carried out using a BD Biosciences FACSCalibur (Oxford, U.K.).
2.4. Immunization and Ag-specific ELISA
Mice were immunized by the i.p. route with 200 μl containing 1x108 of SRBC in PBS (Colorado Serum, Denver, CO). Cells were washed three times with PBS and then diluted to the working concentration for immunization. A day before immunization with SRBC, mice were pre-injected with control anti-Ova IgG1 mAb, or mAb 4B2. Detection of Ab to SRBC was conducted by ELISA essentially as described by Heyman et al. (Heyman et al., 1984). To calculate relative units, the mean OD at 405 nm from triplicate wells was compared with a standard curve of OD measurements of titrated standard high titer serum.
2.5. Collagen-induced arthritis (CIA)
To induce CIA, we adapted with minor modifications a protocol described previously (Del Nagro et al., 2005). Briefly, DBA/1j mice (6–8 wk old) were immunized by intradermal injection at the base of the tail with 100 μg of bovine CII (50 μl; Chondrex) in 50 μl of CFA (Sigma-Aldrich). A booster injection of 200 μg of bovine CII in PBS was given i.p. on day 21. A day before the initial bovine CII immunization, mice were pre-injected with 2 mg of control IgG1, mAb 4B2, or PBS. A 3-point scale was used to assess arthritis for each paw: 0 = normal joint; 1 = slight inflammation and redness; 2 = severe erythema and swelling affecting the entire paw; and 3 = deformed paw or joint with ankylosis, joint rigidity, and loss of function. The maximum score of 12 for each animal was based on the total score for all four paws. Detection of anti-CII Abs in the sera of mice was performed by standard ELISA. Peripheral blood serum was isolated from mice by retro-orbital bleed. CII-specific Abs were captured from serum using either 10 μg/ml bovine CII or mouse CII-coated (Chondrex) 96-well plates (BD Biosciences) and were detected with alkaline phosphatase-conjugated rat anti-mouse IgG, IgM, IgG2a, IgG2b, IgG1, and IgG3 secondary Abs (Southern Biotechnology Associates) applied at a 1/2000 dilution. p-Nitrophenyl phosphate (Southern Biotechnology Associates) substrate was used for visualization at 405 nm using a Versamax microplate reader (Molecular Devices).
2.6. Western blot analysis
Isolated splenocytes were lysed on ice for 20 min in a buffer containing 0.5% Triton X-100, 0.5% Chaps, 20 mM Tris-HCl (pH 7.5), 1 mM EDTA, 10 μg/ml leupeptin and protease inhibitor cocktail (Roche Molecular Biochemicals). Lysates were cleared by centrifugation at 8000xg for 15 min. Following separation by 3–8% Tris-Acetate gel (Life Technologies) in SDS-running buffer, the proteins were transferred to a nitrocellulose membrane. The membrane was blocked overnight with 5% non-fat milk dissolved in PBS. The membrane was washed in PBS with 0.1% Tween (T-PBS) and then probed with biotinylated 7E9 (7E9-b) 1–2 hours in 2% milk/T-PBS, washed and then incubated with streptavidin conjugated horseradish-peroxidase (ST/HRP). A positive signal was visualized using the ECL system (Perkin Elmer).
2.7. Anti-CR2 ELISA
Immulon-2B plates (Dynatech) were coated over night at 4°C with 5 μg/ml of recombinant mouse CR2(SCR1–4) purified protein (Huang et al., 2008) in PBS. Wells were blocked with 1% BSA in PBS for 1 hour at RT. Serial dilutions of serum samples in PBS or hybridoma culture supernatants were applied to wells. After incubation and washing, bound Abs were detected using AP-conjugated anti-mouse IgG Abs, followed by p-nitrophenyl phosphate (NPP) (Sigma-Aldrich) at 1 mg/ml. Plates were read at 405 nm. For C3dg-biotin binding and competition analyses, recombinant C3dg-biotin was pre-incubated for 30 min in BSA/PBS with titrated amounts of SA-labeled HRP, and following this was added to the ELISA plate that had been pre-bound with CR2(SCR1–4). After 1 hour incubation plates were washed in PBS, and NPP was added.
3. Results
3.1. Generation and characterization of mouse anti-mouse CR2/CR1 monoclonal antibody
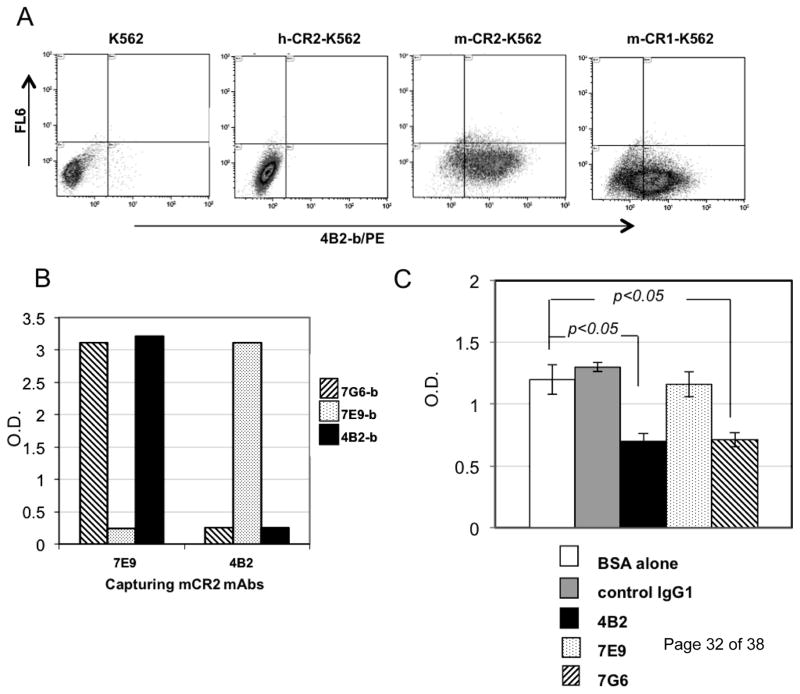
To generate new mAbs, we used as an immunogen recombinant mouse soluble CR2 protein consisting of the first 4 N-terminal SCRs (CR2 (SCR1–4)) that are encompassed by amino acid residues 1–257 of the mature protein. The recombinant protein was expressed in the Chinese hamster ovary (CHO) cell line and purified by affinity purification on a mAb 7G6-Sepharose column (Atkinson et al., 2005;Huang et al., 2008). To generate mAbs to what is a “foreign” protein, Cr2−/− mice were immunized with the antigen, and splenocytes were fused with the X63Ag8.653 myeloma cell line. The resulting hybridomas were screened by ELISA for binding to the mouse CR2 (SCR1–4) protein. Positive clones were assessed by flow cytomentry for the binding with full length mouse CR2 expressed on transfected human K562 cells. One such mAb designated 4B2 (IgG1, κ light chain) was cloned and isolated and found to bind specifically to mouse CR2 and CR1 proteins but not to human CR2 (Fig.1A).
Fig. 1. mAb 4B2 specifically binds to mouse CR2 and CR1 and blocks binding of C3d to CR2.
