Abstract
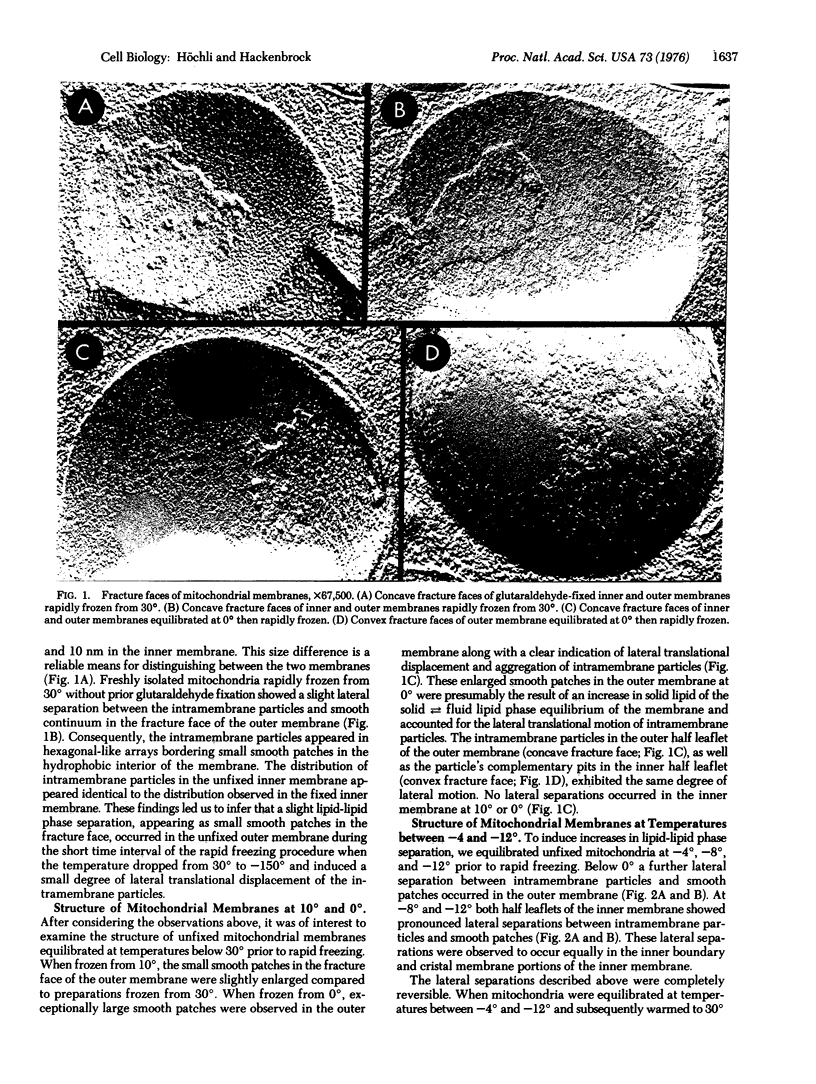
The fracture faces of frozen rat liver mitochondria reveal that intramembrane particles can be induced to under go long-range lateral translational motion and aggregation, which parallel the appearance of large, particle-free smooth patches in the hydrophobic interior of the two mitochondrial membranes. These lateral separations were observed under conditions that induce thermotropic lipid-lipid phase separations. Low temperature-induced lateral separation occurred between the intramembrane particles (integral proteins) and smooth patches (bilayer lipid) at temperatures between about 10 and -12 degrees in the outer membrane and between about -4 and -12 degrees in the inner, energy transducing membrane.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blazyk J. F., Steim J. M. Phase transitions in mammalian membranes. Biochim Biophys Acta. 1972 Jun 20;266(3):737–741. doi: 10.1016/0006-3002(72)90019-4. [DOI] [PubMed] [Google Scholar]
- Capaldi R. A., Green D. E. Membrane proteins and membrane structure. FEBS Lett. 1972 Sep 15;25(2):205–209. doi: 10.1016/0014-5793(72)80486-1. [DOI] [PubMed] [Google Scholar]
- Capaldi R. A. Identification of the major enzymic activities of the mitochondrial inner membrane in terms of their migration in sodium dodecyl sulfate polyacrylamide gel electrophoresis. Arch Biochem Biophys. 1974 Jul;163(1):99–105. doi: 10.1016/0003-9861(74)90459-7. [DOI] [PubMed] [Google Scholar]
- Chen Y. S., Hubbell W. L. Temperature- and light-dependent structural changes in rhodopsin-lipid membranes. Exp Eye Res. 1973 Dec 24;17(6):517–532. doi: 10.1016/0014-4835(73)90082-1. [DOI] [PubMed] [Google Scholar]
- Colbeau A., Nachbaur J., Vignais P. M. Enzymic characterization and lipid composition of rat liver subcellular membranes. Biochim Biophys Acta. 1971 Dec 3;249(2):462–492. doi: 10.1016/0005-2736(71)90123-4. [DOI] [PubMed] [Google Scholar]
- Edidin M. Rotational and translational diffusion in membranes. Annu Rev Biophys Bioeng. 1974;3(0):179–201. doi: 10.1146/annurev.bb.03.060174.001143. [DOI] [PubMed] [Google Scholar]
- Fleischer S., Fleischer B., Stoeckenius W. Fine structure of lipid-depleted mitochondria. J Cell Biol. 1967 Jan;32(1):193–208. doi: 10.1083/jcb.32.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulik-Krzywicki T., Rivas E., Luzzati V. Structure et polymorphisme des lipides: étude par diffraction des rayons X du systéme formé de lipides de mitochondries de coeur de boeuf et d'eau. J Mol Biol. 1967 Jul 28;27(2):303–322. doi: 10.1016/0022-2836(67)90022-8. [DOI] [PubMed] [Google Scholar]
- Hackenbrock C. R. States of activity and structure in mitochondrial membranes. Ann N Y Acad Sci. 1972 Jun 20;195:492–505. [PubMed] [Google Scholar]