(A) K562 cells transfected with whole length human CR2 (h-CR2), mouse CR2 (m-CR2) and mouse CR1 (m-CR1) proteins were analyzed by flow cytometry for binding by mAb 4B2. (B) Cross competition analysis wherein mAb 4B2 was assessed by ELISA for its ability to block binding of the previously described inhibitory rat anti-mouse CR2/CR1 mAb 7G6 to CR2 (SCR1–4). For the study, mouse CR2 (SCR1–4) protein was captured by either the non-cross-inhibitory rat anti-mouse CR2/CR1 mAb7E9 or mAb 4B2, and was detected by using either biotinylated mAb 7G6-b (striped), mAb 7E9-b (dotted), or mAb 4B2-b (black). Capturing mouse CR2 with mAb 7E9 did not affect binding of either mAbs 7G6 or 4B2 to recombinant protein. However, when mouse CR2 was captured by mAb 4B2, only biotinylated mAb 7E9 was able to detect the recombinant protein, and the mAb 7G6 epitope was blocked by 4B2 binding. (C) ELISA plate was coated with mouse CR2(SCR1–4) protein and incubated with BSA alone (unfilled) or purified mAbs: control IgG1 (gray), 7E9 (dotted), 7G6 (striped), and 4B2 (black). Plates were washed and biotinylated human C3dg that had been cross-linked by streptavidin labeled with horseradish peroxidase (C3dg-b/ST/HRP) was added. mAb 4B2 blocked the interaction between mouse CR2 and C3dg similarly to mouse CR2 blocking rat mAb 7G6.. * p<0.05.
The mAb 4B2 was found to bind to the same or overlapping epitope of the published inhibitory rat anti-mouse mAb 7G6 antibody (Fig. 1B). This was demonstrated by the finding that when the non-inhibitory anti-CR2/CR1 mAb 7E9 was used as a capturing antibody both mAbs 4B2 and 7G6 were able to bind mouse CR2. In contrast, when mAb 4B2 was used to capture the mouse CR2 antigen, only mAb 7E9 and not mAb 7G6 was not able to bind to the protein.
Formal demonstration of inhibition of ligand binding was sought by assessing the effects of mAbs on binding to the mouse CR2(SCR1–4) protein by recombinant biotinylated human C3dg that had been cross-linked using streptavidin and labelled with horseradish peroxidase (C3dg-b/ST/HRP). In this experiment, ELISA wells were coated with mouse CR2(SCR1–4) protein and either pre-incubated with control IgG1, anti-mouse CR2/CR1 mAbs (7G6, 7E9 or 4B2), or 1% BSA in PBS. After the plates were washed, the C3dg-b/ST/HRP was added to determine if the applied mAbs would block binding to CR2. It was found in this C3dg competition assay that mAb 4B2 blocked binding of C3dg to mouse CR2(SCR1–4) in a similar manner as mAb 7G6 (Fig.1C), further supporting that the two mAbs recognize a functionally similar site.
3.2. In vivo injection of mAb 4B2 does not induce B cell death but leads to substantial down modulation of CR2 and CR1 levels
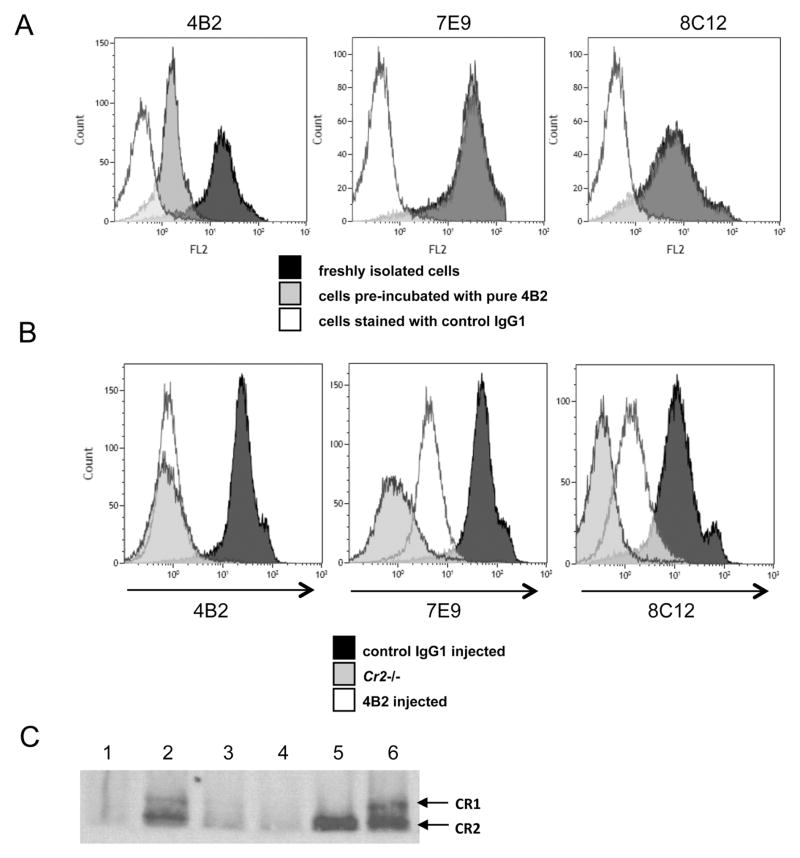
To determine the effects of mAb 4B2 on splenic B cell CR2 and CR1 receptor levels, we first studied freshly isolated splenocytes. When these cells were pre-incubated in vitro with purified mAb 4B2 either at 4°C or at 37°C, no changes in the levels of CR2 or CR1 expression on B cells was found as measured as by staining with mAb 7E9 (binding mouse CR2 and CR1) and 8C12 (specific for mouse CR1) (Fig. 2A). As anticipated, pre-incubation with mAb 4B2 did decrease staining with labelled mAb 4B2 (left). In contrast, when mAb 4B2 was injected in vivo, the intensity of staining by mAb 8C12 was substantially reduced (Fig. 2B). An even more dramatic peak shift was found for mAb 7E9 staining, suggesting the possibility that mouse CR2 protein was down regulated or shed from the B cell surface in a more pronounced manner than CR1 (Fig. 2B). The down modulation of CR2/CR1 by mAb 4B2 was long lasting, as a similar staining phenotype was obtained not only after 24 hours but also 7 days after mAb 4B2 injection (data not shown).
Fig. 2. In vivo injection of mAb 4B2 down modulates mouse CR2 and CR1 expression.
(A) Freshly isolated mouse B cells either untreated (black histogram) or pre-incubated with mAb 4B2 (gray histogram) were probed with labeled mAb 4B2 (left histogram), mAb 7E9 (middle) or mAb 8C12 (right). As a negative control, mice were stained with control IgG1 (unfilled histogram). Incubation of splenocytes in vitro with mAb 4B2 did not cause either CR2 or CR1 protein down regulation, as freshly isolated cells and cells pre-incubated with mAb 4B2 were overlapping for staining with both mAb 7E9 (middle) and mAb 8C12 (right). (B) Mice injected in vivo with 1 mg/mouse of control IgG1 (black histogram) or 4B2 (unfilled) were assayed 24 hours later for the levels of mouse CR2 and CR1 on B cells. B220+ cells were probed with labelled mAb 4B2 (left picture), mAb 7E9 (middle) and mAb 8C12 (right). As a negative staining control, splenocytes from Cr2−/− mice (gray histogram) were used. Anti-mouse CR1 mAb 8C12 showed 4–5 times reduction in intensity, pointing to substantial mouse CR1 down regulation or loss from the surface. mAb 7E9 staining was also substantially decreased, although the relative levels of binding to mouse CR2 and CR1 could not be precisely determined. (C) Protein lysates made from 5x106 splenocytes were separated on tris-acetate gel without using denatured reagents in sample buffer and SDS running buffer. Proteins were transferred to nitrocellulose membrane, and Western blot analysis was performed with biotinylated mAb 7E9 to detect both CR1 and CR2 proteins. Lanes are: (1) splenocytes from a Cr2−/− mouse, (2) WT mouse 24 hours after control IgG1 injected mouse, (3) or 24 hours after mAb 4B2 injection, (4) or after 7 days mAb 4B2 injection. (5) Mouse CR2 transfected K562 cell line, (6) and WT mouse 7 days after control IgG1 injection.
To confirm that mAb 4B2 did cause down modulation of CR2 and CR1 from the B cell surface and the flow cytometric results did not just reflect a conformational change altering mAb 8C12 and 7E9 binding, we prepared lysates from isolated splenocytes of mAb 4B2 treated and control mice and analyzed the level of CR2 and CR1 by Western blot analysis with mAb 7E9. Very little mouse CR2 and CR1 was detected in splenocyte lysates prepared from mice injected with mAb 4B2 either 24 hours or 7 days later, confirming that receptors greatly decrease after mAb 4B2 engagement (Fig.2C).
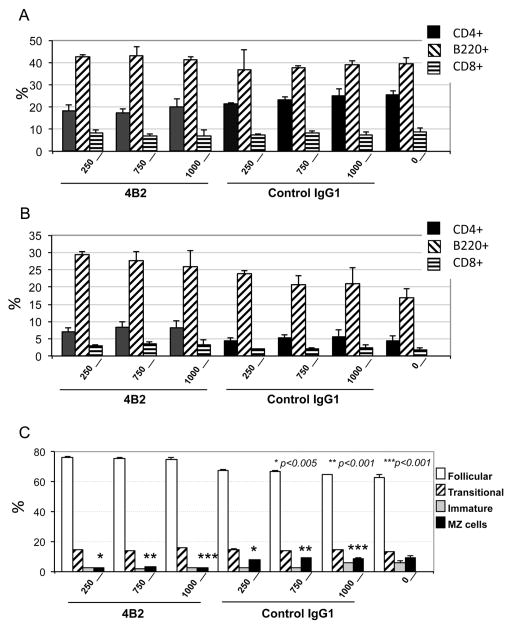
Despite the modulation of mouse CR2 and CR1 expression, treatment with mAb 4B2 did not result in apparent splenic or peripheral blood B cell death or elimination. Specifically, we were not able to detect a decrease in the B cell percentage 24 hours following injection of different amounts of mAb 4B2 (from 250 to 1000 μg/mouse) in naïve WT mice (Fig. 3A), or 7 days later (data not shown). In addition, in mice treated with mAb 4B2 followed by an antigen injection, type II bovine collagen (CII) in adjuvant (Fig. 3B), no changes in B cell percentages were observed in peripheral blood (data not shown). To determine whether mAb 4B2 injection induced changes in spleen cell subpopulations, we performed CD24/CD23 staining of B220+ cells to separate follicular (CD24low, CD23high), marginal (CD24 lowCD23-), transitional (CD24high,CD23high) and immature (CD24high, CD23-) cells. Based on this staining, we found that mAb 4B2 injection modestly altered the relative percentage of the marginal zone (MZ) B cell sub-population. This MZ B cell reduction was observed when either mice were injected with mAb alone or with mAb followed by antigen in adjuvant, and the reduction was detected both 24 hours and 7 days later (Fig. 3C, and data not shown).
Fig. 3. Treatment in vivo with mAb 4B2 does not induce B cell elimination.
(A) Mice were injected with increasing (250, 750 or 1000 μg per mouse) doses of mAb 4B2, control IgG1, or with PBS alone. After 24 hours splenocytes were studied by flow cytometry to quantitative the percentage of CD4+ cells (filled bar), B220+ cells (cross hatched), and CD8+ cells (horizontal stripes). (B) Mice were injected similarly as in (A) at day 0. One day later mice were immunized by intra-dermal injection with 100 μg of bovine CII. Seven days after immunization splenocytes were assayed for CD4, CD8 and B220+ cell percentages. As in (A), no B cell elimination was detected. (C) Injection with mAb 4B2 diminishes the relative marginal zone (MZ) B cell sub-population. Mice were injected as in (B) with mAb 4B2 or control IgG1, or with PBS, followed by CII intradermal injection. Seven days after immunization spleens were analyzed for splenic B cell subpopulations. B220+ cells were analyzed for follicular (unfilled bar), transitional (striped), immature (filled gray), and MZ cells (filled black). A significant 50–70% reduction in the MZ B cell subpopulation was observed in mice pre-injected with mAb 4B2.
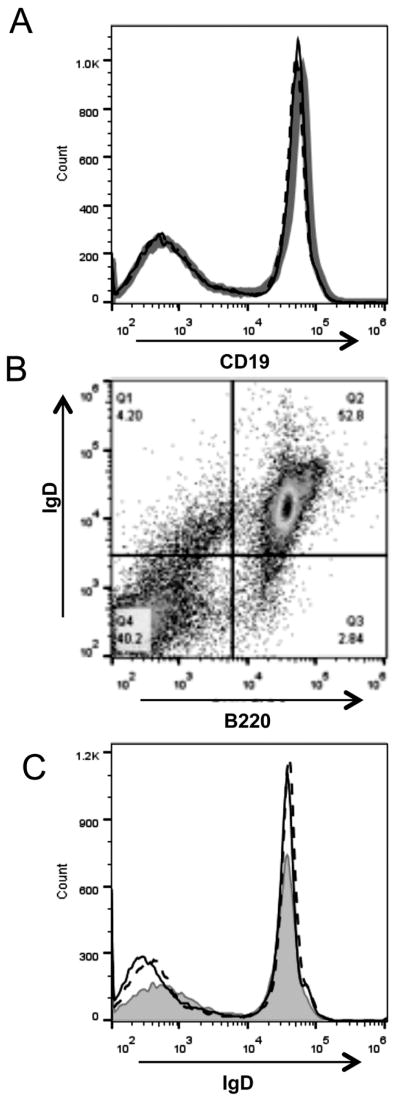
Notably, the down regulation of mouse CR2 and CR1 from the B cell surface after in vivo mAb 4B2 injection did not affect mouse CD19 expression on B cells. Specifically, the level of expression of CD19 on splenic B cells was indistinguishable in mice injected with mAb 4B2 as compared to control IgG1 (Fig. 4A). The same was true for the B cell receptor protein IgD. All B220+ cells were IgD+ in mice injected with mAb 4B2 (Fig.4B), and the level of IgD expression on B cells was not altered (Fig. 4C). Similarly, mAb 4B2 injection did not affect CD19 and IgD expression on peripheral blood B cells (data not shown).
Fig. 4. Injection of mAb 4B2 has no effect on expression of CD19 or IgD levels.
(A) Overlaying histograms represent staining for CD19 on B cells from mice 24 hours following injection with IgG1 control (gray line) or mAb 4B2 (black line). Dotted line represents staining of peripheral blood cells for CD19 1 week after mAb 4B2 injection. (B) Representative staining for IgD and B220 on B cells from mice injected with mAb 4B2. (C) Overlaying histograms represent staining for IgD on B220+ B cells from mice 24 hours following injection with IgG1 control mAb (gray line) or mAb 4B2 (black line). Dotted line represents staining for IgD 1 week after mAb 4B2 injection.