- Hackenbrock C. R. Ultrastructural bases for metabolically linked mechanical activity in mitochondria. I. Reversible ultrastructural changes with change in metabolic steady state in isolated liver mitochondria. J Cell Biol. 1966 Aug;30(2):269–297. doi: 10.1083/jcb.30.2.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackenbrock C. R. Ultrastructural bases for metabolically linked mechanical activity in mitochondria. II. Electron transport-linked ultrastructural transformations in mitochondria. J Cell Biol. 1968 May;37(2):345–369. doi: 10.1083/jcb.37.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmon H. J., Hall J. D., Crane F. L. Structure of mitochondrial cristae membranes. Biochim Biophys Acta. 1974 Sep 16;344(2):119–155. doi: 10.1016/0304-4157(74)90002-1. [DOI] [PubMed] [Google Scholar]
- Jost P. C., Capadil R. A., Vanderkooi G., Griffith O. H. Lipid-protein and lipid-lipid interactions in cytochrome oxidase model membranes. J Supramol Struct. 1973;1(4):269–280. doi: 10.1002/jss.400010404. [DOI] [PubMed] [Google Scholar]
- Jost P. C., Griffith O. H., Capaldi R. A., Vanderkooi G. Evidence for boundary lipid in membranes. Proc Natl Acad Sci U S A. 1973 Feb;70(2):480–484. doi: 10.1073/pnas.70.2.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIELLEY W. W., BRONK J. R. Oxidative phosphorylation in mitochondrial fragments obtained by sonic vibration. J Biol Chem. 1958 Jan;230(1):521–533. [PubMed] [Google Scholar]
- Kleemann W., Grant C. W., McConnell H. M. Lipid phase separations and protein distribution in membranes. J Supramol Struct. 1974;2(5-6):609–616. doi: 10.1002/jss.400020508. [DOI] [PubMed] [Google Scholar]
- Kleemann W., McConnell H. M. Lateral phase separations in Escherichia coli membranes. Biochim Biophys Acta. 1974 Apr 29;345(2):220–230. doi: 10.1016/0005-2736(74)90260-0. [DOI] [PubMed] [Google Scholar]
- Ladbrooke B. D., Williams R. M., Chapman D. Studies on lecithin-cholesterol-water interactions by differential scanning calorimetry and X-ray diffraction. Biochim Biophys Acta. 1968 Apr 29;150(3):333–340. doi: 10.1016/0005-2736(68)90132-6. [DOI] [PubMed] [Google Scholar]
- Singer S. J., Nicolson G. L. The fluid mosaic model of the structure of cell membranes. Science. 1972 Feb 18;175(4023):720–731. doi: 10.1126/science.175.4023.720. [DOI] [PubMed] [Google Scholar]
- Sjöstrand F. S., Baraas L. A new model for mitochondrial membranes based on structural and on biochemical information. J Ultrastruct Res. 1970 Aug;32(3):293–306. doi: 10.1016/s0022-5320(70)80010-7. [DOI] [PubMed] [Google Scholar]
- Speth V., Wunderlich F. Membranes of Tetrahymena. II. Direct visualization of reversible transitions in biomembrane structure induced by temperature. Biochim Biophys Acta. 1973 Feb 16;291(3):621–628. doi: 10.1016/0005-2736(73)90467-7. [DOI] [PubMed] [Google Scholar]
- Verkleij A. J., Ververgaert P. H., van Deenen L. L., Elbers P. F. Phase transitions of phospholipid bilayers and membranes of Acholeplasma laidlawii B visualized by freeze fracturing electron microscopy. Biochim Biophys Acta. 1972 Nov 2;288(2):326–332. doi: 10.1016/0005-2736(72)90253-2. [DOI] [PubMed] [Google Scholar]
- Wunderlich F., Wallach D. F., Speth V., Fischer H. Differential effects of temperature on the nuclear and plasma membranes of lymphoid cells. A study by freeze-etch electron microscopy. Biochim Biophys Acta. 1974 Nov 27;373(1):34–43. doi: 10.1016/0005-2736(74)90102-3. [DOI] [PubMed] [Google Scholar]