3.3. Prolonged modulation of mouse CR2 and CR1 after treatment with mAb 4B2
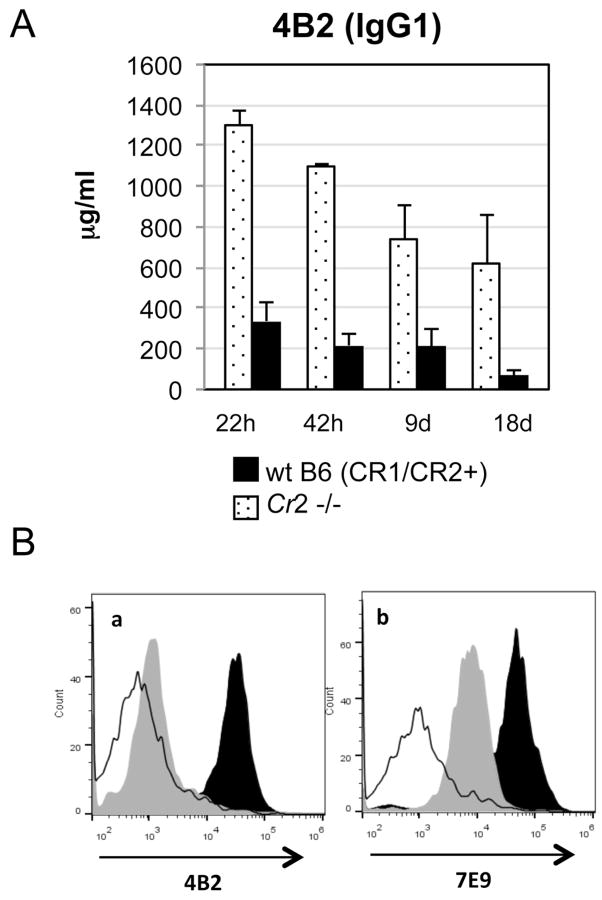
To measure the circulatory half-life of mAb 4B2 and the role of endogenous receptors in its clearance, WT C57BL/6 and Cr2−/− mice were treated with 2 mg/mouse of mAb 4B2, and serial blood samples were acquired after 22 hours, 42 hours, 9 days and 18 days. Serum samples were assayed for mAb 4B2 levels by ELISA using mouse CR2(SCR1- 4) as the substrate. In CR2/CR1 sufficient mice, the level of mAb 4B2 drops substantially as observed 22 hours after injection. However, the levels remain substantial in these mice even at day 18 after injection. This drop in concentration of 4B2 in blood was CR2/CR1 dependent, as Cr2−/− mice lacking receptors demonstrated only gradually decreasing levels of circulating mAb 4B2 to 50 % of the original level after 18 days (Fig.5A).
Fig. 5. Durable effect of in vivo injection with mAb 4B2.
(A) WT (black) and Cr2−/− (dotted) C57BL/6 mice were injected with 2 mg of 4B2 antibody, and peripheral blood samples were acquired 22 hours, 42 hours, 9 days and 18 days later. The concentration of injected 4B2 mAb in processed mouse serum samples was measured by ELISA using mouse CR2(SCR1–4) protein as the substrate. The reduction in mAb 4B2 levels at 22 hours was CR2/CR1 dependent, as Cr2−/− mice did not demonstrate this same drop in concentration. (B) Comparison of CR2 and CR1 down modulation by mAb 4B2 in DBA/1j mice injected with control IgG1 (black histogram) or mAb 4B2 (gray histogram) demonstrates at least a six week duration of receptor inhibition. Blood B220+ cells were analyzed by flow cytometry with labeled mAb 4B2 for the presence of the mAb 4B2 epitope (a) on CR2/CR1 that is exposed and free of previously injected mAb 4B2, as compared to non-cross-reactive labeled mAb 7E9 (b) for the level of total CR2/CR1 on the B cell surface. As a negative control for CR2/CR1 staining, Cr2−/− mice were studied (unfilled histogram).
To determine the durability of inhibition of mouse CR2 and CR1, and thus the frequency by which mAb 4B2 would need to be utilized in an autoimmune disease model, DBA/1j mice were injected with mAb 4B2, and peripheral blood B cells were assayed each week for labelled mAb 4B2 and mAb 7E9 staining. In this study, even at week 6 after injection with 2 mg of mAb 4B2 (Fig 5B), the labeled mAb 4B2 still was not able to bind to its epitope on CR2 or CR1 (a). Staining with the non-cross-reactive mAb also showed a continued reduction in CR2/CR1 levels (b).
3.4. Treatment with mAb 4B2 blocks the humoral immune response to a T-dependent antigen
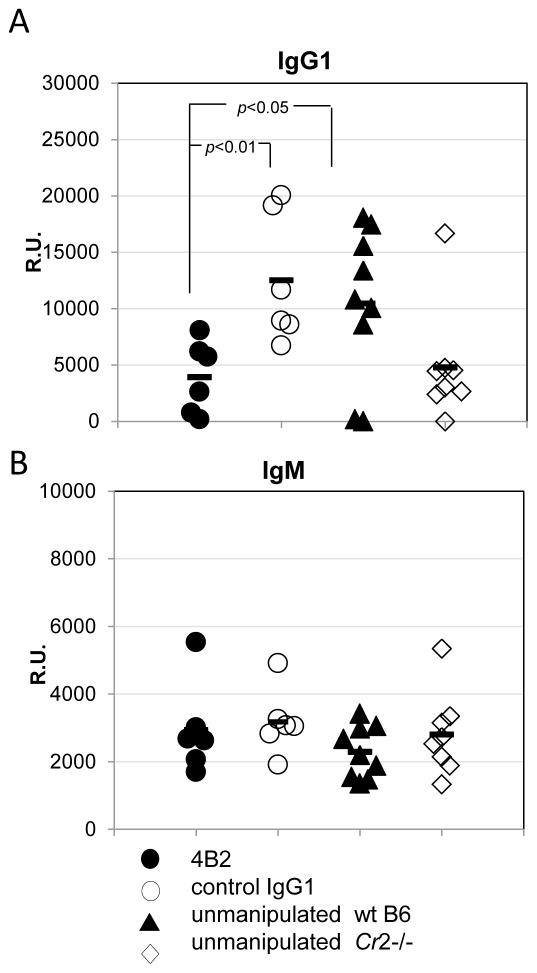
As a model T-dependent antigen that is known to be dependent upon CR2/CR1 function (Rutemark et al., 2012;Fang et al., 1998;Gustavsson et al., 1995;Carlsson et al., 2009;Molina et al., 1996), sheep red blood cells (SRBC) were chosen. After pre-injection of mice with either control IgG1 or mAb 4B2 (1.5 mg/mouse), 1x108 cells/mouse of SRBC were injected. Two and half weeks later mice were bled and sera were assayed for anti-SRBC antibody. Mice pre-injected with mAb 4B2 demonstrated a markedly reduced level of anti-SRBC IgG1 that was comparable to the level of anti-SRBC IgG1 which developed following immunization of Cr2−/− mice (Fig.6A). At the same time the mAb 4B2 treatment demonstrated no effect on anti-SRBC IgM levels, which was similar to the results in Cr2−/− mice (Fig. 6B).
Fig. 6. Injection of 4B2 mAb reduces the humoral immune response to the T dependent antigen SRBC.
WT C57BL/6 mice were pre-injected i.p. with 2 mg/mouse of 4B2 mAb (filled circle), isotype control IgG1 (unfilled circle) or left un-manipulated (filled triangle). Mice were immunized the following day with 1x108 of SRBC injected i.p.. 20 days after immunization the levels of anti-SRBC IgG1 (A) and IgM (B) were analyzed by ELISA with SRBC substrate. As a control Cr2−/− mice (unfilled diamond) were immunized with the same amount of SRBC.
In addition to evaluating the effects of mAb 4B2 on the humoral immune response to foreign antigens, we also utilized ELISA to determine whether the injection of 2 mg of mAb 4B2 was generating an immunological response to 4B2 and thus inducing an anti-idiotypic antibody. We were not able to detect any anti-4B2 Ig in circulating blood (data not shown).
3.5. Pre-injection of mAb 4B2 reduced the severity of arthritis and autoantibody levels in the mouse CIA model
To determine if 4B2 mAb can effect autoimmune disease development, a CR2/CR1-dependent CIA model was chosen (Del Nagro et al., 2005;Kuhn et al., 2008). In this model mice were treated with a single injection of 2 mg/mouse of mAb 4B2 or isotype IgG1control antibody a day before bovine CII immunization. A booster injection with bovine CII was performed on day 21. A single injection was chosen based on half-life determination shown above.
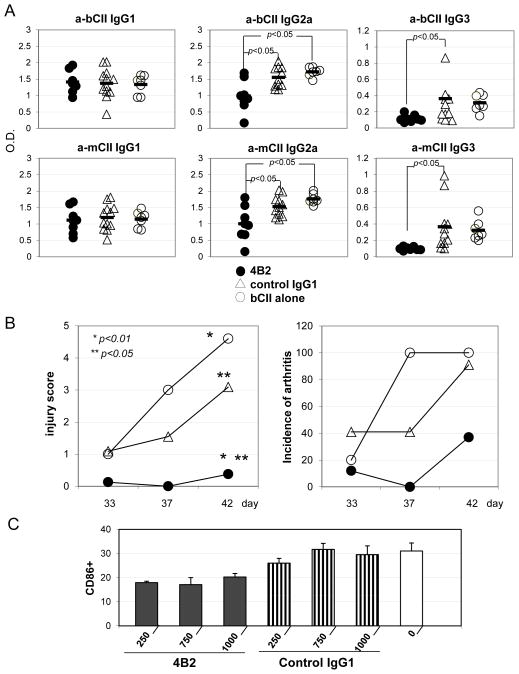
The analyses of blood for anti-bovine (Fig. 7A, upper row) or anti-mouse (Fig. 7A, lower row) CII isotype-specific antibodies showed that mice developed similar levels of IgG1 in each group of mice (Fig. 7A, left two graphs). However, the levels of pathogenic IgG2a antibodies, including autoantibodies to mouse CII, decreased in mice treated with mAb 4B2 (Fig. 7A, middle graphs). Similarly, mice injected with mAb 4B2 demonstrated substantial reductions in IgG3 anti-CII levels (Fig.7A, right graphs).
Fig. 7. Pre-injection of mAb 4B2 prevented arthritis development in the mouse CIA model.
(A) Induction of CIA was performed as described in Material and Methods. Before bovine CII injection, DBA/1j mice received and i.p. injection of 2 mg/mouse of mAb 4B2 (filled circle), control IgG1 (unfilled triangle) or PBS (unfilled circle). Mice were bled at day 40, and serum samples were assayed for the presence of anti-bovine CII (upper row) and anti-mouse CII (lower row) IgG1 (left two graphs), IgG2a (middle two graphs) or IgG3 (right two graphs). (B) Starting from day 33 after the first immunization with bovine CII mice were monitored for signs of arthritis, and an injury score based on a 3 level scale was assigned to each mouse as described in Material and Methods (left). The percentage of mice that developed arthritis is shown in the right graph. (C) Percentage of CD86+ cells in splenocytes of B220+ population was determined by flow cytomentry of mice undergoing CIA 7 days after the first bovine CII injection. 1 day before CII injection the mice were pre-injected with different doses (250, 750, 1000ug/mouse) of mAb 4B2 (black) or control IgG1 (striped vertically) or just PBS (unfilled).
In parallel, mice were monitored for arthritis development (Fig. 7B). Mice that were pre-injected with mAb 4B2 demonstrated a markedly reduced severity of arthritis (Fig.7B, left), as well as a reduced incidence and prevalence of arthritis (Fig. 7B, right). Immunized mice were also bled and peripheral blood B cells were analyzed for activation markers (Fig. 7C). It was found that B cells from mice pre-injected with mAb 4B2 demonstrated a reduction in CD86 expression. CD86 expression is up-regulated after B cell activation and together with CD80 is a key molecule in T cell priming and activation (Lanier et al., 1995;Hathcock et al., 1994).
We also performed a CIA experiment where IgG1 control mAb and mAb 4B2 were injected twice, once before first immunization with bovine CII and at day 21 before the booster bovine CII immunization. Similarly as in the case of a single mAb 4B2 pre-injection shown above, reduction in severity of arthritis and reduction in anti-CII IgG2a antibody levels were observed in mice pre-injected with mAb 4B2 mAb as compared to the two other groups (PBS or control IgG1) (data not shown).
4. Discussion
In the current report we describe a new inhibitory mouse anti-mouse CR2/CR1-reactive mAb, designated 4B2, generated from B cells obtained from a CR2 protein-immunized Cr2−/− mouse. The mAb is species specific and does not bind human CR2. The antibody is directed to either the same or a closely overlapping epitope as the previously described inhibitory rat-anti- mouse CR2/CR1 mAb 7G6 (Kinoshita et al., 1988;Kinoshita et al., 1990). However, because mAb 4B2 is a mouse antibody, it is capable of circulating in the mouse for longer period of time without generating an anti-rat Ab response, or without an apparent anti-idiotype Ab response.
The binding of mAb 4B2 to an epitope on a shared epitope on mouse CR2 and CR1 blocks C3dg ligand binding in vitro and modulates humoral immune responses in vivo. The mechanism of inhibition is likely to be multi-factorial, though. While binding of the mAb to CR2/CR1 on freshly-isolated splenocytes does not induce changes in receptor expression, when the mAb is injected in vivo it leads to substantial decreases in the expression level of the receptors. That is apparent not only from flow cytometric studies but was also visualized by Western blot analysis, confirming that the decrease in mAb 7E9 binding is not simply due to steric inhibition. Also, mAb 4B2 binding to mouse CR1 also affected the expression of the receptor, as assessed by flow cytometry with the CR1-specific mAb 8C12.
While the precise mechanism of the process is not known that leads to decreased B cell receptor expression, based on published data it is likely disappearance of the receptor cell surface is due to partial internalization induced by mAb 4B2 (Hess et al., 2000;Tessier et al., 2007) and/or shedding (Fremeaux-Bacchi et al., 1996;Hoefer et al., 2008;Masilamani et al., 2003) that also accompanies CR2 engagement by C3d ligand, or B Cell Receptor (BCR) activation.
Importantly, in vivo blockade and decreased expression is long lasting, as labelled mAb 4B2 was not able to bind its epitope as long as six weeks after injection, which was associated with decreased mAb 7E9 staining. However, we are not able to answer the question yet what is the exact mechanism of these effects, and this issue requires further study. Importantly, treatment with mAb 4B2 does not appreciably decrease the total number of B cells, as we did not detect B cell death or a B cell reduction in either naïve mice or with injection of a foreign antigen. Also, no change in the levels of CD19 or IgD was found. However, we did detect a relative reduction in MZ B cells after mAb 4B2 treatment. This effect was related to mAb 4B2 alone, as we observed it in mice injected with only the mAb in the absence of foreign antigen. This effect is consistent with prior reports (Whipple et al., 2004) where injection of mAb 7G6 induced MZ B cell down regulation that was directly related to anti-CR2/CR1 mAb injection, as the deficiency in mouse CR2/CR1 proteins does not result in MZ B cell changes (Pozdnyakova et al., 2003;Donius et al., 2013).
Further support for the validity of the use of mAb 4B2 is that the level of IgG antibody responses to the T-dependent foreign SRBC antigen was reduced to a similar level as in Cr2−/− mice. The level of anti-SRBC IgM was not decreased, as it was observed previously in immunized by SRBC Cr2−/− mice (Molina et al., 1996;Carlsson et al., 2009). Finally, we performed an experiment wherein we confirmed that treatment with mAb 4B2 ameliorated development of the foreign anti-bovine CII antibody response as well as the anti-mouse CII autoantibody response and development of clinical arthritis. These results are similar to those in Cr2−/− mice, where a reduced severity and incidence of arthritis was shown (Del Nagro et al., 2005;Kuhn et al., 2008). We also further explored the mechanism of the reduction in CIA development in mice treated with mAb 4B2 by showing that B cells from mice demonstrated a reduction in CD86 expression, a T cell stimulatory molecule, and an effect which would likely prevent T cells from being properly activated by an antigen.
In sum, we present herein a new mouse anti-mouse CR2/CR1 C3d ligand blocking mAb as a new tool to study the effects of chronic complement receptor inhibition in murine models of human diseases. Based on the resent data that the receptors may be expressed on other cells, for example on neuronal cells lineage and involved in neurogenesis and brain recovery after trauma (Moriyama et al., 2011;Neher et al., 2014), mAb 4B2 antibody can also serve as a tool to study these processes in vivo in chronic models.
Highlights.
We generated a new monoclonal mouse anti-mouse complement receptor type 2/complement receptor type 1 (CR2/CR1) antibody, designated mAb 4B2.
mAb 4B2 antibody blocks binding of the C3dg ligand to CR2.
In vivo injected mAb 4B2 induces partial down regulation of CR1 and a more substantial elimination of CR2 from B cells without inducing B cell death or elimination.
In vivo injection of mAb 4B2 antibody decreases humoral immune responses to T dependent antigens.
mAb 4B2 can be used at various time points in murine chronic models of human autoimmune and inflammatory diseases to assess the role of receptor-ligand interactions in the further evolution of disease.
Acknowledgments
The work was supported by an Alliance for Lupus Research Investigator Award and NIH R21 AI105717.
Abbreviations
- Ab
Antibody
- mAb
monoclonal antibody
- CR
complement receptor
- i.p
intra-peritoneal injection
- wt
wildtype
- SRBC
sheep red blood cells
- SA
streptavidin
- IC
immune complexes
- mCR1
mouse complement receptor type 1
- mCR2
mouse complement receptor type 2
- SCR
short consensus repeat
- CIA
collagen induced arthritis
Footnotes
Disclosure
All of the authors declare that they have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ahearn JM, Fearon DT. Structure and function of the complement receptors, CR1 (CD35) and CR2 (CD21) Adv Immunol. 1989;46:183–219. doi: 10.1016/s0065-2776(08)60654-9. [DOI] [PubMed] [Google Scholar]
- 2.Atkinson C, Song H, Lu B, Qiao F, Burns TA, Holers VM, Tsokos GC, Tomlinson S. Targeted complement inhibition by C3d recognition ameliorates tissue injury without apparent increase in susceptibility to infection. J Clin Invest. 2005;115:2444–2453. doi: 10.1172/JCI25208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baiu DC, Prechl J, Tchorbanov A, Molina HD, Erdei A, Sulica A, Capel PJ, Hazenbos WL. Modulation of the humoral immune response by antibody-mediated antigen targeting to complement receptors and Fc receptors. J Immunol. 1999;162:3125–3130. [PubMed] [Google Scholar]
- 4.Boackle SA, Holers VM, Chen X, Szakonyi G, Karp DR, Wakeland EK, Morel L. Cr2, a candidate gene in the murine Sle1c lupus susceptibility locus, encodes a dysfunctional protein. Immunity. 2001;15:775–785. doi: 10.1016/s1074-7613(01)00228-x. [DOI] [PubMed] [Google Scholar]
- 5.Bradbury LE, Goldmacher VS, Tedder TF. The CD19 signal transduction complex of B lymphocytes. J Immunol. 1993;151:2915–2927. [PubMed] [Google Scholar]
- 6.Buhl AM, Pleiman CM, Rickert RC, Cambier JC. Qualitative regulation of B cell antigen receptor signaling by CD19: Selective requirement for P13-kinase activation, inositol-1,4,5-trisphosphate production and Ca2+ mobilization. J Exp Med. 1998;186:1897–1910. doi: 10.1084/jem.186.11.1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carlsson F, Getahun A, Rutemark C, Heyman B. Impaired antibody responses but normal proliferation of specific CD4+ T cells in mice lacking complement receptors 1 and 2. Scand J Immunol. 2009;70:77–84. doi: 10.1111/j.1365-3083.2009.02274.x. [DOI] [PubMed] [Google Scholar]
- 8.Carroll MC. The role of complement and complement receptors in induction and regulation of immunity. Annu Rev Immunol. 1998;16:545–568. doi: 10.1146/annurev.immunol.16.1.545. [DOI] [PubMed] [Google Scholar]
- 9.Carroll MC. The role of complement in B cell activation and tolerance. Adv Immunol. 2000;74:61–88. doi: 10.1016/s0065-2776(08)60908-6. [DOI] [PubMed] [Google Scholar]
- 10.Carter RH, Fearon DT. CD19: lowering the threshold for antigen receptor stimulation of B lymphocytes. Science. 1992;256:105–107. doi: 10.1126/science.1373518. [DOI] [PubMed] [Google Scholar]
- 11.Chen ZM, Koralev SB, Gendelman M, Carroll MC, Kelsoe G. Humoral immune responses in Cr2−/− mice: enhanced affinity maturation but impaired antibody persistence. J Immunol. 2000;164:4522–4532. doi: 10.4049/jimmunol.164.9.4522. [DOI] [PubMed] [Google Scholar]
- 12.Del Nagro CJ, Kolla RV, Rickert RC. A critical role for complement C3d and the B cell coreceptor (CD19/CD21) complex in the initiation of inflammatory arthritis. J Immunol. 2005;175:5379–5389. doi: 10.4049/jimmunol.175.8.5379. [DOI] [PubMed] [Google Scholar]
- 13.Dempsey PW, Allison ME, Akkaraju S, Goodnow CC, Fearon DT. C3d of complement as a molecular adjuvant: Bridging innate and acquired immunity. Science. 1996;271:348–350. doi: 10.1126/science.271.5247.348. [DOI] [PubMed] [Google Scholar]
- 14.Donius LR, Handy JM, Weis JJ, Weis JH. Optimal germinal center B cell activation and T-dependent antibody responses require expression of the mouse complement receptor Cr1. J Immunol. 2013;191:434–447. doi: 10.4049/jimmunol.1203176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fang Y, Xu C, Holers VM, Molina H. Expression of complement receptors 1 and 2 on follicular dendritic cells is necessary for the generation of a normal antigen-specific IgG response. J Immunol. 1998;160:5273–5279. [PubMed] [Google Scholar]
- 16.Fearon DT, Carroll MC. Regulation of B lymphocyte responses to foreign and self-antigens by the CD19/CD21 complex. Annu Rev Immunol. 2000;18:393–422. doi: 10.1146/annurev.immunol.18.1.393. [DOI] [PubMed] [Google Scholar]
- 17.Fingeroth JD. Comparative structure and evolution of murine CR2. The homolog of the human C3d/EBV receptor (CD21) J Immunol. 1990;144:3458–3467. [PubMed] [Google Scholar]
- 18.Fingeroth JD, Benedict MA, Levy DN, Strominger JL. Identification of murine complement receptor type 2. Proc Natl Acad Sci USA. 1989;86:242–246. doi: 10.1073/pnas.86.1.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fischer E, Delibrias C, Kazatchkine MD. Expression of CR2 (the C3dg/EBV receptor, CD21) on normal human peripheral blood T lymphocytes. J Immunol. 1991;146:865–869. [PubMed] [Google Scholar]
- 20.Fleming SD, Shea-Donohue T, Guthridge JM, Kulik L, Waldschmidt TJ, Gipson MG, Tsokos GC, Holers VM. Mice deficient in complement receptors 1 and 2 lack a tissue injury-inducing subset of the natural antibody repertoire. J Immunol. 2002;169:2126–2133. doi: 10.4049/jimmunol.169.4.2126. [DOI] [PubMed] [Google Scholar]
- 21.Fremeaux-Bacchi V, Bernard I, Maillet F, Mani JC, Fontaine M, Bonnefoy JY, Kazatchkine MD, Fischer E. Human lymphocytes shed a soluble form of CD21 (the C3dg/Epstein-Barr virus receptor, CR2) that binds iC3b and CD23. Eur J Immunol. 1996;26:1497–1503. doi: 10.1002/eji.1830260714. [DOI] [PubMed] [Google Scholar]
- 22.Gustavsson s, Kinoshita T, Heyman B. Antibodies to murine complement receptor 1 and 2 can inhibit the antibody response in vivo without inhibiting T helper cell induction. J Immunol. 1995;154:6524–6528. [PubMed] [Google Scholar]
- 23.Hathcock KS, Laszlo G, Pucillo C, Linsley P, Hodes RJ. Comparative analysis of B7–1 and B7–2 costimulatory ligands: expression and function. J Exp Med. 1994;180:631–640. doi: 10.1084/jem.180.2.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Henson SE, Smith D, Boackle SA, Holers VM, Karp DR. Generation of recombinant human C3dg tetramers for the analysis of CD21 binding and function. J Immunol Meth. 2001;258:97–109. doi: 10.1016/s0022-1759(01)00471-9. [DOI] [PubMed] [Google Scholar]
- 25.Hess MW, Schwendinger MG, Eskelinen EL, Pfaller K, Pavelka M, Dierich MP, Prodinger WM. Tracing uptake of C3dg-conjugated antigen into B cells via complement receptor type 2 (CR2, CD21) Blood. 2000;95:2617–2623. [PubMed] [Google Scholar]
- 26.Heyman B, Holmquist G, Borwell P, Heyman U. An enzyme linked immunosorbent assay for measuring anti-sheep erythrocyte antibodies. J Immunol Methods. 1984;68:193–204. doi: 10.1016/0022-1759(84)90150-9. [DOI] [PubMed] [Google Scholar]
- 27.Heyman B, Wiersma EJ, Kinoshita T. In vivo inhibition of the antibody response by a complement receptor-specific monoclonal antibody. J Exp Med. 1990;172:665–668. doi: 10.1084/jem.172.2.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hoefer MM, Aichem A, Knight AM, Illges H. Modulation of murine complement receptor type 2 (CR2/CD21) ectodomain shedding by its cytoplasmic domain. Mol Immunol. 2008;45:2127–2137. doi: 10.1016/j.molimm.2007.12.015. [DOI] [PubMed] [Google Scholar]
- 29.Holers VM. Complement receptors and the shaping of the natural antibody repertoire. Springer Sem Immunopath. 2005;26:405–423. doi: 10.1007/s00281-004-0186-y. [DOI] [PubMed] [Google Scholar]
- 30.Huang Y, Qiao F, Atkinson C, Holers VM, Tomlinson S. A novel targeted inhibitor of the alternative pathway of complement and its therapeutic application in ischemia/reperfusion injury. J Immunol. 2008;181:8068–8076. doi: 10.4049/jimmunol.181.11.8068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Iida K, Nadler L, Nussenzweig V. Identification of the membrane receptor for the complement fragment C3d by means of a monoclonal antibody. J Exp Med. 1983;158:1021–1033. doi: 10.1084/jem.158.4.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jacobson AC, Weis JJ, Weis JH. Complement receptors 1 and 2 influence the immune environment in a B cell receptor-independent manner. J Immunol. 2008;180:5057–5066. doi: 10.4049/jimmunol.180.7.5057. [DOI] [PubMed] [Google Scholar]
- 33.Kalli KR, Ahearn JM, Fearon DT. Interaction of iC3b with recombinant isotypic and chimeric forms of CR2. J Immunol. 1991;147:590–594. [PubMed] [Google Scholar]
- 34.Kaye J, Bagley J, Malkowski D, Iacomini J. The role of complement receptors in production of antibodies specific for Galalpha1,3Gal. Transplantation. 2004;77:314–316. doi: 10.1097/01.TP.0000101008.13282.FD. [DOI] [PubMed] [Google Scholar]
- 35.Kinoshita T, Fujita T, Tsunoda R. Expression of complement receptors CR1 and CR2 on murine follicular dendritic cells and B lymphocytes. In: Imai Y, Tew JG, Hoefsmit ECM, editors. Dendritic cells in lymphoid tissues. Elsevier Science Publishers; 1991. pp. 271–276. [Google Scholar]
- 36.Kinoshita T, Lavoie S, Nussenzweig V. Regulatory proteins for the activated third and fourth components of complement (C3b and C4b) in mice. II Identification and properties of complement receptor type 1 (CR1) J Immunol. 1985;134:2564–2570. [PubMed] [Google Scholar]
- 37.Kinoshita T, Takeda J, Hong K, Kozono H, Sakai H, Inoue K. Monoclonal antibodies to mouse complement receptor type 1 (CR1). Their use in a distribution study showing that mouse erythrocytes and platelets are CR1-negative. J Immunol. 1988;140:3066–3072. [PubMed] [Google Scholar]
- 38.Kinoshita T, Thyphronitis G, Tsokos GC, Finkelman FD, Hong K, Sakai H, Inoue K. Characterization of murine complement receptor type 2 and its immunological cross-reactivity with type 1 receptor. Intl Immunol. 1990;2:651–659. doi: 10.1093/intimm/2.7.651. [DOI] [PubMed] [Google Scholar]
- 39.Kuhn KA, Cozine CL, Tomooka B, Robinson WH, Holers VM. Complement receptor CR2/CR1 deficiency protects mice from collagen-induced arthritis and associates with reduced autoantibodies to type II collagen and citrullinated antigens. Mol Immunol. 2008;45:2808–2819. doi: 10.1016/j.molimm.2008.01.036. [DOI] [PubMed] [Google Scholar]
- 40.Kulik L, Chen K, Huber BT, Holers VM. Human complement receptor type 2 (CR2/CD21) transgenic mice provide an in vivo model to study immunoregulatory effects of receptor antagonists. Mol Immunol. 2011;48:883–894. doi: 10.1016/j.molimm.2010.12.019. [DOI] [PubMed] [Google Scholar]
- 41.Kurtz CB, O'Toole E, Christensen SM, Weis JH. The murine complement receptor gene family. IV Alternative splicing of Cr2 gene transcripts predicts two distinct gene products that share homologous domains with both human CR2 and CR1. J Immunol. 1990;144:3581–3591. [PubMed] [Google Scholar]
- 42.Lanier LL, O'Fallon S, Somoza C, Phillips JH, Linsley PS, Okumura K, Ito D, Azuma M. CD80 (B7) and CD86 (B70) provide similar costimulatory signals for T cell proliferation, cytokine production, and generation of CTL. J Immunol. 1995;154:97–105. [PubMed] [Google Scholar]
- 43.Luxembourg AT, Cooper NR. Modulation of signaling via the B cell antigen receptor by CD21, the receptor for C3dg and EBV. J Immunol. 1994;153:4448–4457. [PubMed] [Google Scholar]
- 44.Lyubchenko T, dal Porto J, Cambier JC, Holers VM. Co-ligation of the B cell receptor with complement receptor type 2 (CR2/CD21) using its natural ligand C3dg: activation without engagement of an inhibitory signaling pathway. J Immunol. 2005;174:3264–3272. doi: 10.4049/jimmunol.174.6.3264. [DOI] [PubMed] [Google Scholar]
- 45.Masilamani M, Kassahn D, Mikkat S, Glocker MO, Illges H. B cell activation leads to shedding of complement receptor type II (CR2/CD21) Eur J Immunol. 2003;33:2391–2397. doi: 10.1002/eji.200323843. [DOI] [PubMed] [Google Scholar]
- 46.Molina H, Holers VM, Li B, Fang YF, Mariathasan S, Goellner F, Strauss-Schoenberger J, Karr RW, Chaplin DD. Markedly impaired humoral immune response in mice deficient in complement receptors 1 and 2. Proc Natl Acad Sci USA. 1996;93:3357–3361. doi: 10.1073/pnas.93.8.3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Molina H, Kinoshita T, Inoue K, Carel JC, Holers VM. A molecular and immunochemical characterization of mouse CR2: Evidence for a single gene model of mouse Complement Receptors 1 and 2. J Immunol. 1990;145:2974–2983. [PubMed] [Google Scholar]
- 48.Molnar E, Erdei A, Prechl J. Novel roles for murine complement receptors type 1 and 2 I. Regulation of B cell survival and proliferation by CR1/2. Immunol Lett. 2008a;116:156–162. doi: 10.1016/j.imlet.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 49.Molnar E, Prechl J, Erdei A. Novel roles for murine complement receptors type 1 and 2 II. Expression and function of CR1/2 on murine mesenteric lymph node T cells. Immunol Lett. 2008b;116:163–167. doi: 10.1016/j.imlet.2007.12.010. [DOI] [PubMed] [Google Scholar]
- 50.Moriyama M, Fukuhara T, Britschgi M, He Y, Narasimhan R, Villeda S, Molina H, Huber BT, Holers M, Wyss-Coray T. Complement receptor 2 is expressed in neural progenitor cells and regulates adult hippocampal neurogenesis. J Neurosci. 2011;31:3981–3989. doi: 10.1523/JNEUROSCI.3617-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Neher MD, Rich MC, Keene CN, Weckbach S, Bolden AL, Losacco JT, Patane J, Flierl MA, Kulik L, Holers VM, Stahel PF. Deficiency of complement receptors CR2/CR1 in Cr2(−)/(−) mice reduces the extent of secondary brain damage after closed head injury. J Neuroinflammation. 2014;11:95. doi: 10.1186/1742-2094-11-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Phan TG, Grigorova I, Okada T, Cyster JG. Subcapsular encounter and complement-dependent transport of immune complexes by lymph node B cells. Nat Immunol. 2007;8:992–1000. doi: 10.1038/ni1494. [DOI] [PubMed] [Google Scholar]
- 53.Pozdnyakova O, Guttormsen HK, Lalani FN, Carroll MC, Kasper DL. Impaired antibody response to group B streptococcal type III capsular polysaccharide in C3- and complement receptor 2-deficient mice. J Immunol. 2003;170:84–90. doi: 10.4049/jimmunol.170.1.84. [DOI] [PubMed] [Google Scholar]
- 54.Prechl J, Molnar E, Szekeres Z, Isaak A, Papp K, Balogh P, Erdei A. Murine CR1/2 targeted antigenized single-chain antibody fragments induce transient low affinity antibodies and negatively influence an ongoing immune response. Adv Exp Med Biol. 2007;598:214–225. doi: 10.1007/978-0-387-71767-8_15. [DOI] [PubMed] [Google Scholar]
- 55.Prechl J, Tchorbanov A, Horvath A, Baiu DC, Hazenbos W, Rajnavolgyi E, Kurucz I, Capel PJ, Erdei A. Targeting of influenza epitopes to murine CR1/CR2 using single-chain antibodies. Immunopharmacology. 1999;42:159–165. doi: 10.1016/s0162-3109(99)00025-9. [DOI] [PubMed] [Google Scholar]
- 56.Prodeus A, Goerg S, Shen L, Pozdnyakova OO, Alicot EM, Goodnow CC, Carroll MC. A critical role of complement in regulation of self-reactive B cells. Mol Immunol. 1998a;35:338. doi: 10.1016/s1074-7613(00)80669-x. (abst) [DOI] [PubMed] [Google Scholar]
- 57.Prodeus A, Goerg S, Shen LM, Pozdnyakova OO, Chu L, Alicot EM, Goodnow CC, Carroll MC. A critical role for complement in maintenance of self-tolerance. Immunity. 1998b;9:721–731. doi: 10.1016/s1074-7613(00)80669-x. [DOI] [PubMed] [Google Scholar]
- 58.Reid RR, Woodcock S, Prodeus AP, Austen J, Kobzik L, Hechtman HB, Moore FD, Jr, Carroll MC. The role of complement receptors CD21/CD35 in positive selection of B-1 cells. Curr Top Microbiol Immunol. 2000;252:57–65. doi: 10.1007/978-3-642-57284-5_7. [DOI] [PubMed] [Google Scholar]
- 59.Reid RR, Woodstock S, Shimabukuro-Vornhagen A, Austen WG, Jr, Kobzik L, Zhang M, Hechtman HB, Moore FD, Jr, Carroll MC. Functional activity of natural antibody is altered in Cr2-deficient mice. J Immunol. 2002;169:5433–5440. doi: 10.4049/jimmunol.169.10.5433. [DOI] [PubMed] [Google Scholar]
- 60.Ross GD, Medof ME. Membrane complement receptors specific for bound fragments of C3. Adv Immunol. 1985;37:217–267. doi: 10.1016/s0065-2776(08)60341-7. [DOI] [PubMed] [Google Scholar]
- 61.Rutemark C, Bergman A, Getahun A, Hallgren J, Henningsson F, Heyman B. Complement receptors 1 and 2 in murine antibody responses to IgM-complexed and uncomplexed sheep erythrocytes. PLoS One. 2012;7:e41968. doi: 10.1371/journal.pone.0041968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Szomolanyi-Tsuda E, Seedhom MO, Carroll MC, Garcea RL. T cell-independent and T cell-dependent immunoglobulin G responses to polyomavirus infection are impaired in complement receptor 2-deficient mice. Virology. 2006;352:52–60. doi: 10.1016/j.virol.2006.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tedder TF, Clement LT, Cooper MD. Expression of C3d receptors during human B cell differentiation: immunofluorescence analysis with the HB-5 monoclonal antibody. J Immunol. 1984;133:678–683. [PubMed] [Google Scholar]
- 64.Tedder TF, Zhou LJ, Engel P. The CD19/CD21 signal transduction complex of B lymphocytes. Immunol Today. 1994;15:437–442. doi: 10.1016/0167-5699(94)90274-7. [DOI] [PubMed] [Google Scholar]
- 65.Tessier J, Cuvillier A, Glaudet F, Khamlichi AA. Internalization and molecular interactions of human CD21 receptor. Mol Immunol. 2007;44:2415–2425. doi: 10.1016/j.molimm.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 66.Weis JJ, Tedder TF, Fearon DT. Identification of a 145,000 Mr membrane protein as the C3d receptor (CR2) of human B lymphocytes. Proc Natl Acad Sci USA. 1984;81:881–885. doi: 10.1073/pnas.81.3.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Whipple EC, Ditto AH, Shanahan RS, Gatesman JJ, Little SF, Taylor RP, Lindorfer MA. Low doses of antigen coupled to anti-CR2 mAbs induce rapid and enduring IgG immune responses in mice and in cynomolgus monkeys. Mol Immunol. 2007;44:377–388. doi: 10.1016/j.molimm.2006.02.032. [DOI] [PubMed] [Google Scholar]
- 68.Whipple EC, Shanahan RS, Ditto AH, Taylor RP, Lindorfer MA. Analyses of the in vivo trafficking of stoichiometric doses of an anti-complement receptor 1/2 monoclonal antibody infused intravenously in mice. J Immunol. 2004;173:2297–2306. doi: 10.4049/jimmunol.173.4.2297. [DOI] [PubMed] [Google Scholar]
- 69.Wu X, Jiang N, Fang YF, Xu C, Mao D, Singh J, Fu YX, Molina H. Impaired affinity maturation in Cr2−/− mice is rescued by adjuvants without improvement in germinal center development. J Immunol. 2000;165:3119–3127. doi: 10.4049/jimmunol.165.6.3119. [DOI] [PubMed] [Google Scholar]